Abstract
Human papillomavirus (HPV) positive tonsillar squamous cell carcinoma (TSCC) is associated with a favorable clinical outcome. However, the HPV detected in a given tumor may be causal (driver HPV) or an incidental bystander (passenger HPV). There is a need to discriminate these forms of HPV in TSCCs to understand their impact on HPV as a biomarker for use in TSCC patient management. This study has compared the polymerase chain reaction (PCR), chromogenic in situ hybridization (CISH), and p16INK4a immunohistochemistry in the assessment of HPV status in TSCC. Archival specimens of TSCC from thirty patients were investigated. HPV was detected by PCR in 25/30 (83.3%) tumors; HPV16 (70.0%) and HPV52 (6.7%) were the most common types. HPV was corroborated by CISH in 22/25 (88.0%) specimens; integrated HPV was implicated by the presence of punctate signals in each of these cases. p16INK4a staining was found in 20/22 (90.9%) HPV PCR positive samples; two PCR/CISH HPV positive cases were p16INK4a negative and two HPV negative samples were p16INK4a positive. These data suggest that a minority of HPV positive TSCCs are positive for passenger HPV and that two or more assays may be required for diagnosing driver HPV status. Further studies are required to exam whether oropharyngeal tumors positive for passenger HPV have a less favorable prognosis than tumors that are driver HPV positive. The clinical significance of TSCCs that test HPV negative/p16INK4a positive, PCR and CISH HPV positive/p16 INK4a negative, or PCR HPV positive/p16 INK4a and CISH negative, also requires further investigation.
Keywords: Tonsillar carcinoma, Human papillomavirus (HPV), p16INK4a
Introduction
Human papillomavirus (HPV) infections are accepted etiologic agents for at least a subset of tonsillar squamous cell carcinomas (TSCC). Moreover, HPV status is important in TSCC patient management since HPV positive tumors may have a favorable prognosis [1–10]. The prevalence of HPV (mostly HPV16) in TSCC varies from 29 to 93% [1–5, 11–14]; the highest values have been reported using PCR methods; lower ones have generally been found following in situ hybridization or immunohistochemical (IHC) assays [15]. Episomal and integrated HPV DNA have been reported in TSCC [6, 7]. HPV has also been reported in cases of chronic tonsillitis, tonsillar hyperplasias, and normal tonsillar mucosa [16].
There is a need for a standardized approach for HPV detection in tonsillar and other head and neck tumors [17]. In this study, TSCCs from Vermont, USA have been investigated by PCR, biotinyl-tyramide-based chromogenic in situ hybridization (CISH), and p16INK4a IHC to assess HPV biomarker status.
HPVs are epitheliotropic DNA viruses. There are at least 120 HPV types and alpha genera such as 16, 18, 35, and 52 are classified as high-risk because of their frequent association with cervical carcinomas, whereas low-risk HPV genotypes such as 6 and 11 are more commonly associated with anogenital condylomas. The circular HPV genome is divided into 8 open reading frames (ORFs) which code for proteins controlling early (E) and late (L) viral functions (E1, E2, E4, E5, E6, E7, L1, and L2). The HPV E6 and E7 oncoproteins are linked to the expression of a malignant phenotype. These two proteins act in conjunction to immortalize and transform squamous epithelial cells [18]. HPV integration into the host cell genome typically involves disruption of the E2 ORF (that is involved in HPV genome expression) and may lead to the unregulated expression of E6 and E7 proteins [18]. Such findings, together with numerous clinical studies, have established the causal link between HPV infection and anogenital, and head and neck carcinomas, respectively [1, 2, 4–9, 12–14, 19].
p16INK4a is a tumor suppressor protein that plays a crucial role in cell cycle regulation. p16INK4a prevents phosphorylation of the retinoblastoma protein (pRB) by inhibiting cyclin-dependent kinases CDK4 and CDK6. In turn, this non-phosphorylated pRB binds the transcription factor E2F, thereby preventing E2F stimulation of cell progression into S phase. In contrast, phosphorylated pRB results in unbound E2F, which promotes cell cycle progression and also p16INK4a transcription. In HPV infected cells, the oncoprotein encoded by the E7 gene from high-risk HPV abrogates host cell pRB. Since pRB is no longer available to bind E2F, there is S phase stimulation and p16INK4a over-expression. p16INK4a IHC is widely used as an aid for diagnosing cervical intraepithelial neoplasia (CIN) grade. In the tonsil, p16INK4a immunoreactivity has been demonstrated in 35–56% of TSCCs; staining is usually absent in non-HPV associated oropharyngeal SCC [4, 6, 20].
