Abstract
Aims/Hypothesis
Bile acid sequestrants (BAS) reduce plasma glucose levels in type II diabetics and in murine models of diabetes but the mechanism herein is unknown. We hypothesized that sequestrant-induced changes in hepatic glucose metabolism would underlie reduced plasma glucose levels. Therefore, in vivo glucose metabolism was assessed in db/db mice on and off BAS using tracer methodology.
Methods
Lean and diabetic db/db mice were treated with 2% (wt/wt in diet) Colesevelam HCl (BAS) for 2 weeks. Parameters of in vivo glucose metabolism were assessed by infusing [U-13C]-glucose, [2-13C]-glycerol, [1-2H]-galactose and paracetamol for 6 hours, followed by mass isotopologue distribution analysis, and related to metabolic parameters as well as gene expression patterns.
Results
Compared to lean mice, db/db mice displayed an almost 3-fold lower metabolic clearance rate of glucose (p = 0.0001), a ∼300% increased glucokinase flux (p = 0.001) and a ∼200% increased total hepatic glucose production rate (p = 0.0002). BAS treatment increased glucose metabolic clearance rate by ∼37% but had no effects on glucokinase flux nor total hepatic or endogenous glucose production. Strikingly, BAS-treated db/db mice displayed reduced long-chain acylcarnitine content in skeletal muscle (p = 0.0317) but not in liver (p = 0.189). Unexpectedly, BAS treatment increased hepatic FGF21 mRNA expression 2-fold in lean mice (p = 0.030) and 3-fold in db/db mice (p = 0.002).
Conclusions/Interpretation
BAS induced plasma glucose lowering in db/db mice by increasing metabolic clearance rate of glucose in peripheral tissues, which coincided with decreased skeletal muscle long-chain acylcarnitine content.
Introduction
Type 2 diabetes is a major health problem worldwide [1]. The predominant features of type 2 diabetes entail increased fasting blood glucose levels, increased plasma triglycerides and LDL-cholesterol levels, as well as disturbed peripheral glucose utilization [2]–[4]. The use of bile acid sequestrants (BAS) for lowering of LDL-cholesterol levels is well established [5]–[7]. More recently, Colesevelam HCl, a BAS, has been indicated by the FDA to improve glycemic control in patients with type 2 diabetes [8]–[10]. So far, however, the actual changes in hepatic and/or peripheral glucose metabolism upon BAS supplementation are not understood.
Bile acids are synthesized from cholesterol in the liver. Upon secretion into bile, bile acids function to emulsify fats in the small intestine. Most of the secreted biliary bile acids are reabsorbed in the ileum (enterohepatic circulation). Sequestrants interfere with the enterohepatic circulation of bile acids by binding them in the intestine, thereby inhibiting their reabsorption and promoting their fecal loss. As a consequence, the liver increases bile acid synthesis and subsequently cholesterol uptake from the circulation thereby reducing LDL-cholesterol levels [11], [12].
To date, there is a lack of understanding how BAS reduce plasma glucose levels. However, a large body of research suggests that bile acids modulate hepatic glucose metabolism via signaling pathways mediated by the nuclear receptor NRH1H4 (Fxr) in diabetes [13]–[19]. Fxr is expressed in the liver, intestine, adrenal gland and kidney [20], and it acts to inhibit de novo bile acid synthesis when activated by bile acids in the liver [21]. Paradoxically, both agents that inhibit de novo bile acid synthesis, such as bile acids themselves and synthetic Fxr ligands [14], [22], as well as agents that increase de novo bile acid synthesis such as BAS, were shown to reduce plasma glucose levels in diabetic mice [23], [24]. Thus, regulation of bile acid-mediated changes in blood glucose levels remains elusive.
We therefore questioned whether BAS-induced changes in hepatic carbohydrate fluxes are responsible for the observed reduction in plasma glucose levels in db/db mice. To test this, we treated healthy lean mice and obese, diabetic db/db mice with Colesevelam HCl. Applying an in vivo infusion protocol of stable isotopes followed by mass isotopologue distribution analysis (MIDA), we first characterized specific disruptions of whole body glucose turnover and hepatic glucose metabolism in db/db mice. We then tested the hypothesis that BAS restores disrupted hepatic glucose fluxes thereby mediating the previously observed reduction in blood glucose levels in diabetic mice. In view of the strong interaction between glucose and fatty acid metabolism, we additionally tested the effect of BAS on levels of lipids and intermediates of fatty acid metabolism in liver and muscle.
Materials and Methods
Animals and diets
Ten week old male lean C57BL/6J and obese, diabetic db/db mice on a C57BL6/J background (B6.Cg-m +/+ Leprdb/J) were purchased from Charles River Laboratories (L'Arbresle, France and Brussels, Belgium, respectively). Mice were housed in a temperature controlled (21°C) room with a dark-light cycle of 12 h each. For all animal experiments the principles of laboratory animal care (NIH publication no. 85–23, revised 1985) were followed. The Ethics Committee for Animal Experiments of the University Groningen, the Netherlands, approved experimental procedures (Experiment ID: DEC5030 University of Groningen).
One week after arrival at the animal facility, 8 db/db (db) and 6 lean (L) mice were put on a diet containing standard laboratory chow (RMH-B; Arie Blok, Woerden, The Netherlands) supplemented with 2% (wt/wt) Colesevelam HCl (Daiichi Sankyo, Inc., Parsippany, NJ, USA) for 2 weeks. Another 8 db/db and 6 lean mice remained on standard laboratory chow. Body weights and food intake were recorded every other day. Mice were fitted with a permanent catheter in the right atrium via the jugular vein, as described previously [25]. Mice were allowed to recover from surgery for 6 days.
Materials The following isotopes were used: 2-13-C glycerol (99% 13C atom percent excess), [1-2H]-galactose (98% 2H atom percent excess) (Isotec, Miamisburg, Ohio, USA), [U-13C]-glucose (99% 13C atom percent) (Cambridge Isotope Laboratories, Andover, Mass., USA). All reagents and chemicals used were reagent pro analysis grade. Blood spots and urine were collected on Schleicher and Schuell No. 2992 filter paper (Schleicher and Schuells, ‘s Hertogenbosch, The Netherlands). Infusates were freshly prepared and sterilized at the day before the experiment.
