Abstract
Lymphoepithelial carcinoma (LEC) is a rare malignancy. Histologically, it is an undifferentiated carcinoma with an intermixed reactive lymphoplasmacytic infiltrate. Herein, we report two cases of LEC in the head and neck region that presented to Oulu University Hospital. Our first case is a 30-year-old man with LEC in the left maxillary sinus. The second case is a 49-year-old man with LEC in the soft palate and uvula with regional lymph node metastases at diagnosis. In addition, a systematic review of the literature from 1980 to 2010 was performed with MEDLINE and cross-references were searched manually. Case reports and clinical series of oral, oropharyngeal, nasal, and paranasal sinus LECs were reviewed revealing a total of 110 cases. Most of the oral cases were found in the tonsils (n = 29), oropharynx (n = 19), and in oral mucosa (n = 18), while sinonasal cases (n = 40) were mainly in the paranasal sinuses and nasal cavity. From 37 case reports, including ours, the median age was 58 and 62 years for sinonasal and oral/oropharyngeal LECs, respectively. Oral and oropharyngeal LECs have a 70.0% tendency to metastasize and 16.6% spread locally. In contrast, none of the nasal and paranasal LECs metastasized, but 60% spread locally. Epstein-Barr virus (EBV) had been detected in 87.5% of all tested LEC cases. Treatment of LECs, during the last decade, has largely consisted of surgery, combined with radiotherapy or chemoradiation. Although local spread or nodal metastases are fairly common at the time of diagnosis, the mortality rate of adequately treated LEC patients is low.
Keywords: Oral pathology, Mouth, Palatal uvula, Nasal cavity, Paranasal sinuses, Systematic review, Case report
Introduction
Lymphoepithelial carcinoma (LEC) is a rare subtype of oral carcinoma. The World Health Organization (WHO) has defined it as “a poorly differentiated squamous cell carcinoma or histologically undifferentiated carcinoma accompanied by a prominent reactive lymphoplasmacytic infiltrate, morphologically similar to nasopharyngeal carcinoma” [1]. LEC is separated from nasopharyngeal carcinoma (NPC) by its location [2] and clinical outcome [3]. LEC has been diagnosed in the oral cavity, oropharynx, nasal cavity, and paranasal sinuses, and in numerous other organs of the head and neck region. Salivary gland-associated LEC is considered to be a type of salivary gland neoplasm [4] and it has been studied in well-documented case reports and reviews [5–9]. Immunohistochemistry shows strong staining for cytokeratin in the epithelial component and for CD45 among the lymphoplasmacytic infiltrate. Epstein-Barr virus (EBV) is associated with LEC in the majority of cases from endemic regions, i.e. Southeast Asia [10, 11]. In the English language literature, approximately 70 cases of oral and oropharyngeal and 40 cases of nasal and paranasal sinus LECs have been previously described either as single case reports or clinical series [10, 12–34]. Herein, we report two cases and systematically review the literature of LECs in the oral cavity, oropharynx, nasal cavity, and paranasal sinus. Our main focus is to compare the etiology and clinical features of the disease in these anatomic locations.
Materials and Methods
Two cases of LEC occurring in the oral cavity and maxillary sinus from the Oulu University Hospital Pathology Department were retrieved. Patient files were examined and histopathologic slides were re-evaluated.
A systemic review of the English literature was performed using MEDLINE (January 1980–December 2010) and cross-references were searched manually. Articles of oral, oropharyngeal, nasal, and paranasal sinus LECs were searched by using the following synonyms: lymphoepithelial carcinoma, undifferentiated carcinoma, undifferentiated carcinoma with lymphocytic stroma, undifferentiated carcinoma of nasopharyngeal type, lymphoepithelioma-like carcinoma and lymphoepithelioma [1, 35]. Subject heading terms for anatomic location were: facial neoplasms, mouth neoplasms, gingival neoplasms, lip neoplasms, palatal neoplasms, salivary gland neoplasms, tongue neoplasms, otorhinolaryngologic neoplasms, and nose neoplasms.
Inclusion criteria were:
Case reports, prospective or retrospective studies with case reports or case series.
Histologic confirmation of lymphoepithelial carcinoma.
Exclusion criteria were:
LEC in major salivary gland, lacrimal gland, or skin.
Lymphoepithelial carcinomas, lymphoepitheliomas, and lymphoepithelial-like carcinomas of the nasopharynx and larynx.
Case Reports
Case 1
A previously healthy 30-year-old man had been sent to the Oulu University Hospital Department of Cranial and Maxillofacial Surgery due to periapical inflammation in the upper left first and second molars. For 5 months prior to admission, he had experienced mild pain and discomfort in the left upper molars, so he underwent endodontic treatment of this first molar. However, during this treatment, he experienced increased pain and both of the molars became movable. His past medical history was notable for surgery 10 years earlier due to a cyst in the left maxillary sinus.
