Abstract
Objective
To compare test performance of hemoglobin A1c (HbA1c) for detecting diabetes/prediabetes for US adolescents vs. adults.
Study design
Individuals were defined as having diabetes (fasting plasma glucose (FPG) ≥126 mg/dl; 2-hour plasma glucose (2-hr PG) ≥200 mg/dl) or prediabetes (100≤FPG<126 mg/dl; 140≤2-hr PG<200 mg/dl). HbA1c test performance was evaluated using receiver operator characteristic (ROC) analyses.
Results
Few adolescents had undiagnosed diabetes (n=4). When assessing FPG to detect diabetes, an HbA1c of 6.5% had sensitivities of 75.0% (30.1–95.4) and 53.8% (47.4–60.0) and specificities of 99.9% (99.5–100.0) and 99.5% (99.3–99.6) for adolescents and adults, respectively. Additionally, when assessing FPG to detect diabetes, an HbA1c of 5.7% had sensitivities of 5.0% (2.6–9.2) and 23.1% (21.3–25.0) and specificities of 98.3% (97.2–98.9) and 91.1% (90.3–91.9) for adolescents and adults, respectively. ROC analyses suggested that HbA1c is a poorer predictor of diabetes (AUC 0.88 vs. 0.93), and prediabetes (FPG: AUC 0.61 vs. 0.74) for adolescents compared with adults. Performance was poor regardless of using FPG or 2-hr PG measurements.
Conclusions
Use of HbA1c for diagnosis of diabetes and prediabetes in adolescents may be premature, until information from more definitive studies is available.
Keywords: HbA1c, diabetes, prediabetes, overweight, obese, children
In 2009, an International Expert Committee consisting of experts from the American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation was convened to assess the role of the Hemoglobin HbA1c (HbA1c) for the diagnosis of diabetes.1 Traditionally, for both adolescents and adults, a diagnosis of diabetes was determined based on a fasting plasma glucose (FPG) level or a 2-hour plasma glucose (2-hr PG) level after a 75 gram load of glucose.2 Both tests require that individuals be fasting, an inconvenience in the clinical setting that may lead to lower testing rates and possible under-diagnosis of diabetes in the population.3 HbA1c is a measure of longer-term glycemia which does not require fasting, and at specific thresholds has been associated with increased rates of diabetes complications (i.e. retinopathy).2 Therefore, the American Diabetes Association recommended that HbA1c be preferentially used for diagnosis of diabetes in the clinical setting, phasing out both the FPG and the 2-hr PG measurements. According to the new guidelines, asymptomatic individuals would be classified as having diabetes if they had HbA1c values of 6.5% or greater on two separate occasions. Individuals with an HbA1c ≥6.0% (International Expert Committee recommendations)1 or HbA1c ≥5.7% (ADA recommendations)4 are considered to be at increased risk for diabetes and targeted for diabetes prevention interventions.
The committee recommended that HbA1c testing also be used for diagnostic purposes in asymptomatic adolescents; however it is unclear whether similar HbA1c cut points are appropriate for the pediatric and the adult populations. Both of the HbA1c thresholds selected were based on studies performed exclusively in adults,5, 6 without consideration of studies from the pediatric population. Therefore, the objective of our study was to assess the utility of the new HbA1c guidelines for diagnosis of diabetes among asymptomatic US adolescents from a nationally representative sample.
Methods
Our data source was the National Health and Nutrition Examination Surveys (NHANES 1999–2006), a cross-sectional, nationally representative examination study of the US civilian non-institutionalized population. NHANES has a stratified multistage probability sampling design,7 which oversamples adolescents, non-Hispanic black and Mexican-American individuals to provide reliable statistical estimates.
We focused on individuals who had both FPG and HbA1c measures, and for a subanalysis of individuals with a 2-hr PG and HbA1c. Both FPG and the 2-hr PG have limitations for identifying diabetes due to poor concordance8 and lack of reproducibility.9, 10 However, because abnormal levels of FPG or 2-hr PG were the recommended tests for diagnosis of diabetes and we use them as the best method to compare with HbA1c. Procedures regarding assessment of fasting status, blood collection, sample processing, and analysis of FPG and 2-hr PG in NHANES have been described in previous publications.11, 12 HbA1c was measured using two High Performance Liquid Chromatography systems (Primus Corporation, Kansas City, MO and Tosoh Medics, Inc., San Francisco, CA, respectively), which were standardized to the reference method used for the Diabetes Control and Complications Trial.13
Of the 21056 and 6873 subjects aged 12–79 years from NHANES 1999–2004 and NHANES 2005–2006, respectively, we evaluated individuals with both HbA1c and FPG measures during the morning examination after fasting for a minimum of 8 hours. We excluded subjects who were pregnant at the time of the exam (n=1350) or who reported a previous diagnosis of diabetes (n=1824). We analyzed data on 1156 overweight and obese adolescents aged 12–18 years as the ADA recommends that only overweight and obese adolescents be screened,14 and compared them with 6751 adults aged 19–79 years.4 Our adult population included normal weight as well as overweight/obese individuals given that adults regardless of weight can be targeted for screening.4 Furthermore, we also conducted our analyses on a subpopulation of adults aged 45–79 given the recommendation to screen adults 45 years and older. Because these findings were similar to those for our entire adult population, the results are not shown. In addition, we analyzed data from a subsample of 267 adolescents and 1476 adults who had 2-hr PG measures from an oral glucose tolerance test (OGTT) during NHANES 2005–2006.
Our outcomes of interest were diabetes and prediabetes, based on definitions using both FPG, which was available for all study years, as well as 2-hr PG, which was available for NHANES 2005–2006. Individuals were classified as having diabetes if they had an FPG ≥126 mg/dl or prediabetes if they had an FPG ≥100 mg/dl and <126 mg/dl. In the 2-hr PG subsample, individuals were classified as having diabetes if they had a 2-hr PG ≥200 mg/dl or prediabetes if they had a 2-hr PG ≥140 mg/dl and <200 mg/dl. To evaluate the International Expert Committee and the ADA’s new recommendations, we first calculated sensitivity, specificity, positive and negative predictive values15 at an HbA1c threshold of 6.5% for diabetes and thresholds of 6.0% (International Expert Committee) and 5.7% (ADA) for prediabetes, separately for adolescents and adults.
