Abstract
Snail transcription factor induces epithelial-mesenchymal transition (EMT) in which the epithelial cells downregulate cell-cell adhesion genes such as E-cadherin and upregulate mesenchymal genes such as vimentin, leading to increased invasion and migration. Very little is known about the role of Snail in cellular adhesion to the extracellular matrix. We hypothesized that Snail will lead to decreased cellular adhesion to fibronectin and collagen I matrix through integrin regulation, concomitant with increased cell migration. Androgen-independent C4-2 cells, an aggressive subline of androgen-dependent LNCaP cells, exhibited increased cell migration on fibronectin and collagen I as compared to LNCaP cells, which was reversed by Snail knockdown in C4-2 cells. ARCaP and LNCaP prostate cancer cells stably transfected with Snail displayed decreased adhesion and increased cell migration on fibronectin and collagen I as compared to control Neo-transfected cells, which was reversed by Snail knockdown. Flow cytometry analysis revealed a decrease in α5, α2 and β1 integrin expression in ARCaP Snail-transfected cells that was reversed in Snail knockdown cells. We also observed an increase in ERK phosphorylation in ARCaP Snail-transfected cells as compared to control ARCaP-Neo cells, and inhibition of the MAPK pathway with UO126 inhibitor in ARCaP Snail-transfected cells abrogated Snail-mediated decrease in cell adhesion and reinduced α5, α2 and β1 integrin expression. Collectively, these studies define a new role for Snail transcription factor in cell adhesion to the ECM, which may be mediated by MAPK signaling, and may be crucial for cell detachment and subsequent metastasis.
Key words: snail, cell adhesion, integrin, extracellular matrix, prostate cancer
Introduction
Prostate cancer is a common type of cancer that is found in men of older ages. In 2009 there were an estimated 192,280 men diagnosed and 27,360 that died from the disease.1 Those numbers are expected to increase to 217,730 diagnosed and 32,050 deaths in 2010.1
Cell adhesion plays an integral role in cell communication and regulation, in the development and maintenance of tissues, stimulating signals that regulate cell differentiation, the cell cycle and cell survival.2–4 To perform these functions, cells must bind to other cells or various molecules in the extracellular matrix (ECM).5
Studies have shown that prostate cancer as well as several other types of cancer such as breast, ovarian, colon and esophageal all display a loss of cellular adhesion to the ECM or each other as cells become cancerous and subsequently metastatic.6–10 The extracellular matrix is a complex structural and functional network of proteins and proteoglycans that can interact simultaneously with multiple cell surface receptors.11,12 The majority of these proteins are glycosylated, including a wide variety of collagens, laminins, fibronectin and elastins.12 Integrins mediate the adhesion of cells to ECM proteins and endothelial surfaces while also playing a role in the type of surrounding interactions a cell will have with other cells.13–16 Specifically, expression of integrin molecules has been shown to be aberrant in melanoma, breast and prostate cancer.17
For example, the prostate cancer cell lines DU145, PC3 and LNCaP have shown aberrant expression of numerous integrins.17–19 The subunits α3, α4, α5 and α7 are downregulated across several cancers while the α2 integrin displays an irregular expression pattern.20 The α2 integrin is downregulated in prostate cancer however it is upregulated in lymph node metastasis.20 This pattern of integrin expression is similar in several cancers.
Integrin binding also induces signaling; however, changes in integrin expression have been associated with changes in integrin signaling. Specifically, prostate cancer integrin signaling has led to an increase in the FAK, MAPK and AKT signaling pathways.20
Snail is a member of the zinc finger transcription factor that induces Epithelial Mesenchymal Transition (EMT).21 This EMT process is characterized by a loss of epithelial cell traits and a gain of mesenchymal cell traits.21 Specifically, there is a loss of the epithelial marker E-cadherin while a noticeable increase in vimentin is displayed.21 Metastatic cancers have been seen to go through this EMT process as they move from a primary site to a secondary site that includes having a loss of cell adhesion.21 Although the role of Snail in cell-cell adhesion is well studied, there is scarce data on the role of Snail in cell-ECM adhesion. Snail has been seen to increase both cell detachment and attachment by altering the expression of integrins in MDCK and A431 cells.22
In our present study, Snail was overexpressed and knocked down in several prostate cancer cell lines in order to determine the role of Snail on cellular adhesion and migration on the ECM in prostate cancer cells. The results showed that an overexpression of Snail in prostate cancer cells reduced cellular adhesion while increasing migration to fibronectin and collagen I, possibly through increased MAPK signaling and decreased α5, α2 and β1 integrin cell surface expression.
Results
Snail transcription factor expression in prostate cancer cell lines.