Clinical examination revealed a fistula in the buccal gingival sulcus and movement of the first and second upper left molars. The patient reported having bloody nasal discharge the night before. By panoramic radiograph (see Fig. 1), a primary infection-related lesion in the left maxilla was suspected, although a malignancy couldn’t be ruled out. A buccal surgical opening was performed revealing a wide area of bone loss on the alveolar ridge and that the upper sinus wall was intact. (Fig. 2a). Three teeth (dd. 25, 26 and 27) were extracted due to the shortage of supporting alveolar bone. A hemorrhagic tumor mass from the sinus was removed (Fig. 2b) and a biopsy was taken.
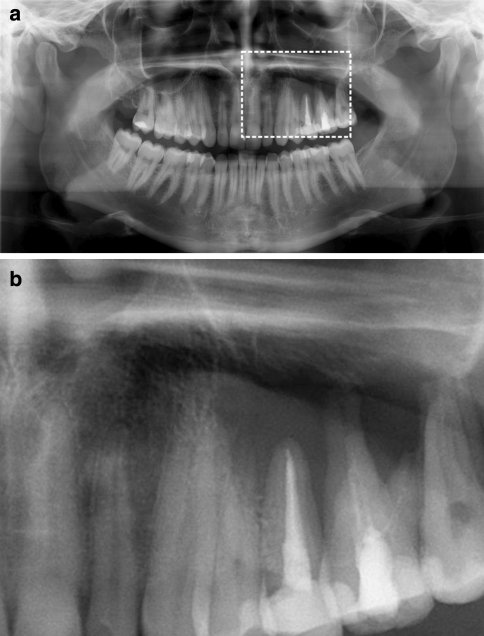
Fig. 1.
a Panoramic radiograph of case 1. A periapical diffuse radiolucency in the left maxilla extends to the bifurcation of the first molar and to marginal bone in interdental spaces between the second premolar and first molar. b Enlarged view of the orthopantomograph of the area marked in a
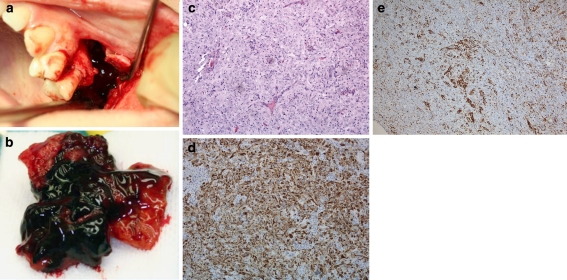
Fig. 2.
a Photograph of the left maxilla operation area of case 1. b Tumor mass from the left maxillary sinus. c The tumor is comprised of undifferentiated cells without connective tissue stroma. Varying degree of cellular and nuclear pleomorphism is noted on various sections. Intermixed are scattered small lymphoid cells (H&E, 40×). d Strong staining for cytokeratin verifies the epithelial origin of the tumour (AE1/AE3, 40×). e The lymphoplasmacytic infiltrate is highlighted by immunohistochemical staining with CD45 (20×)
In the hematoxylin and eosin (H&E) stained sections, invasive irregular strands and islands of epithelial cells were seen. Cell nuclei were vesicular, hyperchromatic, and relatively similar in size. Cell borders were indistinct, yielding a syncytial pattern (Fig. 2c). Epithelial cell islands were surrounded by a lymphoid infiltrate comprised predominantly of small lymphocytes. Tumor cells were positive for AE1/AE3 cytokeratin and lymphocytes for CD45 (Fig. 2d, e). EBV and p16 immunohistochemistry (IHC) were negative, as well as in situ hybridization (ISH) for EBV encoded RNAs (EBERs). A diagnosis of LEC was confirmed.
The sinus cavity was examined by scoping, which showed no evidence of residual tumor. Adjuvant therapy included chemoradiation with a dose of 2.0 Gray (Gy) five times a week up for a total dose of 70 Gy, and Cisplatin (80 mg) once a week for 6 weeks. Eleven months after treatment, the patient was without any evidence of LEC recurrence.
Case 2
A 49-year-old man with a previous history of heavy alcohol and tobacco use was referred to the Oulu University Hospital Otorhinolaryngology Department for consultation due to a suspicious mass in the uvula. He had a medical history of insulin-dependent diabetes, diabetic retinopathy, microalbuminuria, neuropathy, and pancreatitis, which occurred as 3 separate bouts about 20 years earlier. For 6 months prior to presentation, he had been experiencing a persistent cough with the presence of blood clots in his mouth.
