Abstract
Little is known about the chemical coding of the brain neuronal circuitry activated by nociceptive signals of visceral origin. We characterized brain nuclei activated during isovolumetric phasic distension of the proximal colon (10 ml, 30 s on/off for 10 min) in conscious male rats, using Fos as a marker of neuronal activation and dual immunohistochemistry to visualize co-localization of Fos expression and oxytocin (OT), arginine-vasopressin (AVP), corticotrophin-releasing factor (CRF) or tyrosine hydroxylase (TH). Proximal colon distension, compared with sham distension, induced a robust increase in Fos-like immunoreactive (IR) neurons in the paraventricular nucleus (PVN), supraoptic nucleus (SON) and accessory neurosecretory nuclei of the hypothalamus, nucleus of the solitary tract (NTS) and ventrolateral medulla (VLM), and to a lower extent, in the locus coeruleus (LC) and Barrington nucleus. Fos-like immunoreactivity in the PVN after colon distension of identified in 81% of OT-IR, 18% AVP-IR and 18% CRF-IR neurons, while in the SON it represented 36% of OT-IR and 16% AVP-IR. Catecholaminergic cell groups in the pons (LC) and medulla (VLM, NTS) were also activated by proximal colon distension. Of the TH-IR neurons in VLM and NTS, 74% and 42% respectively were double labeled. These results indicate that colon distension stimulates OT-, AVP- and CRF-containing hypothalamic neurons, likely involved in the integration of colonic sensory information to modulate autonomic outflow and pain-related responses. Activation of medullary catecholaminergic centers might reflect the afferent and efferent limbs of the functional responses associated to visceral pain.
Keywords: Colon distension, visceral pain, oxytocin, vasopressin, tyrosine hydroxylase, corticotrophin-releasing factor
1. Introduction
Enhanced colonic and rectal sensitivity to distension is frequently encountered as a clinical manifestation of irritable bowel syndrome (IBS) (Bouin et al., 2002; Drossman et al., 2002; Verne et al., 2003). In an attempt to develop experimental models that recapture this feature, the distension of the rectum and distal colon (colorectum) in rats has been extensively used to assess visceral pain and the underlying mechanisms of colonic sensitivity (Mayer et al., 2008; Ness and Gebhart, 1990). Processing of signals from visceral organs recruits neurons and transmitters in the brain that are important mechanisms regulating adaptive autonomic, neuroendocrine and pain-related responses. We previously used a model of proximal colon distension in conscious rats to study the neuronal pathways activated during noxious visceral mechanical stimulation (Martinez et al., 1998; Martinez et al., 2006). This model uses a chronically implanted balloon in the proximal half of the colon, thus avoiding acute procedures of balloon insertion under anesthesia that cause interfering effects on neuronal activation (Martinez et al., 2006; Ness and Gebhart, 1988). After phasic distension of the proximal colon, neuronal activation, demonstrated by Fos-like immunoreactivity (IR), was found significantly increased in specific brain areas and spinal cord lumbosacral segments (Martinez et al., 1998; Martinez et al., 2006). Main brain nuclei activated by distension of the proximal colon were located in the hypothalamus [paraventricular nucleus (PVN) and supraoptic nucleus (SON)] and the brainstem [nucleus tractus solitarius (NTS), ventrolateral medulla (VLM) and locus coeruleus (LC)-Barrington nucleus complex] (Martinez et al., 2006). These areas represent, respectively, centers of integration of neuroendocrine responses and the origin of efferent autonomic pathways involved mainly in the control of visceral and cardiovascular functions (Madden and Sved, 2003; Ness et al., 1999; Pan et al., 1999; Sawchenko et al., 1996; Sawchenko and Swanson, 1982; Weston et al., 2003). The PVN contains multiple neurotransmitters and neuronal hormones, including corticotropin-releasing factor (CRF), oxytocin (OT) and arginine-vasopressin (AVP), directly involved in responses to stress and visceral functions (Leng et al., 1999; Sawchenko et al., 1996; Swanson and Sawchenko, 1983). Moreover, the PVN receives prominent inputs from catecholaminergic neurons in the brainstem, such as the VLM and LC, which are involved in sympathetic regulation of cardiovascular and visceral functions (Cunningham and Sawchenko, 1988), and were also activated during colonic distension (Card et al., 2006; Martinez et al., 2006; Pan et al., 1999).
Although colonic distension is a commonly used experimental model to study visceral sensitivity, the related neurotransmitters associated to the brain pathways activated during the process have not been explored. In the present study, we used a dual immunohistochemical method for coexistence of the c-fos gene protein and specific neuropeptides (namely OT, AVP and CRF) or the neurotransmitter-synthesizing enzyme for catecholamines, tyrosine hydroxylase (TH), to localize and characterize the chemical coding of the neurons activated by proximal colon distension in conscious rats.
2. Results
Animals recovered well from the surgical procedure involved in the positioning the balloon into the proximal colon and resumed normal feeding and defecation behaviors during the 48 h period thereafter. Isovolumetric phasic distension of the proximal colon by inflating the balloon with 10 ml of air for 30 s on/30 s off for 10 min resulted in a mean intra-balloon pressure of 87 ± 4 mmHg (distension pressure oscillated between 78 and 100 mmHg). This mechanical stimulation in awake unrestrained rats induced behavioral responses consistent with the induction of pain (concave arching of the back, stretching of the torso and licking of the abdominal wall) (Al Chaer et al., 2000; Giamberardino et al., 1995). Control animals (sham distension) showed exploratory activity or slept during the experimental time.
2.1. Distension of the proximal colon induces Fos-IR in specific brain nuclei
The response to the proximal colon distension procedure and the pattern of distribution of Fos-IR neurons were essentially the same as previously reported by us, while few Fos-IR neurons appeared in the same areas in rats undergoing sham distension (Figs. 1-3 and 5) (Martinez et al., 2006). Relative abundance of TH-, OT-, AVP- and CRF-IR neurons were similar in the sham distension and proximal colon distension groups. In the present study, we focused on hypothalamic, medullary and pontine areas responsive to proximal colon distension and known to contain large populations of TH-, OT-, AVP- and CRF-IR neurons.
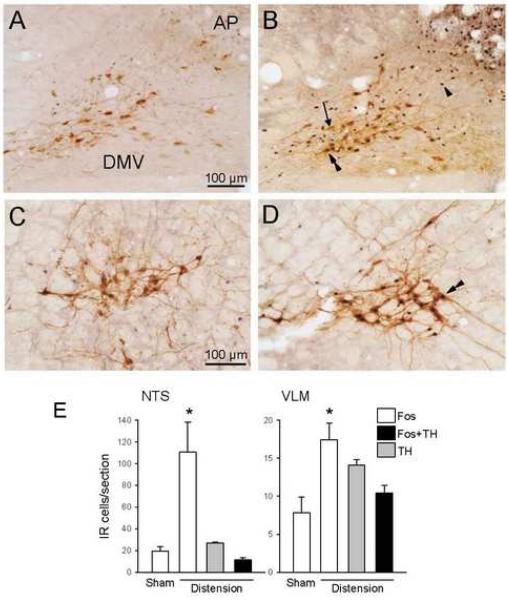
Fig. 1.