We then created receiver operator characteristic (ROC) curves evaluating the test performance of various HbA1c level cut points for detecting diabetes and prediabetes for adolescents vs. adults. ROC analysis is a formal method of assessing the trade-offs between sensitivity and specificity at various test cut points or thresholds,16 providing a measure of diagnostic accuracy called area under the curve (AUC). Tests with an AUC close to 0.5 have very poor discrimination, whereas tests with an AUC close to 1.0 have excellent discrimination.17 We also tested the equality of AUC for adolescents vs. adults. For the ROC analyses for prediabetes, we combined the outcomes of prediabetes and diabetes.
Statistical analyses were performed using Stata 9 (Stata Corporation, College Station, TX, USA) which applies the appropriate sampling weights to adjust for the complex multi-cluster sample design. We used Taylor series linearization for variance estimation. For description of the sample which required the availability of survey weights, we reported demographics separately for NHANES 1999–2004 and 2005–2006. However, for assessing test performance, we combined individuals across all surveys.
Results
The demographic characteristics of the overall study population, which includes weighted estimates of the proportion of individuals with prediabetes and previously undiagnosed diabetes based on FPG or 2-hr PG levels, are presented in Table I. Compared with adults, few adolescents had undiagnosed diabetes, and there were no cases identified with the 2-hr PG (Table II). Although there were substantially more adolescents with prediabetes (based on either FPG or 2-hr PG measures) than with undiagnosed diabetes, prediabetic adolescents were still fewer in number than prediabetic adults (Table II).
Table 1.
Characteristics of the study population (n(weighted %))
| Adolescents (12–18 years)* | Adults (19–79 years) | |||||
|---|---|---|---|---|---|---|
| Fasting plasma glucose | 2-hour PG | Fasting plasma glucose | 2-hour PG | |||
| Survey | 1999–2004 (n=874) |
2005–2006 (n=282) |
2005–2006 (n=267) |
1999–2004 (n=5139) |
2005–2006 (n=1612) |
2005–2006 (n=1476) |
| Sex | ||||||
| Male | 467 (56.1) | 129 (48.1) | 123 (47.8) | 2610 (49.7) | 860 (51.8) | 794 (48.9) |
| Female | 407 (43.9) | 153 (51.9) | 144 (52.2) | 2529 (50.3) | 752 (48.2) | 682 (51.1) |
| Race | ||||||
| White | 185 (58.0) | 56 (58.1) | 52 (58.1) | 2501 (72.8) | 772 (72.3) | 715 (72.1) |
| Black | 288 (17.5) | 93 (17.3) | 87 (17.9) | 1008 (10.0) | 377 (10.8) | 329 (10.7) |
| Mexican American | 338 (13.3) | 114 (16.4) | 110 (15.1) | 1229 (7.4) | 332 (7.8) | 316 (8.1) |
| Other | 63 (11.2) | 19 (8.2) | 18 (8.9) | 401 (9.8) | 131 (9.1) | 116 (9.1) |
| Age (years) | ||||||
| 19–29 | - | - | - | 1206 (20.6) | 417 (20.8) | 358 (21.0) |
| 30–39 | - | - | - | 900 (21.4) | 287 (19.8) | 268 (21.6) |
| 40–49 | - | - | - | 958 (23.0) | 315 (23.6) | 295 (22.2) |
| 50–59 | - | - | - | 704 (17.1) | 219 (17.8) | 210 (17.5) |
| 60–69 | - | - | - | 811 (10.8) | 216 (10.8) | 200 (10.8) |
| 70–79 | - | - | - | 560 (7.1) | 158 (7.2) | 145 (6.9) |
| Weight status** | ||||||
| Normal weight | - | - | - | 1749 (36.4) | 531 (34.4) | 484 (34.5) |
| Overweight | 422 (48.8) | 131 (49.6) | 121 (47.2) | 1780 (34.8) | 520 (32.1) | 479 (31.3) |
| Obese | 452 (51.2) | 151 (50.4) | 146 (52.8) | 1535 (28.8) | 545 (33.5) | 505 (34.2) |
| Glucose tolerance status | ||||||
| Normal | 756 (86.5) | 215 (71.8) | 247 (92.3) | 3452 (69.5) | 959 (61.8) | 1158 (80.5) |
| Impaired fasting glucose/glucose tolerance (prediabetes) | 115 (12.9) | 66 (28.1) | 20 (7.7) | 1506 (27.7) | 594 (35.1) | 227 (14.3) |
| Undiagnosed Diabetes | 3 (0.6) | 1 (0.1) | 0 (0.0) | 181 (2.8) | 59 (3.1) | 91 (5.2) |
Describes demographic characteristics for only overweight and obese children in the sample.
Proportions are calculated based only on overweight and obese children.
Table 2.
Test performance characteristics of an HbA1c threshold of 6.5% for detecting diabetes according to fasting plasma glucose (FPG) or 2-hour plasma glucose (2-hr PG), and HbA1c thresholds of 6.0% and 5.7% for detecting prediabetes according to FPG or 2-hr PG in adolescents and adults.
| Criterion | Age Group | Total n | Cases n | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV | NPV |
|---|---|---|---|---|---|---|---|
| Diabetes (HbA1c 6.5%) | |||||||
| Fasting plasma glucose |
Adolescents | 1156 | 4 | 75.0% (30.1–95.4) |
99.9% (99.5–100.0) |
75.0% | 99.9% |
| Adults | 6751 | 240 | 53.8% (47.4–60.0) |
99.5% (99.3–99.6) |
79.1% | 98.3% | |
| 2-hour plasma glucose |
Adolescents | 267 | 0 | - | - | - | - |
| Adults | 1476 | 91 | 30.8% (22.2–40.9) |
99.6% (99.2–99.9) |
84.8% | 95.6% | |
| Prediabetes (HbA1c 6.0%) | |||||||
| Fasting plasma glucose |
Adolescents | 1156 | 181 | 1.1% (0.3–3.9) |
99.4% (98.7–99.7) |
25.0% | 84.4% |
| Adults | 6751 | 2100 | 11.4% (10.1–12.9) |
94.7% (94.0–95.3) |
49.3% | 70.3% | |
| 2-hour plasma glucose |
Adolescents | 267 | 20 | 0.0% (0.0–16.1) |
99.6% (97.7–99.9) |
0.0% | 92.5% |
| Adults | 1476 | 227 | 13.2% (9.4–18.2) |
93.0% (91.4–94.3) |
25.4% | 85.5% | |
| Prediabetes (HbA1c 5.7%) | |||||||
| Fasting plasma glucose |
Adolescents | 1156 | 181 | 5.0% (2.6–9.2) |
98.3% (97.2–98.9) |
34.6% | 84.8% |
| Adults | 6751 | 2100 | 23.1% (21.3–25.0) |
91.1% (90.3–91.9) |
54.0% | 72.4% | |
| 2-hour plasma glucose |
Adolescents | 267 | 20 | 0.0% (0.0–16.1) |
97.6% (94.8–98.9) |
0.0% | 92.3% |
| Adults | 1476 | 227 | 26.9% (21.5–33.0) |
87.2% (85.2–88.9) |
27.6% | 86.8% | |
Table II shows estimates of test performance of an HbA1c cut point of 6.5% for detecting diabetes and cut points of 6.0% and 5.7% for detecting prediabetes as defined by FPG or 2-hr PG, in adolescents and adults. For detecting diabetes, an HbA1c cut point of 6.5% resulted in a higher point estimate for sensitivity for adolescents compared with adults; however, the confidence intervals for sensitivity in adolescents were quite large, related to the small number with diabetes (n=4). Estimates of specificity were similar for both groups, and PPV was lower and NPV was higher for adolescents due to their lower prevalence of disease.