On clinical examination, a solid tumor mass, approximately 2 cm in diameter, was found (Fig. 3a). The oral and nasal mucosa, as well as the larynx and nasopharynx, appeared normal, and no cervical lymph node enlargement was found on palpation. The tumor was removed with diathermy and sent for histopathologic evaluation.
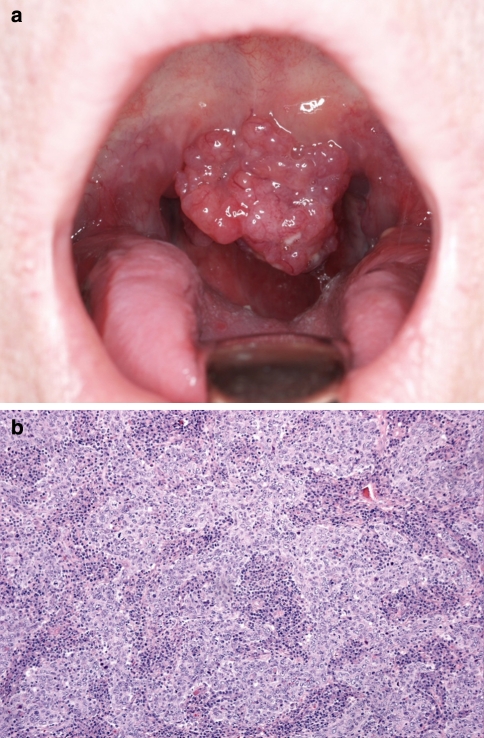
Fig. 3.
a Preoperative picture of the solid tumor involving the soft palate and uvula (Case 2). b Histologic section shows undifferentiated epithelial cells and lymphocytic stroma (HE, 40×)
H&E stained sections revealed syncytial islands of epithelial cells with large, vesicular nuclei, and prominent surrounding lymphocytic stroma (Fig. 3b). The squamous epithelium above the tumor was intact. Immunohistochemistry for AE1/AE3 was positive on tumor cells. EBV IHC and ISH (EBERs) were negative. IHC for p16 expressed focal positivity in the surface epithelium while tumor cells were negative. The final pathologic diagnosis was LEC. Since a surgical resection margin of approximately 2 mm had been taken, a second operation was performed immediately to ensure a marginal status of at least 5 mm. Ultrasound examination was also performed revealing enlarged cervical lymph nodes and nodal metastases were confirmed by fine needle aspiration cytology.
Adjuvant therapy included chemotherapy with cetuximab (400 mg/m2), and focused radiation to the primary site and neck, without further surgical neck dissection. However, due to strong cutaneous side-effects, chemotherapy had to be interrupted after three doses. Intensity modulated radiation therapy using simultaneously integrated boost (IMRT-SIB) for region specific doses was administered at the primary site and on positive cervical lymph nodes in 35 fractions, as follows: total doses of 70 Gy for lymph node metastases; uvula, and neck regions II and III with 63 Gy; and neck region IV with 56 Gy. Ten months after treatment, the patient was without any evidence of disease.
Systematic Review
880 titles and abstracts retrieved from the MEDLINE database were manually searched for relevant topics. 24 articles were found to fit our criteria. Altogether, 110 cases of oral, oropharyngeal and sinonasal LECs, including one case originating from the lacrimal duct, have been previously published as case reports and clinical series in the English literature from 1980 to 2010. Anatomic sites for oral and oropharyngeal LECs included the tonsils (n = 29), oropharynx (n = 19), oral mucosa (n = 18), minor salivary glands (n = 3), and the mandible (n = 1). Sinonasal cases (n = 40) were found in the paranasal sinuses and nasal cavity.
We found 5 case reports of LEC arising in the paranasal sinuses and nasal cavity and these are summarized in Table 1. Data from these reports revealed an age range of 33–64 years, with a median of 58 years. The 5 cases involved 3 males and 2 females. The clinical signs and symptoms varied according to the anatomic site of the tumor. The time span from the first symptoms to diagnosis varied from 2 to 6 months (median 2.5 months). Three cases spread locally but none had metastases. Three cases were positive for EBV out of four cases tested. Patient follow-up varied from 12 to 36 months, with a median of 30 months. None of the patients died during follow-up.
Table 1.