Animal Experiments
The infusion experiment was performed in conscious mice, as described previously [25]. Mice were fasted for 4 hours (03:00–07:00 am) and then housed in metabolic cages to allow frequent collection of urine and blood spots on filter paper. Mice were infused with a sterile solution containing [U-13 C]-glucose (13.9 µmol/ml), [2-13C]-glycerol (160 µmol/ml), [1-2H]-galactose (33 µmol/ml) and paracetamol (1.0 mg/ml) at a rate of 0.6 ml/h. Before and during the experiment, small blood samples were obtained via tail bleeding to allow for the determination of plasma glucose. Blood was immediately centrifuged and stored at −20°C until analysis. Blood spots were collected on filter paper before the start of the infusion and hourly afterwards until 6 h after the start of the infusion. Blood spots were air dried and stored at room temperature until analysis. Hourly urine samples were collected on filter paper, air dried and stored at room temperature until analysis. At the end of the experiment, animals were anesthetized with isoflurane and a small blood sample was collected via orbital puncture for the determination of insulin.
Mice were allowed to recover and five days after the infusion experiment. Mice were then fasted for 7 hours (03:00–10:00 h) and terminated by heart puncture under isoflurane anesthesia. A large blood sample was collected in heparin-containing tubes, immediately centrifuged and stored at −20°C until analysis. Liver was excised, weighed, snap frozen and stored at −80°C until further analysis. Gastrocnemius and plantaris muscle as well as epididymal white adipose tissue were collected, frozen in liquid N2 and stored at −80°C until further analysis.
Measurement and Analysis of Mass Isotopologue Distribution Analysis by GC-MS Analytical procedures for extraction of glucose in bloodspots and paracetamol-glucuronide from urine filter paper strips and derivatization of the extracted compounds and GC-MS measurements of derivatives were all performed according to Van Dijk et al [26], [27]. The measured fractional distribution was corrected for natural abundance of 13C by multiple linear regression as described by Lee et al. [28]to obtain the excess mole fraction of mass isotopologues due to incorporation and dilution of infused labeled compounds, i.e., [2-13C]-glycerol, [U-13C]-glucose and [1-2H]-galactose. This distribution was used in mass isotopologues distribution analysis (MIDA) algorithms of isotope incorporation and dilution according to Hellerstein et al. [29] as described by Van Dijk et al [26], [27]. Rate of glucose disposal, metabolic clearance rate, hepatic glucose production rates as well as the hepatic glucose fluxes are part of MIDA.
Determination of metabolite concentrations
Commercially available kits were used to determine plasma levels of insulin (Mercodia, Uppsala, Sweden), triglycerides, total cholesterol, free cholesterol and NEFA (Wako Chemicals, Neuss, Germany).
Plasma HOMA-index was calculated multiplying the blood glucose levels by the plasma insulin levels at 6 h of MIDA infusion and dividing the product by 22.5 [30]. Hepatic glycogen and glucose-6-phosphate content were determined as previously described [25]. Hepatic lipids were determined in liver homogenates by commercially available kits for triglycerides and total cholesterol (Wako Chemicals, Neuss, Germany) after lipid extraction as described by Bligh and Dyer [31]. Plasma acylcarnitines were determined according to the method of Chase et al [32] as described by Derks et al. [33]. Profiles of long-chain acylcarnitines (C16∶0, C16∶1, C18∶0, C18∶1 and C18∶2) in muscle and liver homogenates (15% (w/v) in PBS) were determined according to the method of Gates [33].
mRNA levels
Total RNA was isolated from liver using TRI-reagent (Sigma, St. Louis, MO) according to the manufacturers' protocol. cDNA was produced as described by Plösch and coworkers [34]. Real-time PCR was performed on a 7900HT FAST real-time PCR system using FAST PCR master mix and MicroAmp FAST optical 96 well reaction plates (Applied Biosystems Europe, Nieuwerkerk ad IJssel, The Netherlands). Primer and probe sequences have been published before (www.labpediatrics.nl) and deposited in the RTprimerDB. PCR results were normalized to 18S-rRNA abundance.
Statistics
All values are represented as mean ± standard deviation. Statistical significance was assessed using the Mann-Whitney-U-test (SPSS 12.0.1 for Windows). P-values were corrected for multiple comparison errors. Statistical significance was accepted for a p<0.05.
Results
Bile acid sequestration reduced plasma glucose values in db/db mice
Previously, it has been observed that Colesevelam HCl treatment lowers plasma glucose concentrations in db/db mice compared to untreated counterparts [24]. To confirm and elaborate these findings, basal parameters related to glucose metabolism were determined in lean and db/db mice treated with the bile acid sequestrant (BAS) for two weeks. As expected, BAS-treatment significantly lowered blood glucose levels of diabetic mice (Table 1). No effects of BAS on body weight, liver weight or liver weight/body weight ratio were observed (Table 1). BAS treatment had no effect on 4 h fasted plasma insulin levels in db/db mice, while a 30% (non-significant) reduction of plasma insulin levels occurred at the end of the MIDA infusion experiment (Table 1). The HOMA-index was significantly improved in sequestrant-treated diabetic mice compared to untreated counterparts. Moreover, we observed a non-significant decrease in plasma NEFA levels, a more than 50%, however non-significant, reduction, in plasma 3-hydroxybutyrate levels with no apparent effect on plasma lactate concentrations (Table 1). Untreated lean and db/db mice displayed similar plasma triglyceride levels, which decreased in both models upon BAS-treatment. Plasma cholesterol levels were slightly, but significantly, elevated in untreated db/db mice compared to untreated lean mice: we hypothesize this to be due to decreased activity of FXR which leads to an increase in hepatic ApoA1 expression as shown in a previous study [35]. Apparently, this effect was stronger in db/db mice than in lean mice because no significant effect was observed in the latter. We hypothesize this to be due to decreased activity of FXR which leads to an increase in ApoA1 confirming data in a previous study. Apparently, this effect was stronger in db/db mice than WT because no significant effect was observed in WT mice. Consistent with our previous observations [24], liver triglyceride contents were significantly increased in sequestrant-treated lean and db/db mice compared to controls, (Table 1). Diabetic mice had significantly higher hepatic glycogen contents compared to lean mice while, surprisingly, hepatic glucose-6-phosphate contents was decreased in the db/db genotype, BAS treatment had no effect herein (Table 1). Additionally, hepatic levels of free fatty acids were increased ∼10-fold in db/db mice with no effect of BAS (Table 1).