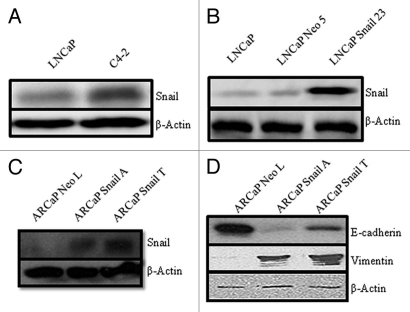
Snail has been shown to play a pivotal role in the progression of cells going through EMT.24 To examine the role of Snail in cancer progression we first evaluated the expression of Snail in different cancer cell lines, including LNCaP cells that are androgen-sensitive and C4-2, a subline of LNCaP that are more aggressive. We observed an increase in Snail expression in C4-2 as compared to LNCaP cells (Fig. 1A). However, we did not observe differences in E-cadherin expression nor vimentin expression between LNCaP and C4-2 cells (data not shown); therefore, we do not believe the LNCaP-C4-2 model represents an EMT model despite increased Snail expression in C4-2 cells as compared to LNCaP cells. Next, we examined a Snail overexpression model using LNCaP cells that have previously been shown to undergo EMT as shown by relocalization of E-cadherin from the cell membrane to the cytosol, and increased vimentin expression.23 Western blot analysis demonstrated that LNCaP Snail transfected cells (LNCaP Snail 23) express more Snail than the empty vector (LNCaP Neo 5) cells (Fig. 1B). Additionally we transfected androgen-independent ARCaP prostate cancer cells with Snail and observed increased Snail expression in Snail clones (Fig. 1C). We confirmed that Snail overexpression in ARCaP cells led to EMT as evidenced by decreased E-cadherin and increased vimentin expression by western blot analysis in ARCaP-Snail A and Snail T clones as compared to ARCaP-Neo L control (Fig. 1D).
Figure 1.
Western blot analysis for Snail expression in prostate cancer. Snail expression was determined by western blot analysis in (A) LNCaP and C4-2 cell line or (B) LNCaP parental, control LNCaP cells transfected with an empty Neo vector (LNCaP Neo 5) and LNCaP cells transfected stably with Snail cDNA (LNCaP Snail 23) or (C) ARCaP cells stably transfected with empty vector (ARCaP Neo L) or Snail cDNA (Snail A and Snail T). (D) Expression of EMT markers, E-cadherin and vimentin, was examined by western blot analysis in ARCaP Neo or Snail clones. Actin was utilized as a loading control. Results are representative of three independent experiments.
Stable knockdown of Snail in ARCaP-Snail and C4-2 prostate cancer cells.
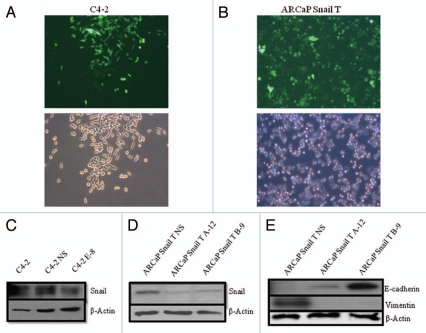
We next wanted to analyze the effects of downregulation of Snail. We performed stable knockdown of Snail in ARCaP cells overexpressing Snail (ARCaP Snail T) and C4-2 cells using shRNA constructs. Monitoring GFP revealed high transfection efficiency in both cell lines (Figs. 2A and B). Western blot analysis showed that in C4-2 cells, the E-8 Snail shRNA construct led to decreased Snail expression as compared to parental C4-2 and non-silencing control (C4-2 NS) (Fig. 2C). When Snail was knocked down in ARCaP-Snail T cells, the A-12 and B-9 Snail shRNA constructs decreased Snail expression as compared to non-silencing control (ARCaP Snail T NS) (Fig. 2D). Moreover, Snail knockdown in ARCaP Snail T cells reverted EMT as shown by reinduction of E-cadherin and inhibition of vimentin protein expression (Fig. 2E). Therefore utilizing shRNA lentiviral vector constructs against Snail in the Snail-overexpression, ARCaP Snail T cell line, as well as in C4-2 cells resulted in an efficient and effective knockdown of Snail expression.
Figure 2.
