Abstract
Objective
We studied health utilities in patients with type 1 diabetes to understand potential differences in health utilities as function of age, type of respondent (self-report vs. proxy-report), and method of assessment (direct versus indirect).
Research Design and Methods
We elicited self-reported health utilities for adults (n=213) and children (n=238) with type 1 diabetes, and by parent proxy-report (n=223) for overall quality of life (Health Utilities Index [HUI] Mark 3 and experienced time-tradeoff [TTO] questions) and hypothetical complication states (TTO questions).
Results
Mean health utilities for overall quality of life (QOL) ranged from 0.81 to 0.91. Children had significantly higher overall QOL compared with adults (0.89 vs. 0.85, p<0.01) by HUI, but had no significant difference in QOL by TTO. There were no significant differences in QOL between child self-report and parent proxy-report. Utilities were higher for HUI vs. TTO for parent proxy-report (p<0.01) but not for adult or child self-report. Utilities for hypothetical complication states were lower than for current QOL. Values were lower for stroke (0.34-0.53), end stage renal disease (0.47-0.55), and blindness (0.52-0.69) than for amputation (0.73-0.82) and angina (0.74-0.80). Complication utilities for parent proxy-report were higher compared with adult self-report for most hypothetical complication states.
Conclusions
Individuals with type 1 diabetes with few complications report a relatively high QOL; however, future end stage complications are rated as having a significant impact on QOL. Differences in utilities by age, self-report vs. proxy-report, and method raise important questions about whose utilities should be used in economic analyses.
Introduction
Type 1 diabetes places individuals at risk for serious microvascular complications such as retinopathy, neuropathy, and nephropathy, as well as macrovascular complications such as cardiovascular disease and stroke.(1) Because of the significant impairment associated with these complications, multiple treatments and supportive devices have been developed to reduce the risk of developing type 1 diabetes and its complications, and to improve the quality of life (QOL) associated with type 1 diabetes.(2, 3)
A comprehensive analysis of a new technology assesses not only the clinical effectiveness, but also the cost effectiveness of the treatment. The most widely accepted methodology for assessing cost effectiveness in such cases is cost-utility analysis (CUA).(4) With CUA, health outcomes are expressed in terms of quality-adjusted-life-years (QALYs), which measure both length and QOL. Use of QALYs allows clinicians and policymakers to systematically evaluate and compare the impact of health interventions on length and QOL. In order to calculate QALYs, numeric measures of QOL, termed health utilities, are needed to capture the value of various health states.
To date, empirically-derived health utility data for health states associated with type 1 diabetes across the age spectrum have been lacking.(5) Recent studies evaluating health preferences for diabetes derived their utility estimates almost exclusively from individuals with type 2 diabetes,(6, 7) which may not be generalizable to individuals with type 1 diabetes. Although a few studies have elicited utilities from persons with type 1 diabetes, they did not include children or proxy respondents,(8-14) relied solely on indirect elicitation methods, such as the Short-Form 36, the Quality of Well Being index, or the EuroQoL five dimensions.(8-11) were based on a smaller number of respondents,(12-14) or did not elicit specific utilities for diabetes complication states.(12-14)
There is a paucity of health utility data from children and adults with type 1 diabetes. Because a majority of individuals develop the disease during childhood, a large number of interventions are being conducted solely in pediatric populations, or in populations consisting of children as well as adults, thus posing multiple dilemmas for investigators conducting cost-effectiveness analyses. Standardized methods for assessing health utilities have been developed primarily for adult populations, but it is unclear whether adult utilities should be applied to children as well. Because type 1 diabetes may impact QOL differently for children versus adults, it is critical to understand how health utilities may differ between the two groups. Furthermore, because proxy respondents are often used to measure health utilities for younger children, it is important to understand whether health utilities might differ between child self-report and proxy report. We therefore had two overarching goals for this study; (1) to provide health utilities specific to individuals with type 1 diabetes both for overall QOL and for complication states; and (2) to use type 1 diabetes as a disease paradigm for understanding potential differences in health utilities as function of age, type of respondent (self-report vs. proxy-report), and method of assessment (direct versus indirect). We hypothesized that children with type 1 diabetes would report higher health utilities than adults, that health utilities generated by proxy-report would be similar to those generated by child self-report, and that direct versus indirect methods would yield similar health utility estimates.
Research Design and Methods
We conducted face-to-face interviews with individuals (>8 years) with type 1 diabetes and their parents/guardians (proxy-report) who enrolled in the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring trial. Further details of the trial have been described in previous publications.(2, 15, 16), and utilities for the treatment arm have been used for a cost-effectiveness analysis of the trial.(17) At baseline, indirect and direct methods were used to assess health utilities for overall QOL with diabetes, and direct methods were used to assess health utilities for hypothetical diabetes complication states. Utilities for control and intervention cohorts were combined for this analysis.