Double immunohistochemical staining of Fos- and TH-IR in the NTS (A and B) and VLM (C and D) and cell count (cells per section) in the same areas (E) 60 min after phasic proximal colon distension for 10 min (B and D) or sham distension (A and C) in conscious rats. Fos-IR is localized in nuclei and appears as dark blue-black dots and TH-IR is found in the cytoplasm as brown color. Arrow: TH-IR; arrowhead: Fos-IR; arrowheads: Fos/TH-IR; AP: area postrema; DMV: dorsal motor nucleus of the vagus. Data are mean±SEM; n=5; *P<0.05 vs sham distension group.
Fig. 3.
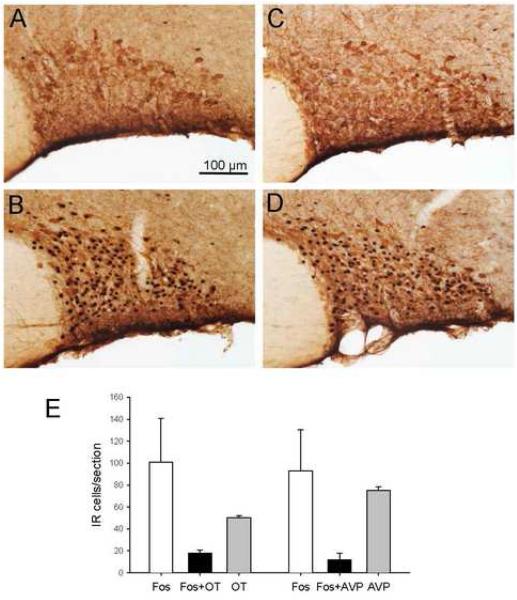
Double immunohistochemical staining of Fos- and OT-IR (A-D) or AVP-IR (E and F) in the PVN of the hypothalamus 60 min after phasic proximal colon distension for 10 min (A-C and E) or sham distension (D and F) in conscious rats. The inserts in A and E are magnification of the framed areas. A: Fos- and OT-IR double labeling in the medial magnocellular (mm) division and dorsal cap (dc). B and D: Fos- and OT-IR double-labeled neurons in the lateral magnocellular (lm), ventral (v), medial parvocellular (mp), dorsal cap (dc) and periventricular (pe) divisions of the PVN. C: Fos- and OT-IR double labeling in the posterior (p) PVN. Note the almost complete absence of double-stained cells. E and F: Fos- and AVP-IR double labeling in the PVN. Bar in B indicates the same scale in all panels while the scale in A corresponds to the two inserts.
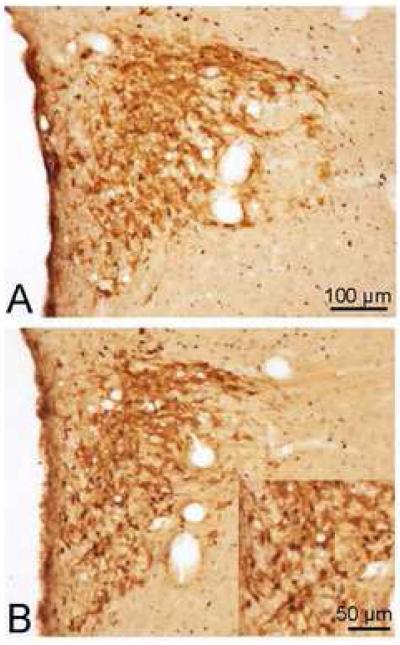
Fig. 5.
Double immunohistochemical staining of Fos- and OT-IR (A and B) or AVP-IR (C and D) in the SON of the hypothalamus 60 min after phasic proximal colon distension for 10 min (B and D) or sham distension (A and C) in conscious rats. Bar = 100 μm. E: Cell counting (cells per section) in the SON.
2.2. Fos and Fos/TH-dual immunoreactive neurons in the medulla and pons
The areas in the medulla with the most pronounced neuronal activation 1 h after the 10 min-period of phasic distension (10 ml, 30s off/on) of the proximal colon in conscious rats were the AP (data not shown, for a more detailed description see Martinez et al, 2006), NTS (111±28 vs 19±4 Fos-IR cells/section in the sham distension group, P<0.05; Fig. 1B) and VLM (17±2 vs 8±2 Fos-IR cells/section in the sham distension group, P<0.05; Fig.1D). The majority of Fos-IR neurons in the NTS were localized in the medial subnucleus, along with a few cells in the subnucleus gelatinosa (Fig. 1B), the commissural, ventral and ventrolateral subnuclei. No Fos-IR neurons were observed after colon distension in the dorsal motor nucleus of the vagus (DMV) (Fig. 1B). The TH-positive cells in this A2/C2 area were observed in the medial subnucleus and subnucleus gelatinosa of the NTS and the caudal DMV (Fig. 1A). Fos/TH-double-stained cells were located predominantly in the NTS (Fig. 1B), but not in the DMV. Within the NTS, most of the double-stained neurons were confined to the medial subnucleus with a few cells in the subnucleus gelatinosa, smaller in size with a scanty cytoplasm that was hardly distinguished in double immunostained sections. Overall, in the NTS, 42.2±7.0% of TH-IR neurons expressed Fos, and 11.5±1.1% of Fos-IR neurons had TH-IR neuronal somata (Fig. 1 E). Fos-IR neurons in the VLM (Fig. 1D) were found in the entire rostro-caudal column including the C1/A1 area. The TH-IR staining clearly delineated the VLM cell group (Fig. 1C and D). After colon distension, 74.0 ± 6.2% of TH-positive neurons were also Fos-IR and 62.6±3.6% of Fos-IR neurons showed TH-IR (Fig. 1E).
