Abstract
The glandular odontogenic cyst (GOC) is now a relatively well-known entity with recent reviews indicating over 100 cases reported in the English literature. The GOC’s importance relates to the fact that it exhibits a propensity for recurrence similar to the odontogenic keratocyst, and that it may be confused microscopically with central mucoepidermoid carcinoma (CMEC). Numerous histopathologic features for the GOC have been described, but the exact microscopic criteria necessary for diagnosis have not been universally accepted. Furthermore, some of the microscopic features of GOC may also be found in dentigerous, botryoid, radicular, and surgical ciliated cysts. The purpose of this multicenter retrospective study is to further define the clinical, radiographic, and microscopic features of GOC, to determine which microscopic features may be helpful for diagnosis in problematic cases, to determine the most appropriate treatment, and to determine if GOC and CMEC share a histopathologic spectrum. In our series of 46 cases, the mean age at diagnosis was 51 years with 71% of cases in the 5th–7th decades. No gender predilection was noted. 80% of cases occurred in the mandible, and 60% of the lesions involved the anterior regions of the jaws. Swelling/expansion was the most common presenting complaint, although some cases were asymptomatic. Radiographically, most cases presented as a well-defined unilocular or multilocular radiolucency involving the periapical area of multiple teeth. Some lesions displayed a scalloped border. Cases also presented in dentigerous, lateral periodontal, and “globulomaxillary” relationships. The canine area was a common location for maxillary cases. All cases were treated conservatively (enucleation, curettage, cystectomy, excision). Follow-up on 18 cases revealed a recurrence rate of 50% (9/18), with 6 cases recurring more than once (range of follow-up: 2 months to 20 years; average length of follow-up: 8.75 years). The mean interval from initial treatment to first recurrence was 8 years, and from first recurrence to second recurrence was 5.8 years. Two cases recurred three times and the interval from second to third recurrence was 7 years (exact interval only documented in one case). All cases exhibited eosinophilic cuboidal (hobnail) cells, a feature not specific for GOC, but necessary for diagnosis, in our opinion. Univariate analysis indicated several features that are most helpful in distinguishing GOC from GOC mimickers in problematic cases, including: (1) the presence of microcysts (P < 0.0001); (2) epithelial spheres (P < 0.0001); (3) clear cells (P = 0.0002); (4) variable thickness of the epithelial cyst lining (P = 0.0002); and (5) multiple compartments (P = 0.006). Stratified analysis indicated that when microcysts are present, epithelial spheres and multiple compartments are still significant, and clear cells are marginally significant in distinguishing GOCs from GOC mimickers. The presence of microcysts (P = 0.001), clear cells (P = 0.032), and epithelial spheres (P = 0.042) appeared to be most helpful in distinguishing GOC associated with an unerupted tooth from dentigerous cyst with metaplastic changes. There were no statistically significant differences microscopically between GOCs that recurred and those that did not. The presence of 7 or more microscopic parameters was highly predictive of a diagnosis of GOC in our series (P < 0.0001), while the presence of 5 or less microscopic parameters was highly predictive of a non-GOC diagnosis (P < 0.0001). Islands resembling mucoepidermoid carcinoma (MEC-like islands) were identified in the cyst wall of three cases, only one of which had follow-up (no evidence of disease at 74 mo.); therefore, at this time insufficient information is available to determine whether GOC and CMEC share a histopathologic spectrum or whether MEC-like islands in GOCs are associated with more aggressive or malignant behavior.
Keywords: Glandular odontogenic cyst, Sialo-odontogenic cyst, Central mucoepidermoid carcinoma, Odontogenic cysts
Introduction
In 1987, Padayachee and van Wyk [1] published two cases of unusual odontogenic cysts, which possessed features of botryoid odontogenic cyst and central mucoepidermoid carcinoma, and proposed the term “sialo-odontogenic cyst” for these lesions. In 1988, Gardner and colleagues [2] described eight additional cases, preferring the term “glandular odontogenic cyst” (GOC). This moniker was also adopted by the World Health Organization [3] in 1992, reflecting the theory that these cysts were most likely of odontogenic rather than salivary gland origin. Odontogenic origin has also been confirmed immunohistochemically by several investigators [4–8]. GOC is a rare lesion comprising only 0.2% of all odontogenic cysts [9, 10].