Indirect Measurement
For indirect measurement of health utilities, all subjects and parents of children 8-18 years completed the Health Utilities Index (HUI) Mark 3, an 8 item self-administered questionnaire which assesses health related QOL.(18) Levels of functioning are measured across a variety of attributes, including vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain, and responses are transformed into a utility score.
Direct Measurement
The Time-Trade-Off (TTO) method was selected over other methods because of its unique balance of relative understandability, sensitivity to preference, and use in prior preference studies of patients with diabetes.(19, 20) Individuals were read a description of a specific health state and then were asked to give their preference for years of life with that health state compared with a shorter period of time in perfect health. The response frame for the time tradeoff exercise was linked to life expectancy for different age groups. Younger children had a longer time frame (e.g. 40 years) to consider than older adults (e.g. 20 years), but the iterative approach to finding the equilibrium point was identical. Individuals aged 8-15 years began by indicating their health preference for 50 years living with the health state versus 50 years in perfect health; the time frame was 40 years for individuals 16-24 years, 30 years for individuals 25-35 years, and 20 years for individuals 35 years and older. A ping-pong method was utilized to arrive at the point of indifference where time in the current health state and decreased time in perfect health were equally desirable;(21) this point was then used to calculate the utility score (e.g., if 25 years of life in perfect health equals 50 years with diabetes, the utility would be 0.50).
To evaluate overall QOL (experienced TTO), individuals were asked to think about their current health with diabetes. To evaluate QOL associated with diabetes complications (complication TTO), standardized hypothetical state descriptions were used. Hypothetical states have been repeatedly used in TTO studies related to diabetes treatments and complications, allowing for an evaluation of patients' perceptions of complication states, particularly in populations with a low rate of complications. The functional and symptomatic experience of living with specific diabetes complications (blindness, end-stage renal disease [ESRD], chronic angina/myocardial infarction, stroke, and lower extremity amputation) was described with no specific age attached to the scenario. (Appendix).(7) Complication health utilities were elicited for adult self-report, but as well for parent proxy-report. Parents must serve as proxy decision-makers for their children when considering enrollment in interventional studies that may prevent complications, underscoring the need for assessing health utilities by proxy-report. Experienced and complication utility data were collected from all subjects and parents with the following exceptions: (1) subjects <15 years of age were excluded from the experienced TTO questionnaire, as it was unclear whether they would be able to cognitively complete the questionnaires; and (2) subjects <19 years of age were excluded from the hypothetical complication scenario TTO questionnaires due to concerns about the sensitive nature of the health state descriptions.
Statistical Analysis
All analyses were performed using Stata 10. To describe the distribution of utilities, mean, median, standard deviation (SD) and interquartile range were calculated. We compared health utilities (1) for adults vs. children; (2) for parent proxy-report vs. child self-report; and (3) for direct (TTO) vs. indirect methods (HUI). We also compared parent proxy-report vs. adult self-report for complication states only. Rank sum tests were used to compare utilities for independent samples and sign rank tests were used to compare paired data (i.e. parent-proxy vs. child self-report). We also performed Spearman correlations of health utilities for parent proxy-report versus child self-report using the HUI, and for HUI vs. Experienced TTO. Finally, we assessed the reliability of the TTO and HUI for the control group at baseline and 6 months later. Because of multiple comparisons, significance was defined as a p value < 0.01 for all comparisons.
Results
Table 1 describes the sample and their demographic/clinical characteristics. Mean baseline HbA1c was 7.5 ± 0.9%, reflecting the relatively good control of the cohort overall. As expected, duration of diabetes was longer and rates of complications were higher for adults compared with children. Overall, 12% (n=52) of subjects had at least one major complication.
Table 1. Demographics and Clinical Characteristics of the Study Population.
| Children (n=238) | Adults (n=213) | |
|---|---|---|
| Mean Age (years) | 13.7 ± 3.1 years | 38.8 ± 13.6 years |
| Female (% (n)) | 51.7% (n=123) | 58.7% (n=125) |
| Race (% (n)) | ||
| White | 92.4% (n=220) | 96.2% (n=205) |
| Black | 1.7% (n=4) | 1.4% (n=3) |
| Asian | 0.8% (n=2) | 0.5% (n=1) |
| Other | 5% (n=12) | 1.9 % (n=4) |
| Mean duration (years) | 6.4 ± 3.4 | 21.6 ± 12.5 |
| Complications (% (n)) | ||
| Hypertension | 0.4% (n=1) | 18.3% (n=39) |
| Hypercholesterolemia | 0.8% (n=2) | 32.4% (n=69) |
| Cardiovascular Disease | 0.4% (n=1) | 7.0% (n=15) |
| Renal disease | 1.3% (n=3) | 8.0 % (n=17) |
| Neurologic disease | 1.7% (n=4) | 3.8% (n=8) |
| Retinopathy | 0% | 6.1% (n=13) |
All adults completed the health utilities questionnaires for overall QOL, and the majority (93%) of hypothetical complication state questionnaires. Most children 8-18 years completed the HUI (97%, n=231); a smaller proportion of children 15-18 years completed the experienced TTO (82%, n=95). Subjects with missing data tended to be younger, with mean ages of 9.7 years (n=7) for the HUI and 14.5 years (n=21) for the experienced TTO. The majority of parents completed the HUI (n=223) and TTO (n=221) questionnaires; a smaller proportion (74-78%) completed the complication state questionnaires.