Summary of the literature review of sinonasal lymphoepithelial carcinoma (LEC) including our case (case 1)
| Report | Age/sex | Tumor site/size | Duration of symptoms | Local spread | Metastases | Treatment | EBV | Follow up | Relapse or residual |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 30/M | Maxillary sinus | 5 mo | Yes | No | SRC | – | 11 mo | No |
| Trabelsi [12] | 58/M | Nasal septum/30 × 50 mm | 2 mo | No | No | CR + R neck | n/a | 12 mo | No |
| Tam [13] | 61/F | Nasolacrimal duct/2.3 × 1.3×1.3 cm | 6 mo | Yes | No | SR | + | 33 mo | No |
| Jung [14] | 64/F | Maxillary sinus/5 × 5 cm | 3 mo | Yes | No | SRC | – | 36 mo | No |
| Hajiioannou [15] | 33/M | Nasal cavity, ethmoid sinus and cavity | 2 mo | Yes | No | CR | + | n/a | n/a |
| Wöckel [16] | 56/M | Meatus of nose | n/a | No | No | S | + | n/a | n/a |
EBV Epstein-barr virus, M male, F female, mo months, S surgery, R radiotherapy, C chemotherapy, + positive, − negative, n/a not available
A total of 30 cases of LEC in the oral cavity and oropharynx have been previously published and are summarized in Table 2. The age range of the patients was between 11 and 80 years, with a median of 62 years. Thirteen (43%) cases involved males and 17 (57%) females, respectively. The clinical signs and symptoms varied based on the anatomic site of the tumor. Time from the onset of the first symptoms to diagnosis ranged from 1 to 36 months (median 3.5 months). Five cases (17%) were locally spreading and 21 (70%) had metastases, most of them to regional lymph nodes. Nine out of 12 tested cases (75%) was positive for EBV. Follow up time varied from 2 to 190 months (median 35 months). Seven patients died of disease during the follow up period (one of those refused adequate treatment).
Table 2.
Summary of the literature review of oral and oropharyngeal lymphoepithelial carcinoma (LEC) including our case (case 2)
| Report | Age/sex | Tumor site/size | Duration of symptoms | Local spread | Metastases | Treatment | EBV | Follow up | Relapse or residual, metastases |
|---|---|---|---|---|---|---|---|---|---|
| Case 2 | 49/M | Soft palate and uvula/2 × 2 cm | 6 mo | No | Neck | SR + R neck | – | 10 mo | No |
| Merz [17] | 73/F | Base of tongue | 2 mo | No | n/a | RC | n/a | n/a | n/a |
| Shet [18] | 11/M | Mandible | 3 mo | Yes | Neck | CR | + | 36 mo | No |
| Mahomed [19] | 73/M | Lower lip midline/1 × 1 cm | 4 mo | No | No | S | – | 20 mo | No |
| Lee [20] | 66/M | Tonsil, R, 2 × 3 × 4.5 cm | 4 mo | Yes | Lung | CR | n/a | 11 mo | No |
| Hsiung [21] | 50/F | Minor salivary gland in right buccal area/T1 | n/a | n/a | No | SR | n/a | 116,5 mo | n/a |
| Lu [22] | 50/F | Minor salivary gland, cheek/2 × 1.6 × 1 cm | 12 mo | No | No | SR | + | 120 mo | No |
| Wakisaka [23] | 62/M | Buccal mucosa, tongue base | 2 mo | Yes | Nodal | SR + R neck | n/a | 20 mo | No |
| Chow [24] | 58/M | Palatinum/2 cm | 18 mo | No | Neck | R + R neck | + | 30 mo | No |
| 56/F | Palatinum/1.5 cm | 12 mo | No | No | R + R neck | + | 12 mo | No | |
| 80/F | Retromolar region | 1 mo | No | Neck | Patient refused | + | 34 mo | Died | |
| Hidaka [25] | 60/M | Tonsil, right | 3 mo | No | Neck | CR + R neck | – | 22 mo | No |
| Ahuja [26] | 66/F | Soft palate | n/a | No | Neck | n/a | + | n/a | n/a |
| 63/M | Roof of the oral cavity/2,5 cm | 36 mo | n/a | n/a | n/a | + | n/a | n/a | |
| 47/F | Palatinum | n/a | Yes | Yes | n/a | + | n/a | n/a | |
| 51/M | Nasal cavity and palatinum | n/a | Yes | No | n/a | + | n/a | n/a | |
| Worley [27] | 69/F | Subepithelial mass,buccal mandibula/0.5 cm | 3 mo | No | Neck | SR | – | 12 mo | No |
| Evans [28] | 68/F | Soft palate and uvula,minor salivary glands | 1 mo | No | Neck | R + R neck | n/a | n/a | No |
| Bansberg [29] | 51/F | Tongue base, R/T2 | n/a | No | N2 | R | n/a | 63 mo | Died |