Stable knockdown of Snail. Snail was stably knocked down by transfection with various Snail shRNA constructs (A-12, B-9 or E-8) or non-silencing control (NS), and selection done with puromycin. Pictures were taken under fluorescence and brightfield to show transfection efficiency after 21 days for (A) C4-2 cells with Snail knockdown and (B) ARCaP-Snail T with Snail knockdown. Western blot analysis was performed to examine Snail expression in (C) C4-2 cells transfected with the Snail shRNA knockdown construct (E-8) and non-silencing control (NS); (D) ARCaP Snail T cells transfected with the Snail shRNA knockdown constructs (A-12, B-9) and NS control. (E) Expression of EMT markers, E-cadherin and vimentin, was examined by western blot analysis in ARCaP SnailT cells with stable Snail knockdown. Actin was utilized as a loading control. Results are representative of three independent experiments. Mag X10.
Overexpression of Snail leads to a reduced cell adhesion to fibronectin and collagen.
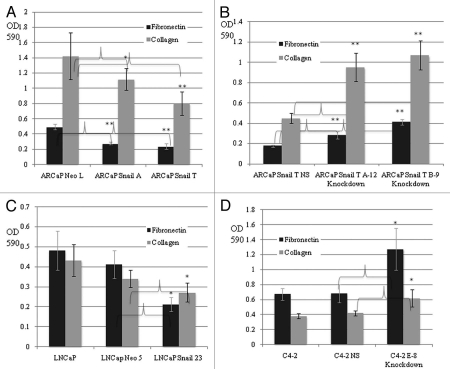
In order to investigate whether Snail plays a role in cell adhesion to the ECM in prostate cancer cells we performed a cell adhesion assay. Cellular adhesion was measured using two different ECMs, fibronectin and collagen I. We observed decreased cell adhesion to fibronectin and collagen I in ARCaP Snail-overexpression cell lines (Snail A and Snail T) as compared to control ARCaP Neo L after 20 min (Fig. 3A) or 60 min (results not shown). Moreover, ARCaP Snail T cells with Snail knockdown (Snail T A-12 and B-9) displayed increased cell adhesion to fibronectin and collagen I as compared to the ARCaP Snail T non-silencing control (Fig. 3B). Similarly, we saw a noticeable decrease in cellular adhesion to fibronectin and collagen I for LNCaP cells overexpressing Snail (LNCaP Snail 23) in relation to empty vector control (LNCaP Neo 5) and parental LNCaP cells (Fig. 3C). Interestingly, LNCaP parental cell lines showed very little difference in adhesion to fibronectin and collagen I than their derivative C4-2 cells (Sup. Fig. 1). However, when Snail was knocked down in C4-2 cells (C4-2 E-8) there was increased adhesion to fibronectin and collagen I as compared to parental C4-2 and C4-2 non-silencing control (Fig. 3D). Overall, we observed that ARCaP and LNCaP cells overexpressing Snail resulted in decreased adhesion to fibronectin and collagen I than the control cell lines. Conversely, knockdown of Snail in ARCaP Snail-T and C4-2 cells results in an increased cell adhesion potential. This result supports the idea that Snail leads to decreased cell adhesion to fibronectin and collagen I in select prostate cancer cells.
Figure 3.
Snail decreases cell adhesion to fibronectin and collagen I. Cell adhesion assays were performed on fibronectin- or collagen I for 20 min, followed by staining of adherent cells with crystal violet, solubilization of stain with Sorenson solution. Subsequent reading of OD at 590 nm are shown for (A) ARCaP prostate cancer cell lines overexpressing Snail (Snail A and T) and control (Neo L); (B) ARCaP Snail T with knockdown of Snail (A-12 and B-9) and compared to NS control; (C) LNCaP prostate cancer cell line overexpressing Snail (Snail 23) and its control (Neo 5); (D) C4-2 cells with Snail knockdown (E-8) and NS control. The results are representative of experiments done in triplicate at least two times. Data represent mean ± SD, *p < 0.05, **p < 0.001.
Overexpression of Snail leads to increased cell migration in prostate cancer cell lines.