Over the last two decades, numerous case reports and short series have been reported with the most recent review tabulating 111 cases in the English literature [11]. Therefore, the GOC, although rare, is now a relatively well known entity. However, even though the microscopic features of GOC have been described in detail, there is no consensus on how many features are necessary for diagnosis. In addition, some of the features described for GOCs may also be seen in other jaw cysts, such as dentigerous cysts, botryoid odontogenic cysts, radicular cysts, and surgical ciliated cysts. Accordingly, it is sometimes difficult to discern whether a particular cyst having some but not all of the described features represents a true GOC or perhaps another cyst with GOC-like features. This can be especially challenging when dealing with a dentigerous cyst exhibiting metaplastic changes. In addition, GOCs are sometimes multicompartmentalized microscopically, and these cases in particular may be misinterpreted as central mucoepidermoid carcinoma (CMEC) of the jaws. Rarely, GOCs may have numerous small islands resembling mucoepidermoid carcinoma in the cyst wall, raising the question of whether GOC and CMEC share a histopathologic spectrum, and it is not known whether this finding is associated with more aggressive or malignant behavior. Only two publications have addressed the subject of microscopic criteria for diagnosis of GOC previously: Kaplan et al. [11, 12] proposed major and minor microscopic criteria for GOC based on the frequency of each feature in reported cases from the literature.
The purpose of this study is to present the clinical, radiographic, and histopathologic features of 46 new cases of GOC with particular emphasis on microscopic parameters necessary for diagnosis, especially in problematic cases. We also sought to determine the biologic behavior, the most appropriate treatment, and to determine if GOC and CMEC share a histopathologic spectrum.
Materials and Methods
Pathology records at each of the five institutions were searched for all cysts coded as glandular odontogenic cyst, sialo-odontogenic cyst, botryoid odontogenic cyst, or other cysts described as having some features of glandular odontogenic cyst. As a result of this search, 67 cases were submitted for review by the five study participants. Clinical and radiographic information was tabulated at each institution. Patient privacy was maintained in accordance with institutional review board approval at each facility. Hematoxylin and eosin stained glass slides were reviewed for each case by each of the five study participants. In some cases, special stains were available for review to include mucicarmine, alcian blue, and periodic acid-schiff. Each study participant recorded the presence or absence of 10 microscopic parameters for each case, and made a determination of whether in his opinion the case was diagnostic of GOC or not. Cases with discrepancies of opinion in this regard were reviewed by two of us (CBF and RBB) and a final determination was made. In all cases, the decision of whether to accept or reject the lesion as GOC was based on interpretation of the diagnostic criteria set forth by Gardner et al. [2] and Kramer et al. [3]. After this review, the cases were separated into two groups based on the diagnosis: 46 of the cases were diagnosed as “GOC” and 21 cases as “non-GOC”. It should be emphasized that all of these “non-GOC” cases contained some of the microscopic features of GOC and were submitted as possible GOC cases by the study participants. Therefore, we decided to use this group of “GOC mimickers” in a statistical comparison with our bona fide GOC cases to determine if it is possible to delineate which and how many microscopic features are necessary for diagnosis, especially in problematic cases where the pathologist is unsure whether the case represents a true GOC versus a cyst with metaplastic changes mimicking a GOC. Univariate comparisons of the microscopic parameters for each of the two groups (GOC versus non-GOC) was accomplished using chi-square and Fisher’s exact tests to determine whether there was any statistical significance between the groups regarding the presence of each microscopic parameter. Similar comparisons were made for GOCs in a dentigerous relationship versus dentigerous cysts with metaplastic changes, and for GOCs that recurred versus those that did not. We also performed a stratified analysis to determine if those parameters that were significant on univariate analysis, were still significant when microcysts were present (SPSS, version 17.0).