| 66/F | Tongue base, L/T1 | n/a | No | N1 | SR | n/a | 87 mo | Died, unrelated cause | |
| 44/F | Tonsil, L/T2 | n/a | No | N0, M1 | R | n/a | 2 mo | Died | |
| 78/F | Tonsil, L/T3 | n/a | No | N2a | R | n/a | 34 mo | Died, lung | |
| 45/F | Tonsil, L/T1 | n/a | No | No | R | n/a | 8 mo | Died, L neck, liver | |
| 62/F | Tongue base, L/T1 | n/a | No | N1 | R | n/a | 120 mo | No | |
| 65/M | Tongue base, R/T1 | n/a | No | N2b | R + S neck | n/a | 108 mo | No | |
| 40/F | Tonsil, L/T2 | n/a | No | no | R | n/a | 190 mo | No | |
| 64/M | Tongue base, M/T2 | n/a | No | N2 | R | n/a | 180 mo | No | |
| 28/F | Tongue base, R/T2 | n/a | No | N1 | R | n/a | 46 mo | Died, tongue, lung | |
| 65/M | Tonsil, R/T3 | n/a | No | N2a | R | n/a | 120 mo | No | |
| 75/M | Tongue base, L/T2 | n/a | No | N2a | R | n/a | 18 mo | Died, R neck, liver | |
| 53/M | Tonsil, R/T1 | n/a | No | N2a | SR + S neck | n/a | 64 mo | No |
EBV Epstein-barr virus, M male, F female, mo months, S surgery, R radiotherapy, C chemotherapy, + positive, − negative, n/a not available
Six available case series of oral cavity, oropharynx, nasal cavity, and paranasal sinus LECs without detailed patient information are summarized in Table 3. In two case series, all LECs were found to be EBV positive [10, 31]. Kilijanienko et al. [33] tested EBV serology from 7 patients with tonsillar LECs and found that 4 of these had high titers. EBV had been studied by in situ hybridization, immunohistochemistry and serology [11, 30, 33].
Table 3.
Clinical series of lymphoepithelial carcinoma of the oral cavity, oropharynx, and sinonasal region
| Report | Cases | Mean age | Sex | Site | EBV | Treatment | Survival |
|---|---|---|---|---|---|---|---|
| Singhi [30] | 22 | n/a | n/a | Oropharynx: 19, other sites: 3 | None | n/a | From 2 to 180 (median 23 months), 21 disease free, one refused treatment and died |
| Jeng [31] | 13 | 58 | Male: 9, female: 4 | Sinonasal regio | All pos. | S/R/SR/RS/SRC/No | 61% disease free |
| Zong [10] | 20 | 46.3 | Male: 15, female: 5 | Nasal cavity: 17, maxillary sinus: 3 | All pos. | n/a | n/a |
| Dubey [32] | 34 | n/a | n/a | Maxillary sinus: 1 nasal cavity: 1, other sites 32 | n/a | R | 100% of two sinonasal cases |
| Klijanienko [33] | 18 | 55.5 | Male: 13, female: 5 | Tonsils: 18 | Pos. 4/Tested 7 | R | 10 year 77% alive |
| Möller [34] | 3 | n/a | n/a | Tonsillas or pharyngopalatine arch | n/a | n/a | n/a |
EBV Epstein-barr virus, n/a not applicable, pos positive, S surgery, R radiotherapy, C chemotherapy
Discussion
In the English literature from 1980 to 2010, a total of 110 cases of LEC in the oral, oropharyngeal, and sinonasal regions were found. We add two more cases, one in the palatal uvula and the other in the maxillary sinus, increasing the total number of reported oral and sinonasal LEC cases to 112.
The terms lymphoepithelial carcinoma, and nasopharyngeal-type undifferentiated carcinoma (NPUC) have been used synonymously for undifferentiated carcinomas (UC). Most of these tumors are virally related—EBV in the nasopharynx and sinonasal tract tumors [10, 31] and HPV in the case of the oropharyngeal tumors [30].
A highly aggressive type of undifferentiated carcinoma is sinonasal undifferentiated carcinoma (SNUC) that should be differentiated from other types of carcinoma. SNUC is EBV negative and has a unique aggressive clinical disease course. The potential histopathologic differentiation from LEC and NPC is largely made by the prominent tumor necrosis and apoptosis seen in SNUC. Moreover, the nuclei lack the syncytial quality that is seen in LEC and NPC [1]. Although our Case 1 showed local destruction of the alveolar bone, this was in a limited area, and the histopathology did not support the diagnostic criteria of SNUC.