Table 1. The effects of bile acid sequestration (BAS) on morphological, plasma and hepatic parameters in lean and diabetic mice.
| L | L BAS | db | db BAS | |
| Body weight (g) | 23.9±1.1 | 24.9±0.9 | 37.9±3.2† | 39.7±3.5† |
| Liver weight (g) | 1.14±0.08 | 1.19±0.09 | 1.74±0.24† | 1.93±0.27† |
| Liver Weight/Body weight (%) | 4.7±0.2 | 4.7±0.2 | 4.6±0.6 | 4.9±0.7 |
| Plasma Parameters | 21.3±0.9 | 20.9±0.8 | 21.9±3.1 | 20.9±3.1 |
| 4 hour fasted blood glucose (mmol/L) | 8.3±0.6 | 8.8±0.9 | 27.7±4.4† | 19.3±4.8* † |
| Blood glucose at 6 hour of MIDA infusion (mmol/L) | 9.2±0.5 | 9.7±1.0 | 32.7±3.9 # | 23.1±3.9* † |
| 4 hour fasted plasma insulin (mU/L) | 5.7±1.5 | 7.2±1.4 | 44.1±26.3† | 49.8±19.3† |
| Plasma insulin at 6 hour of MIDA infusion (mUl/L) | 6.7±1.8 | 7.5±1.6 | 66.6±23.3† | 48.0±18.4† |
| HOMA Index | 1.0±0.3 | 1.2±0.3 | 34.8±10.1† | 16.9±6.7* † |
| NEFA | 300±109 | 393±92 | 697±149† | 555±149 |
| Lacate (mmol/L) | 8.4±1.1 | 8.3±1.3 | 6.8±1.5 | 8.6±1.8 |
| 3-Hydroxybutyrate (mM) | 0.28±0.16 | 0.28±0.16 | 1.5±0.95* | 0.70±0.27 |
| Triglycerides (mmol/L) | 0.74±0.16 | 0.52±0.11* | 0.99±0.14 | 0.67±0.14† |
| Total cholesterol (mmol/L) | 2.3±0.3 | 2.3±0.2 | 2.8±0.1† | 3.2±0.4* † |
| Liver Parameters | ||||
| Triglycerides (µmol/L) | 16±6 | 31±14* | 44±11† | 59±21* † |
| Glycogen (µmol/g liver) | 62±45 | 47±30 | 175±21† | 216±37† |
| Glucose-6-Phosphate (nmol/mg liver) | 0.69±0.18 | 0.74±0.18 | 0.40±0.16† | 0.42±0.10† |
| Free fatty acids (nmol/g liver) | 366±225 | 597±315 | 3079±1503† | 4406±2013† |
7 h fasted parameters (unless otherwise stated) in lean mice (L), lean mice supplemented with BAS (L BAS), db/db (db) and db/db mice supplemented with BAS (db BAS). Data are shown as means ± SD;
*p<0.05 vs. same genotype,
p<0.05 vs. lean same condition.
Bile acid sequestration increased metabolic clearance of glucose without affecting hepatic glucose production
BAS promotes specific changes in hepatic cholesterol and bile acid synthesis [24]. To gain insight in hepatic glucose metabolism upon BAS-treatment, in vivo glucose metabolism was studied in lean and db/db mice after 2 weeks of BAS treatment. First, whole body and hepatic glucose metabolism in db/db mice was characterized.
The peripheral tissue disposal of plasma glucose (also regarded as the amount of glucose taken up by tissues) was significantly higher in db/db mice compared to lean mice (93.1.±13.1 vs. 117.0±14.6 µmol.kg−1.min−1, lean vs. db/db, Table 2), but the metabolic clearance rate (volume of blood cleared from glucose) was significantly lower in db/db mice (9.9±1.3 vs. 3.6±0.7 ml.kg−1.min−1, lean vs. db/db, Table 2), because the plasma glucose concentrations (amount of glucose per volume) were almost 3 times higher in db/db compared to lean mice (9.2±0.5, 32.7±3.9 mmol/l, lean vs. db/db, Table 1).
Table 2. Effects of BAS on in vivo hepatic glucose metabolism.
| L | LBAS | db | db BAS | |
| Rate of plasma glucose: | ||||
| Peripheral tissue disposal (µmol/kg/min) | 93±13 | 107±9.2 | 117±13† | 119±8† |
| Metabolic clearance rate (ml/kg/min) | 9.9±1.3 | 10.7±0.6 | 3.6±0.7† | 5.3±0.3† * |
| Contributions to endogenous glucose production rate (µmol/kg/min) | ||||
| De novo glucose-6-phosphate synthesis to glucose | 60±7 | 64±8 | 66±6 | 56±8* |
| Glycogen to glucose | 27±6 | 37±5* | 49±8† | 61±6† * |
| Endogenous glucose production rate | 87±13 | 101±9 | 115±14† | 117±6† |
| Contributions to hepatic glucose production rate (µmol/kg/min) | ||||
| Endogenous glucose production rate | 87±13 | 101±9 | 115±14† | 117±6† |
| Glucose cycling rate | 31±4 | 38±3 | 129±53† | 156±53† |
| Total hepatic glucose production rate | 118±17 | 139±17 | 244±54† | 273±58† |
| Flux rates (µmol/kg/min) | ||||
| Glucokinase | 47±5 | 56±7 | 150±58† | 184±54† |
| Glucose-6-Phosphate de novo synthesis | 86±6 | 87±9 | 74±11† | 61±7† * |
I n vivo parameters of hepatic glucose metabolism during the last 3 hours of the infusion experiment in lean mice (L), lean mice supplemented with BAS (L BAS), db/db mice (db) and db/db mice supplemented with BAS (db BAS) are depicted in the table, the graphic Graphic S1 illustrates the individual glucose fluxes for a better understanding of the table in a schematic manner. The second panel of the table entitled “Contributions to endogenous glucose production rate” shows the contribution of de novo glucose-6-phosphate synthesis to glucose (displayed as fluxes e+b in Graphic S1) and of glycogen to glucose (displayed as fluxes c+b Graphic S1) which altogether make up the endogenous glucose production rate. The third panel entitled “Contributions to hepatic glucose production rate” takes into account the glucose cycling rate and thus shows the contributions of the endogenous glucose production rate and the glucose cycling rate (which consists of the cycling of glucose: 1. from glucose to glucose-6-phosphate (depicted as a in the graphic, the glucokinase flux) and back (depicted as b in Graphic S1, the glucose-6-phosphatase flux); 2. Glucose-6-phosphate to glycogen and back, fluxes c+d in Graphic S1; and 3. Gluconeogenesis, flux e in the graphic (glycolysis is also a part of this but cannot be measured in the present set up) to the total hepatic glucose production rate which equals the flux rate through glucose-6-phosphatase. The lower panel shows the flux rates through glucokinase (a in the graphic) and the rate of glucose-6-phosphate de novo synthesis (c+e in Graphic S1). Each value represents the mean ± SD;
*p<0.05 vs. same genotype untreated;
p<0.05 vs. L same condition.