Results for GOC Cases
Demographics
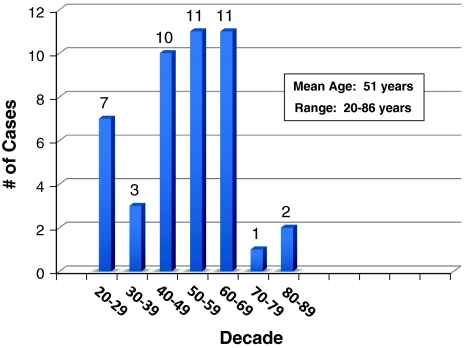
The mean age at diagnosis was 51 years with a range of 20–86 years (N = 45). As indicated in the histogram (Fig. 1), 32 (71.1%) of the cases were diagnosed in the 5th, 6th, and 7th decades.
Fig. 1.
Age distribution of glandular odontogenic cyst
No predilection with regard to gender was noted with 22 cases occurring in males and 23 in females (N = 45).
The majority of cases occurred in Caucasians (25, 80.6%), with 4 cases (12.9%) in Blacks, and 2 cases (6.5%) in Hispanics (N = 31).
Anatomic Location
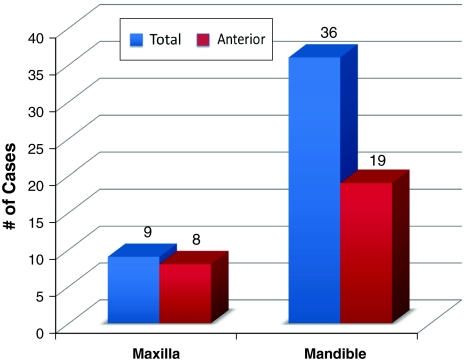
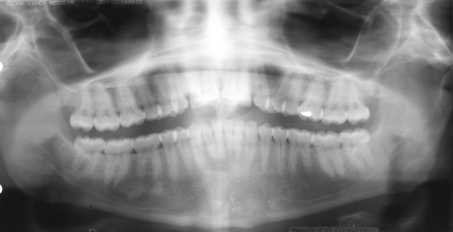
Thirty-six cases (80%) were located in the mandible and 9 (20%) were located in the maxilla (N = 45). Of the mandibular cases, 19 of 34 (55.9%) were located in the anterior mandible with 2 cases specifying only “mandible” for location. For the maxillary cases, 8 of 9 (88.9%) were located in the anterior maxilla, with 7 cases associated with the root of a canine tooth (Fig. 2).
Fig. 2.
Anatomic site of glandular odontogenic cyst
Signs and Symptoms
Swelling/expansion was recorded in 20 cases, pain/discomfort in 5 cases, infection in 2 cases, and paresthesia in 1 case, while 9 cases were listed as asymptomatic. No information was available on signs and symptoms for 16 cases.
Radiographic Features
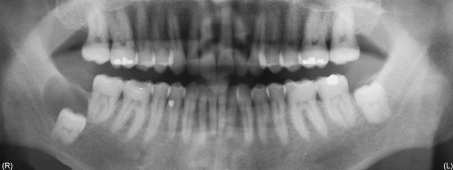
Radiographs and/or radiographic descriptions were available in 41 cases, with selected examples shown in Figs. 3, 4, 5, 6. Unilocular radiolucencies were recorded in 27 cases, while 14 cases were multilocular. Scalloped borders were also noted in many cases. Eight cases were associated with an unerupted tooth (dentigerous relationship), 3 mimicked lateral periodontal cyst, and 2 were located between the roots of a maxillary lateral incisor and canine (“globulomaxillary” relationship). Cortical perforation was noted in 3 cases, root divergence in 2 cases, and root resorption in 1 case.
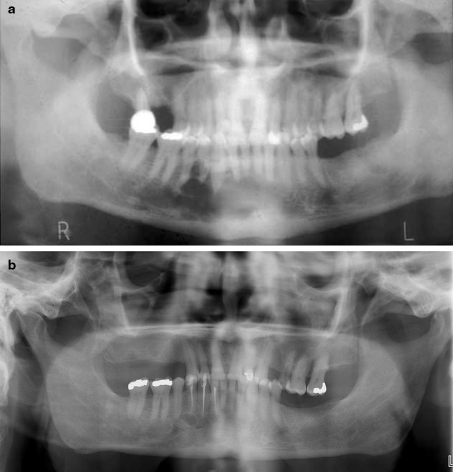
Fig. 3.
a Radiographic appearance of GOC. Multilocular radiolucency in anterior mandible with scalloping of periphery and extension between tooth roots (Case 16). This lesion recurred three times. b Recurrence of GOC shown in a. Lesion recurred for the third time, 14 years after initial conservative excision
Fig. 4.