Utilities for Experienced Overall Quality of Life
Health utility scores for overall QOL for adult self-report (≥19 years), parent proxy-report (8-18 years), and child self-report (8-18 years) are shown in Figure 1 and Table 2. Health utilities as measured by the HUI were relatively high in all three groups. For overall current QOL, anywhere from 14% to 39% of individuals reported the maximum health utility (i.e. perfect health).
Figures 1a and 1b.
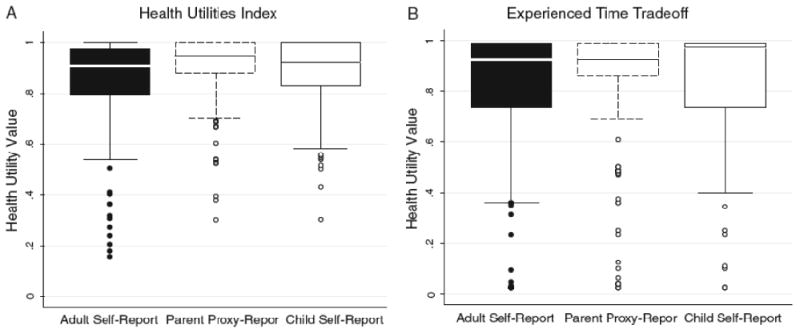
Boxplots of health utilities for overall quality of life for adult subjects by self-report (≥19 years), pediatric subjects by parent proxy-report (8-18 years), and pediatric subjects by self-report (8-18 years)
Table 2.
Health utilities for adult subjects by self-report ((≥19 years), child subjects by parent proxy-report (8-18 years), and child subjects by self-report (8-18 years).
| Adult Self-Report (≥19 yrs) (n=213) |
Parent Proxy-Report (Parents of children 8-18 years) (n=223) |
Child Self-Report (8-18 years for HUI n=231) (15-18 yrs for TTO, n=95) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) |
Median (25th%-75th %) |
% at Maximum Value (n) | Mean (SD) |
Median (25th%-75th %) |
% at Maximum Value (n) | Mean (SD) |
Median (25th%-75th %) |
% at Maximum Value (n) | |
| Current Overall Quality of Life | |||||||||
| HUI | *0.85 (0.17) | 0.91 (0.79-0.97) | 14 (30) | †0.91 (0.12) | 0.95 (0.88-1.00) | 35 (78) | *0.89 (0.12) | 0.92 (0.83-1.00) | 29 (68) |
| Experienced TTO | 0.81 (0.25) | 0.92 (0.73-0.98) | 33 (70) | †0.84 (0.23) | 0.92 (0.86-0.98) | 39 (86) | 0.81 (0.26) | 0.97 (0.73-0.98) | 31 (29) |
| Hypothetical Complication States | |||||||||
| Blindness | §0.52 (0.33) | 0.50 (0.23-0.83) | 22 (40) | §0.69 (0.29) | 0.79 (0.48-0.98) | 22 (40) | - | - | - |
| End Stage Renal Disease | §0.47 (0.32) | 0.48 (0.23-0.73) | 8 (16) | §0.55 (0.33) | 0.50 (0.25-0.86) | 15 (26) | - | - | - |
| Angina | 0.74 (0.28) | 0.85 (0.61-0.98) | 23 (48) | 0.81 (0.23) | 0.90 (0.73-0.98) | 31 (56) | - | - | - |
| Stroke | §0.34 (0.31) | 0.23 (0.03-0.56) | 4 (9) | §0.53 (0.33) | 0.50 (0.25-0.86) | 13 (23) | - | - | - |
| Amputation | §0.73 (0.30) | 0.88 (0.50-0.98) | 27(57) | §0.82 (0.24) | 0.94 (0.78-0.98) | 38 (68) | - | - | - |
Statistically significant differences in utilities comparing child self-report with adult self-report (p<0.01)
Statistically significant differences in utilities for general quality of life comparing the HUI with the TTO (p<0.01).
Statistically significant differences in utilities for hypothetical complication states comparing parent proxy-report with adult self-report for blindness and stroke (p<0.001), end stage renal disease (p<0.01), and amputation (p<0.01).
Comparisons by age
Compared with adults, children had significantly higher utilities (0.89 vs. 0.85, p<0.01) using the HUI. There were no significant differences using the TTO.