For detecting prediabetes based on FPG, an HbA1c cut point of 6.0% resulted in a lower estimate of sensitivity for adolescents compared with adults. As with diabetes, specificity was similar for both groups, and again, PPV was lower and NPV was higher for adolescents compared with adults, due to the low prevalence of prediabetes among adolescents. For detecting diabetes based on FPG, an HbA1c cut point of 5.7% resulted in slightly higher estimates of sensitivity, slightly lower estimates of specificity, and a slightly higher PPV. HbA1c under-diagnosed prediabetes even more severely based on 2-hr PG results compared with FPG results.
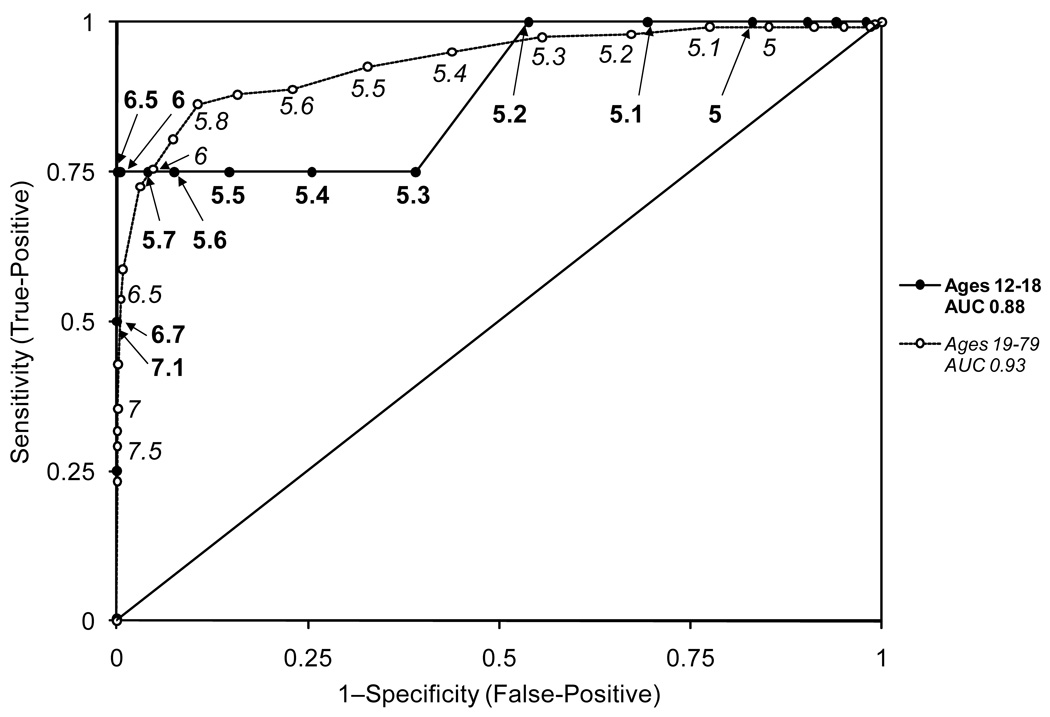
Figure 1 shows the ROC curves for predicting diabetes (FPG ≥126 mg/dl) in adolescents and adults at various thresholds of HbA1c. HbA1c was not as good a predictor of diabetes for adolescents (AUC 0.88 (95% CI 0.66–1.00)) compared with adults (AUC 0.93 (95% CI 0.91–0.95)) although the difference was not statistically significant (p=0.68).
Figure 1.
Receiver operator characteristic curves of various thresholds of HbA1c for predicting diabetes (defined using fasting plasma glucose ≥126 mg/dl) in adolescents (AUC 0.88 (95% CI 0.66–1.00)) and adults (AUC 0.93 (95% CI 0.91–0.95)).
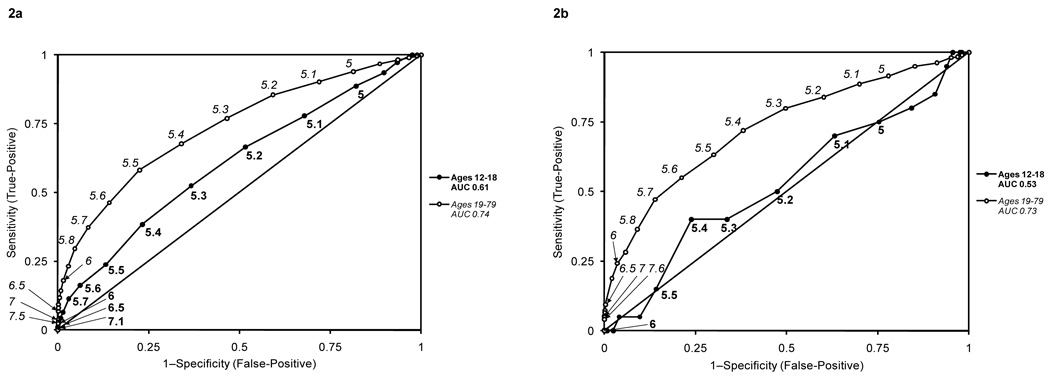
Figure 2 shows the ROC curves for predicting prediabetes using either FPG or 2-hr PG in adolescents and adults at various thresholds of HbA1c. HbA1c was a poor predictor of prediabetes for adolescents compared with adults using either FPG (AUC 0.61 (95% CI 0.56–0.65) (adolescents) vs. 0.74 (95% CI 0.72–0.75) (adults),p<0.01) or 2-hr PG (AUC 0.53 (95% CI 0.39–0.67) (adolescents) vs. 0.73 (95% CI 0.70–0.76) (adults), p<0.01).
