Abstract
Interest in utilizing magnetic nanoparticles (MNP) for biomedical applications has grown considerably over the past two decades. This excitement is driven in large part by the success of MNPs as contrast agents in magnetic resonance imaging (MRI). The recent investigative trend with respect to cancer has continued down a diagnostic path, but has also turned toward concurrent therapy – giving rise to the distinction of MNPs as potential “theranostics”. Here we review both the key technical principles of MNPs and the ongoing advancement toward a cancer theranostic MNP. Recent progress in diagnostics, hyperthermia treatments, and drug delivery are all considered. We conclude by identifying current barriers to clinical translation of MNPs and offer considerations for their future development.
Introduction
The beginning of nanotechnology is often attributed to a 1959 talk, entitled “There’s Plenty of Room at the Bottom”, by renowned physicist Richard Feynman at an American Physical Society meeting held at the California Institute of Technology. In his presentation, Dr. Feynman challenged his audience to consider “manipulating and controlling things on a small scale.” Prior to this point in history, little discussion is found on the application of materials in the “nano” size range (or lower). The use of nanotechnology, however, can be traced back several millennia, with examples that include “nanocosmetics” used by ancient Egyptian pharaohs, and nanocrystal-containing hair dyes used by Greeks and Romans [1]. It was also recently discovered that the famous Damascus blades, forged during 1100 to 1700 AD, contained carbon nanotubes and cementite nanowires that imbued them with extraordinary mechanical properties and a sharp cutting edge [2]. Additionally, gold nanoparticles can be found in the stained-glass windows of medieval churches, giving them their colorfully décor [1]. While nanoparticles have been utilized for thousands of years, it is only recently that man has obtained an intricate understanding of these materials and their unique properties. This advancement of knowledge has, today, enabled the production of nanomaterials varying in size, shape, and/or composition[3].
Nanotechnology strictly encompasses the engineering and utilization of materials having at least one dimension smaller than 100 nanometers. The behavior of particles in this size range cannot be solely described with either classical or quantum mechanics [4]. In a looser sense, the field of nanotechnology seeks to make use of the unique material properties that are displayed by matter sized somewhere between the “molecular” (~ 0.1 nm) and “bulk” (100’s of nanometers) thresholds. High surface energies, due to a predominance of atoms located at the particle surface, and quantum effects give rise to unique chemical, physical, and optical properties not observed in materials at other length scales. These distinct properties of nanoparticles have only recently been applied to problems in biomedical research, including cancer. The large surface-to-volume ratio of nanomaterials allows for relatively high loading of different functional ligands on a single platform. Not surprisingly, interest in applying nanoparticles for drug delivery has grown significantly over the past 10 years and includes a rising interest in the use of magnetic nanoparticles (MNP). Marked attention to MNPs can be directly attributed to their unique magnetic properties. Indeed, magnetic functionality has been exploited to render the MNP a dual diagnostic tool (primarily in magnetic resonance imaging - MRI) and targetable drug carrier for therapy — a so-called “theranostic”. In this article, we briefly review recent progress in the development of MNPs for cancer theranostics. We first examine routes of nanoparticle synthesis and the key properties of MNPs that make them attractive for cancer applications. We also discuss the pharmacokinetics and biodistribution of MNPs, in addition to highlighting recent applications under study with respect to cancer diagnosis and therapy. We conclude by examining current challenges for clinical translation of MNPs and offer considerations for the future.
Techniques for synthesis of MNPs
Although materials containing cobalt or nickel have also been investigated, MNPs comprised of an iron-oxide core (usually magnetite - Fe3O4 or maghemite - γ-Fe2O3) are the most extensively studied for biomedical applications due to their more favorable toxicity profile. These MNPs are typically coated with a material showing good biocompatibility (e.g. polysaccharide, synthetic polymer, lipid, protein, or silane linker) — a composite morphology often referred to as “core-shell structure” as shown in Figure 1A. Coatings can both stabilize MNPs in physiologic fluids and provide functionality for additional modifications. Several techniques for MNP synthesis have been developed to permit control of particle size and shape. Production routes have been reviewed elsewhere in detail [5], but a few are briefly discussed here. A common, simple synthesis strategy involves the aqueous precipitation of iron salts,[6] with in situ or post-synthesis addition of coating material. Precipitation within reverse microemulsions has also been successfully executed. While this method does produce MNPs of narrower size distribution over aqueous precipitation, microemulsion is generally beset with low yields [7]. Recently, thermal decomposition of organometallic compounds has gained considerable attention [8]. This technique offers good control over the final particle size, shape, and crystal structure compared to other methods. The reaction, however, occurs in organic solvent containing hydrophobic stabilizers, necessitating additional surface modifications to nanoparticles to impart aqueous stability. It should be noted that MNP size and surface properties (e.g. charge, hydrophobicity) have important implications for nanoparticle behavior including colloidal stability, ease of coupling ligands, and acceptable pharmacokinetic performance. Therefore, a proper tuning of both size and surface character is essential in the development of MNPs as a successful in vivo platform.
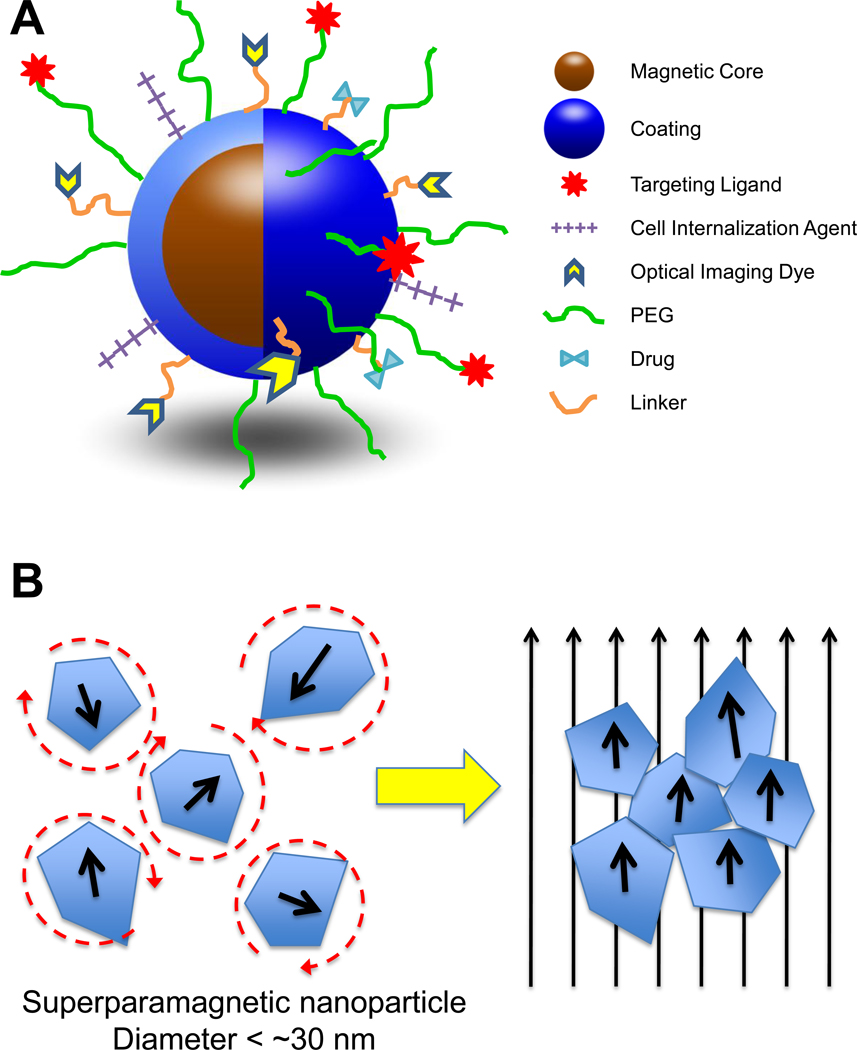
Figure 1.