Materials and Methods
Following Institutional Review Board (Fletcher Allen Health Care/University of Vermont) approval, an electronic data search was conducted of pathology archives from the years 2000 to 2007 to identify patients with TSCC. Basic demographic information, including patient age and gender, was compiled, and a brief review of available patient information was conducted. The selected surgical pathology cases were then reviewed, and corresponding paraffin-embedded tissue blocks were retrieved.
DNA Extraction
Ten 5 μm sections of formalin-fixed, paraffin-embedded (FFPE) tissues were cut and placed into 1.5 mL tubes. Tissues were dewaxed by xylene washes, followed by ethanol rinses, and then air dried. DNA was extracted from the specimens using a proteinase K and column purification method according to supplier instructions (DNeasy Tissue Kit, Qiagen, Valencia, CA).
HPV Genotyping
PCR by amplification of a β-globin fragment ~200 base pairs (bp) in size was performed to confirm DNA extract amenability to PCR [21]. DNA samples (~100 ng) were assayed for HPV by touchdown GP5+/6+ PCR, as previously described [22]. Following PCR, an aliquot of the product was screened for the presence of a ~140 bp amplicon by agarose gel electrophoresis. Detection of this fragment was taken as evidence of HPV positivity. HPV genotyping of the ~140 bp fragments was performed by dot blot hybridization [22].
Chromogenic In Situ Hybridization
CISH utilizing tyramide amplification was performed as previously described [23, 24]. Samples were hybridized using a biotinylated Wide Spectrum HPV DNA Probe Cocktail that detects HPVs 6, 11, 16, 18, 31, 33, 35, 45, 51, and 52 (Dako North America Inc., Carpinteria, CA). Hybridization events were demonstrated using a GenPoint™, Catalyzed Signal Amplification system according to supplier instructions (Dako North America Inc., Carpinteria, CA) using 3,3′-diaminobenzidene (DAB) or 3′-amino-9-ethylcarbazole (AEC). Positive CISH signal patterns were reviewed and classified as follows: (1) diffuse, when nuclei were completely stained (indicative of episomal HPV); (2) punctate, when distinct dot-like intra-nuclear signals were noted (indicative of integrated HPV); and as (3) mixed diffuse and punctate [25]. FFPE invasive cervical carcinoma (ICC) specimens were used as HPV positive controls; negative control test were performed using ICC specimens and omitting the HPV probe from the hybridization mix.
p16INK4a Immunohistochemistry (IHC)
p16INK4a IHC was performed using Clone JC8, Lab Vision, (Fisher Scientific, Pittsburgh, PA). FFPE slide sections 5 μm thick were dewaxed, and then incubated in 10 mM sodium citrate pH 6.0 at 98°C for 20 min and then allowed to cool down for a further 20 min. After buffer rinses, slides were immersed in 3% hydrogen peroxide in methanol for 20 min to block endogeneous peroxidase. After further buffer washes, slides were immersed for 20 min in serum-free protein block (Dako North America Inc., Carpinteria, CA). Following additional buffer washes, the tissue sections were incubated at room temperature for 30 min with the p16INK4a antibody diluted 1:30. p16INK4a staining was subsequently demonstrated following buffer washes with application of the EnVision™ polymer system (Dako North America Inc., Carpinteria, CA) and staining with DAB. FFPE cervical carcinoma specimens were used as positive controls. Negative controls were performed substituting the p16INK4a antibody with a mouse IgG antibody directed against a non-mammalian protein.
Results
TSCCs from 30 consecutive patients (25 male, 5 female) were available for study (Table 1). Patient ages ranged from 40 to 81 year (mean 55.6, SD 11.2). HPV was detected by PCR in 25/30 (83.3%) TSCCs. HPV16 was the most common HPV type, being detected in 21/30 (70.0%) cases, followed by HPV52 (2/30 [6.7%]), HPV18 (1/30 [3.3%]), and HPV 11 (1/30 [3.3%]).
Table 1.