Radiographic appearance of GOC. Large well-defined radiolucency in left posterior mandible (Case 5)
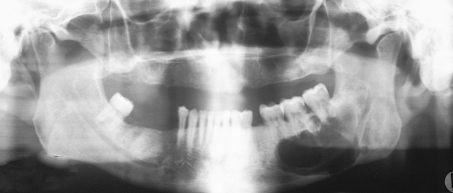
Fig. 5.
Radiographic appearance of GOC. Oval radiolucency in anterior maxilla between canine and first premolar with root divergence (Case 67)
Fig. 6.
Radiographic appearance of GOC. Well-defined radiolucency associated with unerupted tooth #32 (Case 66). This cyst contained 8 of 10 microscopic parameters described in GOC
Treatment
Information on treatment was recorded in 25 cases. All cases were treated conservatively with procedures described as enucleation, curettage, excision, cystectomy, and peripheral ostectomy.
Follow-up
Follow-up was obtained for 18 of the 46 cases. The average length of follow-up was 8.75 years and the range of follow-up was 2 months to 20 years. Nine of the 18 cases with follow-up recurred for a recurrence rate of 50%. If all 46 cases are included, the recurrence rate was 19.6%, which can be considered to represent a minimum recurrence rate for GOC. The average time interval from initial treatment to first recurrence was 8 years (range: 3–13 years). Six cases recurred twice. Specific time interval information was available for 5 of these cases with the second recurrence an average of 5.8 years after the first recurrence (range 2–8 years). Two cases recurred 3 times with specific time information available for only one case, with the third recurrence 7 years after the second recurrence. The panograph of one of the recurrent cases is shown in Fig. 3b.
Microscopic Parameters
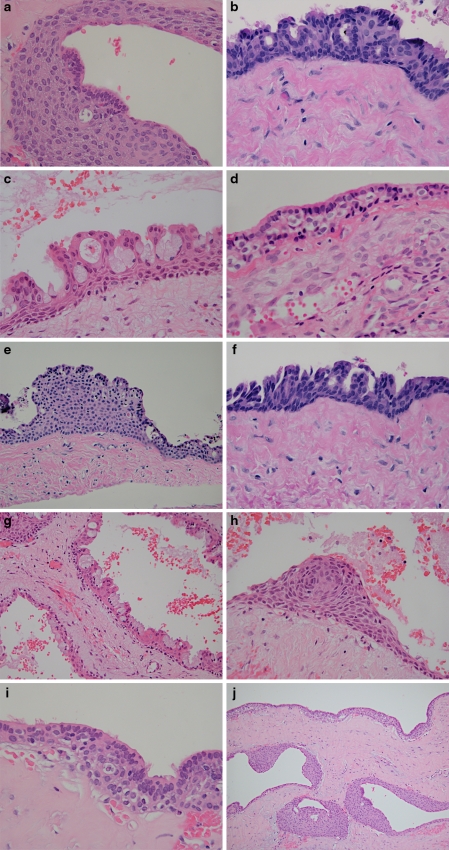
The presence or absence of ten microscopic parameters was recorded for each of the 67 cases. The selection of these parameters was based on and adapted from previously reported microscopic features for GOC [2, 3, 7, 8]. Examples of these parameters are shown in Fig. 7 and the total number of cases displaying each parameter is shown in Table 1.
Surface eosinophilic cuboidal cells, also called “hobnail cells”. These cells are present on the surface of the cyst lining and resemble cuboidal cells of the reduced enamel epithelium that lines dental follicles and dentigerous cysts (Fig. 7a).
Intraepithelial microcysts or duct-like spaces lined by a single layer of cuboidal to columnar cells similar to surface cells. Sometimes the microcysts are lined by mucous goblet cells. These microcysts may contain mucous pools, eosinophilic material, or may appear to be empty. In areas, the microcysts may open onto the surface of the lining epithelium (Fig. 7b).