Figure 2.
Receiver operator characteristic curves of various thresholds of HbA1c for predicting prediabetes in adolescents and adults. Prediabetes was defined either by a fasting plasma glucose ≥100 mg/dl [AUC 0.61 (95% CI 0.56–0.65) (adolescents) vs. 0.74 (95% CI 0.72–0.75) (adults)] (2a) or 2-hour plasma glucose ≥140 mg/dl [AUC 0.53 (95% CI 0.39–0.67) (adolescents) vs. 0.73 (95% CI 0.70–0.76) (adults)] (2b).
The summary of test performance characteristics predicting diabetes and prediabetes across HbA1c thresholds are shown in Table III (available at www.jpeds.com).
Table III.
Test performance characteristics of specific HbA1c thresholds for detecting diabetes according to fasting plasma glucose (FPG) and prediabetes according to FPG or 2-hour plasma glucose (2-hr PG) in adolescents and adults. At certain HbA1c thresholds there were no individuals with those HbA1c values.
| Diabetes (FPG), Adolescents | Diabetes (FPG), Adults | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c Threshold |
Sensitivity (True- Positive Rate) |
Specificity (True- Negative Rate) |
Positive Likelihood Ratio |
Positive Predictive Value |
Negative Predictive Value |
Sensitivity (True- Positive Rate) |
Specificity (True- Negative Rate) |
Positive Likelihood Ratio |
Positive Predictive Value |
Negative Predictive Value |
| 3.6 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.04 | |
| 3.8 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.04 | 1.00 |
| 4.0 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 0.04 | 1.00 | |
| 4.1 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 1.00 | 0.00 | 1.00 | 0.04 | 1.00 |
| 4.2 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.04 | 1.00 |
| 4.3 | 1.00 | 0.00 | 1.00 | 0.00 | 1.00 | 1.00 | 0.00 | 1.00 | 0.04 | 1.00 |
| 4.4 | 1.00 | 0.01 | 1.01 | 0.00 | 1.00 | 1.00 | 0.01 | 1.01 | 0.04 | 1.00 |
| 4.5 | 1.00 | 0.01 | 1.01 | 0.00 | 1.00 | 1.00 | 0.01 | 1.01 | 0.04 | 0.98 |
| 4.6 | 1.00 | 0.02 | 1.02 | 0.00 | 1.00 | 0.99 | 0.02 | 1.01 | 0.04 | 0.98 |
| 4.7 | 1.00 | 0.03 | 1.04 | 0.00 | 1.00 | 0.99 | 0.03 | 1.02 | 0.04 | 0.99 |
| 4.8 | 1.00 | 0.06 | 1.06 | 0.00 | 1.00 | 0.99 | 0.05 | 1.04 | 0.04 | 0.99 |
| 4.9 | 1.00 | 0.10 | 1.11 | 0.00 | 1.00 | 0.99 | 0.09 | 1.09 | 0.04 | 1.00 |
| 5.0 | 1.00 | 0.17 | 1.20 | 0.00 | 1.00 | 0.99 | 0.15 | 1.16 | 0.04 | 1.00 |
| 5.1 | 1.00 | 0.31 | 1.44 | 0.00 | 1.00 | 0.99 | 0.23 | 1.28 | 0.05 | 1.00 |
| 5.2 | 1.00 | 0.46 | 1.86 | 0.01 | 1.00 | 0.98 | 0.33 | 1.46 | 0.05 | 1.00 |
| 5.3 | 0.75 | 0.61 | 1.92 | 0.01 | 1.00 | 0.98 | 0.44 | 1.75 | 0.06 | 1.00 |
| 5.4 | 0.75 | 0.74 | 2.94 | 0.01 | 1.00 | 0.95 | 0.56 | 2.17 | 0.07 | 1.00 |
| 5.5 | 0.75 | 0.85 | 5.11 | 0.02 | 1.00 | 0.93 | 0.67 | 2.82 | 0.09 | 1.00 |
| 5.6 | 0.75 | 0.93 | 10.05 | 0.03 | 1.00 | 0.89 | 0.77 | 3.87 | 0.12 | 0.99 |
| 5.7 | 0.75 | 0.96 | 18.38 | 0.06 | 1.00 | 0.88 | 0.84 | 5.56 | 0.17 | 0.99 |
| 5.8 | 0.75 | 0.98 | 37.57 | 0.12 | 1.00 | 0.86 | 0.89 | 8.13 | 0.23 | 0.99 |
| 5.9 | 0.75 | 0.99 | 72.00 | 0.20 | 1.00 | 0.80 | 0.93 | 11.00 | 0.29 | 0.99 |
| 6.0 | 0.75 | 1.00 | 172.80 | 0.38 | 1.00 | 0.75 | 0.95 | 16.05 | 0.37 | 0.99 |
| 6.1 | 0.75 | 1.00 | 287.99 | 0.50 | 1.00 | 0.73 | 0.97 | 23.96 | 0.47 | 0.99 |
| 6.2 | 0.75 | 1.00 | 432.01 | 0.60 | 1.00 | 0.67 | 0.98 | 31.92 | 0.54 | 0.99 |
| 6.3 | - | - | - | - | - | 0.63 | 0.99 | 53.90 | 0.67 | 0.99 |
| 6.4 | - | - | - | - | - | 0.59 | 0.99 | 73.56 | 0.73 | 0.98 |
| 6.5 | 0.75 | 1.00 | 863.98 | 0.75 | 1.00 | 0.54 | 0.99 | 102.93 | 0.79 | 0.98 |
| 6.6 | - | - | - | - | - | 0.49 | 1.00 | 151.15 | 0.85 | 0.98 |
| 6.7 | 0.50 | 1.00 | 575.99 | 0.67 | 1.00 | 0.46 | 1.00 | 165.79 | 0.86 | 0.98 |
| 6.8 | - | - | - | - | - | 0.43 | 1.00 | 254.03 | 0.90 | 0.98 |
| 6.9 | - | - | - | - | - | 0.39 | 1.00 | 255.03 | 0.90 | 0.98 |
| 7.0 | - | - | - | - | - | 0.35 | 1.00 | 288.24 | 0.91 | 0.98 |
| 7.1 | 0.50 | 1.00 | - | 1.00 | 1.00 | 0.34 | 1.00 | 313.94 | 0.92 | 0.98 |
| 7.5 | - | - | - | - | - | 0.29 | 1.00 | 474.78 | 0.95 | 0.97 |
| 8.0 | - | - | - | - | - | 0.23 | 1.00 | 379.82 | 0.93 | 0.97 |
| 8.4 | - | - | - | - | - | 0.20 | 1.00 | 664.69 | 0.96 | 0.97 |
| 9.0 | - | - | - | - | - | 0.16 | 1.00 | 1000.00 | 0.97 | 0.97 |