Summary of HPV type and chromogenic in situ hybridization (CISH) and p16INK4a immunohistocehmical (IHC) staining patterns in tonsillar squamous cell carcinoma (TSCC) specimens
| TSCC patients (n = 30) | HPV Type | CISH staining Pattern | p16INK4a IHC staining Pattern |
|---|---|---|---|
| 1 (3.3%) | 11 | Negative | Patchy |
| 12 (40%) | 16 | Punctate | Diffuse |
| 2 (6.7%) | 16 | Punctate | Negative |
| 3 (10%) | 16 | Punctate and diffuse | Diffuse |
| 1 (3.3%) | 16 | Negative | Diffuse |
| 2 (6.7%) | 16 | Punctate | Not done |
| 1 (3.3%) | 16 | Negative | Not done |
| 1 (3.3%) | 18 | Punctate and Diffuse | Diffuse |
| 2 (6.7%) | 52 | Punctate | Diffuse |
| 1 (3.3%) | Negative | Not done | Diffuse |
| 1 (3.3%) | Negative | Not done | Patchy |
| 2 (6.7%) | Negative | Not done | Negative |
| 1 (3.3%) | Negative | Not done | Not done |
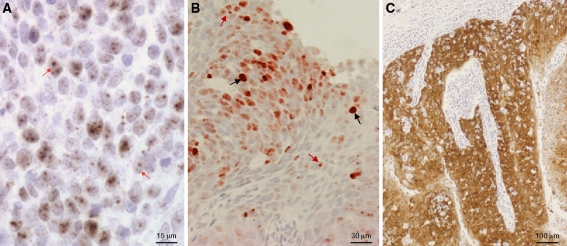
HPV was detected by CISH in 22/25 (88.0%) PCR positive TSCC patients, including 21 punctate signals (Fig. 1a) and two punctate and diffuse signals (Fig. 1b) (no tumors showed only diffuse signals). CISH was not performed on the 5 PCR negative TSCCs. Two p16INK4a IHC staining patterns were noted: (1) Diffuse: >~75% tumor cells demonstrated nuclear and cytoplasmic staining (Fig. 1C); and (2) Patchy: <~30% tumors cells demonstrated staining. Staining intensity was moderate to intense in all cases. p16INK4a was positive in 22/26 (84.6%) TSCC patients [20 with diffuse staining, 2 with patchy staining, 4 negative (Table 1)]; insufficient tissue was available for IHC assay of 4/30 TSCCs). p16INK4a was positive in two TSCC cases that were HPV negative by PCR (one showed diffuse and one patchy staining), and negative in two cases that were HPV positive by both PCR and CISH (both punctuate signals). Two PCR positive (HPV16 and HPV11) but CISH negative samples, were IHC positive (diffuse and patchy staining, respectively).
Fig. 1.
a Human papillomavirus (HPV) chromogenic in situ hybridization (CISH) of an HPV16 positive tonsillar squamous cell carcinoma demonstrating punctate signals (red arrows). b Human papillomavirus (HPV) chromogenic in situ hybridization (CISH) of an HPV16 positive tonsillar squamous cell carcinoma demonstrating punctate (red arrows) and diffuse signals (black arrows). c Diffuse p16INK4a staining of the tonsillar squamous cell carcinoma
Discussion
There has been an increase in TSCC incidence [5, 26]. To account for this trend, it has been suggested there may be an epidemic of viral-induced TSCC [14]. In this study, we compared PCR, biotinyl-tyramide-based CISH, and p16INK4a IHC for the assessment of HPV status in tonsillar lesions. The finding that 25/30 (83%) TSCC patients were HPV positive by PCR assay, which was corroborated by CISH for 23/25 (92.0%) patients, is consistent with ‘epidemic’ HPV-induced TSCC in Vermont, USA. However, race may also be a factor, since HPV related oropharyngeal carcinomas may be significantly more common in Caucasian than African Americans [27]. In the state of Vermont, African-Americans account for 1.0% of the state population compared to 12.9% nationally [28].
In addition to confirming the role of HPV in tonsillar carcinomas and the potential efficacy of HPV vaccination in reducing the incidence of this disease, the findings raise important issues concerning the criteria required for making a clinical diagnosis of HPV positive TSCC. The terms ‘driver’ and ‘passenger’ HPV are appropriate in this context. Driver HPV is causally significant in a lesion, whereas passenger HPV refers to a bystander or opportunistic infection that is incidental to tumor pathogenesis and which may be present in only a minority of cells within a tumor mass; as such, PCR in particular, may be inadequate for establishing that HPV detected in a tumor is also the cause of that tumor [29]. For example, HPV has been detected in normal tonsillar mucosa as well as in tonsillar hyperplasia and tonsillitis [9], and has been reported in up to 81.1% of normal oral cavity scrapes by PCR [16]. Therefore, the possibility exists that HPV detected by PCR in some TSCC could be background rather than driver HPV. Since the sensitivity of the PCR technique supports the detection of either driver or passenger HPV [22, 29], the clinical significance of ‘PCR-only HPV positive’ TSCC demands further study.