Apocrine snouting of hobnail cells. Sometimes the hobnail cells demonstrate “pinching off” of the surface similar to decapitation secretion seen in cells that line apocrine gland ducts (Fig. 7c).
Clear or vacuolated cells. These cells contain clear cytoplasm and may be present in the basal and/or parabasal layers. The clear cytoplasm is due to glycogen in some cases. In areas of attenuated cyst lining, clear basal cells may be directly subjacent to the surface eosinophilic cuboidal cells (Fig. 7d).
Variable thickness of the cyst lining. This was recorded as positive only if marked variability in the thickness of the cyst lining was present (Fig. 7e).
Papillary projections or “tufting” into the cyst lumen. These papillary projections sometimes are formed by several microcysts opening onto the surface of the cyst lining, but may also be formed independent of microcysts (Fig. 7f).
Mucous goblet cells. These cells may be present singly or in small clusters on the surface or within the cyst lining. They may also line microcysts (Fig. 7g).
Epithelial spheres or plaque-like thickenings. These are identical to those seen in lateral periodontal cysts or botryoid odontogenic cysts. Sometimes the epithelium in these plaques exhibits swirling or spherule formation (Fig. 7h).
Cilia. These are true cilia on the surface of eosinophilic cuboidal cells, and are distinct from apocrine snouting (Fig. 7i).
Multiple compartments. Multiple cystic spaces similar to those seen in botryoid odontogenic cysts (Fig. 7j).
Fig. 7.
Microscopic parameters in GOC. a Eosinophilic cuboidal cells seen focally in cyst lining (H&E stain, orig. mag × 200). b Numerous microcysts lined by eosinophilic cuboidal cells. Cilia are also noted (H&E stain, orig. mag × 400). c Cyst lining with several surface eosinophilic cuboidal cells exhibiting apocrine snouting. Microcysts and papillary projections formed adjacent to “open” microcysts are also noted (H&E stain, orig. mag × 400). d Clear (vacuolated) cells located in basal and parabasal layers (H&E stain, orig. mag × 400). e Variable thickness of cyst lining (H&E stain, orig. mag × 200). f Papillary projections (“tufting”) of epithelial lining (H&E stain, orig. mag × 400). g Numerous mucous goblet cells on surface of cyst lining. Multiple compartments are also evident (H&E stain, orig. mag × 200). h Epithelial sphere (plaque-like thickening) exhibiting swirling (spherule formation) (H&E stain, orig. mag × 400). i Cyst lining with numerous clear cells, surface eosinophilic cuboidal cells, and cilia (H&E stain, orig. mag × 400). j Multiple cystic compartments (H&E stain, orig. mag × 100)
Table 1.
Microscopic parameters in glandular odontogenic cyst
| Parameter | No. & % of cases (N = 46) |
|---|---|
| Eosinophilic cuboidal cells | 46 (100%) |
| Microcysts | 44 (95.7%) |
| Apocrine snouting | 42 (91.3%) |
| Clear (vacuolated) cells | 41 (89.1%) |
| Variable thickness | 41 (89.1%) |
| Tufting (papillary projections) | 39 (84.8%) |
| Mucous cells | 33 (71.7%) |
| Epithelial spheres | 31 (67.4%) |
| Multiple compartments | 29 (63.0%) |
| Cilia | 10 (21.7%) |
Statistical Comparison
The results of the univariate and stratified analyses are shown in Tables 2, 3, 4. In addition, the relationship between the number of parameters and the diagnosis of GOC is shown in Table 5. A P value less than 0.05 was considered statistically significant.
Table 2.
Microscopic parameter comparison between GOCs and non-GOCs
| Parameter | GOC (N = 46) | Non-GOC (N = 21) | P value* |
|---|---|---|---|
| Eosinophilic cuboidal cells | 46 (100%) | 20 (95.2%) | 0.313 |
| Microcysts | 44 (95.7%) | 3 (14.3%) | <0.0001 |
| Apocrine snouting | 42 (91.3%) | 16 (76.2%) | 0.126 |
| Clear (vacuolated) cells | 41 (89.1%) | 9 (42.9%) | 0.0002 |
| Variable thickness | 41 (89.1%) | 9 (42.9%) | 0.0002 |
| Tufting (papillary projections) | 39 (84.8%) | 14 (66.7%) | 0.112 |
| Mucous cells | 33 (71.7%) | 13 (61.9%) | <0.602 |
| Epithelial spheres | 31 (67.4%) | 2 (4.8%) | <0.0001 |
| Multiple compartments | 29 (63.0%) | 3 (14.3%) | <0.006 |
| Cilia | 10 (21.7%) | 3 (14.3%) | 0.740 |
GOC glandular odontogenic cyst
*P < 0.05 was considered significant and P < 0.001 was considered highly significant
Table 3.