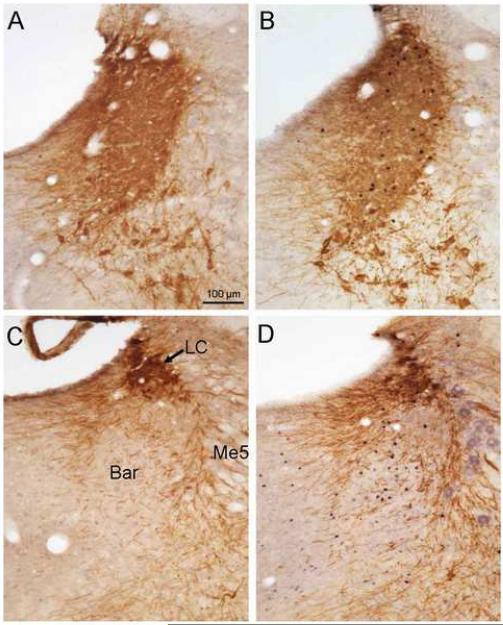
In the pons, proximal colon distension increased Fos expression in the LC and, to a lesser extent, in the Barrington nucleus (Fig. 2). Most of the Fos-IR nuclei were found in TH-IR neurons, however, the processes and cell bodies of TH-IR neurons were compact and heavily stained, making the cell counting difficult (Fig. 2B). In the Barrington nucleus, Fos-IR cells formed a characteristic cluster surrounded by dense TH-positive processes, while no double-stained cells were observed (Fig. 2D).
Fig. 2.
Double immunohistochemical staining of Fos- and TH-IR in the pons 60 min after phasic proximal colon distension for 10 min (B and D) or sham distension (A and C) in conscious rats. A and B show the locus coeruleus (LC) while C and D show the Barrington nucleus (Bar). Me5: mesencephalic nucleus of trigeminal nerve (5th cranial nerve; the staining in panel D is non-specific).
2.3. Fos and Fos/OT- and Fos/AVP-dual immunoreactive neurons in the hypothalamus
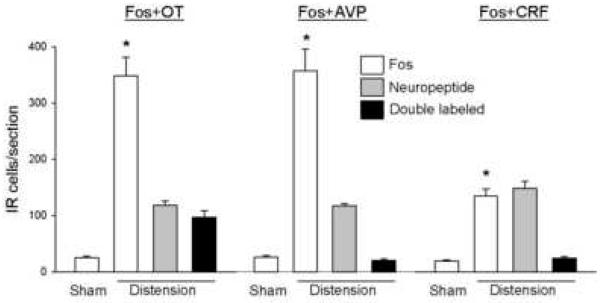
Proximal colon distension increased Fos-IR to 347±34 cells/section in the PVN and 93±37 in the SON compared with 25±4 and 4±1 cells/section (P<0.05) in sham distension rats, respectively. In the PVN, numerous Fos-IR neurons were observed in the medial parvocellular part and the medial and lateral magnocellular parts (Fig. 3 A, B and D) compared to sham distension (Fig. 3D,F). Fos-IR neurons in the medial parvocellular part were heterogeneous in size and had irregular nuclear profiles, while those in the magnocellular subdivisions were more homogeneous in size with large round nuclei. Besides these locations, some Fos-IR cells were in the dorsal cap and ventral and posterior subnuclei. Similarly, a few of Fos-IR cells were found in the periventricular nucleus (Fig. 3A-C). Fos/OT-IR neurons within the PVN were mostly located in the medial magnocellular part where they aggregate into a compact cell cluster (Figs. 3A and B). Fos/OT-IR neurons were also observed in the lateral magnocellular part, however a large variation among rats was observed in this region. The dorsal cap and ventral part of the PVN had a moderate number of Fos/OT-IR double labeled neurons and a few were scattered in the medial parvocellular part and the periventricular nucleus, close to the wall of the third ventricle (Fig. 3A and B). In the posterior part of the PVN, almost no double-labeled cells were observed, although the OT-IR ones were still relatively abundant (Fig. 3C). Overall, within the PVN, the colon distension-induced Fos/OT-coexisting neurons represented 80.8±7.4% of total OT-IR and 27.6±1.1% of Fos-IR cells (Fig 4). The major location of AVP-IR neurons was the magnocellular portion of the PVN, including both the medial and the lateral parts, where most of the Fos/AVP-coexisting cells were found (Fig. 3E). In the medial parvocellular, dorsal cap and ventral part as well as the periventricular nucleus, only scattered Fos/AVP-IR cells were observed (Fig. 3E). Compared with Fos/OT, Fos/AVP-double labeled cells were less abundant, although both Fos-IR and AVP-IR neurons were densely distributed in the same areas (Fig. 3E). The double-labeled cells in response to distension of the proximal colon represented only 5.7±0.5% of Fos-IR and 17.6±1.7% of AVP-IR cells (Fig 4).
Fig. 4.
Cell counts (cells per section) of Fos- and OT-IR or AVP-IR in the PVN 60 min after phasic proximal colon distension for 10 min in conscious rats. Data are mean±SEM; n=5; *P<0.05 vs sham distension group.
In the SON, after colon distension, Fos-IR neurons were evenly distributed except for the ventral zone where they were sparse (Fig. 5B and D). The labeled neuronal nuclei in the SON were large and round in similar size. Both Fos/OT- and Fos/AVP-IR neurons were present in the SON. Fos/AVP-IR neurons were evenly distributed within the SON (Fig. 5D) while Fos/OT neurons were more abundant in the dorsal part (Fig. 5B). Overall, 30±7.4% and 10.5±1.7% of Fos-IR neurons were double-stained with OT- and AVP-IR respectively, representing 36.4±5.2% of OT-IR and 16.2±5 % of AVP-IR neurons expressing Fos-IR (Fig. 5E).
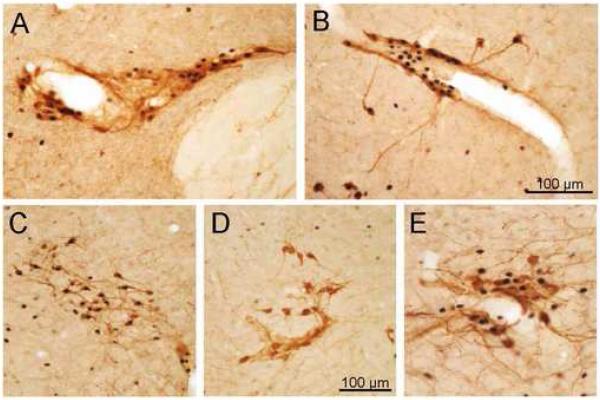
In addition, Fos-IR was also found in small clusters of neurons in the so-called accessory neurosecretory nuclei, mainly the nucleus circularis, in the middle area of the medial hypothalamus, the posterior fornical nucleus, around the fornix, and scattered neuronal groups in the lateral hypothalamus (Fig. 6B-E). These Fos-positive cell groups showed co-localization with OT- (Fig. 6A-D) and AVP-IR (Fig. 6E). In general, Fos/OT double-labeled cells were more abundant than those with Fos/AVP co-existence, and in some of cases nearly all OT-IR neurons were Fos-positive. Often, the double stained cells were observed surrounding the blood vessels (Fig. 6A, B and E).
Fig. 6.
Double immunohistochemical staining of Fos- and OT-IR (A-D) or AVP-IR (E) in the accessory neurosecretory nuclei of the hypothalamus 60 min after phasic proximal colon distension for 10 min (A-C and E) or sham distension (D) in conscious rats. A: parafornical nucleus; B-E: lateral hypothalamus. Double-labeled neurons were often found surrounding the blood vessels (A, B and E).