(A) Schematic representation of core-shell structure of MNPs and multi-functional surface decoration. MNPs consist of an iron oxide core coated with a biocompatible material (e.g. polysaccharide, lipid, proteins, small silane linkers). Functional groups on the surface of coatings are often used to link ligands for molecular targeting, cellular internalization, optical imaging, enhanced plasma residence, and/or therapy. The number of different moieties that decorate the MNP surface impart its multi-functional, “theranostic” character. (B) Illustration of superparamagnetic MNP response to applied magnetic fields. MNPs are comprised of rotating crystals that align with the direction of an applied magnetic field. Crystal reorientation provides for the high magnetic susceptibilities and saturation magnetizations observed with this material. The circular dashed lines around the superparamagnetic nanoparticles on the left illustrate the randomization of their orientation, due to temperature effects, in the absence of a magnetic field.
Magnetic properties of MNPs
Several forms of magnetism exist in nature, with superparamagnetism appearing to be the mode preferred for MNPs applied to biomedical problems. Superparamagnetism occurs when particles are small enough for thermal fluctuations to cause random flipping of magnetic moments. The randomization in orientation of these magnetic moments results in an average magnetization of zero, in the absence of an applied magnetic field. The characteristic time from one moment-flip to the next is called the Néel relaxation time and is approximated by the Néel-Arrhenius relation in Equation 1:
| (1) |
where τ0 is the time between flip attempts and is typically in the range of 10−9 – 10−12 s depending on material, K is the magnetic anisotropy energy density, V is the particle volume, kB is the Boltzmann constant, and T is the temperature [9]. From Equation 1, it is observed that the relaxation time decreases, or flipping frequency increases, as the particle size gets smaller. The required size limit to achieve superparamagnetism can vary with material composition, but is typically < 30 nm for iron oxide crystals. Considering this threshold, MNPs with cores larger than 20–30 nm typically consist of clusters of multiple, smaller iron-oxide domains rather than a single, large crystal. Superparamagnetic MNPs are ideal for in vivo use as the presence of attractive forces between neighboring MNPs (from permanent or remnant magnetization) could lead to the formation of large aggregates, which are more easily cleared from circulation (see discussion below) and pose greater risks for vascular embolism. Additional attractive properties of superparamagnetic MNPs include a greater magnetic susceptibility and magnetic saturation, when exposed to an external magnetic field, compared to paramagnetic materials [10]. High magnetic susceptibility is the result of a reorientation of individual, high-magnetization iron oxide crystals responding to the applied field, as illustrated in Figure 1B. As discussed later, the high magnetic susceptibility of MNPs is paramount in their function as imaging agents in MRI.
Pharmacokinetics, biodistribution, and toxicity of MNPs in vivo
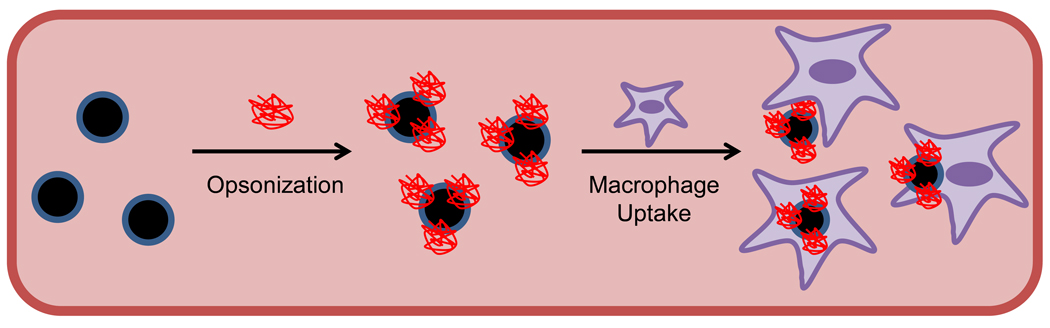
In vivo applications of MNPs generally rely on the passive transport of nanoparticles to the intended target via the bloodstream. It is, therefore, important to consider MNP circulation pharmacokinetics when designing MNP-based systems. The two most important factors that determine MNP pharmacokinetics are nanoparticle surface character and hydrodynamic size [11]. Interplay of these properties with the reticuloendothelial system (RES) – the primary physiologic mechanism responsible for nanoparticle clearance from circulation – determines plasma lifetime. In RES clearance (Figure 2), circulating opsonin proteins adsorb to nanoparticle surfaces, which are then recognized and removed from the bloodstream by tissue macrophages. For example, strong interactions with components in plasma and/or on cellular surfaces results in very short circulation times for cationic MNPs [12]. With respect to size, it is generally agreed that nanoparticles of hydrodynamic diameter 10–100 nm are pharmacokinetically optimal for in vivo applications [13]. Nanoparticles smaller than 10 nm are subject to tissue extravasation and renal clearance, whereas those larger than 100 nm are quickly opsonized and eliminated from the circulation via the RES. Rate of clearance, however, can also be reduced by modification of nanoparticle surfaces with coatings that resist RES interactions. Indeed, polyethylene glycol (PEG) has been a hallmark solution to many pharmacokinetic problems, including those for recently developed MNPs [14–17]. PEG chains linked to nanoparticles (Figure 1A) provide steric resistance to opsonization and macrophage uptake processes, permitting prolonged plasma residence. PEG has been primarily applied to nanoparticles possessing hydrodynamic diameters of 10–100 nm, but has also been investigated with particles of greater size [18, 19]. While in vitro studies have shown that PEG-grafted MNPs with diameters > 100 nm could potentially have enhanced circulation properties, such behavior is not often validated with in vivo work. Our recent investigations with larger (170 nm hydrodynamic diameter) PEG-modified MNPs, though, demonstrated that these nanoparticles possessed a circulation half-life of almost 12 hours in a rat, and could be visualized in tumors by MRI through 24 hours (Figure 3) [20]. While the preferred MNP size and surface character depends on the desired target and delivery strategy employed, the independent variation of these two nanoparticle properties can modulate MNP pharmacokinetics to achieve optimal performance. MNPs with longer circulation half-lives have greater exposure to target tissues, potentially improving nanoparticle utility.
Figure 2.
Reticuloendothelial system (RES) plasma clearance of MNPs. As shown in the conceptual schematic above, opsonin proteins in the circulation adsorb to nanoparticle surfaces, “flagging” them as exogenous materials for plasma clearance. RES tissue macrophages (primarily those of the liver and spleen) recognize flagged MNPs and remove them from the circulation via phagocytosis.