Microscopic parameter comparison between GOCs associated with unerupted teeth and dentigerous cysts with metaplastic changes mimicking GOCs
| Parameter | GOC dentigerous (N = 8) | Dentigerous metaplastic (N = 13) | P value* |
|---|---|---|---|
| Eosinophilic cuboidal cells | 8 (100%) | 12 (92.3%) | 1.000 |
| Microcysts | 8 (100%) | 3 (23.1%) | 0.001 |
| Tufting (papillary projections) | 8 (100%) | 8 (61.5%) | 0.111 |
| Variable thickness | 7 (87.5%) | 5 (38.5%) | 0.067 |
| Mucous cells | 7 (87.5%) | 9 (69.2%) | 0.606 |
| Apocrine snouting | 6 (75%) | 9 (69.2%) | 1.000 |
| Clear (vacuolated) cells | 6 (75%) | 3 (23.1%) | 0.032 |
| Epithelial spheres | 3 (37.5%) | 0 (0%) | 0.042 |
| Multiple compartments | 2 (25%) | 1 (7.7%) | 0.531 |
| Cilia | 2 (25%) | 1 (7.7%) | 0.531 |
GOC glandular odontogenic cyst, GOC dentigerous glandular odontogenic cyst associated with an unerupted tooth, Dentigerous metaplastic dentigerous cyst with metaplastic changes
*P < 0.05 was considered significant and P < 0.001 was considered highly significant
Table 4.
Microscopic parameter comparison between recurrent GOCs and non-recurrent GOCs
| Parameter | GOC recurrent (N = 9) | GOC non-recurrent (N = 9) | P value* |
|---|---|---|---|
| Eosinophilic cuboidal cells | 9 (100%) | 9 (100%) | – |
| Microcysts | 8 (88.9%) | 9 (100%) | 1.000 |
| Clear (vacuolated) cells | 9 (100%) | 9 (100%) | – |
| Variable thickness | 7 (77.8%) | 8 (88.9%) | 1.000 |
| Apocrine snouting | 9 (100%) | 7 (77.8%) | 0.471 |
| Mucous cells | 7 (77.8%) | 7 (77.8%) | 1.000 |
| Epithelial spheres | 8 (88.9%) | 6 (66.7%) | 0.576 |
| Tufting (papillary projections) | 8 (88.9%) | 5 (50%) | 0.294 |
| Multiple compartments | 8 (88.9%) | 4 (44.4%) | 0.131 |
| Cilia | 0 (0%) | 3 (33.3%) | 0.206 |
GOC glandular odontogenic cyst
*P < 0.05 was considered significant and P < 0.001 was considered highly significant
Table 5.
Relationship between number of parameters and diagnosis of GOC
| No. of parameters | Diagnosed as GOC (N = 46) | Diagnosed as non-GOC (N = 21) |
|---|---|---|
| 9 or more | 12 | 0 |
| 8 | 13 | 2a |
| 7 | 18 | 0 |
| 6 | 3 | 1 |
| 5 | 0 | 5 |
| 4 | 0 | 10 |
| 3 | 0 | 1 |
| 2 | 0 | 1 |
| 1 or less | 0 | 1 |
Presence of 7 or more parameters highly predictive of GOC DX (P < 0.0001). Presence of 5 or less parameters highly predictive of non-GOC DX (P < 0.0001)
aSee explanation in discussion section
From these analyses, it can be concluded that:
Microcysts (P < 0.0001), epithelial spheres (P < 0.0001), clear cells (P = 0.0002), variable thickness of the epithelial cyst lining (P = 0.0002), and multiple compartments (P = 0.006) were significantly more often present in cases we diagnosed as “GOC” versus those we diagnosed as “non-GOC” and, therefore, appear to be most helpful in distinguishing GOCs from GOC mimickers in problematic cases (Table 2).