| 9.3 | 0.25 | 1.00 | - | 1.00 | 1.00 | - | - | - | - | - |
| 9.4 | - | - | - | - | - | 0.13 | 1.00 | 868.17 | 0.97 | 0.97 |
| 10.0 | - | - | - | - | - | 0.11 | 1.00 | 732.52 | 0.96 | 0.97 |
| 10.4 | - | - | - | - | - | 0.11 | 1.00 | 705.39 | 0.96 | 0.97 |
| 10.9 | - | - | - | - | - | 0.09 | 1.00 | 596.87 | 0.96 | 0.97 |
| 11.5 | - | - | - | - | - | 0.06 | 1.00 | 1.00 | 0.97 | |
| 11.9 | - | - | - | - | - | 0.04 | 1.00 | 1.00 | 0.97 | |
| 12.4 | - | - | - | - | - | 0.03 | 1.00 | 1.00 | 0.97 | |
| 12.9 | - | - | - | - | - | 0.01 | 1.00 | 1.00 | 0.96 | |
| 13.5 | - | - | - | - | - | 0.01 | 1.00 | 1.00 | 0.96 | |
| 14.0 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.96 | |
| Prediabetes (FPG), Adolescents | Prediabetes (FPG), Adults | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c Threshold |
Sensitivity (True- Positive Rate) |
Specificity (True- Negative Rate) |
Positive Likelihood Ratio |
Positive Predictive Value |
Negative Predictive Value |
Sensitivity (True- Positive Rate) |
Specificity (True- Negative Rate) |
Positive Likelihood Ratio |
Positive Predictive Value |
Negative Predictive Value |
| 3.6 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.35 | |
| 3.8 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.35 | 0.00 |
| 4.0 | 1.00 | 0.00 | 1.00 | 0.16 | 1.00 | 0.00 | 1.00 | 0.35 | 0.50 | |
| 4.1 | 1.00 | 0.00 | 1.00 | 0.16 | 1.00 | 1.00 | 0.00 | 1.00 | 0.35 | 0.71 |
| 4.2 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.35 | 0.73 |
| 4.3 | 1.00 | 0.01 | 1.01 | 0.16 | 1.00 | 1.00 | 0.00 | 1.00 | 0.35 | 0.85 |
| 4.4 | 1.00 | 0.01 | 1.01 | 0.16 | 1.00 | 1.00 | 0.01 | 1.00 | 0.35 | 0.83 |
| 4.5 | 1.00 | 0.01 | 1.01 | 0.16 | 1.00 | 1.00 | 0.01 | 1.01 | 0.35 | 0.82 |
| 4.6 | 1.00 | 0.02 | 1.03 | 0.16 | 1.00 | 0.99 | 0.02 | 1.01 | 0.35 | 0.83 |
| 4.7 | 0.99 | 0.04 | 1.03 | 0.16 | 0.95 | 0.99 | 0.03 | 1.02 | 0.35 | 0.86 |
| 4.8 | 0.97 | 0.07 | 1.04 | 0.17 | 0.93 | 0.98 | 0.06 | 1.05 | 0.36 | 0.87 |
| 4.9 | 0.94 | 0.10 | 1.04 | 0.17 | 0.89 | 0.97 | 0.11 | 1.09 | 0.37 | 0.87 |
| 5.0 | 0.89 | 0.18 | 1.08 | 0.17 | 0.89 | 0.94 | 0.19 | 1.16 | 0.38 | 0.85 |
| 5.1 | 0.78 | 0.32 | 1.15 | 0.18 | 0.88 | 0.90 | 0.28 | 1.26 | 0.40 | 0.84 |
| 5.2 | 0.66 | 0.48 | 1.29 | 0.20 | 0.88 | 0.86 | 0.41 | 1.45 | 0.43 | 0.84 |
| 5.3 | 0.52 | 0.63 | 1.43 | 0.21 | 0.87 | 0.77 | 0.53 | 1.65 | 0.47 | 0.81 |
| 5.4 | 0.38 | 0.77 | 1.65 | 0.24 | 0.87 | 0.68 | 0.66 | 1.99 | 0.51 | 0.79 |
| 5.5 | 0.24 | 0.87 | 1.80 | 0.26 | 0.86 | 0.58 | 0.77 | 2.58 | 0.58 | 0.78 |
| 5.6 | 0.16 | 0.94 | 2.67 | 0.34 | 0.85 | 0.46 | 0.86 | 3.27 | 0.63 | 0.75 |
| 5.7 | 0.11 | 0.97 | 3.80 | 0.42 | 0.85 | 0.37 | 0.92 | 4.45 | 0.70 | 0.73 |
| 5.8 | 0.06 | 0.99 | 4.50 | 0.46 | 0.85 | 0.30 | 0.95 | 6.33 | 0.77 | 0.72 |
| 5.9 | 0.04 | 0.99 | 6.00 | 0.53 | 0.84 | 0.23 | 0.97 | 8.04 | 0.81 | 0.70 |
| 6.0 | 0.03 | 1.00 | 8.75 | 0.63 | 0.84 | 0.18 | 0.99 | 12.02 | 0.86 | 0.69 |
| 6.1 | 0.02 | 1.00 | 10.50 | 0.67 | 0.84 | 0.14 | 0.99 | 17.54 | 0.90 | 0.69 |
| 6.2 | 0.02 | 1.00 | 21.00 | 0.80 | 0.84 | 0.12 | 1.00 | 24.69 | 0.93 | 0.68 |
| 6.3 | - | - | - | - | - | 0.09 | 1.00 | 45.66 | 0.96 | 0.67 |
| 6.4 | - | - | - | - | - | 0.08 | 1.00 | 58.75 | 0.97 | 0.67 |
| 6.5 | 0.02 | 1.00 | 15.75 | 0.75 | 0.84 | 0.07 | 1.00 | 59.57 | 0.97 | 0.67 |
| 6.6 | - | - | - | - | - | 0.06 | 1.00 | 50.14 | 0.96 | 0.67 |
| 6.7 | 0.01 | 1.00 | 10.50 | 0.67 | 0.84 | 0.05 | 1.00 | 58.44 | 0.97 | 0.67 |
| 6.8 | - | - | - | - | - | 0.05 | 1.00 | 51.84 | 0.96 | 0.66 |
| 6.9 | - | - | - | - | - | 0.04 | 1.00 | 47.13 | 0.96 | 0.66 |
| 7.0 | - | - | - | - | - | 0.04 | 1.00 | 41.94 | 0.96 | 0.66 |
| 7.1 | 0.01 | 1.00 | 1.00 | 0.84 | 0.04 | 1.00 | 39.59 | 0.95 | 0.66 | |
| 7.5 | - | - | - | - | - | 0.03 | 1.00 | 32.99 | 0.95 | 0.66 |
| 8.0 | - | - | - | - | - | 0.02 | 1.00 | 26.39 | 0.93 | 0.66 |
| 8.4 | - | - | - | - | - | 0.02 | 1.00 | 46.18 | 0.96 | 0.66 |
| 9.0 | - | - | - | - | - | 0.02 | 1.00 | 71.64 | 0.97 | 0.66 |
| 9.3 | 0.01 | 1.00 | 1.00 | 0.84 | - | - | - | - | - | |
| 9.4 | - | - | - | - | - | 0.01 | 1.00 | 60.33 | 0.97 | 0.66 |
| 10.0 | - | - | - | - | - | 0.01 | 1.00 | 50.90 | 0.96 | 0.66 |
| 10.4 | - | - | - | - | - | 0.01 | 1.00 | 49.02 | 0.96 | 0.66 |