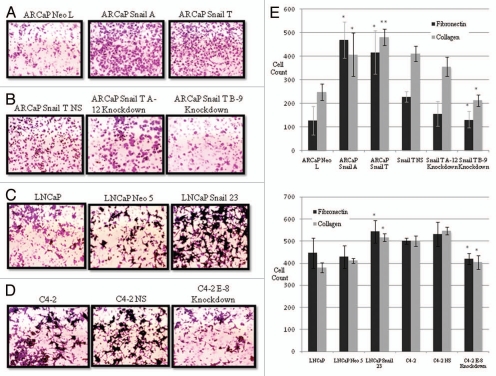
It is known that metastatic cancer cells and cells that undergo EMT become more migratory and this has been associated with Snail expression.25 We investigated migratory potential of cells with overexpression or knockdown of Snail. Using the same fibronectin and collagen I matrices that were used in the prior cell adhesion assays, we conducted migration assays using Boyden chambers. As expected, ARCaP cells overexpressing Snail (Snail A and T) were more migratory on collagen I compared to Neo control (Fig. 4A). Concomitantly we saw a reduction of migratory ability on collagen I in ARCaP Snail T cells with Snail knockdown (ARCaP Snail T A-12 and B-9) (Fig. 4B). Similarly, we saw an increase in cell migration on collagen I, in LNCaP cells overexpressing Snail (LNCaP Snail 23) as compared to parental LNCaP and control LNCaP Neo 5 (Fig. 4C). We also saw a decrease in migration on collagen I, when Snail was knocked down in C4-2 cells (C4-2 E-8) as compared to parental C4-2 and C4-2 non-silencing control (C4-2 NS) (Fig. 4D). Similar images were obtained for cell migration on fibronectin (data not shown). The results for cell migration on fibronectin and collagen I are represented quantitatively in Figure 4E. Our results indicate that an increase in Snail expression leads to an increase in migration on fibronectin and collagen, which can be reversed by Snail knockdown.
Figure 4.
Snail increases migration to fibronectin and collagen I. Cell migration was performed on collagen I coated inserts in a Boyden chamber. Cells that had migrated were stained with crystal violet and images taken for (A) ARCaP cells overexpressing Snail (Snail A and Snail T) or control ARCaP Neo L, (B) ARCaP Snail T with knockdown of Snail (A-12 and B-9) or control (NS), (C) parental LNCaP, Snail overexpressing LNCaP cells (LNCaP Snail 23) or control (LNCaP Neo 5), (D) parental C4-2 cells or C4-2 cells with Snail knockdown (E-8) or control NS. (E) Cell migration was performed on fibronectinor collagen I-coated inserts. Subsequently, the cells that had migrated were stained with crystal violet and cells counted and graphed (the experiment was done in triplicate and five fields counted for each well). The results are representative of experiments done in triplicate at least two times. Data represent mean ± SD, *p < 0.05, **p < 0.001.
Snail transcription factor overexpression reduces integrin expression in ARCaP cells.
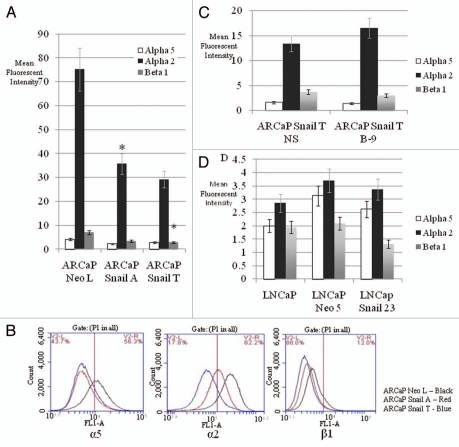
Our data suggests that Snail has a negative effect on the ability of prostate cancer cells to adhere to fibronectin and collagen I. Therefore flow cytometry was used to determine if there is any variation with the expression of the α5 integrin for fibronectin, α2 integrin for collagen and β1 integrin expression across our cell lines. Data showed that the overexpression of Snail in ARCaP cell lines led to a decreased expression of the α5, α2 and β1 integrins (Fig. 5A and B). There also appears to be a trend towards recovery of α2 integrin expression once Snail is knocked down in ARCaP Snail T cells (Fig. 5C). However, Snail knockdown in ARCaP Snail T cells did not significantly alter α5 or β1 levels (Fig. 5C). Additionally there was a trend towards decreased α5, α2 and β1 levels in the Snail-transfected LNCaP cells (LNCaP Snail 23) as compared to LNCaP Neo control, although it was not statistically significant (Fig. 5D). Snail knockdown in C4-2 cells did not lead to any significant increase in α5, α2 and β1 integrin expression (data not shown). Our results suggest that Snail reduces α5, α2 and β1 integrin expression in ARCaP and possibly LNCaP cells.
Figure 5.
Snail reduces α5, α2 and β1 integrin expression in prostate cancer cells. Surface integrin expression was examined by flow cytometry using α5, α2 or β1 antibodies. (A) Mean fluorescent intensity was plotted for ARCaP Snail-overexpressing cells (Snail A and Snail T) or control (NeoL). (B) Integrin expression profile is shown for α5, α2 and β1 for ARCaP Snail-overexpressing cells (Snail A and Snail T) or control (NeoL). (C) Mean fluorescent intensity is shown for ARCaP Snail T with Snail knockdown (B-9) or NS control or (D) parental LNCaP, LNCaP cells stably transfected with Snail (LNCaP Snail 23) or control LNCaP Neo 5. Experiments were done in triplicate at least twice. Data represent mean ± SD, *p < 0.05.