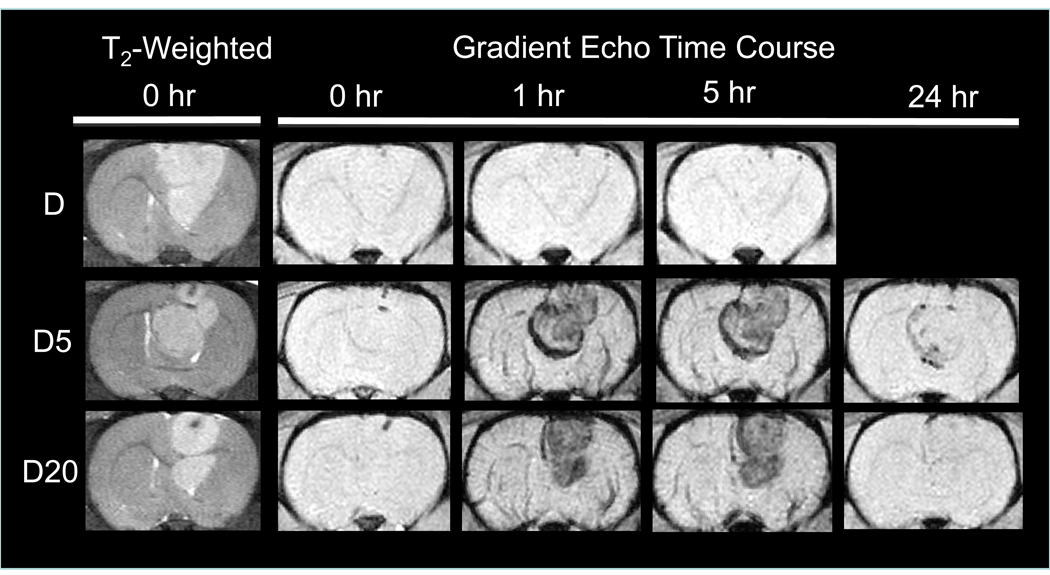
Figure 3.
Brain MRI time course of cross-linked starch coated, PEG-modified MNPs in male Fisher 344 rats bearing 9L-glioma brain tumors (12 mg Fe/kg) (Reproduced with permission) [20]. Baseline T2-weighted, fast spin echo images clearly indicate the positioning of the tumor (hyperintense region) in the brain. T2*-weighted, gradient echo images provide qualitative information about MNP presence in the brain/tumor. Sustained negative contrast (hypointensity) in the tumor confirms that D5 (cross-linked starch coated MNP modified with 5000 MW PEG) and D20 (cross-linked starch coated MNP modified with 20000 MW PEG) reside far longer in plasma and provide greater exposure to tumor when compared to D (unmodified, parent starch MNP). Indeed, some hypointensity can still be observed in tumors through 24 h with the PEGylated MNPs.
Biodistribution of MNPs in tissues is also an important consideration, both with respect to the success of targeting and potential for off-target toxicities [21]. While targeting success is dependent on the particular targeting strategy utilized, the majority of administered MNPs often distribute, not surprisingly, in tissues of the RES – most notably in the liver and spleen [21–25]. In the liver, uptake occurs predominately in Kupffer macrophage cells located in sinusoids, whereas both macrophage and mechanical filtration processes are responsible for distribution in the spleen [13, 25]. Significant accumulation of MNPs in the liver, spleen, and/or other off-target tissues raises important concerns regarding the potential for toxicity. Still, many developed MNPs are well tolerated, with some MNPs showing no measurable median lethal dose (LD50) [26]. Toxicity in tissues, though, is highly dependent on characteristics of the MNP administered, including: synthesis procedures; type and charge characteristics of the coating; purity; toxicity of any attached drug cargo; route of administration, and/or the extent of tissue distribution [25–27]. Therefore, the multi-variable dependence of toxicity on different MNP properties necessitates thorough toxicological assessment of each new MNP formulation, despite the fact that others (even those similar) may have been previously shown to be non-toxic. Liver functionality tests (LFTs), cytokine detection, histology, lipid hydroperoxide (LHPO – a biomarker for tissue oxidative stress) levels, and blood counts have all been shown useful in assessing toxicity in vivo [16, 22–24].
Targeting MNPs to tumors
Passive targeting by the enhanced permeability and retention (EPR) effect
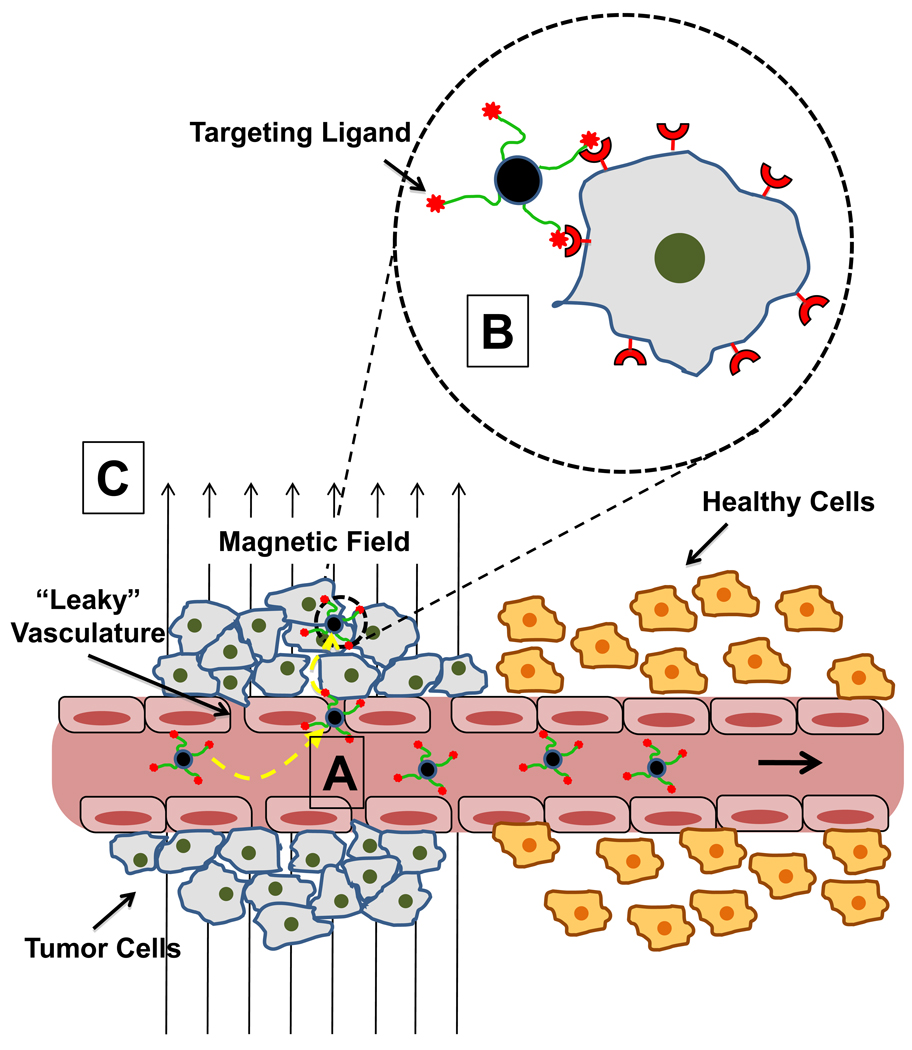
Due to their “nano” size, MNPs can be accumulated in many tumor tissues passively via the enhanced permeability and retention (EPR) effect [28]. As shown in Figure 4, the compromised, “leaky” vasculature characteristic of a solid tumor permits passive extravasation of MNPs from the circulation into the tumor interstitium, where they are retained due to poor lymphatic drainage. Indeed, particles of up to 1 µm in size have been shown to extravasate into the tumor space for some types of cancer [28]. In contrast, endothelial cells of normal tissue vessels are closely packed and present a greater barrier for MNP penetration. This difference in vascular permeability provides a means for tumor selective accumulation of MNPs.
