Abstract
N-cadherin is a homophilic cell adhesion molecule that plays important roles in many aspects of neuronal development. In order to better understand the function of N-cadherin mediated cell-cell contact in activity-dependent dendrite development, we generated a number of new tools. EC1, consisting of the first extracellular domain of N-cadherin, can specifically inhibit N-cadherin, but not E-cadherin, mediated cell-cell contact, both when overexpressed in neurons and added as a purified protein. Ncad-HA is an extracellularly epitope-tagged version of N-cadherin that, when overexpressed under the activity-independent pCS2-min promoter, can be used to assay surface N-cadherin level following various manipulations. These tools are likely to be very useful for studying the function of N-cadherin in multiple aspects of neural circuit development.
Key words: N-cadherin, dendrite development, neuronal activity, homophilic interaction, cell-cell contact, activity-independent expression vector
Pre-Existing Reagents for Studying N-Cadherin Extracellular Function
N-cadherin is a homophilic cell adhesion molecule of the type I classical cadherin family, first identified for its high level of expression in neural tissue.1 It contains five extracellular cadherin (EC) domains that mediate cis- and trans-interactions with cadherins of like subtypes in a calcium-dependent manner.2 Expressed in both axons and dendrites from very early stages of neuronal development,3 N-cadherin has been shown to play important roles in many aspects of neuronal development, including axonal and dendritic morphogenesis, synaptogenesis, spine morphologenesis and synaptic plasticity (reviewed in refs. 4–6).
The importance of N-cadherin mediated cell-cell interaction in neural development was first demonstrated using antibodies directed against its extracellular region. Monoclonal antibodies to its N-terminal epitopes disrupted calcium-dependent adhesion between neural cells in the mouse embryo or between N-cadherin expressing L cells,1,7,8 while use of a polyclonal antibody demonstrated its role in mediating neurite outgrowh on astrocytic surfaces.9 Domain swap studies and monoclonal antibodies generated against E-, P- and N-cadherins mapped the specificity of their homophilic binding to the N-terminal 113 amino acids of the mature protein,7 approximating to the first EC (EC1) domain.
Subsequently, high-resolution structures of the N-cadherin extracellular region and mutagenesis studies demonstrated a critical role of the EC1 domain in mediating cell-cell adhesion and in determining homotypic specificity (reviewed in refs. 10 and 11). Residues particularly worth noting include the highly conserved tryptophan 2 (W2) residue and histidine-alanine-valine (HAV) motif (Fig. 1).10 In the strand-swap binding model of classical cadherins, first visualized in high resolution crystal structures of N-cadherin EC1, the side chain of W2 intercalates into the hydrophobic pocket of the opposing cadherin, thereby “swapping” the N-terminal β-strands (A-strand) of paired EC1 domains.12 Mutating the second tryptophan to alanine (W2A) or other conserved residues within the hydrophobic pocket inhibited N-cadherin mediated cell-cell interaction.13 In contrast to identification of the W2 residue from threedimensional crystal structure, the HAV motif was first proposed as the “adhesion sequence” from primary sequence by virtue of being conserved in all classical cadherins.14 Structural studies showed that the HAV sequence is partially buried and obscured, but that interestingly, its middle alanine residue forms part of the hydrophobic pocket containing W2.12,13 Linear and cyclic forms of the HAV peptide have been shown to block N-cadherin mediated cell adhesion and neurite outgrowth.14,15 However, since the HAV motif is present in all classical cadherins, its specificity for N-cadherin and not other classical cadherins remained in question. This was partly addressed by incorporating flanking sequences, as addition of E-cadherin flanking sequences failed to inhibit N-cadherin mediated adhesion. Interestingly, though, several of the peptides incorporating N-cadherin flanking sequences also failed to inhibit adhesion.15 In another study, 17mer peptides of HAV motifs in the context of N-cadherin or E-cadherin flanking residues inhibited synaptic plasticity to similar degrees and did not have additional effects when co-applied, suggesting that possibly each peptide inhibited both N-cadherin and E-cadherin function.16
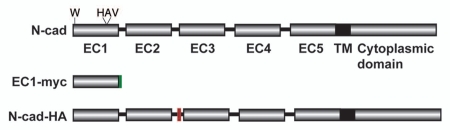
Figure 1.