A strongly increased hepatic glucose cycling brought about by a massively increased glucokinase flux was observed in db/db compared to lean mice (46.7±4.5 vs. 150.2±58.3 µmol.kg−1.min−1, lean vs. db/db, Table 2). In db/db mice de novo synthesis of glucose-6-phosphate was significantly decreased when compared to lean mice (86.0±6.0 µmol.kg−1.min−1 vs. 73.7±10.9 µmol.kg−1.min−1, lean vs. db/db, p<0.05, Table 2). Endogenous glucose production (excluding glucose cycling) was slightly, but significantly, higher (87.3±13.0 vs. 115.0±14.0 µmol.kg−1.min−1, lean vs. db/db, p<0.05, Table 2) whereas total hepatic glucose output (including glucose cycling) was massively higher in db/db compared to lean mice (118.0±16.6 µmol/kg−1.min−1 vs. 243.5±54. µmol/kg−1.min−1 lean and db/db mice, respectively (Table 2).
BAS treatment had differential effects on whole body glucose metabolism in lean and db/db mice. Irrespective of the decreased plasma glucose concentration, BAS treatment had no effect on the flux through glucokinase in livers of db/db mice. Since the glucokinase flux remained significantly high, glucose cycling remained significantly higher in treated db/db mice when compared to treated lean mice. In treated db/db mice the rate of endogenous glucose production did not change and the rate of total hepatic glucose output tended to increase, albeit non-significantly (Table 2). Although BAS was not effective to reduce rates of hepatic glucose consumption or production, we did observe a significant decrease of de novo glucose-6-phosphate synthesis in treated db/db mice (87.4±9.4 µmol/kg−1.min−1 vs. 61.2±7.4 µmol/kg−1.min−1, lean vs. db/db mice, p<0.05, Table 2). This accounted for the significantly decreased contribution of newly synthesized glucose-6-phosphate towards plasma glucose (Table 2). Since the flux through glucokinase and glucose cycling remained significantly high, these decreases of de novo glucose-6-phosphate synthesis and newly synthesized glucose-6-phosphate partitioning towards glucose did not translate into a decreased total glucose output in db/db mice.
Importantly, while the peripheral glucose disposal (amount of glucose taken up by tissues) was not different in BAS-treated db/db mice compared to untreated db/db mice, significantly more blood was cleared of glucose (metabolic clearance rate) in BAS-treated db/db mice (∼37%, Table 2) as blood glucose concentrations were significantly reduced in -treated db/db mice compared to untreated db/db mice (∼30%, Table 1) indicating a higher affinity of peripheral tissues to glucose in BAS treated db/db mice. Increased glucose uptake of glucose will decrease the glucose concentration until a new steady state is reached at lower glucose concentrations when there is again a balance between plasma glucose appearance and disappearance.
To assess whether changes in hepatic glucose metabolism were in parallel with changes in gene expression patterns, expression levels of genes involved in hepatic glucose metabolism in untreated lean and db/db mice were compared. Compared to lean mice, in db/db mice expression levels of glucose transporter 2, glucokinase, glucose-6-phosphate hydrolase as well as glucose-6-phosphatase were increased, while expression levels of genes of β-oxidation like acetyl CoA-carboxylase 2 and pyruvate dehydrogenase, were decreased and no difference was observed in the expression of the gene encoding phospho-enol-pyruvate carboxykinase nor in glycogen phopshorylase, glycogen synthase, pyruvate kinase and carnitine palmitoyltransferase 1. (Table 3). Additionally, expression levels of these genes were measured in both models following BAS-treatment. BAS treatment had differential effects on expression of genes involved in hepatic glucose metabolism. In lean mice, expression of glucokinase was increased upon BAS treatment whereas expression levels remained high in db/db mice. Quite surprisingly, expression of the glycolytic enzyme pyruvate kinase was strongly increased and phospoenolpyruvate carboxylase was decreased upon treatment in db/db mice whereas expression levels remained unaffected in lean mice upon treatment. (Table 3). Acetyl CoA-carboxylase 2 expression levels of treated db/db mice increased to that observed in lean control mice. In addition, hepatic gene expression levels of fibroblast growth factor 21 (FGF21), which has lately gained attention for its remarkable in vivo actions on glucose metabolism, were measured [36]. Surprisingly, expression levels of FGF21 were 2-fold increased in treated lean and 3-fold increased in treated db/db mice compared to untreated counterparts (Table 3).
Table 3. mRNA expression levels.
| L | LBAS | db | db BAS | |
| Gluconeogenesis | ||||
| Phosphoenolpyruvate carboxylase | 1.0±0.3 | 0.8±0.2 | 1.0±0.2 | 0.7±0.2* |
| Glyogen phosphorylase | 1.0±0.2 | 0.9±0.2 | 1.0±0.1 | 1.0±0.1 |
| Glucose-6-phosphate hydrolase | 1.0±0.2 | 1.2±0.4 | 2.9±1.5† | 3.7±1.1† |
| Glucose-6-phosphatase | 1.0±0.2 | 1.2±0.4 | 1.7±0.3† | 1.8±0.3† |
| Glycogen synthase | 1.0±0.3 | 0.8±0.2 | 0.9±0.1 | 1.0±0.1 |
| Glycolysis | ||||
| Glucose transporter 2 | 1.0±0.3 | 1.2±0.2 | 1.4±0.3† | 1.6±0.2† |
| Glucokinase | 1.0±0.3 | 1.7±0.5* | 1.8±0.6† | 2.3±0.3† |
| Pyruvate kinase | 1.0±0.3 | 0.9±0.3 | 1.1±0.2 | 2.1±0.4† * |
| β-oxidation | ||||
| Acetyl CoA-carboxylase 2 | 1.0±0.2 | 1.1±0.4 | 0.5±0.1† | 1.0±0.2* |
| Carnitine palmitoyltransferase 1 | 1.0±0.6 | 1.2±0.3 | 0.9±0.2 | 0.8±0.1† |
| Pyruvate dehydrogenase | 1.0±0.5 | 0.8±0.5 | 0.5±0.2† | 0.8±0.2† |
| Fibroblast growth factor 21 | 1.0±0.3 | 2.1±1.0* | 0.6±0.2 | 2.6±0.8* |
Hepatic mRNA expression levels of genes involved in hepatic glucose metabolism in lean (L, n = 5), lean mice supplemented with BAS (LBAS, n = 6), db/db mice (db, n = 8) and db/db mice supplemented with BAS (db BAS, n = 8). Expression of genes was normalized to 18S-rRNA levels. 18S-rRNA levels were similar in livers of all animals. Each value represents the mean ± SD;
*p<0.05 vs. same genotype untreated;
p<0.05 vs. L same condition.