Although CISH is less sensitive than PCR, the visualization of HPV DNA signals, especially punctate ones (integrated), in tumor cell nuclei is strong evidence of the detection of driver HPV. However, the non-detection of HPV DNA by CISH cannot be taken as proof of an HPV negative or passenger HPV associated lesion, as it is also possible that the driver HPV DNA load might be below the threshold of CISH detection. The choice of CISH technique employed in this regard is important. The two other studies we are aware of that investigated HPV in TSCC by CISH (non-tyramide-based methods) detected HPV in 35% (PCR not done) and 43% (75% positive by PCR) of the specimens examined [6, 12], respectively. A tyramide-based-CISH study of head and neck tumors demonstrated HPV DNA in 71% of the patients (PCR not done) [30]. A study of 18 head and neck adenosquamous carcinomas that included two tonsillar tumors detected HPV16 E6/E7 RNA in both of these samples using a novel sensitive CISH assay [31]. Indeed, the detection of HPV E6/E7 RNA by CISH may represent an effective test for driver HPV, as not all HPV16 DNA positive TSCCs demonstrate E6/E7 RNA expression: Lindquist et al. found E6/E7 RNA in 50/53 (94%) HPV16 DNA positive TSCCs by RT-PCR [32].
In cervical lesions, p16INK4a IHC staining is widely employed as a surrogate marker for a chronic (driver) HPV infection. The extent to which this relationship can be generalized to tonsillar tissues may be questionable, since 28% of normal tonsil tissue has been found to stain for p16INK4a despite testing HPV negative [33]. Nevertheless, p16INK4a staining has been reported to be both an efficient HPV surrogate marker and a test that has good clinical outcome correlates for TSCCs [6, 10]. However, as with the present study, HPV negative/p16INK4a positive or HPV positive/p16INK4a negative specimens were reported [6, 10]. A study that included 26 p16INK4a positive but HPV negative (by PCR and CISH) oropharyngeal tumors found that these patients had a better survival than patients that were negative for p16INK4a and HPV, suggesting the possibility that p16INK4a immunohistochemistry alone may be sufficient for risk stratification in oropharyngeal SCC [34]. In our study, we also found cases that were HPV positive by PCR and CISH, yet p16INK4a negative. This could occur as a consequence of mutation, deletion, abnormal microRNA expression, or epigenetic events impacting the p16 gene; for example, smoking may increase the risk of p16 methylation [35]. Elucidation of the clinical significance of HPV positive/p16INK4a negative and HPV negative/p16INK4a positive samples will likely require multi-institution collaborative studies to ensure the inclusion of a large cohort of patients from all races.
In summary, although our study is limited by the number of TSCCs available at our institution, the findings are nonetheless sufficient to demonstrate that HPV status assignment is not straightforward as a minority of tumors may be positive for passenger HPV. The clinical importance of discriminating driver from passenger HPV and the means for making this diagnosis requires further investigation. Two or more assays may currently be required: (1) p16INK4a IHC allows a rapid review of possible HPV status and is associated with a favorable prognosis [1–10, 32]; and, (2) the visualization of HPV in TSCC cells by CISH supports the classification of a specimen as driver HPV positive. PCR allows identification of the HPV type present in a TSCC as well as the detection of HPV below the CISH sensitivity threshold. HPV E6/E7 RNA CISH [31] might potentially represent a definitive laboratory test for driver HPV, pending further studies to assess the sensitivity and practical utility of the assay in a clinical setting. Clearly, the diagnostic algorithm also needs to take into account tumor staging and patient history of alcohol and tobacco usage; smokers with TSCC are likely to be at greater risk of tumor recurrence irrespective of HPV status [13]. The clinical significance of non-alcohol/tobacco related HPV negative/p16INK4a positive TSCC, PCR/CISH HPV positive samples that are p16INK4a negative, and, PCR HPV positive samples that are p16 INK4a and CISH negative remains to be fully resolved.