Schematic of N-cadherin and its derived tools. Top line is a domain structure of mature full-length N-cadherin with the critical second tryptophan (W) residue and location of histidine-alanine valine (HAV) peptide illustrated; EC, extracellular domain; TM, transmembrane domain. Middle is EC1-myc, containing the first EC domain of N-cadherin fused to a C-terminal myc tag (green). Bottom is the N-cadherin-HA (N-cad-HA) construct, with an HA tag (red) inserted between EC2 and EC3 domains.
Thus, despite the ability of the HAV peptide to inhibit N-cadherin mediated cell-cell interaction, potential issues with its specificity, together with the high concentration typically required (0.1–1 mM) for its effect,14–17 led us to search for alternatives. Since the EC1 domain is only 50–70% identical among type I classical cadherins, and there is overwhelming evidence for its critical role in mediating homotypic binding (reviewed in refs. 10 and 11), we thought to use the entire EC1 domain to specifically inhibit N-cadherin mediated cell-cell interaction.
EC1: A New Tool for Studying N-Cadherin Mediated Extracellular Interaction
To determine the specificity of the N-cadherin EC1 domain (hereafter referred to as EC1) to N-cadherin and not other classical cadherins, such as E-cadherin, we used bacterially purified EC1 to co-immunoprecipitate binding proteins in lysate from cultured hippocampal neurons or from HEK 293 cells overexpressing N-cadherin or E-cadherin. Our results showed that, in both cell types, purified EC1 bound strongly to N-cadherin and very little or not-at-all to E-cadherin, thus demonstrating its specificity for N-cadherin mediated interaction.18 This result was further supported by colocalization of overexpressed myc-tagged EC1 with HA-tagged N-cadherin on the surface of cultured neurons.18
To investigate the function of N-cadherin mediated cell-cell interaction in dendritic morphogenesis, complementary approaches were used. First, overexpression of a secreted, soluble form of EC1 reduced the total dendrite length and complexity of the neuron that express it.18 Even though EC1 protein can be specifically detected in the medium of neurons overexpressing it, this approach left open the possibility that EC1 could affect dendrite growth intracellularly, prior to its secretion. To overcome this, we overexpressed EC1 in Hela cells co-cultured with neurons and showed that dendrite complexity was specifically reduced in neurons proximal to EC1-expressing Hela cells. Lastly, and perhaps most importantly, addition of bacterially purified EC1 protein reduced total dendrite length in a dose-dependent manner both under control conditions and following elevation of neuronal activity.18 Importantly, the concentration of purified EC1 protein required for inhibiting N-cadherin-dependent function was in the range of 25–125 nM,18 much lower than the concentration of HAV peptide typically used for inhibiting N-cadherin function.14–17
Having demonstrated that N-cadherin mediated extracellular interaction is required for activity-dependent dendrite growth, we next asked whether neuron-neuron interaction is specifically required for this process. This question is interesting especially because astrocytes express a high level of N-cadherin on their surface19 and thus can potentially interact with N-cadherin on neuronal surfaces. This interaction could be important for stabilizing newly formed axons and dendrites during early stages of development, prior to their contact with each other. To address this question, we plated newly dissociated neurons under three types of conditions: (1) at very low density such that neurons have no contacting neighbors; (2) at very low density and on a monolayer of astrocytes; (3) at high density such that neurons can form contacts with other neurons or astrocytes. The results were very striking in that application of purified EC1 protein specifically reduced the dendrite complexity of neurons plated at high density, and not those plated at low density, with or without astrocytes.18 This result demonstrated that neuron-neuron interaction and not neuron-astrocyte interaction is specifically required for dendrite growth. It also raised the possibility of potential differences between N-cadherin expressed in neurons or astrocytes. Assuming that our in vitro result could be generalized, it would suggest that either the structure of N-cadherin (through alternative splicing for example) or its associated complex is distinct between neurons or astrocytes, or alternatively that other transmembrane complexes that also mediate interaction between neurons are not present in astrocytes. Further investigation would be required to distinguish these possibilities. It is worth noting that neurons from sister cultures plated at low density on astrocytes have longer dendrites than those plated without, showing that astrocytes do promote dendrite growth through N-cadherin independent mechanism.