| 10.9 | - | - | - | - | - | 0.01 | 1.00 | 41.48 | 0.96 | 0.66 |
| 11.5 | - | - | - | - | - | 0.01 | 1.00 | 1.00 | 0.65 | |
| 11.9 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.65 | |
| 12.4 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.65 | |
| 12.9 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.65 | |
| 13.5 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.65 | |
| 14.0 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.65 | |
| Prediabetes (2-hr PG), Adolescents | Prediabetes (2-hr PG), Adults | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c Threshold |
Sensitivity (True- Positive Rate) |
Specificity (True- Negative Rate) |
Positive Likelihood Ratio |
Positive Predictive Value |
Negative Predictive Value |
Sensitivity (True- Positive Rate) |
Specificity (True- Negative Rate) |
Positive Likelihood Ratio |
Positive Predictive Value |
Negative Predictive Value |
| 3.8 | - | - | - | - | - | 1.00 | 0.00 | 1.00 | 0.22 | |
| 4.0 | 1.00 | 0.00 | 1.00 | 0.07 | 1.00 | 0.00 | 1.00 | 0.22 | 1.00 | |
| 4.1 | 1.00 | 0.02 | 1.02 | 0.08 | 1.00 | 1.00 | 0.00 | 1.00 | 0.22 | 1.00 |
| 4.2 | - | - | - | - | - | 1.00 | 0.01 | 1.01 | 0.22 | 1.00 |
| 4.3 | - | - | - | - | - | 1.00 | 0.01 | 1.01 | 0.22 | 1.00 |
| 4.4 | 1.00 | 0.02 | 1.02 | 0.08 | 1.00 | 1.00 | 0.01 | 1.01 | 0.22 | 1.00 |
| 4.5 | 1.00 | 0.02 | 1.02 | 0.08 | 1.00 | 0.99 | 0.02 | 1.01 | 0.22 | 0.88 |
| 4.6 | 1.00 | 0.04 | 1.05 | 0.08 | 1.00 | 0.98 | 0.03 | 1.02 | 0.22 | 0.88 |
| 4.7 | 0.95 | 0.06 | 1.01 | 0.08 | 0.94 | 0.98 | 0.05 | 1.03 | 0.22 | 0.90 |
| 4.8 | 0.85 | 0.09 | 0.94 | 0.07 | 0.88 | 0.96 | 0.09 | 1.06 | 0.22 | 0.89 |
| 4.9 | 0.80 | 0.16 | 0.95 | 0.07 | 0.91 | 0.95 | 0.15 | 1.12 | 0.23 | 0.91 |
| 5.0 | 0.75 | 0.25 | 1.00 | 0.07 | 0.92 | 0.92 | 0.22 | 1.17 | 0.24 | 0.90 |
| 5.1 | 0.70 | 0.37 | 1.11 | 0.08 | 0.94 | 0.89 | 0.30 | 1.27 | 0.26 | 0.91 |
| 5.2 | 0.50 | 0.53 | 1.06 | 0.08 | 0.93 | 0.84 | 0.40 | 1.40 | 0.28 | 0.90 |
| 5.3 | 0.40 | 0.66 | 1.19 | 0.09 | 0.93 | 0.80 | 0.50 | 1.61 | 0.31 | 0.90 |
| 5.4 | 0.40 | 0.76 | 1.67 | 0.12 | 0.94 | 0.72 | 0.62 | 1.89 | 0.34 | 0.89 |
| 5.5 | 0.15 | 0.86 | 1.06 | 0.08 | 0.93 | 0.63 | 0.70 | 2.11 | 0.37 | 0.87 |
| 5.6 | 0.05 | 0.90 | 0.51 | 0.04 | 0.92 | 0.55 | 0.79 | 2.60 | 0.42 | 0.86 |
| 5.7 | 0.05 | 0.96 | 1.24 | 0.09 | 0.93 | 0.47 | 0.86 | 3.39 | 0.48 | 0.86 |
| 5.8 | 0.00 | 0.98 | 0.00 | 0.00 | 0.92 | 0.36 | 0.91 | 4.02 | 0.52 | 0.84 |
| 5.9 | 0.00 | 0.99 | 0.00 | 0.00 | 0.92 | 0.28 | 0.94 | 4.89 | 0.57 | 0.83 |
| 6.0 | 0.00 | 1.00 | 0.00 | 0.00 | 0.92 | 0.24 | 0.96 | 6.84 | 0.65 | 0.82 |
| 6.1 | - | - | - | - | - | 0.19 | 0.98 | 9.10 | 0.71 | 0.81 |
| 6.2 | - | - | - | - | - | 0.15 | 0.99 | 10.50 | 0.74 | 0.81 |
| 6.3 | - | - | - | - | - | 0.13 | 1.00 | 29.86 | 0.89 | 0.81 |
| 6.4 | - | - | - | - | - | 0.10 | 1.00 | 23.31 | 0.86 | 0.80 |
| 6.5 | - | - | - | - | - | 0.09 | 1.00 | 36.42 | 0.91 | 0.80 |
| 6.6 | - | - | - | - | - | 0.07 | 1.00 | 41.88 | 0.92 | 0.80 |
| 6.7 | - | - | - | - | - | 0.07 | 1.00 | 76.47 | 0.95 | 0.80 |
| 6.9 | - | - | - | - | - | 0.06 | 1.00 | 65.55 | 0.95 | 0.79 |
| 7.0 | - | - | - | - | - | 0.05 | 1.00 | 1.00 | 0.79 | |
| 7.6 | - | - | - | - | - | 0.05 | 1.00 | 1.00 | 0.79 | |
| 8.0 | - | - | - | - | - | 0.04 | 1.00 | 1.00 | 0.79 | |
| 8.8 | - | - | - | - | - | 0.04 | 1.00 | 1.00 | 0.79 | |
| 9.2 | - | - | - | - | - | 0.03 | 1.00 | 1.00 | 0.79 | |
| 9.6 | - | - | - | - | - | 0.03 | 1.00 | 1.00 | 0.79 | |
| 10.0 | - | - | - | - | - | 0.02 | 1.00 | 1.00 | 0.79 | |
| 10.6 | - | - | - | - | - | 0.02 | 1.00 | 1.00 | 0.79 | |
| 11.2 | - | - | - | - | - | 0.01 | 1.00 | 1.00 | 0.79 | |
| 12.8 | - | - | - | - | - | 0.00 | 1.00 | 1.00 | 0.79 | |
Discussion
When the International Expert Committee recommended using HbA1c to diagnose diabetes in the pediatric population as in adults, it did so without considering the test performance of HbA1c in adolescents. We found that at the recommended HbA1c threshold of 6.5% for diagnosis of diabetes according to FPG in adolescents, the sensitivity estimate (75%) was highly unstable with wide confidence intervals (95% CI (30.1–95.4)). This instability is due, in large part, to the low prevalence of diabetes in the pediatric population. Moreover, our ROC curves for predicting diabetes demonstrate that HbA1c does not appear to have the same level of discrimination for adolescents compared with adults. Taken together, these findings highlight that extrapolation of adult HbA1c testing recommendations to the pediatric population is likely premature.
