Abstract
Menisci are one of the most commonly injured parts of the knee. Conventional surgical interventions are often associated with a long-term increased risk of osteoarthritis. Meniscal tissue engineering utilizes natural or synthetic matrices as a scaffold to guide tissue repair or regeneration in three dimensions. Studies have shown that a diverse cellular response can be triggered depending on the composition of the surrounding extracellular matrix (ECM) components. As such, attempts have been made to replace or repair meniscus defects using tissue grafts or reconstituted ECM components prepared from a multitude of tissues. This commentary summarizes the most recent data on the response of meniscal cells to ECM components, both in vivo and in vitro, and focuses on their potential roles in meniscal repair and regeneration. We also discuss our recent investigations into the interactions of meniscal cells and a self-assembled biomimetic surface composed of meniscal ECM molecules. The biological effects conferred by the biomimetic surface, in terms of cell adhesion, proliferation, gene expression profiles and matrix synthesis, were evaluated. Finally, some suggested directions for future research in this field are outlined.
Key words: meniscus, tissue engineering, extracellular matrix, cell-matrix interactions, chondroitin sulfate, collagen
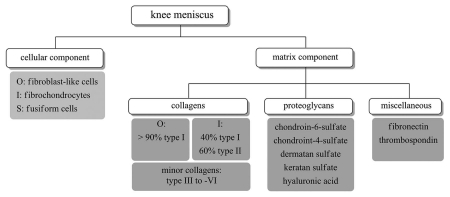
The meniscus is a C-shaped fibrocartilaginous tissue positioned within the knee joint that plays a chondroprotective role through load bearing, shock absorption and joint lubrication.1–3 Normal human menisci is composed of 72% water, 22% collagen and 0.8% glycosaminoglycans (GAGs), with chondroitin-6-sulfate being the major GAG constituent.4 In contrast to articular cartilage, where the predominant collagen is type II, collagen I is expressed throughout the meniscus and accounts for >90% of its collagen content.5 Collagen II, on the other hand, is only detected in the inner region. A small quantity of collagen III, -IV, -V and -VI is also found within the meniscus.5–7 It is suggested that interactions among collagen, proteoglycans and water accounts for the ability of meniscus to resist compressive load.3 The cellular components of meniscal tissue include elongated fibroblast-like cells located at the outer vascular region of the tissue and round fibrochondrocytes that are interspersed within the middle and inner region.8,9 These cells are responsible for synthesizing and maintaining the ECM components, especially the collagen.10,11 A schematic diagram of the cellular and matrix components of the meniscus is shown in Figure 1.
Figure 1.
A schematic diagram showing the cellular and matrix components found in knee menisci. O, outer one-third of the tissue; I, inner two-thirds of the tissue; S, superficial zone.
Menisci are one of the most commonly injured parts of the knee, accounting for a total number of 850,000 surgeries performed in the United States each year.12 Although conventional surgical interventions, including meniscectomy and suture fixation, appear to relieve pain symptom in the short term, long-term follow-ups suggest that these treatments are associated with an increased risk of osteoarthritis.13–15 The bottom line is that none of these procedures initiate repair or regeneration of the injured meniscus. This situation is worsened by the fact that the inner two-thirds of the meniscus is avascular.6,16 Without serum-derived factors to trigger the normal healing process and a provisional scaffold for cell migration into the defect site, cells within this region are deprived of their intrinsic reparative response to injury.17,18
Meniscal tissue engineering utilizes natural or synthetic matrices as a scaffold to guide tissue repair or regeneration in three dimensions. This can be attained via recruitment of cells from adjacent tissues (the meniscal remnant and the synovial membrane), or generation of neo-fibrocartilage in vitro by growing cells in the scaffold prior to implantation. Regardless of the strategies employed, a permissive microenvironment for cell attachment, proliferation and matrix synthesis should be created. Numerous studies have demonstrated the positive effect of growth factors (TGFβ, BMP-2, PDGF, IGF-1, FGF, etc.)19–22 low oxygen tension22,23 and cell-cell interactions24,25 on meniscal tissue regeneration.