Putting our result that only neurons plated at high density exhibit N-cadherin dependent dendrite growth, together with N-cadherin's early expression in neurites and its role in synapse formation (reviewed in refs. 4–6), a most logical deduction is that N-cadherin mediated axonal-dendritic interaction is required for promoting dendrite growth. Additional experiments specifically transfecting EC1 pre- and/or post-synaptically, in combination with time-lapse live imaging, would be required to demonstrate more specifically the nature of the neuron-neuron interaction required for dendrite growth and stabilization. Furthermore, since N-cadherin also affects axonal development,9,17,20–22 it would be interesting to assay the effect of EC1 on axonal growth, independently or in combination with its effect on dendrites.
Summarizing the above points, we believe that EC1 protein provides a valuable tool for studying N-cadherin dependent cell-cell interaction, both in vitro and in vivo. The bacterially purified EC1 protein can be conveniently added to the medium of primary neuronal cultures, slice cultures or cell lines to study the function of N-cadherin mediated cell adhesion in neurite outgrowth and maintenance, synapse formation and stabilization, spinogenesis and synaptic plasticity (Table 1). This manipulation in principle would block N-cadherin mediated interaction in all treated cells. As a complementary approach, secreted, soluble EC1-myc can be overexpressed in individual cells. In addition to further verifying the effects of adding purified EC1, this manipulation would allow one to compare the effect of disrupting N-cadherin mediated interaction in an individual cell rather than the entire cell population (Table 1).
Table 1.
Description of some tools available for studying N-cadherin function
| Tool | Advantages | Potential Uses | Potential Further Development |
| Purified EC1 protein |
|
|
|
| EC1-myc construct |
|
|
|
| N-cadherin-HA construct |
|
|
|
| pCS2min vector |
|
|
|
We demonstrated that the EC1 domain of N-cadherin does not interact with E-cadherin, another highly expressed classical cadherin.18 Additional tests would be required for other cadherins, as well as a direct comparison against the HAV peptide. We note that a relatively low concentration of EC1 is required for blocking N-cadherin mediated effect on dendrite morphogenesis, as compared to the HAV peptide, consistent with it being more specific. As further concurring evidence for the specificity of EC1 protein, its effect on inhibiting dendrite growth can be mimicked by Ncad-Fc, a chimeric protein consisting of the extracellular domain of N-cadherin fused to the immunoglobulin Fc region.18
Generation of an Activity-Independent Surface-Tagged N-Cadherin Overexpression Construct
The last point of the previous section reminded us of another important tool for studying extracellular interaction mediated by transmembrane proteins, namely functional blocking antibodies. In fact, these were the tools originally used to study the function of N-cadherin. However, with the passing of years, many of the antibodies previously used are no longer available, at least not commercially. Of the commercially available antibodies that we were able to obtain, none convincingly labeled endogenous N-cadherin on neuronal surfaces, at least not at the relatively young neuronal age (DIV 6–8 days) that we investigated. This lack of available tools made it difficult for us to assay the effects of elevated neuronal activity on surface N-cadherin level by immunocytochemistry. In order to circumvent this problem, we generated an extracellular epitope-tagged N-cadherin.
Since a key point of our study18 was that elevated neuronal activity increased the surface protein level of N-cadherin, independent of potential transcriptional changes, it is critical that our epitopetagged N-cadherin is expressed in a vector that itself does not respond to changes in activity. Unfortunately, most mammalian expression vectors contain multiple cAMP response elements (CREs) and thus respond to elevated activity with increased gene expression.23 We thus mutated all ten potential CRE sites in the pCS2 vector and linked it to a luciferase reporter to assay whether the mutated pCS2m10 still responded to elevated activity. To our surprise, pCS2m10 still did, and to a similar extent as compared with the original pCS2 vector.18 We thus truncated most of the pCS2 promoter, removing all SP1 sites as well. The resulting pCS2min vector no longer responded to activity elevation with increased gene expression when assayed at both the 4 and 48 h time points, making it a suitable vector for our purpose.18
The pCS2min vector would be useful more generally to any study involving activity manipulation (Table 1). In contrast to the parent pCS2 vector, whose expression is significantly increased following activity manipulation, reflected both in the number of cells expressing the protein of interest (or the morphology marker GFP) and the protein level within each cell, in the case of pCS2min, neither increased significantly. This is especially important for assays of dendrite morphology, where sparse labeling is required to distinguish the GFP-labeled neuron from untransfected neighbors. Furthermore, for some proteins, a very high level of expression could have toxic side effects, making it important to keep the level of overexpression constant between manipulations. Finally, and importantly, despite having a very short promoter, pCS2min expression is reasonably high in neurons, comparable to the parent vector under unstimulated conditions.