2.4. Fos/CRF-dual immunoreactive neurons in the hypothalamus
To reveal CRF immunoreactivity in a reliable manner, animals were pre-treated with a low dose of received colchicine did not show any change in Fos expression in the PVN 24 h later compared with non-treated rats (19±2 vs. 25±2 Fos-IR cells/section in non-treated and colchicine-treated animals, respectively; P>0.05). CRF-IR neurons in the hypothalamus were located in the medial parvocellular part, dorsal cap and ventral and posterior parts of the PVN, accessory neurosecretory nuclei, the arcuate nucleus and the lateral hypothalamic area. After proximal colon distension, Fos-IR neurons were increased to 134±13 cells/section (P<0.05 vs sham distension). Fos/CRF-IR double-labeled neurons were located mostly in the medial parvocellular PVN (Fig. 7) resulting in 17.5±1.1% of Fos-IR neurons double-stained with CRF-IR, while 16.4±3.5% of CRF-IR was co-localized with Fos-IR. Few of double-stained cells were found in rats with sham distension (Fig. 7).
Fig. 7.
Double immunohistochemical staining of Fos- and CRF-IR in the PVN. Animals were treated with icv colchicine 24 h before the distension protocol. A: sham distension; B: proximal colon distension.
3. Discussion
Phasic isovolumetric distension of the proximal colon for a 10-min period at pressures within a noxious range (~90 mmHg) in freely moving rats evoked a marked neuronal activation in specific populations of brain neurons as assessed by Fos expression 1 h later. The most intense Fos expression was observed in the NTS, AP, PVN and SON, while a more modest increase, although still clear, was found in the VLM and the LC-Barrington nucleus complex, a pattern consistent with that reported previously by us under similar conditions (Martinez et al., 2006). Using double labeling, the characterization of these neuronal populations based on their morphological aspects and chemical coding revealed that proximal colonic distension activates medullary and pontine catecholaminergic cell groups and hypothalamic OT-, AVP- and CRF-containing neurons.
It is worthy to remember that Fos is a non-specific indicator of neuronal activation. Therefore, given that gut distension likely produces multiple reflex responses, it is possible that some of the changes in Fos expression observed following colon distension are not a direct consequence of the distension procedure but due to the indirect activation of reflex mechanisms that contribute to the overall responses observed. Nevertheless, extensive previous works using the same (colon distension) (Ness and Gebhart, 1990; Lantéri-Minet et al., 1993; Martinez et al., 1998, 2006; Traub et al., 2002; Mönnikes et al., 2003) or related experimental models of visceral pain (gastric distension or urinary bladder distension) (Birder and de Groat, 1993; Traub et al., 1996; Molinari et al., 2006) largely support the assumption that the neuronal responses observed reflect mainly the activation of pain-related pathways.
In the brainstem, the marked activation of catecholaminergic neurons induced by proximal colon distension encompasses mainly the VLM (A1/C1), NTS (A2/C2) and LC (A6) cell groups. In the VLM and NTS double-labeled Fos and TH neurons represent approximately 74% and 42% of the TH-IR neurons, respectively. While somatic nociceptive stimulation, such as thermal pain, was shown to activate catecholaminergic neurons in the VLM of rats (Pan et al., 1999), the activation of TH-IR neurons in relation to visceral pain stimuli has not been reported. Here we show that a significant subpopulation of brainstem catecholaminergic neurons is responsive to visceral pain-related signals arising from the colon. These catecholaminergic cell groups are interconnected with the PVN and preganglionic sympathetic and parasympathetic neurons in the brainstem and spinal cord (Pan et al., 1999; Tucker et al., 1987). In fact, a major afferent innervation of the PVN originates in noradrenergic ascending fibers arising from the VLM and NTS (Sawchenko and Swanson, 1982). In particular, the parvocellular division of the PVN is mainly innervated by the A1 in the VLM, and A2 in NTS, whereas the A6 noradrenergic group in the LC innervates mainly the periventricular zone (Cunningham, Jr. and Sawchenko, 1988). The descending projections from the VLM to the spinal cord arise mainly from C1 (Cunningham, Jr. et al., 1990; Tucker et al., 1987), and the caudal VLM (C1) and NTS which are reciprocally connected in rats (Yu and Gordon, 1996). Similarly, the LC sends axons to both the DVC and the intermediolateral column in the spinal cord (Westlund and Coulter, 1980; Wotherspoon et al., 1997). The extensive connections of these noradrenergic and adrenergic neurons suggest that the proximal colon distension-induced catecholaminergic neuronal activation might trigger the activation of a complex circuitry involved in the modulation of autonomic functions associated with visceral sensations (Benarroch, 2006). Therefore, the activation of these medullary and pontine catecholaminergic neurons correlates well with the intense Fos-IR observed in the PVN and SON and with the activation of preganglionic parasympathetic neurons in the lumbosacral spinal cord (Martinez et al., 1998).
Consistent with our findings, electrophysiological recordings of LC neurons showed activation of the LC-noradrenergic system during distal colonic or colorectal distension (Cullen et al., 1995; Elam et al., 1986; Lechner et al., 1997; Kosoyan et al., 2005) and norepinephrine (NE) release into in the hippocampus (Saito et al., 2002; Saito et al., 2005) from LC ascending projections that contribute the sole source of norepinephrine (Valentino and Van Bockstaele, 2008). This facilitates processing of stimulus-related information leading to arousal and anxiogenic behavioral responses (Valentino and Van Bockstaele, 2008).
