Abstract
Numerous studies have shown that Akt isoforms promote tumorigenesis by enhancing cancer cell survival and growth, and it is well established that signaling through the Akt upstream regulator PI3-K enhances cancer cell migration. Therefore, it is conventionally accepted that PI3-K/Akt pathway promotes tumor formation and metastasis. A few years ago, studies from several laboratories added a new layer to the pleiotropic effects of Akt function by showing that the Akt1 isoform inhibits breast cancer cell migration and invasion, whereas Akt2 promotes these phenotypes. These studies challenged the dogma and identified non-redundant functions of Akt isoforms in cancer progression. The identification of palladin as an Akt1-specific substrate in our recently published work has exemplified distinct Akt isoform-specific signaling in breast cancer.
Here, we review these findings and discuss the implications for the understanding of the mechanistic basis for designing more effective anti-cancer therapeutics targeting the Akt pathway.
Key words: Akt isoforms, breast cancer, migration, metastasis, palladin
Over the past two decades, numerous studies have revealed a central role of the Akt family of protein kinases (PKBα/Akt1, PKBβ/Akt2 and PKBγ/Akt3) in cellular functions including cell survival, proliferation and growth—processes that are crucial for tumorigenesis.1 Indeed, hyperactivation of Akt signaling due to mutations in PIK3CA, the gene that encodes the p110α catalytic subunit of phosphoinositide 3-kinase (PI3-K), PTEN loss or HER2 amplification is one of the most common features of many human solid tumors as well as hematological malignancies.2,3 Studies in transgenic mice have shown that Akt promotes mammary tumor progression by increasing cell survival.4 Similarly, a constitutively activated Akt1 allele is able to transform fibroblasts in vitro.5 Using a murine lymphoma model, Akt was shown to promote tumor formation and drug resistance via the translational regulators mTOR and eIF4E.6 The critical role of Akt in tumorigenesis is also suggested by the fact that a large number of proteins in the Akt pathway are either oncogenes (e.g., Akt itself, PI3-K, MDM2, cyclin D1) or tumor suppressors (e.g., PTEN, INPP4B, TSC2, p27, PHLPP), and these proteins are frequently deregulated in human cancers, most often by somatic mutations or loss of heterozygosity.7 Based on this notion, there is currently a major effort to develop compounds that target the Akt pathway for therapeutic benefit in cancer and other pathophysiologies. In fact, several Akt inhibitors including triciribine, perifosine and MK-2206 are currently being tested in phase I and II clinical trials.8–10 A few years ago, however, observations from several independent laboratories revealed that, perhaps unexpectedly, Akt isoforms have opposing roles in the regulation of breast cancer cell invasive migration and metastatic dissemination, events that ultimately determine clinical outcome.
In transgenic mouse models, Akt1 has been shown to promote mammary tumor growth, and yet suppress metastasis.11,12 However, experiments using Akt1-deficient mice have not yielded wholly consistent results. In one study, metastasis of mammary tumors was increased in Akt1 knockout mice.12 Yet in a separate study, Akt1 deficiency was found to reduce lung metastases.13 Nonetheless, the majority of the currently available in vivo data do agree with the overall model whereby Akt1 inhibits invasive migration of breast cancer cells. Our own initial studies showed that mechanistically this is in part due to the degradation of Nuclear Factor of Activated T cells (NFAT), a pro-invasion transcription factor.14 Studies from the Brugge laboratory also demonstrated that Akt1 inhibits breast cancer cell motility by attenuating ERK activity,15 whereas Bissell and colleagues showed the same phenotype functioning through the tumor suppressor tuberous sclerosis complex 2 (TSC2).16 Finally, it has been demonstrated that Akt2 upregulates β1 integrins and promotes invasion as well as metastasis of breast cancer cells in vivo.17,18 Although these studies have provided considerable insight into the mechanisms by which Akt isoforms differentially regulate epithelial and cancer cell migration (Fig. 1), the immediate isoform-specific substrates that modulate cell motility in an Akt isoform-specific manner have yet to be identified.
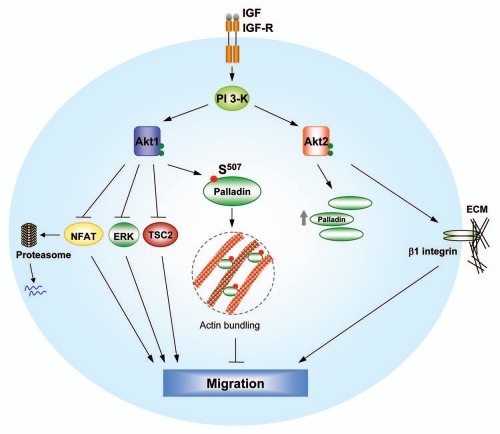
Figure 1.