Bile acid sequestration reduced long-chain acylcarnitine content in muscle and plasma in db/db mice
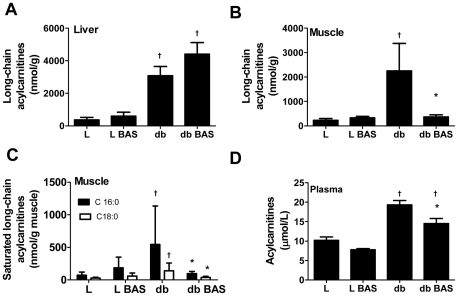
Skeletal muscle is the major site of both glucose and fatty acid uptake and oxidation [4]. It is known that under circumstances of high glucose concentrations muscle favors glucose uptake and oxidation over fatty acid uptake and oxidation [4], while accumulating excess fatty acids, in particular saturated free fatty acid species like stearate and palmitate [37], [38]. Recently, it has been shown that excessive fatty acid oxidation in diabetic mice, results in inefficient oxidation [39]. Concomitantly, high intracellular concentrations of long-chain acylcarnitines, markers of inefficient mitochondrial fatty acid oxidation, were measured. High concentrations of these intermediates can impair the switch to carbohydrate oxidation, a marker of “metabolic flexibility”. To study whether BAS affected fatty acid metabolism, long-chain acylcarnitine contents in liver and skeletal muscle were measured. In db/db mice, both skeletal and liver long-chain acylcarnitines were strongly increased compared to lean mice (Figure 1). BAS treatment had no effect on hepatic long-chain acylcarnitine content in db/db mice (Figure 1A). In contrast, in skeletal muscle of db/db mice a strong reduction of long-chain acylcarnitine content was observed which almost reached the level of untreated lean mice (Figure 1B). Specifically saturated long-chain acylcarnitine species of palmitic (C16∶0) and stearic acid (C18∶0) were affected (Figure 1C). Paralelling the BAS effect on skeletal muscle, BAS treatment reduced the high levels of plasma acylcarnitines (Figure 1D) by reducing both short chain and long-chain acylcarntitines (data not shown). Apparently, increased metabolic clearance of glucose by peripheral tissue is paralleled by changes in long-chain acylcarnitine content of muscle indicative of increased efficiency of mitochondrial fatty acid oxidation.
Figure 1. Effect of BAS on liver, skeletal muscle and plasma acylcarnitines.
Effect of BAS on hepatic long-chain acylcarnitines content (Sum of C16∶0, C16∶1, C18∶0, C18∶1 and C18∶2) (A), skeletal muscle long-chain acylcarnitines content (B), skeletal muscle saturated long-chain acylcarnitines content (C) and plasma acylcarnitine concentration (D) in lean mice (L, (n = 5), lean mice supplemented with BAS (L BAS, n = 4), db/db mice (db, n = 5) and db/db mice supplemented with BAS (db BAS, n = 5). Data are mean ± SD; † p<0.05 vs. L same condition; * p<0.05 vs. same genotype untreated.
Discussion
The leptin receptor deficient db/db mouse is a widely utilized mouse model of type 2 diabetes as it displays several of the features of human type 2 diabetes at 12 weeks of age [40]. However, no characterization describing disturbances of in vivo hepatic glucose metabolism in this mouse model is available. Here, we first show that leptin receptor deficiency results in a variety of alterations of in vivo hepatic glucose metabolism, the most prominent perturbations being the massively increased glucokinase flux and glucose cycling in db/db mice. Secondly, we tested whether the bile acid sequestrant Colesevelam (BAS) induces its reported blood glucose-lowering actions [23], [24] by specific alterations of in vivo hepatic glucose metabolism. Our results, however, demonstrate that BAS treatment increased metabolic clearance of glucose by peripheral tissue without affecting hepatic glucose production. Intrigiungly, we observed a decrease in skeletal muscle long-chain acylcarnitine content in treated db/db mice. We speculate this lowering in skeletal muscle long-chain acylcarnitines to be indicative of an improved skeletal muscle insulin sensitivity and glucose uptake.
A major and novel observation of this study concerns the disturbances in in vivo hepatic glucose metabolism in db/db mice. Most strikingly, glucokinase flux was drastically increased in db/db mice compared to lean mice. Irrespective the dramatic increase of the HOMA index in db/db compared to lean mice, the metabolic clearance of glucose by the liver was essentially not affected. Moreover, a similar ineffectiveness of insulin to modulate hepatic glucose metabolism was apparent from the data on de novo synthesis of glucose-6-phosphate: biosynthesis of glucose-6-phosphate was only slightly decreased (∼20%) in db/db compared to lean mice. Moreover, fluxes through glucokinase and de novo glucose-6-phosphate synthesis were insensitive towards changes in insulin concentration.
Secondly, we studied whole body glucose metabolism. We found the metabolic clearance rate of glucose by peripheral organs was ∼3-fold lower in db/db mice at blood glucose concentrations more than 3 times higher and plasma insulin concentrations ∼7.5 times higher than those of lean mice. It is of interest to compare the hepatic clearance of glucose by glucokinase (as derived by dividing blood glucose by the glucokinase flux) with values obtained for peripheral glucose clearance in db/db mice. In db/db mice, peripheral clearance of glucose was strongly reduced, whereas hepatic clearance of glucose by glucokinase was hardly affected compared to lean mice (5.1±0.7 ml.kg−1.min−1 vs. 4.6±0.7 ml.kg−1.min−1, lean vs. db/db mice, data not shown in the results section), the observed increase in glucose cycling is most likely driven by the extreme hyperglycemia in db/db mice.