Comparisons for self-report versus proxy-report
There were no significant differences between child self-report and parent proxy-report using either HUI or TTO. Correlations between child self-report and parent proxy-report were 0.34 (0.22-0.45) for HUI and 0.31 (0.10-0.50) for experienced TTO, with scatterplots demonstrating considerable variability between parent and child (Figures 2a and 2b). For the HUI, parent-proxy utilities were higher than self-reported child utilities in 41% of cases, equal in 28% of cases, and lower in 31% of cases. For experienced TTO, parent-proxy utilities were higher than self-reported child utilities in 45% of cases, equal in 20% of cases, and lower in 30% of cases.
Figures 2a and 2b.
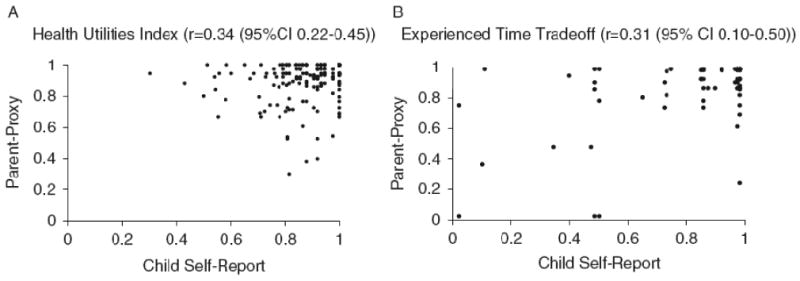
Scatterplots of parent proxy-report health utilities vs. child self-report health utilities (for overall quality of life) using the Health Utilities Index (8-18 years) (n=216) or Experienced Time Tradeoff (15-18 years) (n=80)
Comparisons for HUI versus TTO
We did find that utilities were higher for HUI vs. TTO for parent-proxy report (p<0.01), but not for child or adult self-report. Accordingly, correlations between HUI and TTO were higher for adult self-report (0.16 (95% CI 0.03-0.29)) and child self-report (0.20 (95% CI 0-0.39)) than for parent proxy-report (0.12 (0-0.25)). Again there was considerable individual variability (Figures 3a, 3b, and 3c). Self-reported utilities for HUI were higher than TTO in 51% of cases but were lower in 49% of cases, and parent proxy-reported utilities for HUI were higher than TTO in 59% of cases and lower in 41% of cases.
Figures 3a, 3b, 3c.
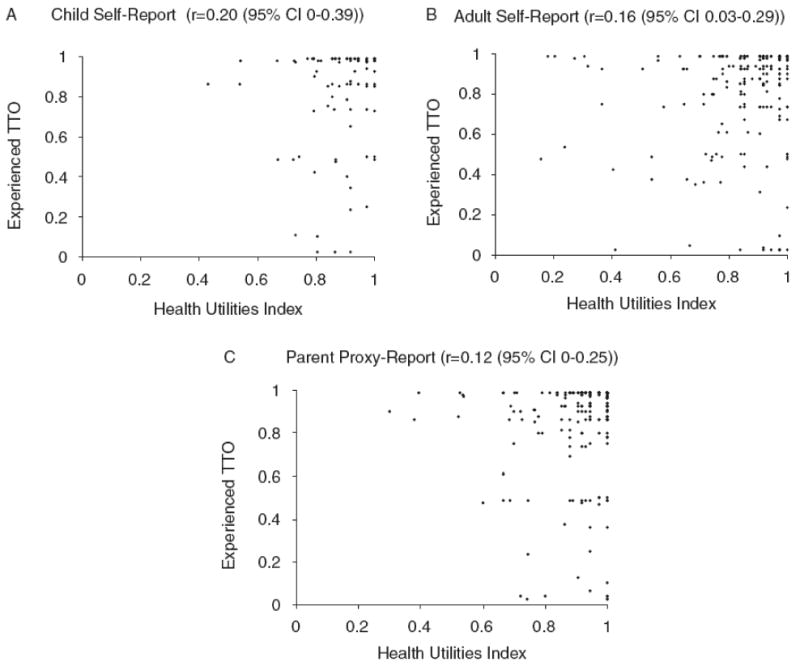
Scatterplots of health utilities using the Health Utilities Index 3 vs. Experienced Time-Trade-off (for overall quality of life) for child self-report (15-18 years) (n=95), adult self-report (n=213), and parent proxy-report (n=221)
We also assessed test-retest reliability comparing baseline and 6 month values (control group only) for experienced TTO and HUI. Correlations for experienced TTO by self-report and proxy-report were 0.67 (95% CI 0.47-0.81) and 0.49 (95% CI 0.33-0.63), respectively. Correlations for HUI by self-report and proxy-report were 0.42 (95% CI 0.25-0.57) and 0.49 (95% CI 0.33-0.63), respectively.