The PVN is the main hypothalamic nucleus integrating the activation of the hypothalamic-pituitary-adrenal (HPA) axis and the neuroendocrine control of visceral autonomic functions. The pattern of Fos expression in the PVN induced by the proximal colonic distension is consistent with the recruitment of magnocellular neurons projecting to the posterior pituitary gland, parvocellular neurons projecting to the median eminence, and descending neurons from the ventral part and dorsal cap projecting to the brainstem and spinal cord (Swanson and Kuypers, 1980; Swanson and Sawchenko, 1983). This is supported by the increase in Fos expression that encompasses various subnuclei of the PVN. This includes the medial parvocellular part showing a consistent and robust increase in Fos immunoreactivity, the dorsal cap, the ventral and posterior subnuclei and the periventricular nuclei, that displayed a more modest Fos expression, and the magnocellular subdivisions in which neuronal activation was clear although with some degree of individual variation, that may reflect differences in visceral sensitivity, but also other systemic influences. Double labeling showed that the majority of oxytocin neurons in the hypothalamus, including those in PVN, SON and accessory neurosecretory nuclei, expressed Fos, indicating that they were directly or indirectly activated by the noxious mechanical stimulation of the proximal colon. In the PVN, as much as 81% of oxytocin neurons were activated, contrasting with less than 18% of vasopressin neurons. These findings indicate that PVN oxytocinergic neurons are prominently activated by visceral nociceptive inputs from the colon. This different OT/AVP ratio in the neuroendocrine activation of the PVN expands previous studies showing a differential activation of OT- and AVP-containing neurons in response to a variety of stimuli, including both visceral and somatic pain (Onaka et al., 1986; Bonaz et al., 1994; Smith and Day, 1994). We previously reported that a mixed somatovisceral stimulation in rats, consisting of laparotomy and 1 min cecal manipulation, induced Fos expression in approximately 33% of oxytocin neurons of the PVN (Bonaz et al., 1994). In addition, several stressors, like physical immobilization, forced swimming, systemic injection of interleukin-1β, or hypertonic salt loading, activated OT-containing neurons in the PVN and SON as a part of the stress response in rats (Ericsson et al., 1994; Giovannelli et al., 1990; Lang et al., 1983). Restraint stress induced Fos in about 40% of oxytocin neurons in the PVN (Miyata et al., 1995), while immunological stress resulted in the activation of 70% and 7% of the OT and AVP neurons, respectively (Ericsson et al., 1994). In response to osmotic stress, 70% of oxytocin neurons were activated (Giovannelli et al., 1990). These observations are consistent with the present results and suggest that OT-containing neurons within the PVN are a key component in the integration of stress-related signals, including visceral pain, while vasopressin neurons seem to participate to a lesser extent.
Whether the activation of OT-containing neurons in the PVN in response to proximal colon distension translates to the release of oxytocin both centrally from cell bodies and dendrites and also peripherally from the axon terminals (Onaka, 2004) needs to be further investigated. However, consistent with a systemic release of oxytocin, there are reports showing that activation of afferent sensory fibers from the vagus and sciatic nerves in rats resulted in the release of oxytocin into the systemic circulation (Stock and Uvnas-Moberg, 1988). Our observation that distension of the proximal colon-induced Fos expression in PVN neurons was largely dependent upon the activation of capsaicin sensitive sensory afferents (Martinez et al., 2006) agrees with similar effects taking place during proximal colon distension. In the periphery, oxytocin has been shown to reduce visceral pain and discomfort in IBS patients during colorectal distension (Louvel et al., 1996; Ohlsson et al., 2005), to stimulate colonic motor function in healthy women (Ohlsson et al., 2004) and to ameliorate colonic inflammation in rats (Iseri et al., 2005). In addition, convergent evidence in the brain established that oxytocin dampened the HPA responses to stressors (Amico et al., 2004). Therefore, it may be argued that the relatively low proportion of CRF-IR co-localized with Fos-IR (16.4±3.5%) within the PVN may reflect a percentage of CRF activated neurons dampened by the local release of oxytocin. Indeed, studies in oxytocin deficient mice showed a higher upregulation of CRF mRNA in the PVN and increased plasma levels of corticosterone compared with wild type animals when exposed to stress (Amico et al., 2004; Nomura et al., 2003).
Oxytocin-containing neurons in the hypothalamus may be also part of the reflex arch involved in the inhibition of gastric motility induced by distension of the proximal colon observed under similar experimental conditions (Martinez et al., 2006). Oxytocin-containing neurons in the PVN project to the DVC and spinal cord at the level of sympathetic preganglionic neurons, where oxytocin receptors are present (Rinaman, 1998; Swanson and Kuypers, 1980; Vaccari et al., 1998). Microinjection of oxytocin into the DVC activates gastric projecting neurons in the NTS and DMV, as identified by gastric distension or vagal stimulation (McCann and Rogers, 1990), and inhibits gastric motility (Rogers and Hermann, 1987) in rats. Moreover, the decrease in gastric motility in response to PVN stimulation is blocked by microinjection of oxytocin receptor antagonists in the DMV (Rogers and Hermann, 1987).
We also observed that nearly one-fifth of AVP-containing neurons in the PVN were activated by the colon distension. Although the ratio of activated neurons was much lower than that of oxytocin, these observations still support a role for vasopressin in the central integration of pain related signals, as previously reported (Gong et al., 1992; Yang et al., 2006; Yang et al., 2007). In particular, central injection of AVP antibodies reversed the increase in pain threshold induced by the injection of glutamate sodium into the PVN of rats (Yang et al., 2006; Yang et al., 2007). Immunological stress elicited by administration of IL-1β resulted also in a relatively low percentage (7%) of activated AVP-containing neurons (Ericsson et al., 1994). Interestingly, mixed psychological and physical stressors like immobilization and forced swimming, failed to affect AVP-containing neurons in rats (Lang et al., 1983). Nevertheless, consistent evidence both in animal models and humans have implicated vasopressin in anxiogenic responses and vasopressin receptors are a recognized target for anxiety-related disorders (Buéno et al., 1992; Griebel et al., 2003; Landgraf, 2006). Whether or not the activation of AVP-containing neurons during noxious colon distension reflects only the anxiogenic component of pain or is more directly related to the integration and processing of pain signals warrants further studies. For instance, there is evidence of cardiovascular effects of vasopressin acting in the brain as a transmitter, in addition to its well-known peripheral vasopressor actions (Milutinović et al. 2006).
CRF and its receptors in the brain play an important role in colorectal distension-elicited anxiogenic responses, ACTH release, and alterations of gut motility and visceral sensitivity in animals and humans (Ferguson et al., 2008; Gué et al., 1997; Martinez et al., 2006; Million et al., 2006; Taché et al., 2004). Indeed, central administration of CRF in rats results in hypersensitivity to colorectal distension (Greenwood-Van Meerveld et al., 2005; Gué et al., 1997) and CRF1 receptor antagonist attenuated colorectal distension (80 mm Hg for 20 min)-induced plasma adrenocorticotropic hormone, anxiety and visceral pain (Saito et al., 2005). As shown in the present study, proximal colon distension in rats induced Fos in CRF-containing hypothalamic neurons, supporting the involvement of CRF in the central processing of visceral pain signals. Similarly, ip injection of acetic acid, a recognized somatovisceral pain model (Martinez et al., 1999), induced c-fos expression in CRF-IR neurons and increased CRF receptor 1 transcription in the PVN of rats (Sinniger et al., 2004), although CRF mRNA levels were not increased (Hwang et al., 2007). In colchicine-treated rats, colon distension elicited an increase in Fos expression, visualized in many CRF neurons in the medial parvocellular division of the PVN, the main source of CRF. However, the magnitude of the Fos response was relatively lower than in non-treated animals. It needs to be pointed out that colchicine treatment acts as a neuronal stressor (Ceccatelli et al., 1989; Kjaer et al., 1994) that might increase Fos expression. However, few Fos-positive cells were observed in the PVN 24 h after colchicine treatment in sham distension animals, in agreement with previous reports (Ceccatelli et al., 1989; Kjaer et al., 1994). Although the procedures seems innocuous, colchicine treatment might not be an optimal method to reveal the complete population of CRF-containing neurons within the PVN. In future studies, the use of more sensitive antibodies could provide a better tool to avoid colchicine treatment.