Figure 4.
Conceptual representation of MNP tumor targeting modalities. (A) EPR effect – Unlike that found in normal tissue, tumor vasculature is “leaky” due to fenestrations and gaps between endothelial cells that result from abnormal angiogenesis. MNPs in circulation can passively extravasate through these gaps and enter the tumor interstitium. Poor lymphatic drainage in some tissues helps retain particles in the tumor space. (B) Molecular targeting – ligands (antibodies, peptides, small molecules, etc.) targeted toward moieties overexpressed/uniquely present on the plasma membrane of tumor cells can be used to actively enhance retention of MNPs at the tumor site and can also help to internalize particles into cells via endocytosis. (C) Magnetic targeting – an external magnetic field can be applied to the tumor region, producing a field gradient across the tumor. The magnetic force produced by the gradient actively attracts particles into the tumor space (through comprised vasculature) and helps with subsequent retention. It should be noted that each form of targeting in the figure can be employed simultaneously depending on the particular strategy utilized.
Active, tumor-specific molecular targeting with ligands
Concurrently with the EPR mechanism, active MNP accumulation and retention can be achieved with molecular targeting, a route based on the use of tumor-selective ligands (illustrated in the dashed circle of Figure 4). Carefully selected ligands, attached to the MNP surface, specifically bind moieties overexpressed, or uniquely present, on tumor cell plasma membranes. Typically, many identical ligands are coupled to each MNP to enable multivalent binding. Multivalency offers the important advantage of cooperative ligand binding that can significantly strengthen MNP retention at tumor cells [29]. By their attachment to the MNP, other coupled cargoes (including therapeutics which are often limited in targeting ligand loading capacity) can also share the benefits of multivalency phenomena. A variety of targeting ligands have been utilized for MNPs depending on the specific target, and these are reviewed extensively elsewhere [26, 29, 30]. More recently studied ligands, though, include peptides (e.g. chlorotoxin [31], RGD [32], lung cancer targeting peptide [33], CREKA [34], bombesin [35], F3 [36, 37], A54 [38], LHRH [39]), antibodies (e.g. Anti-HER2 [40], Anti-EGFR/EGFRvIII [41, 42]), and small molecules (e.g. folate [43]). Molecular targeting has been explored extensively in the development of tumorselective MR imaging agents, showing promise for more therapeutic applications [30]. Attractive macromolecular ligands, though, often require costly synthetic production and complicated chemistries for their attachment to the parent MNP. These limitations certainly make necessary scale-up challenging and could be one major hurdle to advancing these types of agents beyond the laboratory and into the clinical mainstream.
Cellular internalization of nanoparticles may also be desired after targeting, especially in cases where the goal is to deliver anti-tumor agents that exert their effects inside the cell. Several of the previously mentioned molecular targeting ligands can be used to facilitate internalization of MNPs into cells, primarily via endocytosis [29]. Where targeting ligands are not used or are used but do not aid in cellular uptake, highly cationic, cell-penetrating peptides (CPP – e.g. HIV-TAT, MPAP, low molecular weight protamine) or aminated synthetic polymers (e.g. PEI) that ferry their attached cargo into cells have been explored as alternatives [12, 24, 44–46]. While highly efficient at transporting their cargoes across cell membranes, cationic agents do so non-specifically [47]. The extension of cationic functionality to an MNP can also shorten its plasma residence, as discussed above. Therefore, to ensure tumor selectivity and maintain necessary pharmacokinetics, strategies to modulate cationic activity must be considered when choosing to include this type of material in platform designs.
Active targeting with applied magnetic fields
Unlike other nanocarriers, active targeting of MNPs can also be realized by exploiting their responsiveness to an external magnetic field. Referred to as “magnetic targeting”, the gradient (∇ B) produced by an externally applied magnetic field (B) to the tumor region exerts attractive forces on MNPs delivered via the circulation according to Equation 2 [48, 49]:
| (2) |
where χ is the magnetic susceptibility of the magnetic core, Vc is the volume of the core, and μ0 is the magnetic permeability of free space. MNP are retained in tumors when the magnetic force is sufficient to overcome hydrodynamic drag forces exerted on the particle by the blood flow [50]. MNPs optimal for magnetic targeting typically consist of a large core (> 100 nm diameter) of multiple, magnetic iron-oxide domains (a structure necessary to maintain superparamagnetism) [51]. Although larger cores provide for greater magnetic force, as suggested by the volume term in Equation 2, particles with large hydrodynamic diameters have been shown to have substantially faster clearance as discussed above, and, thus, possess limited circulation availability for targeting [12]. One possible approach to minimize increase of overall particle size while still improving the particle’s magnetic response is to increase the magnetic core size and reduce coating thickness [52]. Such a strategy, though, would certainly have to be balanced against a minimum required amount of coating material.
While a number of tumor models have been studied with magnetic targeting [48], most recent work appears to be related to brain cancer. We have explored magnetic brain tumor targeting of MNPs extensively, achieving proof-of-concept with starch and polyethyleneimine (PEI) MNPs after unobstructed, intravascular administration [12, 18, 50, 53, 54]. Recently, another group successfully showed the benefit of coupling focused ultrasound (to temporarily compromise the blood-brain-barrier) to magnetic targeting in achieving enhanced, selective MNP delivery to a brain tumor [55]. Brain tumors can be especially hard to target magnetically as they are positioned deep within the skull at distances where the magnetic flux density drops off substantially [55]. Indeed, attenuated gradients pose the greatest translational challenge for this method. Strategies to overcome magnet barriers are currently under study for cell delivery and may also be useful for tumor targeting. These methods include magnetic resonance targeting (using a MRI system magnet as the source) [56], deeply focused targeting [57], dynamically controlled magnets [27], and magnetic implants [58] to produce localized fields.