Zong et al. [10] published a study of 20 sinonasal lymphoepithelial carcinomas (SNLECs) in Guangzhou China, where the ratio of LEC to NPC is 1: 564. NPCs, major salivary gland-associated LECs, and LEC of the head and neck skin have been reviewed recently [36–38]. These are relatively distinct diseases distinguished by the clinical course and treatment outcome. Before 1980, case reports of LECs had been published using terminology like “lymphoepithelioma Schmincke-Regaud” [35].
The patient in our first case was only 30 years old at the time of diagnosis although sinonasal LEC is commonly diagnosed in patients between 40 and 70 years old [1]. All case reports, including our own, found a mean age of 50 years for sinonasal and 58 years for oral and oropharyngeal LECs. In addition, we found a similar male predilection for sinonasal LECs compared to previously published cases by Tsang et al. [1] which found a ratio of approximately 3:1. In contrast, oral and oropharyngeal cases showed a roughly equal gender distribution. Nasal obstruction and epistasis are frequently reported symptoms in LECs affecting ductal structures and cavities, while painless masses and swellings are commonly reported in the oral cavity.
Previous reports showed that sinonasal LEC prefers spreading locally. Furthermore, none of the sinonasal LECs had neck metastases, similar to our case. In contrast, oral and oropharyngeal LECs metastasize to regional lymph nodes, with metastases present at the time of diagnosis in about 68% of the case reports, including our case. In one case, there was distant metastases to the mediastinum [29].
Epidemiologic associations for LECs with EBV come from Southeast Asia, where both LEC and NPC have been found to be strongly EBV associated [10, 31]. However, there is evidence for the absence of EBV in LEC [14, 19, 25, 27], as seen in our two cases, which tested negative for EBV by IHC and in situ hybridization (EBERs). The sensitivity of the methods for testing EBV in various publications has varied. Either highly sensitive ISH, clearly less sensitive IHC, or rather unspecific EBV serology (indicating only the general EBV infection) have been used across different studies [10, 30, 33]. It has been suggested that IHC for determining EVB infection is actually unnecessary because of it is irrelevant information for therapeutic or prognostic purposes [11, 20, 39]. EBV status has been shown to be more valuable in diagnosing distant tumors witch are suspected to be metastases of NPC [39].
Recently, human papilloma virus (HPV) has also been suggested to be a potential etiological factor for squamous cell carcinomas (SCCs) with similar morphology to EBV-related lymphoepithelial carcinomas [30]. In EBV-negative breast LEC, a high-risk HPV subtype genome was found in breast cancer cells in concert with HPV related morphology of the cell nuclei [40]. Oropharyngeal LECs with HPV etiology might represent the clinically less aggressive subset of this malignancy [30]. Both of our cases were negative for p16 IHC, most likely indicating the absence of HPV-infection as an etiology for these LECs.
In the late eighties, Bansberg and co-authors [29] reported recurrences and metastases of LEC leading to death in 6 patients treated only with radiotherapy. Consequently, surgery, combined with radiotherapy or chemoradiation, later became widely used and most recently reported cases have shown no residual LEC or evidence of relapse. Based on current knowledge, the prognosis for head and neck LECs seems to be rather favorable.
However, the reported follow up time in these cases, and ours, has been rather short (from 2 to 190 months, median of 35) and may not illustrate the true clinical outcome. Late relapses or metastases in previously published cases are less frequently reported and we found no published cases with prolonged follow-up periods.
In summary, we report two cases of LEC with detailed clinical information, and review the English literature of oral and sinonasal LECs since 1980. Our case, like previously published cases, revealed that despite the tendency of LECs to metastasize or spread locally, the prognosis remains rather favorable compared to other poorly differentiated epithelial tumors.
Acknowledgments
The authors are grateful to Atte Kyllönen for helping with the histopathology of the lymphoepithelial carcinoma. Raija Heino helped with the literature search and Antti Raunio, Petri Iso-Kungas and Antti Raappana provided the clinical pictures.