Opposing functions of Akt isoforms in breast cancer cell invasive migration. Akt1 suppresses NFAT, TSC2 and ERK functions directly or indirectly, thereby inhibiting cell motility. By phosphorylating palladin, Akt1 promotes its actin-bundling function and leads to decreased cell migration. In contrast, Akt2 upregulates expression of β1 integrin and enhances breast cancer cell migration and metastasis. Interestingly, Akt2 also promotes stability and mRNA levels of palladin. The molecular mechanisms by which Akt2 regulates palladin remain to be determined.
Intriguingly, Akt isoforms exhibit distinct functions in cancer progression in spite of their high sequence and structural homology.19,20 The considerable efforts currently aimed at developing Akt inhibitors for anti-cancer therapeutics prompted us to investigate the molecular mechanisms by which Akt isoforms differentially regulate breast cancer metastasis. Using a bioinformatics data set generated by Gygi and colleagues,21 we set out to search for candidate substrates that contain an evolutionary conserved sequence that conforms to the Akt phosphorylation motif (RXRXXS/T),22 and furthermore have functions relating to cell architecture and motility. With a combination of biochemical and molecular genetic approaches, we recently identified the actin-bundling protein palladin as the first Akt isoform-specific substrate that contributes to the differential regulation of breast cancer cell migration.23 We showed that wild-type but not S507A mutant palladin is phosphorylated in response to IGF-I stimulation. By using Akt isoform-specific siRNAs and in vitro kinase assays, we reported that Akt1 but not Akt2 directly phosphorylates palladin at Ser507. As palladin has been shown to regulate the organization of the actin cytoskeleton,24 we then investigated the functional role for the Akt and palladin signaling axis in modulating invasive migration. Depletion of palladin by shRNA enhances invasive migration of breast cancer cells and induces abnormal branching morphogenesis in 3D cultures, suggesting an anti-migratory role for palladin. Moreover, Akt1-mediated inhibition of cell migration was rescued by palladin silencing, indicating that palladin is at least one downstream effector which mediates the inhibitory effect of Akt1 on migration. Importantly, the migratory effect induced by palladin silencing was reversed by wild-type but not the S507A mutant, suggesting that phosphorylation of palladin by Akt1 is critical for the inhibition on cell motility. Taken together, these findings not only provide a mechanism that is responsible for the non-redundant functions of Akt isoforms in migration, but also identify an Akt isoform-specific substrate that contributes to cancer progression (Fig. 1). This likely bears important clinical implications, such that in order to maximize efficacy and minimize side effects, it would ultimately be more desirable to target downstream substrates of PI3-K/Akt rather than the kinases themselves for therapeutic intervention. Future efforts aimed at identifying a comprehensive list of Akt isoform-specific substrates and dissecting their functions in tumorigenesis are warranted. In this context, newly available Akt isoform-specific inhibitors and global phosphoproteomic screens from tumor cell lines and tissues should prove to be instrumental towards these goals.
A particularly surprising revelation emerging from our studies is the molecular insight by which Akt isoforms recognize their substrates. Despite more than 100 Akt substrates that have been identified to date, only a few have been shown to be isoform-specific.19,25 The isoform-specificity of these substrates is likely to be cell type-dependent, and in turn be determined by the amount and spatial localization of distinct Akt isoforms, and thus accessibility to different scaffolding proteins in different cell types. In our studies we demonstrated that palladin only interacts with Akt1 but not Akt2.23 More importantly, recombinant Akt1 but not Akt2 phosphorylates palladin in a cell-free system, suggesting that there are one or more molecular determinants on Akt1 that confer substrate specificity. Using chimeras of Akt1 and Ak2 isoforms, we went on to demonstrate that the linker region located between the pleckstrin homology and kinase domains plays a critical role in the association of Akt1 and palladin. Current studies are aimed at evaluating the model that a docking site on Akt isoforms determines substrate recognition. Identification of such docking motif will ultimately facilitate the discovery of novel isoform-specific substrates and further our understanding of the mechanisms of functional selectivity in the PI3-K and Akt pathway.