Growing evidence indicates that, through its dynamic interactions with growth factors and degradative enzymes, the ECM microenvironment plays an essential role in regulating cell behaviors (reviewed in refs. 26–28). As such, attempts have been made to replace or repair meniscus defects using tissue grafts, including periosteal membrane,29 small intestinal submucosa,30 tendon31 and decellularized meniscus.32,33 The main drawback with all of these tissue grafts is that their initial material properties (e.g., strength, geometry, architecture) cannot be tailored to match those of native meniscal tissue. Despite the fact that cell repopulation was evident in most of these cases, the repair process was often incomplete, with continual progression of degenerative changes and graft shrinkage being reported in some cases. The possibility of disease transmission and tissue shortage also limits their applicability. Alternatively, ECM molecules can be extracted from a multitude of tissues and reconstituted according to the desired requirement, thus allowing examination of their individual effects on cell behavior. These molecules are typically presented to cells in the form of three-dimensional (3D) scaffolds, and to a lesser extent, as surface coatings or in a soluble (free) form.
Several candidate ECM molecules, mostly constituting the matrix of the native meniscus, are of interest for meniscal tissue engineering (Table 1). Among these is hyaluronic acid (HA), a non-sulfated GAG component found in the meniscus.34 Improvement in meniscal healing with intra-articular injection of HA solution has been documented in both rabbit and canine models.35,36 The exact mechanism for this therapeutic effect remains unclear, although the suppression of local nitric oxide production and proteoglycan degradation appear to play a role.37,38 In vitro, the solubilized HA exerted a mitogenic response on human meniscal cells in a dose-dependent manner without altering their appearance and chondroitin sulfate secretion.11 In contrast, ovine fibrochondrocytes grown on a HA-based scaffold showed a decrease on growth rate over a 4-week period.39 In our experience, poor cell attachment and proliferation are also consistently observed in rat meniscal cells that are seeded on a bare HA surface. Although no direct comparison is available, it is tempting to speculate that the way in which the HA molecules are presented to the cells might affect the cellular response and hence be related to the reported discrepant effects on meniscal cell behavior.
Table 1.
The response of meniscal cells to ECM components
| ECM components | Cell source | In vitro or in vivo | Treatments | Time | Results | References |
| Soluble (free) form | ||||||
| Hyaluronic acid (HA) | - | Rabbit model with a cylindrical meniscal defect | 1% solution of HA was injected into the joint once per week. | 6 weeks |
|
35 |
| HA | - | Rabbit model with anterior cruciate ligament (ACL) deficiency | The HA was injected into the joint once per week. | 5 weeks |
|
36 |
| HA | Human meniscus | In vitro | Passage 0 (P0) cells were cultured as monolayers in DMEM/F12 with 10% FBS overnight before treated with the solubilized HA. | 5 days |
|
11 |
| Scaffolds | ||||||
| Fibrin clot | - | Canine model with avascular meniscal defect | Circular defects (2 mm in diameter) were filled with an exogenous fibrin clot that had been prepared from the animal. | 6 months |
|
18 |
| Fibrin gel | Autologous fibrochondrocytes | Lapine model with full thickness defect | 2-week in vitro culture of cells before transplantation. | 4 weeks |
|
54 |
| Collagen I (allogenic devitalized meniscus) | Ovine fibrochondrocytes | In vitro | Cells at P3 were seeded onto the allograft and maintained in DMEM with 10% FBS and 1% penicillin and streptomycin. | 4 weeks |
|
55 |
| Collagen I | - | Rabbit model with partial medial meniscectomy | The defect (6–7 mm in length) was filled with a collagen I sponge. | 24 weeks |
|
29 |