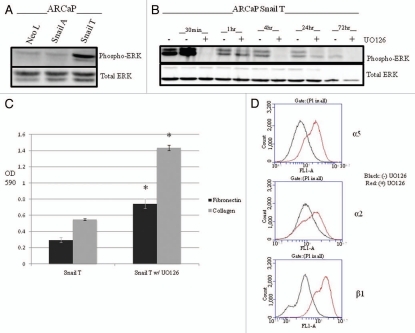
Inhibition of MAPK signaling pathway in ARCaP cells overexpressing Snail leads to increased cellular adhesion and increased α5, α2 and β1 integrin expression.
Prior studies have shown there to be an increase of phosphorylated MAPK in prostate cancer as it becomes more aggressive and into a more androgen-independent state.26 We looked at the expression of phosphorylated MAPK in our Snail-transfected ARCaP cells and found that ARCaP Snail T cells expressed more phospho-ERK1/2 than ARCaP Neo L (Fig. 6A). We also saw an increased expression level of phosphorylated AKT that has also been associated with cancer progression (data not shown). Next, we proceeded to see how the inhibition of these pathways would impact the ability of our cells to adhere as well as the integrin expression. We treated Snail-transfected ARCaP cells with the UO126 MAPK inhibitor for various time points and observed decreased ERK activity within 30 min up to 72 h (Fig. 6B). Next, we performed a cell adhesion assay and flow cytometry in order to determine a role of MAPK in this process. The results showed that in Snail transfected ARCaP cells, inhibition of the MAPK pathway led to increased cellular adhesion to fibronectin and collagen I (Fig. 6C) and increased surface expression of the α5, α2 and β1 integrins (Fig. 6D). However, inhibition of the PI3K pathway with LY294002 did not lead to any significant changes in cell adhesion or integrin expression (data not shown). Therefore, Snail-mediated decrease in cell adhesion and integrin expression may be regulated in part, by the MAPK pathway.
Figure 6.
Snail mediates decreased cell adhesion and integrin expression through the MAPK pathway. (A) Activated ERK (phospho-ERK) was examined in ARCaP cells stably transfected with Snail. ARCaP-Snail T clone expressed higher levels of phospho-ERK as compared to ARCaP Neo L control. (B) ARCaP Snail T cells were treated with UO126 inhibitor for various time points and western blot analysis performed to verify that UO126 inhibited phospho-ERK but did not affect total ERK. (C) Cell adhesion assay was performed on Snail transfected ARCaP Snail T cells with or without UO126 treatment. (D) Surface expression of α5, α2 and β1 integrin was examined by flow cytometry after treatment with UO126. Black = no UO126, Red = treated with UO126. Experiments were done in triplicate at least twice. Data represent mean ± SD, *p < 0.05.
Discussion
In our study we looked at the ability of Snail transcription factor to influence cellular adhesion to the ECM in prostate cancer cells in vitro. Cellular adhesion is the attachment of one cell to another or to the extracellular matrix and is regulated by cellular adhesion molecules, including the immunoglobulin superfamily, cadherins, integrins, occludins, etc.27 Integrins are heterodimeric transmembrane receptors that attach to the extracellular matrix and play a pivotal role in several important cell functions namely proliferation, migration, survival, etc.28 These integrins are key in the life of a cell; however, in several types of cancer the expression of these integrins has become abnormal.20
The present study correlates the expression of Snail with prostate cancer progression, in that, the more aggressive C4-2 cell line expressed more Snail than less aggressive LNCaP cells. Additionally, Snail overexpression in ARCaP cells led to EMT where cells expressed less E-cadherin and more vimentin. We had previously reported that Snail induces EMT in LNCaP and ARCaP cells.23,31 We wanted to extend these studies to specifically study the role of Snail in cell adhesion to ECM.
Our study showed that increasing the levels of Snail in ARCaP and LNCaP prostate cancer cells led to decreased cell adhesion to the ECMs, fibronectin and collagen I. Conversely we saw that knocking down Snail in ARCaP Snail-overexpressing cells (ARCaP Snail T) and C4-2 led to regaining the ability to adhere to these same ECMs.
We noticed that the androgen-independent cell line C4-2 had nominal difference of cellular adhesion to fibronectin and collagen I, when compared to the androgen-dependent LNCaP cell line. This was unexpected since the C4-2 cell line expresses more Snail than LNCaP cells. However once we knocked down Snail in the C4-2 cell line, we observed an increase in cell adhesion. This could be due in part to Snail not being the only gene that regulates cellular adhesion in C4-2 cells. There may be other genes that are involved with its ability to adhere; however, by knocking down Snail, we have shown that it does contribute to adhesion to fibronectin and collagen I. Although Snail is higher in C4-2 cells as compared to LNCaP cells, it may not be sufficient to effect cell adhesion. Conversely, C4-2 cells may be less adherent on alternate matrices.