In summary, we established that noxious phasic distension of the proximal colon in conscious rats for 10 min results in the activation of an important population of catecholaminergic neurons in the brainstem as shown by 74% and 42% of the TH-IR neurons in the VLM and NTS respectively being Fos positive and double labeling with TH-IR and Fos in LC neurons. In addition, in the hypothalamus, noxious colon distension activates a large population of oxytocin containing neurons (81% PVN and 36% in the SON) along with a lower proportion of AVP-IR (18% in the PVN and 16% in the SON). We also observed that 16% of CRF neurons in the PVN were double labeled. The co-localization of Fos expression with OT, TH, CRF and AVP indicates that these neuronal transmitters are important in the central processing of visceral pain signals, and further establishes these mediators as key players in the central integration and modulation of visceral autonomic responses, including pain arising from visceral structures.
4. Experimental procedures
4.1. Animals Preparation
Male Sprague-Dawley rats (280-350 g; Harlan, San Diego, CA) were maintained under conditions of controlled temperature (20-22°C), illumination (12:12 h light/dark cycle, starting from 0600 h) and humidity with food (Purina Lab Chow, Purina Mills, Inc., Richmond, IN) and water ad libitum. All experiments were performed under the VA animal component of research protocol number 95-085-10.
The implantation of a flaccid latex balloon (5×1 cm) attached to polyethylene tubing (PE-50; ID, 0.58 mm; OD, 0.97 mm) into the proximal colon (1 cm distally to the ileocecocolic junction) was performed by surgical approach as detailed in our previous studies (Martinez et al., 1998; Martinez et al., 2006) in non-fasted rats anesthetized with ketamine (75 mg/kg, ip; Ketaset, Fort Dodge Laboratories, Fort Dodge, IO, USA) and xylazine (25 mg/kg, ip; Rompun, Mobay, Shawnee, KS, USA). The PE-50 tubing was exteriorized through the abdominal wall, conducted subcutaneously to the back of the neck, and capped. After surgery, rats were housed in individual cages.
4.2. Distension of the proximal colon
The colonic distension was performed 48 h after the intracolonic implantation of the balloon in non-fasted and freely moving rats maintained in their home cages. Rats were slightly restrained by hand for approximately 1 min to connect the PE-50 catheter through flexible plastic tubing with a two-way stopcock key to a 10-ml syringe and a manometer. Within the next 5 min, mechanical distension of the proximal colon was achieved by inflating the balloon with 10 ml of air in a ramped manner (5-6 s). Then, inflation was repeated at 30 s intervals (30 s on/30 s off) for a 10-min period. Pressure within the balloon during the distension procedure was monitored by connecting the catheter to a manometer, and was determined at the end of each 30 s distension period. Pressure reached during the distension procedure was calculated by subtracting the intrinsic pressure associated with the elasticity of the balloon from the intra-balloon pressure recorded during the distension procedure. The intrinsic pressure of each balloon was measured by inflating the balloon hanging freely in the air with the same volume of air (10 ml) before its placement in the proximal colon, as previously described (Azpiroz and Malagelada, 1985; Martinez et al., 1998; Martinez et al., 2006). In the sham distension group, rats implanted with a latex balloon into the proximal colon were exposed to the similar manipulations as the distension group, but without inflating the balloon.
4.3. Colchicine treatment
For detection of CRF-IR, 7 rats were treated with colchicine via a chronically implanted intracerebroventricular (icv) cannula. The guide cannulae were stereotaxically implanted into the right lateral brain ventricle according to coordinates in the Paxinos and Watson’s atlas (Paxinos and Watson, 1998), i.e. from the bregma posterior −0.8 mm; lateral −1.5 mm and dorsoventral −3.5 mm. Five days later, a balloon was implanted in the proximal colon, as described above. Colchicine (20 μg/rat in 10 μl of sterile isotonic saline; Sigma Chemical Co., San Louis, MO) was administered icv 24 h before the proximal colon distension procedure. The icv injection was performed in lightly restrainted animals using a 28 ga injection cannula, 1 mm longer than the guide cannula, connected to a 50 μl Hamilton syringe by a PE-50 catheter (Intramedic Polyethylene Tubing, Clay Adams, Sparks, MD) filled with distilled water. A small air bubble (1 μl) was drawn at the distal end of the PE-50 catheter to separate the colchicine solution from the water and for visual inspection of the 10 μl injection, which was performed slowly over 30 s period. At the end of the experiments, at the time of euthanasia, the correct location of the cannula into the lateral ventricle was verified by injecting 10 μl of dye (0.1% toluidine blue). Visualization of dye on the wall of the lateral ventricle indicates correctness of the icv injections. Of the rats treated with colchicine, two served as sham distension controls and five were subjected to the colon distension procedure described above.
4.4. Tissue processing and immunohistochemistry
Sixty min after completion of the proximal colon distension, rats were deeply anaesthetized with sodium pentobarbital (70 mg/kg, ip) (Nembutal®, Abbott Laboratories, Chicago, IL) and perfused via the ascending aorta with 50 ml isotonic saline followed by 500 ml of 4% paraformaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer (pH 7.4). Brains were post-fixed for 5 h at 4°C in the same fixative and then cryoprotected in 20% sucrose in 0.1M phosphate buffer overnight at 4°C. Frozen coronal sections of the hypothalamus, pons and medulla were cut at 30 μm in a cryostat and collected in phosphate buffer. The immunoreactivity of Fos plus OT, AVP, CRF or TH was detected by a dual ABC (avidin-biotin-peroxidase complex) immunohistochemical technique as previously. The procedures were as follows: (1) Incubation overnight at 4°C with c-fos antibody (Ab-5, rabbit polyclonal IgG; Oncogene Research Products, Cambridge, MA) at a dilution of 1:10,000 in 0.1 M phosphate buffered saline (PBS) containing 0.3% Triton X-100 and 1% bovine serum albumin (BSA); (2) incubation with biotinylated goat Fab fragment anti-rabbit IgG (1:2,000; Jackson ImmunoResearch Laboratories, Inc; West Grove, PA) 60 min at room temperature; (3) incubation with avidin-biotin-peroxidase complex (1:100; Vectastain ABC kit, Vector Laboratories, Burlingame, CA) 60 min at room temperature. (4) Reaction in a solution containing 2.5% nickel ammonium sulfate, 0.035% 3,3′-diaminobenzidine tetrahydrochloride (DAB) and 0.001% hydrogen peroxide (H2O2) in 0,1 M acetate buffer (pH 6.5). (5) Incubation overnight at 4°C with rabbit anti-OT (1:1,000), anti-AVP (1:2,000) (Peninsula Laboratories, Belmont, CA), anti-CRF (1:10,000; Dr. Vale, the Salk Institute, La Jolla, CA) or mouse anti-TH (1:4,000; Boehringer Mannheim Co., Germany) in 0.3% Triton X-100 and 1% BSA in PBS; steps (6) and (7) were the same as (2) and (3); lastly, the visualization of the immunoreactive products was obtained by reaction with 0.025% DAB and 0.01% H2O2 in Tris buffer at pH 7.6. Then, the sections were air-dried, dehydrated, cleared and cover-slipped.