| Collagen I (CMI, Regen Biologics, USA) | Autologous fibrochondrocytes | Sheep model with meniscectomy | 3-week in vitro culture of cells before transplantation. | 12 weeks |
|
56 |
| Collagen I (Menaflex CMI, Regen Biologics, USA) | - | Human patients with partial medial meniscectomy | The implant was sutured to the meniscus remnant with non-absorbable and an inside-out technique. | 1-year relook |
|
43 |
| Collagen I-GAG | Bovine fibrochondrocytes | In vitro | Cells at P5 were seeded onto the scaffold and maintained in DMEM/F12 containing 10% FBS, 1% penicillin/streptomycin and ascorbic acid. | 3 weeks |
|
44 |
| Collagen II-GAG |
|
|||||
| Collagen II/I,III (Geistlich Biomaterials, Switzerland) | Ovine meniscal cells | In vitro | Cells at P2 were seeded onto the scaffold and maintained in DMEM/F12 containing 10% FBS, 1% penicillin/streptomycin and ascorbic acid. | 4 weeks |
|
39 |
| HA (Hyaff-11, Fidia Advanced Biopolymers, Italy) |
|
|||||
| Surface coatings | ||||||
| Collagen I or aggrecan | Bovine knee fibrochondrocytes | In vitro |
|
1 day | Both coatings:
|
52 |
| Collagen I/II (at a 2:3 ratio) | Rat meniscal cells | In vitro |
|
2 weeks |
|
46 |
| Collagen I/II and chondroitin-6-sulfate |
|
Abbreviations: DMEM/F12, Dulbecco's modified Eagle's/Nutrient Mixture F-12 culture medium; FBS, fetal bovine serum; CMI, collagen meniscus implant.
The most extensively investigated matrix molecule in meniscal tissue engineering thus far is collagen I, owing to its abundance in the tissue and ease of reconstitution. Besides its major role as a structural protein to maintain meniscal integrity, collagen also provides binding sites for adhesion molecules involved in cell attachment or in the organization of the ECM.3,7 Walsh et al.29 reported neo-fibrocartilage formation in a rabbit partial meniscectomy model using a collagen I sponge pre-seeded with bone marrow-derived mesenchymal stem cells (bmMSCs). Without pre-seeding with bmMSCs, the collagen sponge was repopulated only with fibroblastic cells after six weeks. Degenerative changes were still present in both experimental groups, indicating that the mechanical properties of these constructs were overall suboptimal.
Apart from these examples, collagen-based copolymers have also been investigated. In the 1990s, the group of Stone et al.40,41 fabricated a copolymeric collagen scaffold that facilitated cell migration, proteoglycan synthesis and regeneration of meniscus in dogs. The scaffold was then used as a prototype for the development of the ReGen Biologics Menaflex® Collagen Meniscus Implant (formerly CMI®), which is the only commercially available graft for medial meniscus replacement following partial meniscectomy. It is composed mainly of bovine collagen I (>97%) with the remaining components being GAGs (chondroitin sulfate and hyaluronic acid).42 The randomized clinical trial revealed 45–58% of the defect being filled after one year, with the formation of biomechanically competent neo-fibrocartilage in patients with prior meniscal repair.43 The chondroprotective effect of the implant, however, was absent in recently injured patients.
Mueller et al.44 employed various porous collagen-GAG (chondroitin sulfate) matrices as a scaffold to investigate their effect on the growth and biosynthetic activity of seeded calf meniscal cells. Compared to the collagen I-GAG scaffold, the collagen II-GAG matrix displayed greater scaffold integrity and produced higher cell proliferation, as well as GAG production, consistent with a previous finding using canine articular chondrocytes.45 In a collagen-based composite sponge, it was also observed that more cells were attached to the collagen II layer compared to the collagen I/III layer.39 Whether these effects are the result of direct cell-matrix interactions remains to be resolved. Regardless, the differential responses of meniscal cells in these matrices comprised of different collagen types are highly likely to be related to the difference in the structural properties of the resultant scaffold.