Utilities for Hypothetical Diabetes Complications
Compared with health utilities for overall QOL, health utilities for hypothetical complication states were lower (Table 1). The complication considered the most damaging to QOL was stroke, while the least damaging complication was angina. The ranking of complication utilities were similar for adult subjects and parent proxies. The percentage of individuals with health utilities at the maximal value was generally lower for the hypothetical health states vs. overall QOL.
Subgroup Comparisons of Utilities for Hypothetical Diabetes Complication States
For the hypothetical complication states, utilities were more favorably rated by proxy-report than by adult self-report across all states (p<0.001 for blindness and stroke, p<0.01 for end stage renal disease, p=0.03 for angina, and p<0.01 for amputation).
Discussion
This is one of the first studies to provide empirically-derived health utility data for overall QOL and diabetes-specific complications from adults and children with type 1 diabetes and their parents as proxy respondents. We found that mean health utilities for overall QOL for individuals in our study were relatively high and were comparable to two different smaller studies of US and Canadian adults with type 1 diabetes which also used the TTO methodology for generating utilities. These studies reported overall utilities of 0.88 (n=72) (14) and 0.87 (n=85),(12) which were comparable with the utilities from our study. Our study further extends this work with a much larger sample size and a greater variety of respondents, including children and parents as proxy respondents.
Our findings however contrast with the findings of additional studies that have elicited health utilities for individuals with type 1 diabetes. Another study of Canadian adults with type 1 diabetes also used the HUI, estimating an overall QOL health utility of 0.78, versus 0.85 for adults in our study, which may be due to higher complication rates in that population.(22) Similarly, Coffey et al estimated utilities using the Self-Administered Quality of Well-Being index (QWB-SA) in a slightly younger population (mean age 34 years) of individuals with type 1 diabetes, and reported mean health utility scores of 0.63-0.67 for individuals without microvascular, neuropathic, or cardiovascular complications.(9) Finally, Wu et al(8) estimated health utilities of 0.73, 0.68, and 0.64 for adult patients with type 1 diabetes aged <45 years, 45-64 years, and ≥ 65 years, respectively, by predicting QWB scores based on responses to the SF-36, a generic health status assessment instrument that profiles eight health domains for an individual. The characteristics of our population as well as the differing methodologies used for obtaining health utilities may account for some of the differences.
We note that adult individuals in our study had higher levels of overall QOL compared with the general population, with one recent study reporting overall utilities of 0.81 using the HUI3 in a national probability sample of US adults.(23)
Individuals with type 1 diabetes did report lower health utilities when asked to consider hypothetical scenarios of complication states. Huang et al (7) also estimated health utilities using hypothetical complication states for individuals with type 2 diabetes, and found a similar ranking of complication states as in our study. However, our rankings were different from those reported by Coffey et al(9) (lowest to highest: blindness, amputation, stroke, and end stage renal disease), who used regression techniques rather than TTO to estimate the decrement in QWB-SA scores for individuals suffering from specific complications.
We found that health utilities for child self-report (HUI) were higher than for adult self-report, which is consistent with the previous literature demonstrating that health utilities tend to be higher for younger versus older individuals.(24) This makes logical sense given that children with type 1 diabetes, particularly in this cohort, were relatively healthy with lower rates of complications compared with older adults. However, this could also be confounded by disease duration, which was longer for adults versus children.
Although a variety of studies have compared QOL assessments for children with type 1 diabetes with those from their parents as proxy, we are unaware of studies that have compared actual health utilities for this specific population. Consistent with our hypotheses, we did not find significant differences in health utilities between child self-report and parent proxy-report. Other health preference studies of children with chronic diseases, including those with pediatric brain tumors(25) or extremely low-birthweight infants(26) have reported higher utilities for parent proxy-report compared with child self-report, whereas other studies have reported lower utilities.(27)
We found that parent-proxy utilities tended to be higher compared with adult self-report for hypothetical complication states. Parents were asked to think about their child's health for the scenario; these higher scores reflect the fact that parents are generally willing to trade very little time from the length of their child's life. Another possibility is that the longer life expectancy assumed for children compared with adults in the scenarios may have affected their responses. These differences will potentially have implications for future economic analyses of type 1 diabetes. For example, the higher utilities associated with complication states reported by parents would lead to less favorable (higher) absolute estimates of cost-effectiveness for a specific treatment or therapy. Furthermore, the higher utilities could result in smaller estimated benefits of therapies and a ceiling effect whereby improvements in QALYs over the course of an intervention would be underestimated because of higher baseline estimates of QOL.
Consistent with our hypotheses, we found a lack of differences between HUI and TTO for child and adult self-report. However, we did find higher utility scores for HUI vs. TTO for parent proxy-report, which contrasts with the findings of a recent systematic analysis which reported that direct methods tend to result in higher health utilities compared with indirect methods.(28) That study incorporated results from studies of adults from patient groups and the general public, focused on individuals with diseases other than type 1 diabetes, and evaluated additional instruments besides the HUI, which may account for the differences in our findings. Further studies are needed to understand why these differences exist for parent proxy-report.