References
- 1.Tsang WYW, Chan JKC. Lymphoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 18–19. [Google Scholar]
- 2.Sckolnick J, Murphy J, Hunt JL. Microsatellite instability in nasopharyngeal and lymphoepithelial carcinomas of the head and neck. Am J Surg Pathol. 2006;30:1250–1253. doi: 10.1097/01.pas.0000209829.16607.cd. [DOI] [PubMed] [Google Scholar]
- 3.Lountzis NI, Tyler WB, Marks VJ. Primary cutaneous lymphoepithelioma-like carcinoma treated using Mohs micrographic surgery and zinc chloride fixative. Dermatol Surg. 2010;36:564–567. doi: 10.1111/j.1524-4725.2010.01494.x. [DOI] [PubMed] [Google Scholar]
- 4.Ellis GL. Lymphoid lesions of salivary glands: malignant and benign. Med Oral Patol Oral Cir Bucal. 2007;12:E479–E485. [PubMed] [Google Scholar]
- 5.Wang CP, Chang YL, Ko JY, et al. Lymphoepithelial carcinoma versus large cell undifferentiated carcinoma of the major salivary glands. Cancer. 2004;101:2020–2027. doi: 10.1002/cncr.20614. [DOI] [PubMed] [Google Scholar]
- 6.Tian Z, Li L, Wang L, et al. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg. 2010;39:235–242. doi: 10.1016/j.ijom.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Saqui-Salces M, Martinez-Benitez B, Gamboa-Dominguez A. EBV + lymphoepithelial carcinoma of the parotid gland in Mexican Mestizo patients with chronic autoimmune diseases. Pathol Oncol Res. 2006;12:41–45. doi: 10.1007/BF02893430. [DOI] [PubMed] [Google Scholar]
- 8.Wu DL, Shemen L, Brady T, et al. Malignant lymphoepithelial lesion of the parotid gland: a case report and review of the literature. Ear Nose Throat J. 2001;80:803–806. [PubMed] [Google Scholar]
- 9.Zhang Q, Qing J, Wei MW, et al. Clinical analysis of sixteen cases of lymphoepithelial carcinoma of salivary gland. Ai Zheng. 2005;24:1384–1387. [PubMed] [Google Scholar]
- 10.Zong Y, Liu K, Zhong B, et al. Epstein-Barr virus infection of sinonasal lymphoepithelial carcinoma in Guangzhou. Chin Med J (Engl) 2001;114:132–136. [PubMed] [Google Scholar]
- 11.Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995;103:308–315. doi: 10.1093/ajcp/103.3.308. [DOI] [PubMed] [Google Scholar]
- 12.Trabelsi A, Tebra S, Abdelkrim SB, et al. Lymphoepithelial carcinoma of the nasal cavity with EBV Infection in a North African Man. World J Oncol. 2010;1:91–93. doi: 10.4021/wjon2010.04.204w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam YY, Lee LY, Chang KP. Lymphoepithelial carcinoma of the nasolacrimal duct. Otolaryngol Head Neck Surg. 2010;142:144–145. doi: 10.1016/j.otohns.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Jung H, Park SK, Heo KW, et al. Lymphoepithelial carcinoma of the maxillary sinus with orbital invasion. Auris Nasus Larynx. 2009;36:487–490. doi: 10.1016/j.anl.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Hajiioannou JK, Kyrmizakis DE, Datseris G, et al. Nasopharyngeal-type undifferentiated carcinoma (lymphoepithelioma) of paranasal sinuses: rare case and literature review. J Otolaryngol. 2006;35:147–151. doi: 10.2310/7070.2005.5012. [DOI] [PubMed] [Google Scholar]
- 16.Wöckel W, Wernert N. Excessive epithelioid cell granulomatous reaction associated with a lymphoepithelial carcinoma (Schmincke-Regaud) Pathol Res Pract. 1986;181:349–356. doi: 10.1016/S0344-0338(86)80118-2. [DOI] [PubMed] [Google Scholar]
- 17.Merz H, Marnitz S, Erbersdobler A, et al. Schmincke’s tumor, carcinoma of the base of the tongue c T1–2, cN2c M0—a case report. Case Rep Oncol. 2010;3:77–82. doi: 10.1159/000302139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shet T, Arora B, Laskar S, et al. Epstein-barr virus-associated lymphoepithelioma-like carcinoma of mandible. Pediatr Dev Pathol. 2009;12:152–155. doi: 10.2350/07-12-0395.1. [DOI] [PubMed] [Google Scholar]
- 19.Mahomed F, Grayson W. A rare case of lymphoepithelial carcinoma of the lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e49–e52. doi: 10.1016/j.tripleo.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Lee SL, Lee CY, Batniji RK, et al. Unilateral tonsillar lymphoepithelioma with ipsilateral parapharyngeal space involvement: a case report. Ear Nose Throat J. 2007;86:754–755. [PubMed] [Google Scholar]
- 21.Hsiung CY, Huang CC, Wang CJ, et al. Lymphoepithelioma-like carcinoma of salivary glands: treatment results and failure patterns. Br J Radiol. 2006;79:52–55. doi: 10.1259/bjr/17905092. [DOI] [PubMed] [Google Scholar]