Furthermore, the rate of gluconeogenesis was hardly affected by the prevailing high glucose and insulin concentrations in db/db mice. Similar observations were made previously by us in ob/ob mice [25]. It clearly indicates that the increase in blood glucose concentration in both db/db and ob/ob mice is driven by an impaired uptake and metabolism of glucose in peripheral organs rather than by increased hepatic glucose production.
Treatment with BAS has been shown to reduce plasma glucose levels in Type 2 diabetic humans [41], [42] and rodents [23], [24]. We tested whether the glucose-lowering actions of BAS in db/db mice were due to improvement of disturbed hepatic glucose metabolism. Plasma glucose concentration and de novo glucose-6-phosphate synthesis decreased upon BAS treatment in db/db mice. Furthermore, insulin concentration tended to decrease, albeit not significantly, when compared to untreated db/db mice. The glucokinase flux as well as the glucose cycling rate, however, remained invariantly high. Thus, changes in liver glucose metabolism do not mediate the glucose-lowering effect of BAS.
Intriguingly, we also observed an increase in hepatic triglyceride content in BAS treated db/db mice concomitant with an increased hepatic pyruvate kinase expression, likely due to induction of lipogenesis, which we previously described for this model upon BAS treatment [24]. High demand on precursors of cholesterol to bile acids and lipogenesis impairs the TCA cycle. Additionally, phosphoenolpyruvate carboxylase expression was decreased in db/db mice upon BAS. Apparently, the flux of carbons and of the TCA cycle needs to be diminished to deliver enough precursors to these biosynthetic processes.
Lipogenesis is often associated with impaired insulin sensitivity. However, to date the relationship between insulin sensitivity and hepatic triglyceride levels is not straight forward and fatty liver does not necessarily result in insulin resistance. For example, fatty liver induced by an Lxr-agonist resulted in improved whole body insulin sensitivity, lowered blood glucose levels and increased metabolic glucose clearance in ob/ob mice while increasing hepatic triglyceride levels [43]. Moreover, adding an Lxr-agonist to healthy mice induced hepatic steatosis while not affecting blood glucose levels, whole-body insulin sensitivity nor metabolic clearance rate [43]]. Yet, we found that BAS improved peripheral glucose clearance in db/db mice. Interestingly, long-chain acylcarnitine content in db/db skeletal muscle, specifically long-chain acylcarnitines of palmitic and stearic acid, decreased upon BAS treatment. Acylcarnitines are by-products of mitochondrial fatty acid oxidation and are formed upon acyl transfer from acyl-CoA to carnitine. Composition and content of acylcarnitines can reflect both high and low rates of mitochondrial fatty acid oxidation. In inborn errors of mitochondrial fatty acid oxidation, in which fatty acid oxidation is impaired, acylcarnitines typically accumulate in tissues and plasma. Additionally, it has been shown that excessive fatty acid oxidation in diabetic mice results in inefficient oxidation. Increased content of long-chain acylcarnitines in skeletal muscle of db/db mice compared to lean mice could therefore be indicative of impaired oxidation of long-chain fatty acids in skeletal muscle mitochondria [39]. This results in metabolic inflexibility, i.e., the switch to glucose oxidation cannot be made and insulin is unable to stimulate glucose oxidation [44]. BAS treatment resulted in a clear-cut decrease of long-chain acylcarnitine content of skeletal muscle in db/db mice only, nearly to the level that was observed in lean mice. This is suggestive of a more efficient mitochondrial fatty acid oxidation. Concomitantly, a higher metabolic clearance rate of glucose was observed, indicative of an increased ability of the mitochondria to switch to carbohydrate oxidation and indicative of an improved insulin sensitivity. While we report an increased glucose metabolic clearance rate and decreased skeletal muscle content of long-chain acylcarnitines upon BAS treatment in db/db mice, we did not observe an effects of BAS treatment on mRNA expression levels of glucose metabolism related genes in peripheral tissues, such as skeletal muscle and white adipose tissue (Table S1).
We also observed an increased ileal expression of glucagon-like-peptide-1 (data not shown), which has been associated with an improved insulin sensitivity [45]. Concomitantly, we also found a BAS-induced massive malabsorption of dietary fatty acids in lean and db/db mice (data not shown), indicative of an impaired micelle formation and thus an increased amount o fatty acids passing through the distal parts of the intestine upon BAS treatment. It previously has been described that that fatty acids can induce GLP-1 by stimulation of L-cells in the ileum [46], [47]. Indeed, very recent work by Shang et al showed that 8 week dietary addition of BAS in insulin-resistant, diet-induced obese rats led to an increased Glp-1 release during an oral glucose tolerance test while plasma glucose and insulin concentrations decreased compared to untreated insulin-resistant diet-induced obese rats [48]. The question arises how BAS treatment, which interrupts the enterohepatic circulation of bile acids, can exerts its beneficial effects in peripheral tissues. Bile acids have been shown to increase peripheral energy utilization via activation of the bile acid receptor TGR5 [49]. However, in our previous study we showed that BAS treatment does lower the bile acid concentration in plasma by 30% which should lower rather than increase TGR5 signalling. Intriguingly. We observed that BAS induced a 2-fold increase in hepatic FGF21 gene expression in lean and 3-fold increase in db/db mice. Overexpression of FGF21 in livers of db/db or ob/ob mice or administration of recombinant FGF21 to db/db or ob/ob mice or to diabetic Zucker rats have been shown to have beneficial effects on insulin sensitivity and glucose clearance due to FGF21 actions on adipose tissue [36], [50], [51]. In addition to FGF21's action on adipose tissue it has recently also been shown to affect muscle tissue [52]. In this respect, FGF21 might provide a link to communicate changes in liver metabolism to peripheral tissues to allow for metabolic adaptation. However, analysis of mRNA expression levels of genes associated with FGF21 in skeletal muscle and white adipose tissue of lean and db/db mice treated and not treated with BAS did not provide any further clues herein (Table S2). Thus the role of FGF21 as a link to communicate modulations in liver metabolism and peripheral tissues remains largely elusive at the moment.