While it is well known that Akt regulates the physiological function of its targets, Akt-dependent phosphorylation can also modify the stability of a small subset of substrates. For example, Akt phosphorylates the tumor suppressor merlin and the transcription factor Androgen Receptor, leading to their degradation.26,27 Interestingly, although our data indicate that phosphorylation of palladin by Akt1 has no effect on its stability, subsequent studies revealed that Akt2 enhances palladin expression by promoting its stability, as well as upregulating mRNA levels (Fig. 1).28 This is surprising since Akt1, but not Akt2, associates with palladin.23 At the molecular level, it is likely that both Akt kinase function as well as a putative scaffolding activity regulate the contribution of Akt1 and Akt2 signaling with respect to palladin function.
Palladin function has been implicated in human cancer progression and its expression is found to be increased in breast tumor tissues.29 Since our studies have shown that palladin phosphorylation plays an anti-migratory role in breast cancer cells, it may be possible to utilize palladin phosphorylation as a biomarker for breast cancer metastases. We predict that the phosphorylation levels of palladin are lower in invasive breast carcinoma tissues compared to non-invasive carcinoma or normal breast tissues. Indeed, this is in agreement with our observations that whereas the total levels of palladin do not appear to correlate with the relative invasiveness of breast cancer cell lines, palladin phosphorylation is higher in non-tumorigenic, non-invasive MCF10A breast epithelial cells when compared to invasive and metastatic basal-like breast cancer cell lines such as SUM-159-PT and MDA-MB-231.23
There are numerous unanswered questions that stem from the discovery that palladin is an Akt1-specific substrate that controls invasive migration. For example, what is the migratory role of palladin in tumor-associated stromal cells? Our studies have focused on breast epithelial cells and also were conducted using in vitro or cell-based systems. Although Matrigel invasion assays and 3D cultures provide much insight into the molecular mechanisms of palladin regulation by Akt, it is important to demonstrate the physiological relevance of this novel mechanism of Akt1-specific signaling in breast cancer metastasis in vivo, and also determine the function of palladin in stromal cells such as fibroblasts and endothelial cells. Similarly, Akt signaling has been studied from numerous perspectives. However, isoform-specificity issue has arguably remained an under-explored area of work. Do Akt isoforms have distinct functions in other aggressive tumors where the Akt pathway is hyperactive? Finally, over the past few years, increasing number of proteins are demonstrated to both “help and hinder” tumor progression. In addition to Akt1, transforming growth factor β (TGFβ) and WAVE have been shown to be double-faced proteins.30,31 Which aspect the protein functions at a particular moment will likely depend on the nature of the interacting partners and cross-talk pathways that are involved at various stages of tumor progression. Careful dissection of these mechanisms is critically important not only for a better understanding of the complexity of signaling networks that promote carcinoma, but also for providing new insight for the design of new and more effective cancer therapeutics.
Acknowledgments
This work was supported by a National Institutes of Health grant (A.T., CA122099; Y.R.C., T32 CA081156-09) and a generous grant from the Susan G. Komen Breast Cancer Foundation (Y.R.C., PDF0706963).
Abbreviations
- PI3-K
phosphoinositide-3-kinase
- NFAT
nuclear factor of activated T cells
- TSC2
tuberous sclerosis complex 2
- WT
wild-type
- TGFβ
transforming growth factorβ
References
- 1.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 2.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol Cell Biol. 2001;21:2203–2212. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Targeting the phosphatidylinositol-3-kinase/Akt pathway for the treatment of cancer. Curr Opin Investig Drugs. 2005;6:1250–1258. [PubMed] [Google Scholar]
- 9.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation: a 2009 update. Curr Top Med Chem. 2010;10:458–477. doi: 10.2174/156802610790980602. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- 12.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 13.Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci USA. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, et al. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci USA. 2006;103:4134–4139. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, et al. Overexpression of AKT2/protein kinase Bbeta leads to upregulation of beta1 integrins, increased invasion and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 18.Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21:470–476. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, et al. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- 23.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, et al. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- 25.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, et al. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–1207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- 27.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin YR, Toker A. Akt2 regulates expression of the actin-bundling protein palladin. FEBS Lett. 2010;584:4769–4774. doi: 10.1016/j.febslet.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goicoechea SM, Bednarski B, Garcia-Mata R, Prentice-Dunn H, Kim HJ, Otey CA. Palladin contributes to invasive motility in human breast cancer cells. Oncogene. 2009;28:587–598. doi: 10.1038/onc.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meulmeester E, Ten Dijke P. The dynamic roles of TGF-beta in cancer. J Pathol. 2010;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 31.Kurisu S, Takenawa T. WASP and WAVE family proteins: friends or foes in cancer invasion? Cancer Sci. 2010;101:2093–2104. doi: 10.1111/j.1349-7006.2010.01654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]