Despite the fact that we found significant correlations between direct and indirect utilities at the group level, we found poor correlations at the individual level. This discrepancy has been reported by other studies.(29, 30) It has been suggested that this difference is due to the fact that there can be individual variability in a single utility measurement based on TTO, whereas HUI scores remove this individual variability since they are derived from the mean preferences of a large adult community.(30, 31) Another possibility is that HUI scores, which were generated based on an adult population, are not generalizable to older children or proxy respondents.
Our findings raise the critical question of “whose values” and “what methods” should be used for CEA. The formal recommendation for CEA from the societal perspective is that preferences should be assessed for the general population rather than a patient population and through indirect rather than direct methods. However, others have advocated that in cases where patients' preferences represent an important outcome (i.e. randomized trials) preferences should be derived from affected patient populations through direct methods. (4, 30) For pediatric diseases like type 1 diabetes, the answer to this question is even more complicated, as not only the choice of instrument, but also the choice of which respondent (child self-report or parent-proxy) needs to be considered. Currently, there is no universal or recommended standard regarding which utilities should be used for pediatric CEA.(32, 33) Because of differences that we found, we recommend that investigators consider including both sets of utilities in sensitivity analyses. However, it is clear that further research is needed to further develop methods for eliciting health utilities among children and provide standardization of methods for conducting CEA among children.
We do acknowledge limitations of our study. First, because there is no gold standard for measuring preferences, the validity of utility measurements cannot be directly assessed. However, the rank ordering of the complication utilities has face validity and is consistent with the findings of other studies that have used TTO methods.(7) Secondly, the cross-sectional nature of the data is also a limitation. Third, our correlation plots demonstrated substantial variation between utilities derived from the HUI vs. TTO and between children and their parents. However, we did find fair test-retest reliability when comparing utilities at baseline and at 6 months for the control group, suggesting that the differences we found may represent true differences rather than an artifact of poor measurement. Fourth, the significant proportion of individuals with health utility values at the maximum value limits the sensitivity of cost-utility analyses for detecting improvements in QOL over time related to specific treatments or interventions. Finally, we did not administer the complication scenarios to children.
We recognize that the preferences we elicited may not be representative of all individuals with type 1 diabetes, as individuals in this cohort were participants in a randomized controlled trial, were receiving intensive insulin therapy, and had better than average control. However, our findings are relevant for the growing population of patients with type 1 diabetes who are adopting new diabetes technologies to help control their disease.
Strengths of this study include the use of both direct and indirect elicitation methods, and the inclusion of both adults and children with type 1 diabetes, as well as parents of children with type 1 diabetes. Given the differences in health utilities for the hypothetical complication states that we found for parent proxy-report and adult self-report, further studies are needed to explore the unique preferences of children regarding diabetes complication states.
Conclusions
Although individuals with type 1 diabetes report a relatively high QOL, complications of diabetes have a significant impact on the QOL. The differences in health utilities that we found for children vs. adults, self- vs. proxy-report for complication states, and for direct and indirect methods raise important questions about whose utilities should be used in economic analyses. The health utility data generated from this study will be critical for future studies assessing the economic value of current and future interventions targeted at individuals with type 1 diabetes for the health care system. There is no consensus yet as to how to incorporate health values for adults, children, and their caregivers in economic analyses. Further work is needed to explore reasons for these differences and their potential impact on the economic value of various health care interventions for type 1 diabetes.
Health State Descriptions for Overall Diabetes-Related Complications
Blindness
Imagine a life with blindness:
You would not be able to read, see the TV, or drive a car.
You may also need assistance with many day-to-day tasks such as cooking, cleaning, dressing yourself, and bathing.
You may also need assistance taking your medications.
End-stage renal disease
Imagine a life with kidney failure:
You would experience fatigue, bone problems, joint problems, itching, and “restless legs.”
You would need to have dialysis 3X/ week and this procedure usually lasts 3-5 hours. You may need to make changes in your work or home life to maintain this schedule.
During dialysis you are attached to a machine. To do this, a needle is inserted into tubing that has been placed under the skin of your arm. The machine then filters the blood to get rid of waste products.
You may sometimes feel sick or tired for a few hours after you have had dialysis.
Angina
Imagine living with chest pain related to heart disease:
You experience chest pain after walking a block or two but can relieve the pain by stopping or taking medicine.
Your energy level may be low some of the time.
You can bathe and dress yourself, and feed yourself without difficulty.
Stroke
Imagine life after having a severe stroke:
You cannot move the arm or leg on the side that you write with.
You can stand with a leg brace and walk a short distance with help.
You can use a wheel chair. You cannot climb stairs.
You need help to dress, bathe, and use the bathroom. You need help preparing and eating food.