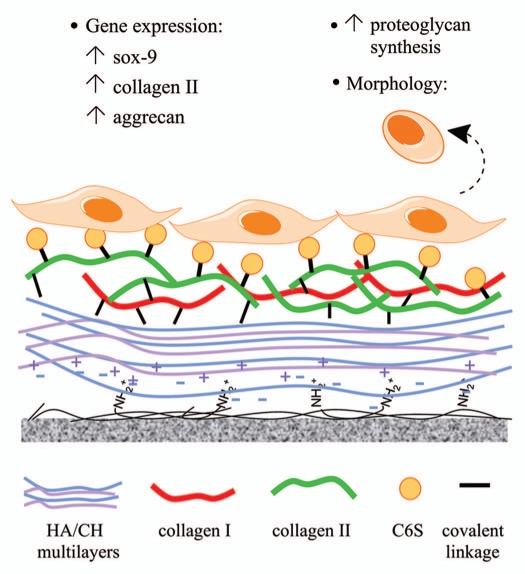
We have recently demonstrated that primary rat meniscal cells rapidly dedifferentiated during monolayer expansion on standard tissue culture plastic; the cells acquired a fibroblastic-like morphology and expressed high levels of collagen I, low levels of collagen II and proteoglycans, a gene expression profile opposite to that of native meniscal tissue.46 We also showed that this process could be reverted by culturing the cells on an engineered surface that is mimetic of the native meniscal ECM microenvironment. The surface is comprised of a precursor coating of hyaluronic acid/chitosan (HA/CH) mutilayers (a bioinert surface) to which major ECM components in the avascular region of the meniscus, namely collagen I/II (at a 2:3 ratio) and chondroitin-6-sulfate, were covalently immobilized (hereafter referred to as the C6S surface) (Fig. 2).
Figure 2.
A schematic diagram showing the interaction of meniscal cells and a biomimetic surface comprised of a precursor hyaluronic/chitosan (HA/CH) film and major ECM molecules found in the native meniscus. C6S, chondroitin-6-sulfate.
In our study, the initial attachment and growth rate was slower for cells seeded on the C6S surface compared to the collagen I/II-coated surface (hereafter referred to as the COL.I/II surface). A previous study, comparing effects of various ECM molecules on adhesion of chondrocytes, ligament cells and mesenchymal stem cells, showed that both collagen I and -II coatings induced greater cell adhesion, while HA, chondroitin sulfate and aggrecan served to inhibit this behavior.47 Enhanced cell adhesion and growth over native collagen was reported by Srivastava et al.48 when chondroitin sulfate was incorporated into collagen films. The absence of this enhancing effect on the meniscal cells, as observed in our study, may be due to the inverse relationship between proliferation and differentiation, in which differentiation (or “redifferentiation” in our case) is usually accompanied by decreased proliferation.49,50 On both surfaces, the cells regained their differentiated appearance, and started to lay down an extensive ECM over time.
In vitro culture of the dedifferentiated meniscal cells on the COL.I/II surface resulted in an upregulation of aggrecan gene (11-fold), although there was no significant effect on their collagen II expression levels. The inclusion of immobilized chondroitin-6-sulfate molecules on the surface (C6S surface) was found to enhance the cellular response, in which a dramatic upregulation of aggrecan (43-fold) and collagen II (8-fold) gene expression was noted. Simultaneously, these cells produced nearly three times more sulfated GAGs/DNA than on the COL.I/II surface. The upregulation of collagen II gene expression in meniscal cells by chondroitin-6-sulfate molecules could have been mediated via Sox-9 [(Sex determining region Y)-box 9] transcription factor, as an accumulation of Sox-9 mRNA preceded the expression of collagen II. In contrast, on the COL.I/II surface, where no upregulation of collagen II gene expression was detected, a low level of Sox-9 mRNA was noted throughout the experimental period. It has been reported that Sox-9 is strongly expressed in mesenchymal condensation preceding cartilage formation, as well as during chondrocyte differentiation in mouse embryos.51 A study by Gunja et al.52 demonstrated that collagen I and aggrecan (a chondroitin sulfate-rich proteoglycan) coatings were able to revert collagen I and cartilage oligomeric matrix protein (COMP, a chondrocytic marker), but not collagen II expression levels in dedifferentiated bovine fibrochondrocytes. Altogether, the findings of both of these investigations highlight the possibility to modulate gene expression in meniscal cells via cell-matrix interactions.