In addition to observing decreased cell adhesion, we also saw increased cell migration for ARCaP and LNCaP Snail overexpressing cells, when compared to the empty Neo vector. Snail knockdown in ARCaP-Snail or C4-2 cells led decreased migration across the matrices in comparison to the non-silencing controls.
Our study also correlated with prior studies that showed that integrin expression is deregulated in cancers.20 One study shows that in prostate cancer the α2 integrin is downregulated in grade I and II tumors while expression in grade III tumors was more heterogeneous; however, α2 was upregulated in lymph node metastasis.29 Another study has shown through flow cytometry that the α4 subunit is only expressed on nontumorigenic cells that were not able to invade across matrigel; however, tumorigenic cells were able to invade.30 We show that Snail overexpression leads to a reduction in α5, α2 and β1 integrin expression in ARCaP Snail and possibly LNCaP cell lines.
Although Snail knockdown in C4-2 cells led to increased cell adhesion to fibronectin and collagen I, this did not correlate with increased expression of α5, α2 and β1 integrins. This could be due to the understanding that different cell lines use different integrins to adhere to their surroundings. Specifically it has been seen that αVβ3, α3β1, α5β1, α6, αIIbβ3, α6β4, α2β1, α4β1 as well as others play a role in the malignant properties of cancer.17 The DU145 prostate cancer cell line has variations in αVβ3, α3β1, α5β1, α6 and αIIbβ3 while PC3 has variability among α3β1, α6β4, α2β1 and α3β1.17 Additionally, it has been shown that there are negligible differences in integrin expression between LNCaP and C4-2 cells.32 This could explain why there was no differences in cell adhesion to fibronectin or collagen I between LNCaP and C4-2 cell lines in our study.
Our study showed conflicting data to another study that showed that Snail overexpression in MDCK and A431 cells led to increased attachment to fibronectin.22 They further showed that Snail overexpression correlated with increased expression of α5 integrin. The differences may be explained by the use of different cell lines, while they used kidney epithelial cells (MDCK) and human epithelial vulva carcinoma (A431), we used prostate cancer cells. Alternatively, they showed increased expression of α5 in protein lysate and at the mRNA level, while we focused on cell surface expression of integrins. Our study and previous studies underscore the complexity of cell-ECM interactions.
We saw that treatment of Snail transfected ARCaP cells with the MAPK inhibitor UO126, led to increased cell adhesion to fibronectin and collagen I. Additionally we saw an increase in α5, α2 and β1 integrins on this same cell line treated with UO126 that would correlate with the stronger adherence observed. We also treated ARCaP Snail T cells with PI3K inhibitor, LY294002, but saw no significant changes to cell adhesion or integrin expression (data not shown). This supports the idea that Snail utilizes the MAPK pathway to reduce cell adhesion to fibronectin and collagen and integrin expression in ARCaP cells.
Overall our results show that Snail negatively regulates cell adhesion in prostate cancer cell lines concomitant with increased cell migration. Snail also negatively regulates α5, α2 and β1 integrin expression in ARCaP cells via the MAPK pathway. We believe these studies may define a novel role for Snail in cell-ECM interactions in human prostate cancer, which is an important step for cells to detach, migrate and subsequently metastasize.
Materials and Methods
Reagents and antibodies.
Antibody for Snail (rabbit monoclonal) was from Cell Signaling Technology (3879S). Antibodies for Maspin (mouse monoclonal) (554292) and E-cadherin (mouse monoclonal) (610182) were from BD Biosciences. Antibodies for Vimentin (mouse monoclonal) (SC6260) and secondary donkey anti goat were from Santa Cruz Biotechnology, Inc. (SC2020). Antibodies for total ERK1/2 (9102) and phospho ERK1/2 (rabbit polyclonal) (9101) were from Cell Signaling Technology, Inc. Antibodies for α5 (CBL497), α2 (CBL477) and β1 (MAB1951) integrins were from Millipore. RNAintro GIPZ lentiviral shRNA starter kit with A-12, B-9, E-8 and non-silencing negative control were from Open Biosystems (Thermo Scientific, RHS4287). G418 sulfate was acquired from Calbiochem (345810). BCA protein assay kit from Thermo Scientific (23227). Nitrocellulose membranes were from Bio-Rad (162-0115). Amersham ECL plus was from GE Healthcare (RPN2132). Secondary antibodies for anti rabbit (NA934V) and anti mouse (NA931V) were from GE Healthcare. Constitutively active Snail cDNA (8SA) was a kind gift from Dr. Mien-Chie Hung (University of Texas, Houston, TX).