You might have difficulty speaking or finding the right words.
Amputation
Imagine a life after you have lost part of your lower leg or foot:
You may be able to walk with an artificial leg, or you may have to use a wheelchair to get around.
You might have some difficulty performing daily tasks such as driving, shopping, or cleaning your house.
The JDRF Continuous Glucose Monitoring Study Group
Clinical Centers: Listed in order of number of patients enrolled with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators:
Diabetes Care Center, University of Washington, Seattle, WA: Irl B. Hirsch, M.D. (PI); Lisa K. Gilliam, M.D., Ph.D. (I); Kathy Fitzpatrick, R.N., M.N., C.D.E. (C); Dori Khakpour, R.D., C.D., C.D.E. (C); Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, M.D. (PI); William V. Tamborlane, M.D. (I); Brett Ives, M.S.N., A.P.R.N. (C); Joan Bosson-Heenan (C); Adult Section, Joslin Diabetes Center, Boston, MA: Howard Wolpert, M.D. (PI); Greeshma Shetty, M.D. (I); Astrid Atakov-Castillo (C); Judith Giusti, M.S., R.D., L.D.N., C.D.E. (C); Stacey O'Donnell, R.N., C.D.E. (C); Suzanne Ghiloni, R.N., C.D.E. (C); Atlanta Diabetes Associates, Atlanta, GA: Bruce W. Bode, M.D. (PI); Kelli O'Neil, C.D.E. (C); Lisa Tolbert, R.N., M.N., C.D.E. (C); Nemours Children's Clinic, Jacksonville, FL: Tim Wysocki, Ph.D. (co-PI); Larry A. Fox, M.D. (co-PI); Nelly Mauras, M.D. (I); Kimberly Englert, R.N. (C); Joe Permuy, M.S.N., A.R.N.P. (C); Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce Buckingham, M.D. (PI); Darrell M. Wilson, M.D. (I); Jennifer Block, R.N., C.D.E. (C); Kari Benassi, R.N., N.P. (C); Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, M.D. (PI); Michael Tansey, M.D. (I); Debra Kucera, A.R.N.P., C.P.N.P. (C); Julie Coffey, A.R.N.P., C.P.N.P. (C); Joanne Cabbage (C); Pediatric Adolescent, and Young Adult Section, Joslin Diabetes Center, Boston, MA: Lori Laffel, M.D., M.P.H., (PI), Kerry Milaszewski, R.N., C.D.E. (C); Katherine Pratt (C); Elise Bismuth, M.D., M.S., (C); Joyce Keady, M.S.N., C.P.N.P. (C); Margie Lawlor, M.S., C.D.E. (C); Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, M.D. (PI); Rosanna Fiallo-Scharer, M.D. (I); Paul Wadwa, M.D. (I); Laurel Messer, R.N., C.D.E. (C); Victoria Gage, R.N. (C); Patricia Burdick (C); Departments of Pediatric Endocrinology and Research and Evaluation, Kaiser Permanente, San Diego and Pasadena, CA: Jean M. Lawrence, Sc.D., M.P.H., M.S.S.A. (co-PI); Robert Clemons, M.D. (co-PI); Michelle Maeva, R.N., C.D.E. (C); Bonnie Sattler, M.S., R.D. (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, M.D., Ph.D.; Katrina J. Ruedy, M.S.P.H.; Craig Kollman, Ph.D.; Dongyuan Xing, M.P.H.; Judy Sibayan University of Minnesota Central Laboratory: Michael Steffes, M.D., Ph.D., Jean M. Bucksa, C.L.S., Maren L. Nowicki, C.L.S., Carol Van Hale, C.L.S., Vicky Makky, C.L.S.
Cost-effectiveness investigators: National Opinion Research Center, University of Chicago: Michael O'Grady, Ph.D.; Elbert S. Huang, M.D., M.P.H.; Anirban Basu, Ph.D.; David O. Meltzer, M.D., Ph.D.; Priya John, M.P.P., Aaron Winn, M.P.P., Kirsten Rhee, B.A. University of Michigan: Joyce M. Lee, M.D., M.P.H.
Juvenile Diabetes Research Foundation, Inc.: Aaron J. Kowalski, Ph.D.
Operations Committee: Lori Laffel, M.D., M.P.H. (co-chair), William V. Tamborlane, M.D. (co-chair), Roy W. Beck, M.D., Ph.D., Aaron J. Kowalski, Ph.D., Katrina J. Ruedy, M.S.P.H.
Data and Safety Monitoring Board: Ruth S. Weinstock, M.D., Ph.D. (chair), Barbara J Anderson, Ph.D.; Davida Kruger, M.S.N., A.P.R.N.; Lisa LaVange, Ph.D.; Henry Rodriguez, M.D.