Tissue engineering holds great promise for meniscal repair and regeneration. Compared to other musculoskeletal tissues, such as cartilage and bone, relatively little is known about the biology of meniscal cells and their interactions with the native microenvironment. A systematic investigation of the effects of ECM components on meniscal cell behavior is thus vital to gaining a greater understanding of cell-material interactions and the requisite material inputs for optimal scaffold design. Another emergent area of interest and importance is the mechanism(s) involved in the response of meniscal cells to ECM molecules. For example, Lee et al.53 have shown that the articular chondrocyte attachment to collagen II, achieved partly via β1 integrin engagement, enhanced subsequent TGFβ-induced cell growth and proteoglycan synthesis. Much work remains to be done in this area with menisci. Last but not least, the effect of mechanical stimulation, at both macroscopic (tissue)54,55 and microscopic (cellular) levels,56 on meniscal cell behavior, is another important parameter to consider when developing a tissue engineered meniscus. Given the complexity of the in vivo microenvironment, an optimal outcome for meniscal tissue engineering will likely require a tailored combination of all of these microenvironmental factors.22,23,55
Acknowledgments
This work was supported by Australian Research Council (ARC) Discovery Grants Scheme and the University of Queensland AIBN Challenge Project Grant Scheme. G.K.T. is supported by the Australian Government Endeavour Postgraduate Award.
Abbreviations
- TGFβ
transforming growth factor-beta
- BMP-2
bone morphogenetic protein-2
- PDGF
platelet-derived growth factor
- IGF-1
insulin-like growth factor-1
- FGF
fibroblast growth factor
- ECM
extracellular matrix
- GAGs
glycosaminoglycans
- Sox-9
(sex determining region Y)-box 9 transcription factor
References
- 1.Lee J, Fu F. The meniscus: basic science and clinical applications. Oper Tech Orthop. 2000;10:162–168. [Google Scholar]
- 2.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–290. [PubMed] [Google Scholar]
- 3.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990:19–31. [PubMed] [Google Scholar]
- 4.Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635–640. doi: 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983;158:265–270. doi: 10.1016/0014-5793(83)80592-4. [DOI] [PubMed] [Google Scholar]
- 6.Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD. Meniscus structure in human, sheep and rabbit for animal models of meniscus repair. J Orthop Res. 2009;27:1197–1203. doi: 10.1002/jor.20869. [DOI] [PubMed] [Google Scholar]
- 7.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990:8–18. [PubMed] [Google Scholar]
- 8.Ghadially FN, Lalonde JM, Wedge JH. Ultrastructure of normal and torn menisci of the human knee joint. J Anat. 1983;136:773–791. [PMC free article] [PubMed] [Google Scholar]
- 9.McDevitt CA, Mukherjee S, Kambic H, Parker R. Emerging concepts of the cell biology of the meniscus. Curr Opin Orthop. 2002;13:345–350. [Google Scholar]
- 10.Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13:548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Nakata K, Shino K, Hamada M, Mae T, Miyama T, Shinjo H, et al. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clin Orthop Relat Res. 2001:208–218. [PubMed] [Google Scholar]
- 12.Ford GM, Hegmann KT, White GL, Jr, Holmes EB. Associations of body mass index with meniscal tears. Am J Prev Med. 2005;28:364–368. doi: 10.1016/j.amepre.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 13.McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88:1549–1556. doi: 10.1302/0301-620X.88B12.18140. [DOI] [PubMed] [Google Scholar]
- 14.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen year follow-up of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178–2187. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 15.Sommerlath KG. Results of meniscal repair and partial meniscectomy in stable knees. Int Orthop. 1991;15:347–350. doi: 10.1007/BF00186875. [DOI] [PubMed] [Google Scholar]