Theranostics
Cancer diagnostics
Over the past twenty years, MNPs have been studied most extensively as contrast agents in MRI [59, 60]. It is well known that the high magnetic susceptibility of MNP cores provide strong enhancement of transverse (T2 and T2*) relaxivity in tissue regions where nanoparticles have localized. Strong relaxivity is manifested on T2-weighted MR images as pronounced negative contrast (hypointensity). Not surprisingly, MNPs have had their greatest clinical success for use in MRI-based investigations, as shown in Table 1 [27]. Due to their significant deposition in the liver, several approved agents (e.g. Feridex and Resovist) have been used in hepatic imaging [59]. Molecular targeting ligands have been coupled to MNPs to explore extending the MR visibility to imaging of other tumors [30, 59, 61], yet wide-spread clinical reports of these agents remains relatively low.
Table 1.
Commercially available MNPs currently FDA approved or in clinical trial (Ref. 26)
| Pre-Clinical Agent | Commercial Name | MR Target | Status |
|---|---|---|---|
| AMI-25 | Ferumoxide, Feridex, Endoderm | Liver | Approved |
| OMP | Abdoscan | Bowel | Approved |
| AMI-121 | Gastromark, Ferumoxsil, Lumirem | Bowel | Approved |
| SHU555A | Resovist | Liver | Approved (EU, Japan, Australia), Phase III (USA) |
| AMI-227 | Combidex, Sinerem, Ferumoxtran | Lymph Node Metastases | Phase III |
| CODE 7228 | Feraheme | Vasculature | Phase II |
Another growing trend in cancer diagnostics is the use of multi-modal imaging agents, including those based on MNPs. In one respect, MNPs have been linked to other imaging moieties and used to validate MR visualization of nanoparticle accumulation in tumors [14, 24]. Each modality in a multi-modal imaging system, though, has its own strength, weakness, and target purpose [61]. The MR visibility of MNPs is a macroscopic tool, used primarily to obtain anatomical (e.g. tumor size, location) information in oncology. MRI, however, is not readily amendable to intravital confirmation of microscopic information such as cellular internalization of MNPs (if desired), specific cancer type, the success of treatments at the cellular level, or the precise location of tumor boundaries after surgical resection; optical modalities (e.g. fluorescence) would likely better serve this purpose [61]. Indeed, several recently developed MNPs possessing properties conducive to optical imaging have been investigated [14, 24, 62]. Optically active MNPs have been synthesized by linking nanoparticles with fluorescent dyes (Figure 1) or by coating MNPs with other inorganic agents (e.g. gold, quantum dots) that exhibit surface plasmon resonance [63]. Moreover, triple functional agents have also been developed, with a recent example showing combined positron emission tomography (PET), MRI, and fluorescence capabilities [64, 65]. MNPs with multi-modality capabilities could give clinicians a powerful probe to use in both tumor diagnosis and monitoring of intervention efficacy.
Novel MNPs are also emerging as cancer detection agents in diagnostic nuclear magnetic resonance (DMR) [66, 67]. In this approach, MNPs are targeted, via conjugated molecular targeting ligands, to a specific cancer biomarker or an overexpressed surface moiety on cancer cells that reside in biological fluids removed from the body. Several MNPs can bind a single target molecule and, due to multivalency, each MNP can participate in more than one binding event. Such behavior leads to self-assembly of monodisperse MNPs into larger clusters. These larger clusters display enhanced T2 relaxivity behavior when compared to unbound MNPs, a change detectable by a NMR spectrometer. Microfluidic and microelectronic chips have been developed to conduct such measurements, with substantially enhanced detection sensitivity (>800-fold) over benchtop systems [66]. Moreover, the sensitivity of NMR for bound MNPs also enables measurement of turbid, complex biological fluids. Such capability could significantly lower sample measurement times compared with currently used ex vivo methods that require substantial specimen preprocessing.
Cancer therapy
Magnetic hyperthermia
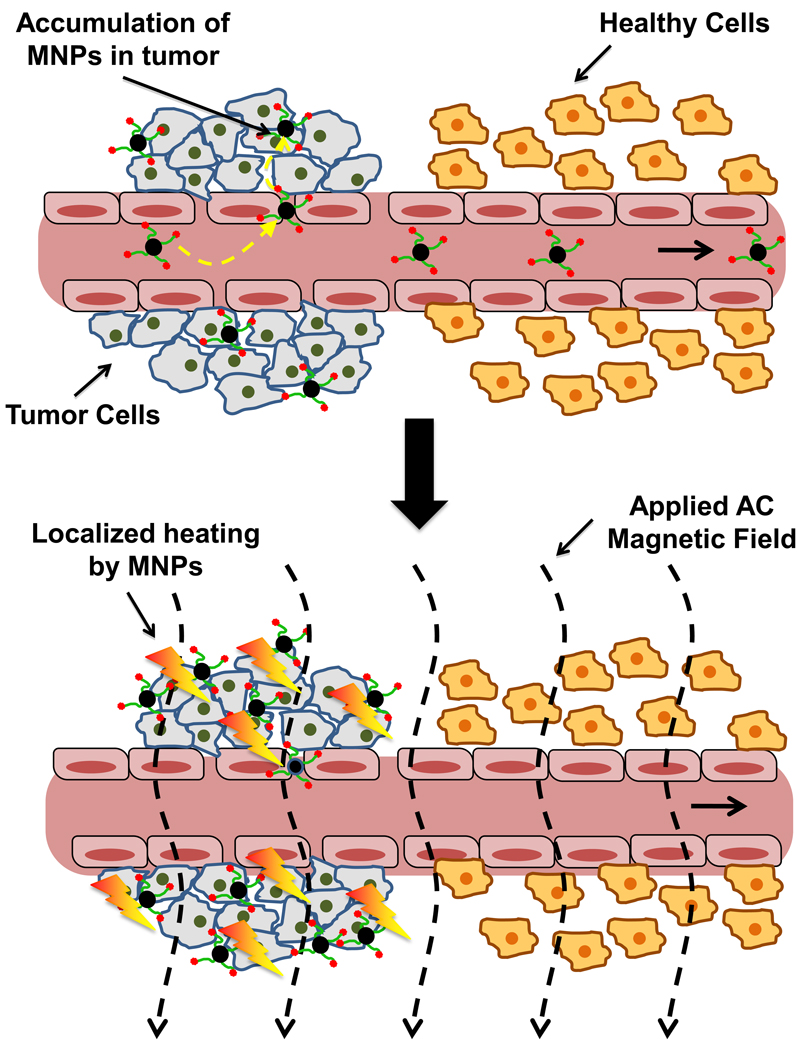
Magnetic hyperthermia, a cancer therapy unique to MNPs, can be used to selectively kill tumor cells via increases in tissue temperature. The technical principles behind magnetic hyperthermia are reviewed extensively elsewhere [51]. Briefly, MNPs that accumulate at the tumor site are exposed to an alternating current (AC) magnetic field, as illustrated in Figure 5. Superparamagnetic MNPs absorb this energy and convert it into heat, due to relaxation of rotating magnetic moments induced by the AC field [68]. Tumors are typically heated to 41°C to 47°C, as cancer tissues have been shown to possess higher heat-sensitivity over normal tissues in this temperature range [69]. While magnetic hyperthermia methods are promising, risk for local overheating (thus, damaging normal tissues) remains the major concern for this therapy modality. To address these challenges, next-generation magnetic oxides (e.g. CoFe2O4, SrFe12O19/γ-Fe2O3 complexes) have been studied to improve heating efficiency (resulting in dose reductions) and introduce self-regulated temperature controls in MNPs [68, 70]. MNPs that also incorporate thermally responsive agents (e.g. hydrogels, thermosensitive polymers, lipids) that aid in specific nanoparticle tumor retention (and reduce diffusion to healthy tissues) or augment hyperthermia treatments with temperature-released chemotherapy have also been recently explored [69, 71–73].