Cell lines.
LNCaP cells were from ATCC. C4-2 and ARCaP cells that were a kind gift from Dr. Leland Chung (Cedar Sinai Medical Center, Los Angeles, CA). Cultured cells were grown in medium (RPMI 1640) supplemented with 10% FBS, penicillin and streptomycin, in a humidified atmosphere at 37°C and 5% CO2. LNCaP cells stably transfected with Snail that display EMT have been described previously in reference 23, and were maintained in 400 µg/ml of G418.
Stable overexpression of Snail.
ARCaP cells grown to 90% confluence in a six-well culture dish were transfected with either 1 µg of Snail cDNA or the empty neomycin vector, using Lipofectamine 2000 (11668-019) according to manufacturer instructions (Invitrogen). Stable transfectants were selected by treatment with 800 µg/ml of G418 and maintained in 400 µg/ml G418. Selected clones were tested for Snail expression by western blot analysis. ARCaP Snail A and ARCaP Snail T are representative Snail overexpressing clones, while ARCaP Neo-L is the representative control.
Stable knockdown of Snail.
Eighteen to 24 h prior to transfection, ARCaP Snail T and C4-2 cells were plated at a 10 × 104 cells per well (Costar, 12-well). The next day, transfection was performed with 1 µg of the different Snail shRNA lentiviral constructs (A-12, B-9 or E-8) or non-silencing control and 5 µl of Arrest-in reagent according to manufacturer instructions. Selection was done with 1 µg/ml of puromycin. Expression was confirmed visually by green fluorescent protein (GFP) within the transduced cells.
Cell adhesion assay.
Ninety-six-well plates were coated with 3.67 µg/µl of rat-tail collagen I or 2.5 µg/cm2 of fibronectin overnight at 4°C. Wells were rinsed with 1x PBS the following day, preheated to 37°C, for surface neutralization. Remaining binding sites were blocked with 0.1% bovine serum albumin (BSA) in PBS for a period of 1 h. Cells were plated with ARCaP-Neo/Snail, ARCaP Snail-T A-12/B-9/Non-silencing (NS) control; parental LNCaP and LNCaP-Neo/Snail; parental C4-2 and C4-2 E-8/NS at 30 × 104 per well. After incubation for 20 or 60 min, cells were treated with percoll flotation medium and percoll fixative for 15 min, washed with PBS and treated with 0.5% crystal violet staining, washed again and allowed to dry overnight. An automated plate reader was used to quantitate cell attachment on the next day, once each well was solubilized with Sorenson solution, and OD read at 590 nm.
Migration assay.
In vitro migratory potentials were assayed in the ARCaP-Neo/Snail, LNCaP-Neo/Snail transfectants, as well as the Snail knockdowns in ARCaP Snail T and C4-2 cells. 5 × 104 cells in 0.1% BSA were plated in the upper chamber of Costar 24-well plates containing 8 µm pore size polycarbonate filter inserts coated with 3.67 µg/µl of rat-tail collagen or 2.5 µg/cm2 of fibronectin, while the lower chamber contained 10% BSA. After 5 h for ARCaP or 24 h for C4-2 or LNCaP transfected cells, cotton swabbing was performed to remove cells that did not migrate and those that had migrated into the lower chamber were collected fixed, stained with crystal violet and counted.
Western blot assay.
Protein lysates were made from the various cell lines by lysing in modified RIPA buffer that included 1 mol/L Tris, 5 mol/L NaCl, 1% Triton X-100, 1 mmol/L sodium orthovanadate and protease inhibitor cocktail (Roche). For western blotting, equal concentrations of protein lysates were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and proteins transferred onto nitrocellulose membrane. Membranes were blocked in 5% milk, and then incubated with primary antibodies at 4°C overnight. Blots were then washed with TBS-T, probed with secondary antibody and visualized with enhanced chemiluminescence reagent ECL + according to the manufacturer's instructions. They were developed on film as well as on LAS 3000.
Flow cytometry.
The α5 (Fibronectin), α2 (Collagen) and β1 integrins were probed for, in each of our cell lines. Cells were plated at 2 × 106 overnight, detached with citric saline, centrifuged down and resuspended in 2% Paraformaldehyde. After this they were rinsed in 1x PBS and suspended in blocking buffer (1% horse serum) and incubated with an antibody against an aforementioned integrin at a 1:500 concentration overnight at 4°C. Next day it was probed with a FITC conjugated secondary antibody and ran on the Accuri C6 Flow cytometer for integrin cell surface expression. Controls were cells incubated without antibody to integrin but with secondary antibody. Experiments were performed three times and representative data displayed. The mean fluorescent intensity was plotted after subtracting control and background.