- 16.Gray JC. Neural and vascular anatomy of the menisci of the human knee. J Orthop Sports Phys Ther. 1999;29:23–30. doi: 10.2519/jospt.1999.29.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Webber RJ, York JL, Vanderschilden JL, Hough AJ., Jr An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am J Sports Med. 1989;17:393–400. doi: 10.1177/036354658901700314. [DOI] [PubMed] [Google Scholar]
- 18.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209–1217. [PubMed] [Google Scholar]
- 19.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng. 2005;11:1141–1148. doi: 10.1089/ten.2005.11.1141. [DOI] [PubMed] [Google Scholar]
- 20.Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27:636–643. doi: 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- 21.Tumia NS, Johnstone AJ. Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-I in vitro. J Bone Joint Surg Br. 2004;86:1077–1081. doi: 10.1302/0301-620x.86b7.13747. [DOI] [PubMed] [Google Scholar]
- 22.Gunja NJ, Athanasiou KA. Additive and synergistic effects of bFGF and hypoxia on leporine meniscus cell-seeded PLLA scaffolds. J Tissue Eng Regen Med. 2010;4:115–122. doi: 10.1002/term.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adesida AB, Grady LM, Khan WS, Hardingham TE. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res Ther. 2006;8:61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunja NJ, Athanasiou KA. Effects of co-cultures of meniscus cells and articular chondrocytes on PLLA scaffolds. Biotechnol Bioeng. 2009;103:808–816. doi: 10.1002/bit.22301. [DOI] [PubMed] [Google Scholar]
- 25.Hoben GM, Hu JC, James RA, Athanasiou KA. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng. 2007;13:939–946. doi: 10.1089/ten.2006.0116. [DOI] [PubMed] [Google Scholar]
- 26.Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 27.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 29.Walsh CJ, Goodman D, Caplan AI, Goldberg VM. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Eng. 1999;5:327–337. doi: 10.1089/ten.1999.5.327. [DOI] [PubMed] [Google Scholar]
- 30.Cook JL, Fox DB, Malaviya P, Tomlinson JL, Farr J, Kuroki K, et al. Evaluation of small intestinal submucosa grafts for meniscal regeneration in a clinically relevant posterior meniscectomy model in dogs. J Knee Surg. 2006;19:159–167. doi: 10.1055/s-0030-1248100. [DOI] [PubMed] [Google Scholar]
- 31.Kohn D. Autograft meniscus replacement: experimental and clinical results. Knee Surg Sports Traumatol Arthrosc. 1993;1:123–125. doi: 10.1007/BF01565466. [DOI] [PubMed] [Google Scholar]
- 32.van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37:2134–2139. doi: 10.1177/0363546509336725. [DOI] [PubMed] [Google Scholar]
- 33.Hommen JP, Applegate GR, Del Pizzo W. Meniscus allograft transplantation: ten-year results of cryopreserved allografts. Arthroscopy. 2007;23:388–393. doi: 10.1016/j.arthro.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Nakano T, Dodd CM, Scott PG. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J Orthop Res. 1997;15:213–220. doi: 10.1002/jor.1100150209. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Takeuchi N, Sagehashi Y, Yamaguchi T, Itoh H, Iwata H. Effects of hyaluronic acid on meniscal injury in rabbits. Arch Orthop Trauma Surg. 1998;117:303–306. doi: 10.1007/s004020050255. [DOI] [PubMed] [Google Scholar]
- 36.Sonoda M, Harwood FL, Amiel ME, Moriya H, Amiel D. The effects of hyaluronan on the meniscus in the anterior cruciate ligament-deficient knee. J Orthop Sci. 2000;5:157–164. doi: 10.1007/s007760050143. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res. 2001;19:500–503. doi: 10.1016/S0736-0266(00)90024-X. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K, Matsuzaka S, Yoshida Y, Miyauchi S, Wada Y, Moriya H. The effects of intraarticularly injected sodium hyaluronate on levels of intact aggrecan and nitric oxide in the joint fluid of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:536–542. doi: 10.1016/j.joca.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Chiari C, Koller U, Kapeller B, Dorotka R, Bindreiter U, Nehrer S. Different behavior of meniscal cells in collagen II/I, III and Hyaff-11 scaffolds in vitro. Tissue Eng Part A. 2008;14:1295–1304. doi: 10.1089/ten.tea.2007.0341. [DOI] [PubMed] [Google Scholar]