- 22.Lu SY, Huang CC, Hsiung CY, et al. Primary lymphoepithelioma-like carcinoma of minor salivary gland: a case report with immunohistochemical and in situ hybridization studies. Head Neck. 2006;28:182–186. doi: 10.1002/hed.20312. [DOI] [PubMed] [Google Scholar]
- 23.Wakisaka N, Murono S, Minato H, et al. A case report: Epstein-Barr virus-associated undifferentiated carcinoma of the tongue base. Auris Nasus Larynx. 2006;33:487–491. doi: 10.1016/j.anl.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Chow TL, Chow TK, Lui YH, et al. Lymphoepithelioma-like carcinoma of oral cavity: report of three cases and literature review. Int J Oral Maxillofac Surg. 2002;31:212–218. doi: 10.1054/ijom.2001.0148. [DOI] [PubMed] [Google Scholar]
- 25.Hidaka H, Nakamura N, Asano S, et al. A case of lymphoepithelioma-like carcinoma arising from the palatine tonsil. Tohoku J Exp Med. 2002;198:133–140. doi: 10.1620/tjem.198.133. [DOI] [PubMed] [Google Scholar]
- 26.Ahuja AT, Teo PM, To KF, et al. Palatal lymphoepitheliomas and a review of head and neck lymphoepitheliomas. Clin Radiol. 1999;54:289–293. doi: 10.1016/S0009-9260(99)90556-1. [DOI] [PubMed] [Google Scholar]
- 27.Worley NK, Daroca PJ., Jr Lymphoepithelial carcinoma of the minor salivary gland. Arch Otolaryngol Head Neck Surg. 1997;123:638–640. doi: 10.1001/archotol.1997.01900060088015. [DOI] [PubMed] [Google Scholar]
- 28.Evans AT, Guthrie W. Lymphoepithelioma-like carcinoma of the uvula and soft palate: a rare lesion in an unusual site. Histopathology. 1991;19:184–186. doi: 10.1111/j.1365-2559.1991.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 29.Bansberg SF, Olsen KD, Gaffey TA. Lymphoepithelioma of the oropharynx. Otolaryngol Head Neck Surg. 1989;100:303–307. doi: 10.1177/019459988910000410. [DOI] [PubMed] [Google Scholar]
- 30.Singhi AD, Stelow EB, Mills SE, et al. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am J Surg Pathol. 2010;34:800–805. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 31.Jeng YM, Sung MT, Fang CL, et al. Sinonasal undifferentiated carcinoma and nasopharyngeal-type undifferentiated carcinoma: two clinically, biologically, and histopathologically distinct entities. Am J Surg Pathol. 2002;26:371–376. doi: 10.1097/00000478-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Dubey P, Ha CS, Ang KK, et al. Nonnasopharyngeal lymphoepithelioma of the head and neck. Cancer. 1998;82:1556–1562. doi: 10.1002/(SICI)1097-0142(19980415)82:8<1556::AID-CNCR18>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Klijanienko J, Micheau C, Azli N, et al. Undifferentiated carcinoma of nasopharyngeal type of tonsil. Arch Otolaryngol Head Neck Surg. 1989;115:731–734. doi: 10.1001/archotol.1989.01860300085023. [DOI] [PubMed] [Google Scholar]
- 34.Möller P, Wirbel R, Hofmann W, et al. Lymphoepithelial carcinoma (Schmincke type) as a derivate of the tonsillar crypt epithelium. Virchows Arch A Pathol Anat Histopathol. 1984;405:85–93. doi: 10.1007/BF00694927. [DOI] [PubMed] [Google Scholar]
- 35.Dohnert G. Lymphoepithelioma Schmincke-Regaud. Virchows Arch A Pathol Pathol Anat. 1971;352:279–284. doi: 10.1007/BF00600677. [DOI] [PubMed] [Google Scholar]
- 36.Wei KR, Yu YL, Yang YY, et al. Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pac J Cancer Prev. 2010;11:29–32. [PubMed] [Google Scholar]
- 37.Abdulla AK, Mian MY. Lymphoepithelial carcinoma of salivary glands. Head Neck. 1996;18:577–581. doi: 10.1002/(SICI)1097-0347(199611/12)18:6<577::AID-HED13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Welch PQ, Williams SB, Foss RD, et al. Lymphoepithelioma-like carcinoma of head and neck skin: a systematic analysis of 11 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:78–86. doi: 10.1016/j.tripleo.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann K, Niedobitek G. Epstein-Barr virus-associated carcinomas: facts and fiction. J Pathol. 2003;199:140–145. doi: 10.1002/path.1296. [DOI] [PubMed] [Google Scholar]
- 40.Kulka J, Kovalszky I, Svastics E, et al. Lymphoepithelioma-like carcinoma of the breast: not Epstein-Barr virus-, but human papilloma virus-positive. Hum Pathol. 2008;39:298–301. doi: 10.1016/j.humpath.2007.08.006. [DOI] [PubMed] [Google Scholar]