References
- 1.Dahlgren L, Mellin H, Wangsa D, et al. Comparative genomic hybridization analysis of tonsillar cancer reveals a different pattern of genomic imbalances in human papillomavirus-positive and -negative tumors. Int J Cancer. 2003;107:244–249. doi: 10.1002/ijc.11371. [DOI] [PubMed] [Google Scholar]
- 2.Dahlstrand H, Nasman A, Romanitan M, et al. Human papillomavirus accounts both for increased incidence and better prognosis in tonsillar cancer. Anticancer Res. 2008;28(2B):1133–1138. [PubMed] [Google Scholar]
- 3.Dahlstrand H, Dahlgren L, Lindquist D, et al. Presence of human papillomavirus in tonsillar cancer is a favourable prognostic factor for clinical outcome. Anticancer Res. 2004;24:1829–1835. [PubMed] [Google Scholar]
- 4.Hafkamp HC, Mooren JJ, Claessen SM, et al. P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod Pathol. 2009;22:686–698. doi: 10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 5.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 6.Kuo KT, Hsiao CH, Lin CH, et al. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod Pathol. 2008;21:376–386. doi: 10.1038/modpathol.3800979. [DOI] [PubMed] [Google Scholar]
- 7.Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–304. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 9.Syrjanen S. HPV infections and tonsillar carcinoma. J Clin Pathol. 2004;57:449–455. doi: 10.1136/jcp.2003.008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittekindt C, Gültekin E, Weissenborn SJ, Dienes HP, Pfister HJ, Klussmann JP. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Adv Otorhinolaryngol. 2005;62:72–80. doi: 10.1159/000082474. [DOI] [PubMed] [Google Scholar]
- 11.Brandsma JL, Abramson AL. Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg. 1989;115:621–625. doi: 10.1001/archotol.1989.01860290079018. [DOI] [PubMed] [Google Scholar]
- 12.Luginbuhl A, Sanders M, Spiro JD. Prevalence, morphology, and prognosis of human papillomavirus in tonsillar cancer. Ann Otol Rhinol Laryngol. 2009;118:742–749. doi: 10.1177/000348940911801010. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 15.Lajer CB, Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118:510–519. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 16.Terai M, Hashimoto K, Yoda K, et al. High prevalence of human papillomaviruses in the normal oral cavity of adults. Oral Microbiol Immunol. 1999;14:201–205. doi: 10.1034/j.1399-302X.1999.140401.x. [DOI] [PubMed] [Google Scholar]
- 17.Braakhuis BJ, Brakenhoff RH, Meijer CJ, et al. Human papillomavirus in head and neck cancer: the need for a standardised assay to assess the full clinical importance. Eur J Cancer. 2009;45:2935–2939. doi: 10.1016/j.ejca.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Syrjänen K, Syrjänen S. Molecular biology of papillomaviruses. In: Syrjänen K, Syrjänen S, editors. Papillomavirus infections in human pathology. Chichester: Wiley; 2000. pp. 11–52. [Google Scholar]
- 19.Bosch FX, Lorincz A, Muñoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roda Husman AM, Snijders PJ, Stel HV, et al. Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br J Cancer. 1995;72:412–417. doi: 10.1038/bjc.1995.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans MF, Adamson CS, Simmons-Arnold L, et al. Touchdown General Primer (GP5+/GP6+) PCR and optimized sample DNA concentration support the sensitive detection of human papillomavirus. BMC Clin Pathol. 2005;5:10. doi: 10.1186/1472-6890-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans MF, Mount SL, Beatty BG, et al. Biotinyl-tyramide-based in situ hybridization signal patterns distinguish human papillomavirus type and grade of cervical intraepithelial neoplasia. Mod Pathol. 2002;15:1339–1347. doi: 10.1038/modpathol.3880698. [DOI] [PubMed] [Google Scholar]
- 24.Evans MF, Aliesky HA, Cooper K. Optimization of biotinyl-tyramide-based in situ hybridization for sensitive background-free applications on formalin-fixed, paraffin-embedded tissue specimens. BMC Clin Pathol. 2003;3(1):2. doi: 10.1186/1472-6890-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper K, Herrington CS, Stickland JE, et al. Episomal and integrated human papillomavirus in cervical neoplasia shown by non-isotopic in situ hybridisation. J Clin Pathol. 1991;44:990–996. doi: 10.1136/jcp.44.12.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the US population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 27.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137:163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Census Bureau: State & County QuickFacts; Vermont 2009. Available from: http://www.quickfacts.census.gov/qfd/states/50000.html. Accessed 7 April 2011.
- 29.Matsukura T, Sugase M. Pitfalls in the epidemiologic classification of human papillomavirus types associated with cervical cancer using polymerase chain reaction: driver and passenger. Int J Gynecol Cancer. 2008;18:1042–1050. doi: 10.1111/j.1525-1438.2007.01157.x. [DOI] [PubMed] [Google Scholar]
- 30.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 31.Masand RP, El-Mofty SK, Ma XJ, et al. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5:108–16. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindquist D, Romanitan M, Hammarstedt L, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klingenberg B, Hafkamp HC, Haesevoets A, et al. p16 INK4A overexpression is frequently detected in tumour-free tonsil tissue without association with HPV. Histopathology. 2010;56:957–967. doi: 10.1111/j.1365-2559.2010.03576.x. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma YT, Collins SI, Young LS, et al. Smoking initiation is followed by the early acquisition of epigenetic change in cervical epithelium: a longitudinal study. Br J Cancer. 2011;104:1500–1504. doi: 10.1038/bjc.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]