Figure 5.
Principle of Magnetic Hyperthermia. Magnetic hyperthermia relies on the preferential accumulation of MNPs at tumors compared to normal tissues. Targeted MNPs delivered to the tumor are exposed to an alternating current (AC) magnetic field, causing nanoparticles to absorb energy by increasing alignment (lower entropy state) with the applied field. This energy is then converted to heat when the particles undergo relaxation. Tumors have been shown to possess thermal hypersensitivity, compared to normal tissues, when heated to temperatures ranging from 41–47°C. The concern for overheating normal tissues remains, however, presenting an important limitation to this method.
Drug delivery
Indeed, the success of MNPs in MRI has generated considerable excitement for their use as drug delivery vehicles. Nanoparticle coatings provide the anchor-points to which drug molecules are coupled to MNPs. Therapeutic moieties can be chemically linked to nanoparticles via bioconjugation (either directly or by molecular cross-linkers), complexed via electrostatic/hydrophobic interactions, or encapsulated in the coating. MNP drug delivery systems for cancer therapy have incorporated traditional small molecules (e.g. paclitaxel, doxorubicin, or methotrexate), radiotherapeutics, and photo-activated agents (e.g. Photofrin) [29, 74]. While studies have demonstrated success to some degree with these agents, the specificity and potency of many macromolecular biotherapeutics (peptides, proteins, genes) for cancer targets could result in more effective treatments, with reduced side effects. While highly effective at the tumor target, macromolecular agents typically possess poor plasma pharmacokinetics, low tissue selectivity, and/or poor plasma membrane penetration necessary for access to the cell’s internal environment. Carefully tailored MNPs can improve plasma pharmacokinetics, achieve tumor selectivity for these agents, and provide a means for their internalization into cells. MNPs are only beginning to emerge as carriers of macromolecular therapy, though, as reports of successful delivery and tumor response in vivo remain scant. A few notable examples have been published, though, including the successful delivery of survivin small interfering RNA (siRNA) to human colorectal cancer xenografts and the delivery of BIRC5 siRNA against human breast cancer xenografts, both in mice [24, 75]. Moreover, EGFRvIII antibody linked MNPs have been shown to improve survival times in glioma-bearing rats [41, 74]. Successful delivery of chlorotoxin (shown to have anti-infiltrative effects against tumor cells [76]) conjugated MNPs to brain tumors was reported in mice [31]. Just recently, the combination of chlorotoxin and siRNA on a single MNP platform with gene silencing capabilities was reported in vitro, showing promise for a future brain tumor therapy [46]. Others have had in vivo success against brain tumors in mice utilizing a “string” of MNPs linked to dendrimers (a “dendriworm”) and anti-EGFRvIII siRNA [77].
Challenges for clinical translation and future considerations
The ultimate goal for any cancer theranostic is to achieve an effective treatment of tumors in humans. While significant progress has been made with respect to MNP platforms, significant knowledge and technical gaps continue to prevent their movement from bench to bedside. Among other factors, FDA approval for use in humans requires extensive safety and toxicology study of any newly developed MNP delivery system. This can be a daunting task as the size, shape, composition, surface properties, drug loading, dose, administration route, biodistribution, and pharmacokinetics of the system are all factors that can affect toxicity profiles. Limitations also exist for targeting efficiency, especially with respect to magnetic targeting. For one, we have observed that embolism of blood vessels can occur depending on MNP stability and geometry of the applied magnetic field. While there has been a greater focus in recent years towards understanding potential safety issues from magnetic targeting, most studies continue to be conducted in vitro – rigorous testing has yet to be conducted in vivo [78, 79]. Another important challenge for successful magnetic targeting of MNPs is the need for improved magnetic field gradients. Current technology allows for magnetic capture of MNPs at only short distances away from the magnet sources, which, in humans, can only be effective for superficial tumors (e.g. skin cancer) and not those in deep-seated tissues (e.g. brain). Additionally, a greater focus of the flux gradient is desirable, since the volume between the magnet and target site is also exposed to the field, which could lead to off-target accumulations. Even with successful targeting of any kind, the lack of homogenous penetration of nanoparticles into the tumor volume might also limit therapeutic outcomes. The heterogeneous nature of tumor vasculature, variability in vessel fenestration size and number, combined with diffusion limitations of MNPs through the tumor interstitial space, result in accumulation of nanoparticles in only small, heterogeneously distributed pockets within the tumor. Inadequate delivery of therapeutics to the entire tumor volume is evidenced by the fact that even the most recent efficacy studies only show some reduction in tumor growth rates and not actual remission. The recent use of externally applied forces, such as ultrasound mentioned above, has shown potential to “open up” the tumor for increased MNP penetration after targeting and, thus, potentially better treatment efficacy [55, 80]. While showing some promise, the successful translation of an MNP-based, cancer drug delivery system continues to proceed slowly; likely due to the need for more interdisciplinary cooperation between diverse scientific fields. The expertise of material scientists and chemists is needed for synthesis of nanoparticles and their coatings. Engineering is required to carry out the challenging task of cost-effectively, mass-producing developed materials, while input from physicists is needed for the development of suitable magnetic field gradients for magnetic targeting. Pharmaceutical scientists are required for in vitro and in vivo evaluation of pharmacokinetics, biodistribution, toxicity, and efficacy of the drug delivery system in pre-clinical development. Contributions from clinicians are necessary for planning and executing human trials. Success with MRI and progress over the past few years, however, has offered some glimmers of hope for eventual therapeutic translation of this technology. Indeed, several diagnostic clinical trials using MNPs have been initiated over the past few years as listed in Table 2. The increasing trend toward in vivo studies in animals and subsequent escalation to clinical trials can be expected to accelerate over the coming years as research institutes develop interdisciplinary centers to address disconnects in the development pipeline.
Table 2.