4.5. Cell counting and statistical analysis
The immunoreactive cells were counted and photographed in a Zeiss Axioskop Microscope. Fos-IR was identified as dark blue-black immunoreactive products deposited in the nuclei, other neuronal markers’ staining appeared in the cytoplasm as brown color. For quantitative assessment, the number of immunoreactive cells was counted unilaterally in 10-20 sections of selected brain nuclei identified with the Paxinos and Watson’s atlas (Paxinos and Watson, 1998) coordinates (mm from bregma): −1.80 to −1.88 for the PVN; −0.92 to −1.40 for the SON; −9.30 to −10.04 for the LC, −12.30 to −14.30 for the VLM and −13.30 to −14.8 for the NTS. In all cases, the nomenclature of the brain nuclei and their cytoarchitectonic subdivisions followed those of Paxinos and Watson (Paxinos and Watson, 1998). In each nucleus, 3 groups of neurons were counted (1) Fos-IR, (2) any of CRF-IR, OT-IR, AVP-IR or TH-IR and (3) double-labeled neurons. Since no consecutive sections were used for the detection of the same neuronal marker, no corrections for double counting were applied. Number of labeled neurons per section in each nucleus was presented as mean ± SEM of 4-5 rats for each group. Differences between means were determined by a Kruskal-Wallis nonparametric analysis of variance (ANOVA) followed by a Dunn’s multiple comparisons test. Statistical comparisons between two groups were performed by a Mann-Whitney U-test. Data were considered significantly different when P < 0.05.
Acknowledgements
This work was supported by the National Institute of Arthritis, Metabolism and Digestive Diseases; Center Grant DDK-P30 41301 (VM, Pilot and Feasibility Award; Animal Core), R01 Grant DK-33061 (YT), P50 AR 049550 (YT, LW) and VA Career Scientist Award (YT). We thank Dr Wylie Vale (Salk Institute, La Jolla, CA) for the generous supply of the rat CRF antibody.
Abbreviations
- AP
area postrema
- AVP
arginine-vasopressin
- CRF
corticotrophin-releasing factor
- DMV
dorsal motor nucleus of the vagus
- DVC
dorsal vagal complex
- IR
immunoreactive or immunoreactivity
- LC
locus coeruleus
- NTS
nucleus of the solitary tract
- OT
oxytocin
- PVN
paraventricular nucleus of the hypothalamus
- SON
supraoptic nucleus
- TH
tyrosine hydroxylase
- VML
ventrolateral medulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Malagelada JR. Intestinal control of gastric tone. Am. J. Physiol. 1985;249:G501–G509. doi: 10.1152/ajpgi.1985.249.4.G501. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Pain-autonomic interactions. Neurol. Sci. 2006;27(Suppl 2):S130–S133. doi: 10.1007/s10072-006-0587-x. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am. J. Physiol. 1993;265:R326–R333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Plourde V, Taché Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J. Comp Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distension testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- Buéno L, Gué M, Delrio C. CNS vasopressin mediates emotional stress and CRH-induced colonic motor alterations in rats. Am. J. Physiol. 1992;262:G427–G431. doi: 10.1152/ajpgi.1992.262.3.G427. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J. Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Villar MJ, Goldstein M, Hokfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JJ, Caropreso DK, Ephgrave KS. Effect of endotoxin on canine gastrointestinal motility and transit. J. Surg. Res. 1995;58:90–95. doi: 10.1006/jsre.1995.1015. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr., Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr., Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J. Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- Elam M, Thoren P, Svensson TH. Locus coeruleus neurons and sympathetic nerves: activation by visceral afferents. Brain Res. 1986;375:117–125. doi: 10.1016/0006-8993(86)90964-9. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert. Opin. Ther. Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamberardino MA, Valente R, de Bigontina P, Vecchiet L. Artificial ureteral calculosis in rats: behavioural characterization of visceral pain episodes and their relationship with referred lumbar muscle hyperalgesia. Pain. 1995;61:459–469. doi: 10.1016/0304-3959(94)00208-V. [DOI] [PubMed] [Google Scholar]
- Giovannelli L, Shiromani PJ, Jirikowski GF, Bloom FE. Oxytocin neurons in the rat hypothalamus exhibit c-fos immunoreactivity upon osmotic stress. Brain Res. 1990;531:299–303. doi: 10.1016/0006-8993(90)90789-e. [DOI] [PubMed] [Google Scholar]
- Gong S, Yin WP, Yin QZ. Involvement of vasopressinergic neurons of paraventricular nucleus in the electroacupuncture-induced inhibition of experimental visceral pain in rats. Sheng Li Xue. Bao. 1992;44:434–441. [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol. Motil. 2005;17:415–422. doi: 10.1111/j.1365-2982.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Stemmelin J, Gal CS, Steinberg R. The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr. Drug Targets. CNS. Neurol. Disord. 2003;2:191–200. doi: 10.2174/1568007033482850. [DOI] [PubMed] [Google Scholar]
- Gué M, Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol. Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Chang HM, Gu ZH, Suzuki R. c-fos gene expression is increased in the paraventricular hypothalamic nucleus of Sprague-Dawley rats With visceral pain induced by acetic acid without detectable changes of corticotrophin-releasing factor mRNA: a quantitative approach with an image analysis system. Anat. Rec. (Hoboken. ) 2007;290:406–413. doi: 10.1002/ar.20495. [DOI] [PubMed] [Google Scholar]
- Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides. 2005;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Kjaer A, Larsen PJ, Knigge U, Moller M, Warberg J. Histamine stimulates c-fos expression in hypothalamic vasopressin-, oxytocin-, and corticotropin-releasing hormone-containing neurons. Endocrinology. 1994;134:482–491. doi: 10.1210/endo.134.1.8275963. [DOI] [PubMed] [Google Scholar]
- Kosoyan HP, Grigoriadis DE, Taché Y. The CRF1 receptor antagonist, NBI-35965, abolished the activation of locus coeruleus neurons induced by colorectal distension and intracisternal CRF in rats. Brain Res. 2005;1056:85–96. doi: 10.1016/j.brainres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Landgraf R. The involvement of the vasopressin system in stress-related disorders. CNS. Neurol. Disord. Drug Targets. 2006;5:167–179. doi: 10.2174/187152706776359664. [DOI] [PubMed] [Google Scholar]
- Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- Lantéri-Minet M, Isnardon P, de Pommery J, Menétrey D. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Krox-24 proteins. Neuroscience. 1993;55:737–753. doi: 10.1016/0306-4522(93)90439-m. [DOI] [PubMed] [Google Scholar]