In conclusion, this study is the first to characterize hepatic glucose fluxes in db/db mice. Glucokinase flux and rates of hepatic glucose output were massively increased in these mice compared to lean mice. Additionally, db/db mice had lower metabolic clearance rates of glucose by peripheral tissues compared to lean mice. Decreased plasma glucose levels upon BAS treatment were mainly attributable to increased metabolic clearance of glucose by peripheral tissues: hepatic glucose output remained unaffected. Interestingly, skeletal muscle long-chain acylcarnitine content was decreased in BAS-treated db/db mice. Increased hepatic FGF21 gene expression levels might play a role in modulating peripheral glucose handling upon BAS-treatment. This hypothesis, however, requires further investigation.
Supporting Information
Skeletal muscle and white adipose tissue mRNA expression levels of genes implicated in glucose metabolism. Skeletal muscle and white adipose mRNA expression levels of genes implicated in glucose metabolism in 7h-fasted lean (L, n = 5), lean mice supplemented with BAS (LBAS, n = 6), db/db mice (db, n = 8) and db/db mice supplemented with BAS (db BAS, n = 8). Expression of genes was normalized to 18S-mRNA levels. 18S-mRNA levels were similar in tissues.
(DOC)
White adipose and skeletal muscle mRNA expression levels of genes associated with FGF21. White adipose and skeletal muscle mRNA expression levels of genes associated with FGF21 in 7h-fasted lean (L, n = 5), lean mice supplemented with BAS (LBAS, n = 6), db/db mice (db, n = 8) and db/db mice supplemented with BAS (db BAS, n = 8). Expression of genes was normalized to 18S-mRNA levels. 18S-mRNA levels were similar in tissues of all animals. Each value represents the mean ± SD; *p<0.05 vs. same genotype untreated; †p<0.05 vs. L same condition.
(DOC)
The graphic illustrates the individual glucose fluxes for a better understanding of Table 2 in a schematic manner. The second panel of the table entitled “Contributions to endogenous glucose production rate” shows the contribution of de novo glucose-6-phosphate synthesis to glucose (displayed as fluxes e+b in the graphic) and of glycogen to glucose (displayed as fluxes c+b in the graphic) which altogether make up the endogenous glucose production rate. The third panel entitled “Contributions to hepatic glucose production rate” takes into account the glucose cycling rate and thus shows the contributions of the endogenous glucose production rate and the glucose cycling rate (which consists of the cycling of glucose: 1. from glucose to glucose-6-phosphate (depicted as a in the graphic, the glucokinase flux) and back (depicted as b in the graphic, the glucose-6-phosphatase flux); 2. Glucose-6-phosphate to glycogen and back, fluxes c+d in the graphic; and 3. Gluconeogenesis, flux e in the graphic (glycolysis is also a part of this but cannot be measured in the present set up) to the total hepatic glucose production rate which equals the flux rate through glucose-6-phosphatase. The lower panel shows the flux rates through glucokinase (a in the graphic) and the rate of glucose-6-phosphate de novo synthesis (c+e in the graphic).
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by an unrestricted research grant of the Groningen Expert Center for Kids with Obesity (GECKO) and DaiichiSankyo (Pirsipanny, NJ). Neither GECKO nor DaiichiSankyo took part in the data collection nor the preparation of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Pathogenesis of type 2 (non-insulin dependent) diabetes mellitus: A balanced overview. Diabetologia. 1992;35(4):389–97. doi: 10.1007/BF00401208. [DOI] [PubMed] [Google Scholar]
- 3.Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav. 2008;94(2):252–8. doi: 10.1016/j.physbeh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle. Obes Rev. 2009;10(2):178–93. doi: 10.1111/j.1467-789X.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 5.Donovan JM, Von Bergmann K, Setchell KD, Isaacsohn J, Pappu AS, et al. Effects of colesevelam HC1 on sterol and bile acid excretion in patients with type IIa hypercholesterolemia. Dig Dis Sci. 2005;50(7):1232–8. doi: 10.1007/s10620-005-2765-8. [DOI] [PubMed] [Google Scholar]
- 6.Davidson MH. The use of colesevelam hydrochloride in the treatment of dyslipidemia: A review. Expert Opinion on Pharmacotherapy. 2007;8(15):2569–2578. doi: 10.1517/14656566.8.15.2569. [DOI] [PubMed] [Google Scholar]
- 7.Insull WJMD. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. Southern Medical Journal. 2006;99(3):257–273. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 8.Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: Glucose and lipid effects. Arch Intern Med. 2008;168(18):1975–83. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31(8):1479–84. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168(14):1531–40. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 11.Onishi T, Sano N, Takikawa H. Effect of colestimide on absorption of unconjugated bile acids in the rat jejunum. J Gastroenterol Hepatol. 2002;17(6):697–701. doi: 10.1046/j.1440-1746.2002.02765.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki Y. Colestimide: The efficacy of a novel anion-exchange resin in cholestatic disorders. J Gastroenterol Hepatol. 2002;17(11):1133–5. doi: 10.1046/j.1440-1746.2002.02860.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279(22):23158–65. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, et al. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278(40):39124–32. doi: 10.1074/jbc.M305079200. [DOI] [PubMed] [Google Scholar]
- 16.Cariou B, Duran-Sandoval D, Kuipers F, Staels B. Farnesoid X receptor: A new player in glucose metabolism? Endocrinology. 2005;146(3):981–3. doi: 10.1210/en.2004-1595. [DOI] [PubMed] [Google Scholar]
- 17.Duran-Sandoval D, Cariou B, Fruchart JC, Staels B. Potential regulatory role of the farnesoid X receptor in the metabolic syndrome. Biochimie. 2005;87(1):93–8. doi: 10.1016/j.biochi.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Duran-Sandoval D, Mautino G, Martin G, Percevault F, Barbier O, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53(4):890–8. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- 19.Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146(3):984–91. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- 20.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–93. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre P, Lien F, Cariou B, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiological Reviews. 2009:147. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 22.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281(16):11039–49. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Ikegami H, Fujisawa T, Nojima K, Kawabata Y, et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56(1):239–47. doi: 10.2337/db06-0353. [DOI] [PubMed] [Google Scholar]