List of recently initiated clinical trials evaluating the utility of MNPs in diagnostic applications*
| Status | Start Date | Study Title | Condition | Sponsor |
|---|---|---|---|---|
| Recruiting | Jan-2010 | Inflammatory Cell Labelling and Tracking With Magnetic Resonance Imaging After Myocardial Infarction | Myocardial Infarction; Inflammation | University of Edinburgh |
| Ongoing | Jul-2008 | Evaluation of Magnetic Nanoparticle Enhanced Imaging in Autoimmune Diabetes | Diabetes Mellitus, Type | Joslin Diabetes Center |
| Recruiting | Jul-2008 | Improved Pre-Operative Staging of Pancreatic Cancer Using Superparamagnetic Iron Oxide Magnetic Resonance Imaging | Pancreatic Cancer | Massachusetts General Hospital |
| Recruiting | Oct-2008 | Assessing Dynamic Magnetic Resonance (MR) Imaging in Patients With Recurrent High Grade Glioma Receiving Chemotherapy | Brain Neoplasms | OHSU Knight Cancer Institute |
| Terminated | Jul-2005 | A Validation Study of MR Lymphangiography Using SPIO, a New Lymphotropic Superparamagnetic Nanoparticle Contrast | Bladder, Genitourinary, and Prostate Cancers | M.D. Anderson Cancer Center |
Data accessed from www.clinicaltrial.gov on December 2010
Acknowledgment
This work was supported by National Institutes of Health (NIH) R01 grants CA114612, NS066945, and a Hartwell Foundation Biomedical Research Award. This work was also partially sponsored by grant R31-2008-000-10103-01 from the World Class University (WCU) project of South Korea and by the National Basic Research Program of China (973 Program) 2007CB935800. Victor C. Yang is currently a participating faculty member in the Department of Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, South Korea. Adam Cole is the recipient of a NIH Pharmacological Sciences and Bio-related Chemistry Training Grant (GM007767 from NIGMS), a University of Michigan Rackham Pre-Doctoral Fellowship, and is currently an American Foundation for Pharmaceutical Education (AFPE) Pre-Doctoral Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors each declare no conflict of interest in this work.
References
- 1.Walter P, et al. Early Use of PbS Nanotechnology for an Ancient Hair Dyeing Formula. Nano Letters. 2006;6:2215–2219. doi: 10.1021/nl061493u. [DOI] [PubMed] [Google Scholar]
- 2.Reibold M, et al. Materials: Carbon nanotubes in an ancient Damascus sabre. Nature. 2006;444:286–286. doi: 10.1038/444286a. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Schmid Gn, Wiley I. Nanoparticles: from theory to application. Wiley-VCH ; John Wiley; 2004. [Google Scholar]
- 5.Roca AG, et al. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D: Applied Physics. 2009;42:224002. [Google Scholar]
- 6.Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Transactions on Magnetics. 1981;17:1247–1248. [Google Scholar]
- 7.Loo A, et al. Synthesis of magnetic nanoparticles in bicontinuous microemulsions. Effect of surfactant concentration. Journal of Materials Science. 2008;43:3649–3654. [Google Scholar]
- 8.Latham AH, Williams ME. Controlling Transport and Chemical Functionality of Magnetic Nanoparticles. Accounts of Chemical Research. 2008;41:411–420. doi: 10.1021/ar700183b. [DOI] [PubMed] [Google Scholar]
- 9.Zelenakova A, et al. Magnetic properties of Fe[sub 2]O[sub 3] nanoparticles embedded in hollows of periodic nanoporous silica. Journal of Applied Physics. 2010;108:034323. [Google Scholar]
- 10.Josephson L, et al. The magnetic properties of some materials affecting MR images. Magnetic Resonance in Medicine. 1991;22:204–208. doi: 10.1002/mrm.1910220208. [DOI] [PubMed] [Google Scholar]
- 11.Yoo JW, et al. Factors that Control the Circulation Time of Nanoparticles in Blood: Challenges, Solutions and Future Prospects. Current Pharmaceutical Design. 2010;16:2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 12.Chertok B, et al. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials. 2010;31:6317–6324. doi: 10.1016/j.biomaterials.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta AK, Wells S. Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. NanoBioscience, IEEE Transactions on. 2004;3:66–73. doi: 10.1109/tnb.2003.820277. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, et al. Thermally cross-linked superparamagnetic iron oxide nanoparticles: Synthesis and application as a dual Imaging probe for cancer in vivo. Journal of the American Chemical Society. 2007;129:12739–12745. doi: 10.1021/ja072210i. [DOI] [PubMed] [Google Scholar]
- 15.Larsen EKU, et al. Size-dependent accumulation of PEGylated silane-coated magnetic iron oxide nanoparticles in murine tumors. Acs Nano. 2009;3:1947–1951. doi: 10.1021/nn900330m. [DOI] [PubMed] [Google Scholar]
- 16.Sun C, et al. PEG-Mediated Synthesis of Highly Dispersive Multifunctional Superparamagnetic Nanoparticles: Their Physicochemical Properties and Function In Vivo. ACS Nano. 2010;4:2402–2410. doi: 10.1021/nn100190v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumagai M, et al. Enhanced in vivo Magnetic Resonance Imaging of Tumors by PEGylated Iron-Oxide-Gold Core-Shell Nanoparticles with Prolonged Blood Circulation Properties. Macromol. Rapid Commun. 2010;31:1521–1528. doi: 10.1002/marc.201000341. [DOI] [PubMed] [Google Scholar]
- 18.Chertok B, et al. Substantiating in vivo magnetic brain tumor targeting of cationic iron oxide nanocarriers via adsorptive surface masking. Biomaterials. 2009;30:6780–6787. doi: 10.1016/j.biomaterials.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yallapu MM, et al. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm Res. 2010;27:2283–2295. doi: 10.1007/s11095-010-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole AJ, et al. Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting. Biomaterials. 2011;32:2183–2193. doi: 10.1016/j.biomaterials.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chertok B, et al. Comparison of Electron Spin Resonance Spectroscopy and Inductively-Coupled Plasma Optical Emission Spectroscopy for Biodistribution Analysis of Iron-Oxide Nanoparticles. Molecular Pharmaceutics. 2010;7:375–385. doi: 10.1021/mp900161h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain TK, et al. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Molecular Pharmaceutics. 2008;5:316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 23.Lee MJ-E, et al. Rapid Pharmacokinetic and Biodistribution Studies Using Cholorotoxin-Conjugated Iron Oxide Nanoparticles: A Novel Non-Radioactive Method. PLoS One. 2010;5:e9536. doi: 10.1371/journal.pone.0009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medarova Z, et al. In vivo imaging of siRNA delivery and silencing in tumors. Nat Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 25.Kunzmann A, et al. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochimica et Biophysica Acta (BBA)-General Subjects. 2010 doi: 10.1016/j.bbagen.2010.04.007. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Gupta AK, et al. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Tran N, Webster TJ. Magnetic nanoparticles: biomedical applications and challenges. Journal of Materials Chemistry. 2010;20:8760–8767. [Google Scholar]
- 28.Maeda H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 29.Veiseh O, et al. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Advanced Drug Delivery Reviews. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Advanced Drug Delivery Reviews. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veiseh O, et al. Specific Targeting of Brain Tumors with an Optical/Magnetic Resonance Imaging Nanoprobe across the Blood-Brain Barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montet X, et al. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guthi JS, et al. MRI-Visible Micellar Nanomedicine for Targeted Drug Delivery to Lung Cancer Cells. Molecular Pharmaceutics. 2010;7:32–40. doi: 10.1021/mp9001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simberg D, et al. Biomimetic amplification of nanoparticle homing to tumors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AL, et al. Synthesis of bombesin-functionalized iron oxide nanoparticles and their specific uptake in prostate cancer cells. Journal of Nanoparticle Research. 2010;12:1599–1608. doi: 10.1007/s11051-009-9681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, et al. A Surface-Charge Study on Cellular-Uptake Behavior of F3-Peptide-Conjugated Iron Oxide Nanoparticles. Small. 2009;5:1990–1996. doi: 10.1002/smll.200900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy GR, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clinical Cancer Research. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 38.Jiang JS, et al. A novel magnetic fluid based on starch-coated magnetite nanoparticles functionalized with homing peptide. Journal of Nanoparticle Research. 2009;11:1321–1330. [Google Scholar]