Having generated a vector suitable for N-cadherin overexpression, we next added an HA epitope-tag to an extracellular region that speculating from its crystal structure was unlikely to affect N-cadherin mediated trans- or cis-interaction. We were lucky in this case, as an HA tag inserted between the EC2 and EC3 domains of N-cadherin did not affect ability of the modified N-cadherin-HA to promote dendrite arborization.18 Furthermore, significant correlation was observed between the level of surface N-cadherin-HA expression and the extend of dendrite arborization under basal conditions and following elevated activity.18 More generally, the pCS2min-N-cadherin-HA construct could be a useful tool for studying the function of surface N-cadherin in other aspects of neuronal development (Table 1). A potentially even more useful tool would be a fluorescent extracellular tag on N-cadherin, possibly even pH sensitive, to investigate the effect of various manipulations on N-cadherin trafficking.
To end on a note of summary and not speculation, we believe that both pCS-2min and the N-cadherin-HA construct generated in our study, as well as the EC1 protein described earlier, independently and together, would be useful for further studies of N-cadherin function in multiple aspects of neuronal development and plasticity (Table 1).
Acknowledgments
I would like to thank Zhu-Jun Tan and members of the Yu laboratory for comments. This work is funded by grant 31021063 from the National Natural Science Foundation of China.
References
- 1.Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- 2.Hatta K, Nose A, Nagafuchi A, Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988;106:873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31:487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ye B, Jan YN. The cadherin superfamily and dendrite development. Trends Cell Biol. 2005;15:64–67. doi: 10.1016/j.tcb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Nose A, Tsuji K, Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990;61:147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- 8.Hatta K, Okada TS, Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci USA. 1985;82:2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- 10.Perez TD, Nelson WJ. In: Cadherin Adhesion: Mechanisms and Molecular Interactions. Behrens J, Nelson WJ, editors. Springer, Berlin: Cell Adhesion; 2005. pp. 3–22. [Google Scholar]
- 11.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1:3053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (N-)cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 14.Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 15.Williams E, Williams G, Gour BJ, Blaschuk OW, Doherty P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J Biol Chem. 2000;275:4007–4012. doi: 10.1074/jbc.275.6.4007. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 17.Doherty P, Rowett LH, Moore SE, Mann DA, Walsh FS. Neurite outgrowth in response to transfected N-CAM and N-cadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 1991;6:247–258. doi: 10.1016/0896-6273(91)90360-c. [DOI] [PubMed] [Google Scholar]
- 18.Tan ZJ, Peng Y, Song HL, Zheng JJ, Yu X. N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci USA. 2010;107:9873–9878. doi: 10.1073/pnas.1003480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilby MJ, Muir EM, Fok-Seang J, Gour BJ, Blaschuk OW, Fawcett JW. N-Cadherin inhibits Schwann cell migration on astrocytes. Mol Cell Neurosci. 1999;14:66–84. doi: 10.1006/mcne.1999.0766. [DOI] [PubMed] [Google Scholar]
- 20.Bekirov IH, Nagy V, Svoronos A, Huntley GW, Benson DL. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus. 2008;18:349–363. doi: 10.1002/hipo.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol. 2000;29:215–223. doi: 10.1023/a:1026515426303. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler DG, Cooper E. Depolarization strongly induces human cytomegalovirus major immediate-early promoter/enhancer activity in neurons. J Biol Chem. 2001;276:31978–31985. doi: 10.1074/jbc.M103667200. [DOI] [PubMed] [Google Scholar]