Alternative HbA1c thresholds to those used in adults may be useful for the pediatric population. The ROC curve for predicting diabetes in adolescents was based on a limited number of individuals, therefore additional analyses with sufficient numbers of adolescents with diabetes are necessary before any firm recommendation can be made as to what constitutes an appropriate HbA1c level for diagnosis of diabetes in this age group. However, we speculate that a lower HbA1c threshold should be explored for adolescents, given that studies have shown that HbA1c increases with age in the population.18 In fact, the committee’s choice of an HbA1c threshold of 6.5% for adults was in part based on the fact that an HbA1c of 6.5% represents just 3 standard deviations above the mean for US adults, as well as the high specificity (99.6%) and reasonable sensitivity (43–44%) at this threshold based on NHANES III and NHANES 1999–2004 data.3
The notion of a lower threshold for adolescents is supported by the findings of one recent study based on the Bogalusa Heart Study, albeit based on FPG criteria. Nguyen et al found that children with an FPG of 86 to 99 mg/dL had a greater than 2-fold risk of developing adult prediabetes and type 2 diabetes compared with children with an FPG less than 86 mg/dL, even after controlling for other traditional cardiometabolic risk factors.19 As a result, ranges that are considered “normal” for adults may in fact be abnormal for children and adolescents. However, we do acknowledge that the majority of the Bogalusa Heart Study participants with elevated FPG in childhood did not continue with prediabetes nor develop diabetes by approximately age 32 years. Therefore, some elevations in FPG in childhood may be explained by the transient insulin resistance that occurs during Tanner stages 2–4 of puberty.20
We also evaluated the performance of the recommended HbA1c thresholds of ≥5.7% or ≥6.0% for detecting individuals with prediabetes based either on a FPG or a 2-hr PG. We found that HbA1c had a much lower sensitivity for adolescents compared with adults using either of the measures. Moreover, HbA1c for predicting prediabetes in adolescents had poor sensitivity over a range of values and was generally a poor marker for detecting adolescents with prediabetes compared with adults, whether diagnosed by FPG (AUC 0.61 (adolescents) vs. 0.74 (adults) or 2-hr PG (0.53 for adolescents vs. 0.73 for adults). Even though HbA1c may be useful for detecting adults with prediabetes, it may not adequately serve as a diagnostic tool for identifying adolescents with prediabetes, given the poor concordance with 2-hr PG and FPG. Even more concerning is that by following the Committee recommendations of phasing out glucose measurements for detecting prediabetes and using HbA1c in its place, a majority of adolescents with prediabetes would be missed.
We acknowledge the practical appeal of using HbA1c over plasma glucose levels for detecting diabetes, especially in the pediatric population. Compared with HbA1c levels, plasma glucose levels are not perfectly stable and are subject to diurnal21 as well as laboratory variation.22 In contrast, the HbA1c test can be obtained non-fasting, is stable at room temperature, and has less day-to-day and within person variability. Furthermore, there has been standardization of HbA1c assays across laboratories,3 and a variety of epidemiologic studies have demonstrated a link between HbA1c levels ≥6.5% and increased rates of diabetic retinopathy among adult populations.23, 24 We also note that because of the high negative predictive values of HbA1c for predicting diabetes and prediabetes, HbA1c may be a clinically useful test to exclude a diagnosis of prediabetes or diabetes for at-risk adolescents.