- 24.Herrema H, Meissner M, van Dijk TH, Brufau G, Boverhof R, et al. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptoralpha-controlled metabolic pathways in mice. Hepatology. 2009 doi: 10.1002/hep.23408. [DOI] [PubMed] [Google Scholar]
- 25.Bandsma RH, Grefhorst A, van Dijk TH, van der Sluijs FH, Hammer A, et al. Enhanced glucose cycling and suppressed de novo synthesis of glucose-6-phosphate result in a net unchanged hepatic glucose output in ob/ob mice. Diabetologia. 2004;47(11):2022–31. doi: 10.1007/s00125-004-1571-8. [DOI] [PubMed] [Google Scholar]
- 26.van Dijk TH, van der Sluijs FH, Wiegman CH, Baller JF, Gustafson LA, et al. Acute inhibition of hepatic glucose-6-phosphatase does not affect gluconeogenesis but directs gluconeogenic flux toward glycogen in fasted rats. A pharmacological study with the chlorogenic acid derivative S4048. J Biol Chem. 2001;276(28):25727–35. doi: 10.1074/jbc.M101223200. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk TH, Boer TS, Havinga R, Stellaard F, Kuipers F, et al. Quantification of hepatic carbohydrate metabolism in conscious mice using serial blood and urine spots. Anal Biochem. 2003;322(1):1–13. doi: 10.1016/j.ab.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee WN, Byerley LO, Bergner EA, Edmond J. Mass isotopomer analysis: Theoretical and practical considerations. Biol Mass Spectrom. 1991;20(8):451–8. doi: 10.1002/bms.1200200804. [DOI] [PubMed] [Google Scholar]
- 29.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis: A technique for measuring biosynthesis and turnover of polymers. Am J Physiol. 1992;263(5 Pt 1):E988–1001. doi: 10.1152/ajpendo.1992.263.5.E988. [DOI] [PubMed] [Google Scholar]
- 30.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 31.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 32.Chace DH, DiPerna JC, Mitchell BL, Sgroi B, Hofman LF, et al. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin Chem. 2001;47(7):1166–82. [PubMed] [Google Scholar]
- 33.Gates SC, Sweeley CC. Quantitative metabolic profiling based on gas chromatography. Clin Chem. 1978;24(10):1663–73. [PubMed] [Google Scholar]
- 34.Plosch T, Kok T, Bloks VW, Smit MJ, Havinga R, et al. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem. 2002;277(37):33870–7. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 35.Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. 2002;109(7):961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Archives of Biochemistry and Biophysics. 2003;419(2):101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Ragheb R, Shanab GML, Medhat AM, Seoudi DM, Adeli K, et al. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: Evidence for PKC activation and oxidative stress-activated signaling pathways. Biochemical and Biophysical Research Communications. 2009;389(2):211–216. doi: 10.1016/j.bbrc.2009.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Shafrir E. Animal models of non-insulin-dependent diabetes. Diabetes Metab Rev. 1992;8(3):179–208. doi: 10.1002/dmr.5610080302. [DOI] [PubMed] [Google Scholar]
- 41.Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann Intern Med. 1994;121(6):416–22. doi: 10.7326/0003-4819-121-6-199409150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL. Results of the glucose-lowering effect of WelChol study (GLOWS): A randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29(1):74–83. doi: 10.1016/j.clinthera.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Grefhorst A, van Dijk TH, Hammer A, van der Sluijs FH, Havinga R, et al. Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. Am J Physiol Endocrinol Metab. 2005;289(5):E829–838. doi: 10.1152/ajpendo.00165.2005. [DOI] [PubMed] [Google Scholar]
- 44.Boden G. Effects of free fatty acids (FFA) on glucose metabolism: Significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111(3):121–4. doi: 10.1055/s-2003-39781. [DOI] [PubMed] [Google Scholar]
- 45.Freeman JS. Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus. Cleve Clin J Med. 2009;76(Suppl 5):S12–9. doi: 10.3949/ccjm.76.s5.03. [DOI] [PubMed] [Google Scholar]
- 46.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, et al. Glucagon-like peptide-1(7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 47.Rocca AS, LaGreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142(3):1148–1155. doi: 10.1210/endo.142.3.8034. [DOI] [PubMed] [Google Scholar]
- 48.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G419–424. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 49.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54(6):1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–27. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, et al. FGF21 is an akt-regulated myokine. FEBS Lett. 2008;582(27):3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Skeletal muscle and white adipose tissue mRNA expression levels of genes implicated in glucose metabolism. Skeletal muscle and white adipose mRNA expression levels of genes implicated in glucose metabolism in 7h-fasted lean (L, n = 5), lean mice supplemented with BAS (LBAS, n = 6), db/db mice (db, n = 8) and db/db mice supplemented with BAS (db BAS, n = 8). Expression of genes was normalized to 18S-mRNA levels. 18S-mRNA levels were similar in tissues.
(DOC)
White adipose and skeletal muscle mRNA expression levels of genes associated with FGF21. White adipose and skeletal muscle mRNA expression levels of genes associated with FGF21 in 7h-fasted lean (L, n = 5), lean mice supplemented with BAS (LBAS, n = 6), db/db mice (db, n = 8) and db/db mice supplemented with BAS (db BAS, n = 8). Expression of genes was normalized to 18S-mRNA levels. 18S-mRNA levels were similar in tissues of all animals. Each value represents the mean ± SD; *p<0.05 vs. same genotype untreated; †p<0.05 vs. L same condition.
(DOC)
The graphic illustrates the individual glucose fluxes for a better understanding of Table 2 in a schematic manner. The second panel of the table entitled “Contributions to endogenous glucose production rate” shows the contribution of de novo glucose-6-phosphate synthesis to glucose (displayed as fluxes e+b in the graphic) and of glycogen to glucose (displayed as fluxes c+b in the graphic) which altogether make up the endogenous glucose production rate. The third panel entitled “Contributions to hepatic glucose production rate” takes into account the glucose cycling rate and thus shows the contributions of the endogenous glucose production rate and the glucose cycling rate (which consists of the cycling of glucose: 1. from glucose to glucose-6-phosphate (depicted as a in the graphic, the glucokinase flux) and back (depicted as b in the graphic, the glucose-6-phosphatase flux); 2. Glucose-6-phosphate to glycogen and back, fluxes c+d in the graphic; and 3. Gluconeogenesis, flux e in the graphic (glycolysis is also a part of this but cannot be measured in the present set up) to the total hepatic glucose production rate which equals the flux rate through glucose-6-phosphatase. The lower panel shows the flux rates through glucokinase (a in the graphic) and the rate of glucose-6-phosphate de novo synthesis (c+e in the graphic).
(DOC)