References
- 1.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Gold MR, Siegel JE, Russell LB, et al. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 5.Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17:895–913. doi: 10.1007/s11136-008-9350-5. [DOI] [PubMed] [Google Scholar]
- 6.Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). U.K. Prospective Diabetes Study Group. Diabetes Care. 1999;22:1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 7.Huang ES, Brown SE, Ewigman BG, et al. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30:2478–2483. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu SY, Sainfort F, Tomar RH, et al. Development and application of a model to estimate the impact of type 1 diabetes on health-related quality of life. Diabetes Care. 1998;21:725–731. doi: 10.2337/diacare.21.5.725. [DOI] [PubMed] [Google Scholar]
- 9.Coffey JT, Brandle M, Zhou H, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25:2238–2243. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 10.Currie CJ, Poole CD, Woehl A, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia. 2006;49:2272–2280. doi: 10.1007/s00125-006-0380-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee AJ, Morgan CL, Morrissey M, et al. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with Type 1 diabetes, Type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22:1482–1486. doi: 10.1111/j.1464-5491.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 12.Landy J, Stein J, Brown MM, et al. Patient, community and clinician perceptions of the quality of life associated with diabetes mellitus. Med Sci Monit. 2002;8:CR543–548. [PubMed] [Google Scholar]
- 13.Chancellor J, Aballea S, Lawrence A, et al. Preferences of patients with diabetes mellitus for inhaled versus injectable insulin regimens. Pharmacoeconomics. 2008;26:217–234. doi: 10.2165/00019053-200826030-00005. [DOI] [PubMed] [Google Scholar]
- 14.Brown GC, Brown MM, Sharma S, et al. Quality of life associated with diabetes mellitus in an adult population. J Diabetes Complications. 2000;14:18–24. doi: 10.1016/s1056-8727(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 15.JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10:310–321. doi: 10.1089/dia.2007.0302. [DOI] [PubMed] [Google Scholar]
- 16.The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378–1383. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang ES, O'Grady M, Basu A, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 33:1269–1274. doi: 10.2337/dc09-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40:113–128. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Huang ES, Jin L, Shook M, et al. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new onset diabetes. Diabetes Care. 2006;29:259–264. doi: 10.2337/diacare.29.02.06.dc05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang ES, Brown SE, Ewigman BG, et al. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007:2478–2483. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21:587–611. doi: 10.1146/annurev.publhealth.21.1.587. [DOI] [PubMed] [Google Scholar]
- 22.Supina AL, Feeny DH, Carroll LJ, et al. Misinterpretation with norm-based scoring of health status in adults with type 1 diabetes. Health Qual Life Outcomes. 2006;4:15. doi: 10.1186/1477-7525-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo N, Johnson JA, Shaw JW, et al. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43:1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 24.Bowker SL, Pohar SL, Johnson JA. A cross-sectional study of health-related quality of life deficits in individuals with comorbid diabetes and cancer. Health Qual Life Outcomes. 2006;4:17. doi: 10.1186/1477-7525-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser AW, Furlong W, Walker DA, et al. Applicability of the Health Utilities Index to a population of childhood survivors of central nervous system tumours in the UK. Eur J Cancer. 1999;35:256–261. doi: 10.1016/s0959-8049(98)00367-0. [DOI] [PubMed] [Google Scholar]
- 26.Saigal S, Stoskopf BL, Feeny D, et al. Differences in preferences for neonatal outcomes among health care professionals, parents, and adolescents. Jama. 1999;281:1991–1997. doi: 10.1001/jama.281.21.1991. [DOI] [PubMed] [Google Scholar]
- 27.Sung L, Young NL, Greenberg ML, et al. Health-related quality of life (HRQL) scores reported from parents and their children with chronic illness differed depending on utility elicitation method. J Clin Epidemiol. 2004;57:1161–1166. doi: 10.1016/j.jclinepi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Arnold D, Girling A, Stevens A, et al. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339:b2688. doi: 10.1136/bmj.b2688. [DOI] [PubMed] [Google Scholar]
- 29.Feeny D, Furlong W, Saigal S, et al. Comparing directly measured standard gamble scores to HUI2 and HUI3 utility scores: group- and individual-level comparisons. Soc Sci Med. 2004;58:799–809. doi: 10.1016/s0277-9536(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 30.Krahn M, Ritvo P, Irvine J, et al. Patient and community preferences for outcomes in prostate cancer: implications for clinical policy. Med Care. 2003;41:153–164. doi: 10.1097/00005650-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Jones AM. The Elgar companion to health economics. Cheltenham, UK; Northampton, MA: Edward Elgar; 2006. [Google Scholar]
- 32.Prosser LA. Current challenges and future research in measuring preferences for pediatric health outcomes. J Pediatr. 2009;155:7–9. doi: 10.1016/j.jpeds.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics. 2005;115:e600–614. doi: 10.1542/peds.2004-2127. [DOI] [PubMed] [Google Scholar]