However, there are disadvantages regarding the use of HbA1c such as racial/ethnic variation in HbA1c levels (i.e. higher levels of HbA1c by 0.4%–0.7% for African-Americans compared with Caucasians)25 and medical conditions that can affect HbA1c levels independent of glucose levels. For example, hemolytic anemia and active bleeding can lead to decreases in erythrocyte age, which lowers HbA1c; this glucose-independent lowering of HbA1c could possibly lead to a missed diagnosis of diabetes. In contrast, iron-deficiency anemia, splenectomy, or aplastic anemia can lead to higher HbA1c levels; this glucose-independent increase in HbA1c could possibly lead to an erroneous diagnosis of diabetes.3 Although the Committee recommends that providers perform glucose tests (FPG or 2-hr PG) rather than HbA1c for patients with these conditions, most pediatric providers in the primary care setting are not familiar with these limitations of HbA1c, potentially leading to diagnostic errors.
Our findings contrast with a recent study suggesting that HbA1c could be a useful marker for identifying adolescents with prediabetes and diabetes.26 They found that optimal sensitivity and specificity to detect type 2 diabetes were, respectively, 99% and 96% at an HbA1c ≥ 6.0%. Their reported levels of sensitivity and specificity were notably higher than our estimates. However, their population of obese adolescents and subgroup of obese insulin-resistant adolescents were referred to an obesity clinic and may have had symptoms of diabetes. Therefore these results are not generalizable to an asymptomatic population-based sample of adolescents in the pediatric primary care setting. Furthermore, their estimates were based on a total of 4 cases of type 2 diabetes out of 468 children. They do not report confidence intervals for their estimates of sensitivity and specificity, but based on the low number of cases, it is likely that the confidence intervals, similar to our study, were quite high.
We do acknowledge limitations to our study. We note that our findings relate to HbA1c testing of asymptomatic, rather than symptomatic, overweight and obese children. We recognize that previous studies have used NHANES data to evaluate the performance of HbA1c for predicting diabetes in adults,5, 6 but we are unaware of studies that have systematically compared its performance for adults compared with children. Despite the fact that FPG was measured in the morning, which maximizes the prevalence of diabetes detected,21 the number of individuals with diabetes in the sample was low, which is related to the overall low prevalence of diabetes among US children and adolescents compared with adults.27
The findings of our study highlight the dilemma of screening for diabetes in adolescents. The prevalence of undiagnosed diabetes in the pediatric population is only 0.02%,27 As a result, any test, not just HbA1c, will have a low positive predictive value for detecting diabetes. The ADA guidelines were published in 2000, when there was believed to be an epidemic of type 2 diabetes in children. However, more recent studies suggest that the epidemic is not as large as was initially anticipated.27–29
Our ability to diagnose undiagnosed diabetes and prediabetes was limited due to the absence of repeat testing of FPG or 2-hr PG on a different day in NHANES. Without a repeat measure, some adolescents or adults who would not have had a confirmatory FPG or 2-hr PG may have been erroneously diagnosed with diabetes or prediabetes. Despite this lack of repeat testing, studies of diabetes prevalence using NHANES have employed similar methods for identifying undiagnosed diabetes and prediabetes.30 Furthermore, studies in both adolescents9 and adults10 suggest that FPG has better reproducibility compared with a 2-hr PG. We do however recognize that one abnormal measurement of HbA1c is not sufficient for diagnosis of diabetes, as additional testing in the clinical setting would likely be needed to confirm the clinical diagnosis.
Acknowledgments
Supported by NIDDK K08DK082386 and the Clinical Sciences Scholars Program at the University of Michigan (to J.L.), T-35 HL 0076909-27 (to E.-L.W.), K23HD057994 (to B.T.), and NHLBI K23HL092060 (E.Y.). The funding sources had no role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the article for publication.
Abbreviations
- HbA1c
Hemoglobin A1c
- FPG
fasting plasma glucose
- 2-hr PG
2-hour plasma glucose
- OGTT
oral glucose tolerance test
- ROC
receiver operator characteristic
- AUC
area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007 30 Suppl 1:S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 3.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2447–2453. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 4.Standards of medical care in diabetes--2010. Diabetes Care. 2010 33 Suppl 1:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187–191. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care. 2007;30:2233–2235. doi: 10.2337/dc07-0585. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed Feb 7, 2007];National Center for Health Statistics, National Health Examination Survey. at Available at: http://www.cdc.gov/nchs/products/elec_prods/subject/nhes3.htm#description1.
- 8.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 9.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93:4231–4237. doi: 10.1210/jc.2008-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39:298–305. doi: 10.1007/BF00418345. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care. 2009;32:342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed July 27th, 2009];NHANES MEC Laboratory Component: Glycohemoglobin. at Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l10_c.pdf.
- 14.ADA. Type 2 diabetes in children and adolescents. Pediatrics. 2000;105:671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 15.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X-h, Obuchowski NA, McClish DK. Statistical methods in diagnostic medicine. New York: Wiley-Interscience; 2002. [Google Scholar]
- 17.Galley HF. Editorial II: Solid as a ROC. Br J Anaesth. 2004;93:623–626. doi: 10.1093/bja/aeh247. [DOI] [PubMed] [Google Scholar]
- 18.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31:1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: the Bogalusa Heart Study. Arch Pediatr Adolesc Med. 2010;164:124–128. doi: 10.1001/archpediatrics.2009.268. [DOI] [PubMed] [Google Scholar]
- 20.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 21.Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. JAMA. 2000;284:3157–3159. doi: 10.1001/jama.284.24.3157. [DOI] [PubMed] [Google Scholar]
- 22.Petersen PH, Jorgensen LG, Brandslund I, De Fine Olivarius N, Stahl M. Consequences of bias and imprecision in measurements of glucose and hba1c for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl. 2005;240:51–60. doi: 10.1080/00365510500236135. [DOI] [PubMed] [Google Scholar]
- 23.McCance DR, Hanson RL, Charles MA, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ. 1994;308:1323–1328. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelgau MM, Thompson TJ, Herman WH, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care. 1997;20:785–791. doi: 10.2337/diacare.20.5.785. [DOI] [PubMed] [Google Scholar]
- 25.Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30:2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah S, Kublaoui BM, Oden JD, White PC. Screening for type 2 diabetes in obese youth. Pediatrics. 2009;124:573–579. doi: 10.1542/peds.2008-2949. [DOI] [PubMed] [Google Scholar]
- 27.Liese AD, D'Agostino RB, Jr, Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 28.Dolan LM, Bean J, D'Alessio D, et al. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr. 2005;146:751–758. doi: 10.1016/j.jpeds.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 29.Goran MI, Davis J, Kelly L, et al. Low prevalence of pediatric type 2 diabetes: where's the epidemic? J Pediatr. 2008;152:753–755. doi: 10.1016/j.jpeds.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]