- 39.Meng J, et al. LHRH-functionalized superparamagnetic iron oxide nanoparticles for breast cancer targeting and contrast enhancement in MRI. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2009;29:1467–1479. [Google Scholar]
- 40.John R, et al. In vivo magnetomotive optical molecular imaging using targeted magnetic nanoprobes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8085–8090. doi: 10.1073/pnas.0913679107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadjipanayis CG, et al. EGFRvIII Antibody-Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Convection-Enhanced Delivery and Targeted Therapy of Glioblastoma. Cancer Res. 2010;70:6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho Y-S, et al. Cetuximab-conjugated magneto-fluorescent silica nanoparticles for in vivo colon cancer targeting and imaging. Cancer Lett. 2010;299:63–71. doi: 10.1016/j.canlet.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Chen TJ, et al. Targeted folic acid-PEG nanoparticles for noninvasive imaging of folate receptor by MRI. Journal of Biomedical Materials Research Part A. 2008;87A:165–175. doi: 10.1002/jbm.a.31752. [DOI] [PubMed] [Google Scholar]
- 44.Suh JS, et al. Efficient labeling of mesenchymal stem cells using cell permeable magnetic nanoparticles. Biochemical and Biophysical Research Communications. 2009;379:669–675. doi: 10.1016/j.bbrc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 45.Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90:604–610. doi: 10.1002/bip.20989. [DOI] [PubMed] [Google Scholar]
- 46.Veiseh O, et al. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. 2010;31:8032–8042. doi: 10.1016/j.biomaterials.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarze SR, et al. In Vivo Protein Transduction: Delivery of a Biologically Active Protein into the Mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 48.Dobson J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006;67:55–60. [Google Scholar]
- 49.Pankhurst QA, et al. Applications of magnetic nanoparticles in biomedicine. J. Phys. D-Appl. Phys. 2003;36:R167–R181. [Google Scholar]
- 50.Chertok B, et al. Glioma selectivity of magnetically targeted nanoparticles: A role of abnormal tumor hydrodynamics. Journal of Controlled Release. 2007;122:315–323. doi: 10.1016/j.jconrel.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goya GF, et al. Magnetic nanoparticles for cancer therapy. Curr. Nanosci. 2008;4:1–16. [Google Scholar]
- 52.Yu F, et al. The magnetophoretic mobility and superparamagnetism of core-shell iron oxide nanoparticles with dual targeting and imaging functionality. Biomaterials. 2010;31:5842–5848. doi: 10.1016/j.biomaterials.2010.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chertok B, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David AE, et al. A combined theoretical and in vitro modeling approach for predicting the magnetic capture and retention of magnetic nanoparticles in vivo. J. Control. Release. 2011 doi: 10.1016/j.jconrel.2011.01.033. In Press, Uncorrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu HL, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riegler J, et al. Targeted magnetic delivery and tracking of cells using a magnetic resonance imaging system. Biomaterials. 2010;31:5366–5371. doi: 10.1016/j.biomaterials.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 57.Huang ZY, et al. Deep magnetic capture of magnetically loaded cells for spatially targeted therapeutics. Biomaterials. 2010;31:2130–2140. doi: 10.1016/j.biomaterials.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 58.Polyak B, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steed stents. Proc. Natl. Acad. Sci. U. S. A. 2008;105:698–703. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrari M, et al. In BioMEMS and Biomedical Nanotechnology. Springer US: 2006. Magnetic Nanoparticles for MR Imaging; pp. 227–237. [Google Scholar]
- 60.Weissleder R, et al. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 61.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foy SP, et al. Optical Imaging and Magnetic Field Targeting of Magnetic Nanoparticles in Tumors. ACS Nano. 2010;4:5217–5224. doi: 10.1021/nn101427t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson TA, et al. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology. 2007;18:8. [Google Scholar]
- 64.Xie J, et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stelter L, et al. Modification of Aminosilanized Superparamagnetic Nanoparticles: Feasibility of Multimodal Detection Using 3T MRI, Small Animal PET, and Fluorescence Imaging. Molecular Imaging and Biology. 2010;12:25–34. doi: 10.1007/s11307-009-0237-9. [DOI] [PubMed] [Google Scholar]
- 66.Lee H, et al. Chip-NMR biosensor for detection and molecular analysis of cells. Nature Medicine. 2008;14:869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H, et al. Ultrasensitive Detection of Bacteria Using Core-Shell Nanoparticles and an NMR-Filter System. Angew. Chem.-Int. Edit. 2009;48:5657–5660. doi: 10.1002/anie.200901791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollert E, et al. Search of new core materials for magnetic fluid hyperthermia: Preliminary chemical and physical issues. Prog. Solid State Chem. 2009;37:1–14. [Google Scholar]
- 69.Purushotham S, Ramanujan RV. Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta Biomater. 2010;6:502–510. doi: 10.1016/j.actbio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Comes Franchini M, et al. Bovine serum albumin-based magnetic nanocarrier for MRI diagnosis and hyperthermic therapy: a potential theranostic approach against cancer. Small. 2010;6:366–370. doi: 10.1002/smll.200901689. [DOI] [PubMed] [Google Scholar]
- 71.Le Renard PE, et al. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials. 2010;31:691–705. doi: 10.1016/j.biomaterials.2009.09.091. [DOI] [PubMed] [Google Scholar]
- 72.Chen YJ, et al. Controlled Release from Bilayer-Decorated Magnetoliposomes via Electromagnetic Heating. ACS Nano. 2010;4:3215–3221. doi: 10.1021/nn100274v. [DOI] [PubMed] [Google Scholar]
- 73.Pradhan P, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release. 2010;142:108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Bhojani MS, et al. Targeted Imaging and Therapy of Brain Cancer Using Theranostic Nanoparticles. Molecular Pharmaceutics. 2010;7:1921–1929. doi: 10.1021/mp100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar M, et al. Image-Guided Breast Tumor Therapy Using a Small Interfering RNA Nanodrug. Cancer Res. 2010;70:7553–7561. doi: 10.1158/0008-5472.CAN-10-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veiseh O, et al. Inhibition of Tumor-Cell Invasion with Chlorotoxin-Bound Superparamagnetic Nanoparticles. Small. 2009;5:256–264. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agrawal A, et al. Functional Delivery of siRNA in Mice Using Dendriworms. ACS Nano. 2009;3:2495–2504. doi: 10.1021/nn900201e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soenen SJH, De Cuyper M. Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine. 2010;5:1261–1275. doi: 10.2217/nnm.10.106. [DOI] [PubMed] [Google Scholar]
- 79.Ying EB, Hwang HM. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Science of the Total Environment. 2010;408:4475–4481. doi: 10.1016/j.scitotenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 80.Chen PY, et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro-Oncology. 2010;12:1050–1060. doi: 10.1093/neuonc/noq054. [DOI] [PMC free article] [PubMed] [Google Scholar]