- Lechner SM, Curtis AL, Brons R, Valentino RJ. Locus coeruleus activation by colon distension: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog. Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Louvel D, Delvaux M, Felez A, Fioramonti J, Bueno L, Lazorthes Y, Frexinos J. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut. 1996;39:741–747. doi: 10.1136/gut.39.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Sved AE. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol. Neurobiol. 2003;23:739–749. doi: 10.1023/A:1025000919468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Thakur S, Mogil JS, Taché Y, Mayer EA. Differential effects of chemical and mechanical colonic irritation on behavioral pain response to intraperitoneal acetic acid in mice. Pain. 1999;81:179–186. doi: 10.1016/s0304-3959(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Mayer E, Taché Y. Proximal colon distension increases Fos expression in the lumbosacral spinal cord and activates sacral parasympathetic NADPHd-positive neurons in rats. J. Comp Neurol. 1998;390:311–321. [PubMed] [Google Scholar]
- Martinez V, Wang L, Taché Y. Proximal colon distension induces Fos expression in the brain and inhibits gastric emptying through capsaicin-sensitive pathways in conscious rats. Brain Res. 2006;1086:168–180. doi: 10.1016/j.brainres.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Bradesi S, Chang L, Spiegel BM, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Oxytocin excites gastric-related neurons in rat dorsal vagal complex. J. Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu V, Wei JY, Rivier J, Vale W, Mayer EA, Taché Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinović S, Murphy D, Japundzić-Zigon N. The role of central vasopressin receptors in the modulation of autonomic cardiovascular controls: a spectral analysis study. Am. J. Physiol. 2006;291:R1579–91. doi: 10.1152/ajpregu.00764.2005. [DOI] [PubMed] [Google Scholar]
- Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res. Bull. 1995;37:391–395. doi: 10.1016/0361-9230(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Molinari C, Sabbatini M, Grossini E, Mary DA, Cannas M, Vacca G. Cardiovascular effects and c-Fos expression in the rat hindbrain in response to innocuous stomach distension. Brain Res. Bull. 2006;69:140–146. doi: 10.1016/j.brainresbull.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Mönnikes H, Rüter J, König M, Grote C, Kobelt P, Klapp BF, Arnold R, Wiedenmann B, Tebbe J,J. Differential induction of c-fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors. Brain Res. 2003;966:253–264. doi: 10.1016/s0006-8993(02)04197-5. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Piper JG, Follett KA. The effect of spinal analgesia on visceral nociceptive neurons in caudal medulla of the rat. Anesth. Analg. 1999;89:721–726. doi: 10.1097/00000539-199909000-00036. [DOI] [PubMed] [Google Scholar]
- Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. J. Neuroendocrinol. 2003;15:1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Ringstrom G, Abrahamsson H, Simren M, Bjornsson ES. Oxytocin stimulates colonic motor activity in healthy women. Neurogastroenterol. Motil. 2004;16:233–240. doi: 10.1111/j.1365-2982.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Truedsson M, Bengtsson M, Torstenson R, Sjolund K, Bjornsson ES, Simren M. Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterol. Motil. 2005;17:697–704. doi: 10.1111/j.1365-2982.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. J. Neuroendocrinol. 2004;16:308–312. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- Onaka T, Hamamura M, Yagi K. Potentiation of vasopressin secretion by footshocks in rats. Jpn. J. Physiol. 1986;36:1253–1260. doi: 10.2170/jjphysiol.36.1253. [DOI] [PubMed] [Google Scholar]
- Pan B, Castro-Lopes JM, Coimbra A. Central afferent pathways conveying nociceptive input to the hypothalamic paraventricular nucleus as revealed by a combination of retrograde labeling and c-fos activation. J. Comp Neurol. 1999;413:129–145. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Orlando: 1998. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J. Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Saito K, Kanazawa M, Fukudo S. Colorectal distension induces hippocampal noradrenaline release in rats: an in vivo microdialysis study. Brain Res. 2002;947:146–149. doi: 10.1016/s0006-8993(02)03007-x. [DOI] [PubMed] [Google Scholar]
- Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distension in rats. Gastroenterology. 2005;129:1533–1543. doi: 10.1053/j.gastro.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog. Brain Res. 1996;107:201–222. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Sinniger V, Porcher C, Mouchet P, Juhem A, Bonaz B. c-fos and CRF receptor gene transcription in the brain of acetic acid-induced somato-visceral pain in rats. Pain. 2004;110:738–750. doi: 10.1016/j.pain.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Smith DW, Day TA. c-fos expression in hypothalamic neurosecretory and brainstem catecholamine cells following noxious somatic stimuli. Neuroscience. 1994;58:765–775. doi: 10.1016/0306-4522(94)90453-7. [DOI] [PubMed] [Google Scholar]
- Stock S, Uvnas-Moberg K. Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiol Scand. 1988;132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br. J. Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Zhai Q, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression in the spinal cord and the visceromotor reflex. Neuroscience. 2002;113:205–211. doi: 10.1016/s0306-4522(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. J. Comp Neurol. 1987;259:591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG. Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J. Comp. Neurol. 2003;460:525–541. doi: 10.1002/cne.10663. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Coulter JD. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res. 1980;2:235–264. doi: 10.1016/0165-0173(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Lopez-Costa JJ, Michael GJ, Priestley JV. Constitutive expression of calmodulin-binding phosphoprotein GAP-43 in rat serotonergic and noradrenergic cell groups which project to the spinal cord. Neurochem. Res. 1997;22:985–993. doi: 10.1023/a:1022474826040. [DOI] [PubMed] [Google Scholar]
- Yang J, Song CY, Liu WY, Lin BC. Only through the brain nuclei, arginine vasopressin regulates antinociception in the rat. Peptides. 2006;27:3341–3346. doi: 10.1016/j.peptides.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Chen JM, Xu HT, Liu WY, Wang CH, Lin BC. Arginine vasopressin is an important regulator in antinociceptive modulation of hypothalamic paraventricular nucleus in the rat. Neuropeptides. 2007;41:165–176. doi: 10.1016/j.npep.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Yu D, Gordon FJ. Anatomical evidence for a bi-neuronal pathway connecting the nucleus tractus solitarius to caudal ventrolateral medulla to rostral ventrolateral medulla in the rat. Neurosci. Lett. 1996;205:21–24. doi: 10.1016/0304-3940(96)12383-1. [DOI] [PubMed] [Google Scholar]