- 40.Stone KR, Rodkey WG, Webber RJ, McKinney L, Steadman JR. Future directions. Collagen-based prostheses for meniscal regeneration. Clin Orthop Relat Res. 1990:129–135. [PubMed] [Google Scholar]
- 41.Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically and biochemically. Am J Sports Med. 1992;20:104–111. doi: 10.1177/036354659202000202. [DOI] [PubMed] [Google Scholar]
- 42.Rodkey WG. Menaflex (TM) Collagen Meniscus Implant: Basic Science. In: Beaufils P, Verdonk R, editors. The Meniscus. New York: Springer-Verlag; 2010. pp. 367–371. [Google Scholar]
- 43.Rodkey WG, DeHaven KE, Montgomery WH, 3rd, Baker CL, Jr, Hormel SE, et al. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J Bone Joint Surg Am. 2008;90:1413–1426. doi: 10.2106/JBJS.G.00656. [DOI] [PubMed] [Google Scholar]
- 44.Mueller SM, Shortkroff S, Schneider TO, Breinan HA, Yannas IV, Spector M. Meniscus cells seeded in type I and type II collagen-GAG matrices in vitro. Biomaterials. 1999;20:701–709. doi: 10.1016/s0142-9612(98)00189-6. [DOI] [PubMed] [Google Scholar]
- 45.Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, et al. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997;38:95–104. doi: 10.1002/(sici)1097-4636(199722)38:2<95::aid-jbm3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Tan GK, Dinnes DL, Butler LN, Cooper-White JJ. Interactions between meniscal cells and a self assembled biomimetic surface composed of hyaluronic acid, chitosan and meniscal extracellular matrix molecules. Biomaterials. 2010;31:6104–6118. doi: 10.1016/j.biomaterials.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya K, Chen G, Ushida T, Matsuno T, Tateishi T. Effects of cell adhesion molecules on adhesion of chondrocytes, ligament cells and mesenchymal stem cells. Mater Sci Eng C. 2001;17:79–82. [Google Scholar]
- 48.Srivastava S, Gorham SD, Courtney JM. The attachment and growth of an established cell line on collagen, chemically modified collagen and collagen composite surfaces. Biomaterials. 1990;11:162–168. doi: 10.1016/0142-9612(90)90149-k. [DOI] [PubMed] [Google Scholar]
- 49.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 51.Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 52.Gunja NJ, Athanasiou KA. Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Res Ther. 2007;9:93. doi: 10.1186/ar2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JW, Qi WN, Scully SP. The involvement of beta1 integrin in the modulation by collagen of chondrocyte-response to transforming growth factor-beta1. J Orthop Res. 2002;20:66–75. doi: 10.1016/S0736-0266(01)00073-0. [DOI] [PubMed] [Google Scholar]
- 54.Natsu-Ume T, Majima T, Reno C, Shrive NG, Frank CB, Hart DA. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10:396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- 55.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol. 2006;290:1610–1615. doi: 10.1152/ajpcell.00529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isoda K, Saito S. In vitro and in vivo fibrochondrocyte growth behavior in fibrin gel: an immunohistochemical study in the rabbit. Am J Knee Surg. 1998;11:209–216. [PubMed] [Google Scholar]
- 58.Maier D, Braeun K, Steinhauser E, Ueblacker P, Oberst M, Kreuz PC, et al. In vitro analysis of an allogenic scaffold for tissue-engineered meniscus replacement. J Orthop Res. 2007;25:1598–1608. doi: 10.1002/jor.20405. [DOI] [PubMed] [Google Scholar]
- 59.Martinek V, Ueblacker P, Braun K, Nitschke S, Mannhardt R, Specht K, et al. Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg. 2006;126:228–234. doi: 10.1007/s00402-005-0025-1. [DOI] [PubMed] [Google Scholar]