Treatment with inhibitor.
T-75 flasks were plated with 1–2 × 106 cells, the day before beginning treatments. Once 75% confluent, cells were treated with either control DMSO or 20 µM of UO126 for specific time points and subsequently washed with PBS before proceeding with follow up experiments.
Acknowledgments
We would like to acknowledge Dr. Cimona V. Hinton for help with editing this manuscript.
Abbreviations
- EMT
epithelial mesenchymal transition
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinase
- ECM
extracellular matrix
- MDCK
madin-darby canine kidney cells
- shRNA
short hairpin RNA
Financial Support
NIH grants 1P20MD002285 (V.O.M.), G12RR03062 (V.O.M.).
Supplementary Material
References
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review 1975–2007. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site 2010. [Google Scholar]
- 2.Humphries MJ. Cell adhesion assays. Methods Mol Biol. 2009;522:203–210. doi: 10.1007/978-1-59745-413-1_14. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010;339:83–92. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, et al. Differential expression of extracellular matrix molecules and the alpha 6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- 5.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peinado H, Quintanilla M, Cano A. Transforming Growth Factor β-1 induces Snail transcription factor in epithelial cell lines. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 7.Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 8.Vergara D, Merlot B, Lucot JP, Collinet P, Vinatier D, Fournier I, et al. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291:59–66. doi: 10.1016/j.canlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and β-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 10.Usami Y, Satake S, Nakayama F, Matsumoto M, Ohnuma K, Komori T, et al. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215:330–339. doi: 10.1002/path.2365. [DOI] [PubMed] [Google Scholar]
- 11.Nagle RB. Role of extracellular matrix in prostate carcinogenesis. J Cell Biochem. 2004;91:36–40. doi: 10.1002/jcb.10692. [DOI] [PubMed] [Google Scholar]
- 12.Kakkad SM, Solaiyappan M, O'Rourke B, Stasinopoulos I, Ackerstaff E, Raman V, et al. Hypoxic tumor microenvironments reduce collagen i fiber density. Neoplasia. 2010;12:608–617. doi: 10.1593/neo.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin avβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries MJ. Insights into integrin-ligand binding and activation from the first crystal structure. Arthritis Res. 2002;4:69–78. doi: 10.1186/ar563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nermut MV, Green NM, Eason P, Yamada SS, Yamada KM. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7:4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphries MJ. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990;97:585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- 17.Koistinen P, Heino J. Integrins in Cancer Cell Invasion. Austin (TX): Landes Bioscience; 2000. [Google Scholar]
- 18.Lal Goel H, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rokhlin OW, Cohen MB. Expression of cellular adhesion molecules on human prostate tumor cell lines. Prostate. 1995;26:205–212. doi: 10.1002/pros.2990260406. [DOI] [PubMed] [Google Scholar]
- 20.Goel HL, Alam N, Johnson IN, Languino LR. Integrin signaling aberrations in prostate cancer. Am J Transl Res. 2009;1:211–220. [PMC free article] [PubMed] [Google Scholar]
- 21.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haraguchi M, Okubo T, Miyashita Y, Miyamoto Y, Hayashi M, Crotti TN, et al. Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J Biol Chem. 2008;283:23514–23523. doi: 10.1074/jbc.M801125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKeithen D, Graham T, Chung LW, Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. 2010;70:982–992. doi: 10.1002/pros.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 25.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse Snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4-2 prostate cancer cells. Cancer Res. 2003;63:1975–1980. [PubMed] [Google Scholar]
- 27.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 28.Takada Y, Ye X, Simon S. The integrins. Genom Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonkhoff H, Stein U, Remberger K. Differential expression of alpha6 and alpha2 very late antigen integrins in the normal, hyperplastic and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993;24:243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- 30.Haywood-Reid PL, Zipf DR, Springer WR. Quantification of integrin subunits on human prostatic cell lines—comparison of nontumorigenic and tumorigenic lines. Prostate. 1997;31:1–8. doi: 10.1002/(sici)1097-0045(19970401)31:1<1::aid-pros1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C, et al. Receptor activator of NFkappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18:858–870. doi: 10.1038/cr.2008.84. [DOI] [PubMed] [Google Scholar]
- 32.Sikes RA, Nicholson BE, Koeneman KS, Edlund NM, Bissonette EA, Bradley MJ, et al. Cellular interactions in the tropism of prostate cancer to bone. Int J Cancer. 2004;110:497–503. doi: 10.1002/ijc.20153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.