For the stratified analysis, multiple compartments appear to be significantly associated with a diagnosis of “GOC”, but only when microcysts are present (P = 0.05). Likewise, clear cells appear to be marginally significantly associated with a diagnosis of “GOC” only when microcysts are present (P = 0.054). In contrast, epithelial spheres appear to be significantly associated with a diagnosis of “GOC” regardless of whether microcysts were present or not (P = 0.03 and P = 0.05, respectively).
When the analysis was restricted to cases associated with unerupted teeth, the presence of microcysts (P = 0.001), clear cells (P = 0.032) and epithelial spheres (P = 0.042) appears to be most helpful in distinguishing GOCs associated with an unerupted tooth from dentigerous cysts with metaplastic changes (Table 3).
There were no statistically significant differences in parameters between recurrent and non-recurrent GOCs (Table 4).
The presence of 7 or more microscopic parameters was highly predictive of a diagnosis of GOC in this series (P < 0.0001) (Table 5).
The presence of 5 or less microscopic parameters was highly predictive of a diagnosis of non-GOC in this series (P < 0.0001) (Table 5).
Discussion
At this time, well over 100 cases of GOC have been reported in the English literature. Many of these have been single case reports; however, several short series and detailed reviews of the literature have also been published [1, 2, 6, 7, 11, 13–26]. Therefore, the GOC, though rare, is now relatively well known, especially among oral and head and neck pathologists. Moreover, the microscopic features of GOC have been well documented, and the most recent World Health Organization classification includes a definition of the GOC and lists numerous characteristic microscopic features of this cyst [3]. However, it is not uncommon in the practice of pathology to encounter jaw cysts that display some but not all the features that have been described in the “classic” GOC. This situation often occurs when the cyst is associated with an unerupted tooth. It is well known that odontogenic cysts, particularly dentigerous cysts, may show metaplastic changes in the cyst lining that overlap with microscopic features described in GOC, such as eosinophilic cuboidal cells, mucous cells, and ciliated cells. [27, 28] Therefore, in such cases, the pathologist may be unsure whether the cyst in question is a true GOC or simply a dentigerous cyst mimicking a GOC. To our knowledge, the question of how many and which microscopic features are necessary for diagnosis of GOC has been previously addressed by only one group of investigators. Kaplan et al. [11, 12] proposed a list of major and minor microscopic criteria for GOC based on the frequency of each feature in reported cases from the literature. Based on their analysis, it was suggested that at least focal presence of each of the following major criteria must be present for diagnosis:
Squamous epithelial lining, with a flat interface with the connective tissue wall, lacking basal palisading
Epithelium exhibiting variations in thickness along the cystic lining with or without epithelial “spheres” or “whorls” or focal luminal proliferation
Cuboidal eosinophilic cells or “hobnail” cells
Mucous (goblet) cells with intraepithelial mucous pools, with or without crypts lined by mucous-producing cells
Intraepithelial glandular, microcystic, or duct-like structures
They also listed the following minor criteria, which support the diagnosis, but are not mandatory:
Papillary proliferation of the lining epithelium
Ciliated cells
Multicystic or multiluminal architecture
Clear or vacuolated cells in the basal or spinous layers
Although these diagnostic criteria have merit, our findings demonstrate several differences with regard to specific microscopic features necessary for diagnosis of GOC. As shown in Table 1, two of our cases diagnosed as GOC did not contain microcysts, five did not display variable thickness of the lining, only 71.7% contained mucous cells, and only 67.4% contained epithelial spheres. Therefore, we do not believe that all of Kaplan and colleagues’ “major criteria” need be present for diagnosis. Our findings suggest it is likely a combination of specific microscopic features, not necessarily corresponding with their major and minor criteria, that appear to be important in making an accurate diagnosis of GOC. By comparing which microscopic parameters were statistically more often present in cases we diagnosed as “GOC” versus those we diagnosed as “non-GOC” (Table 2), it can be seen that eosinophilic cuboidal (hobnail) cells, though necessary for diagnosis, are of little value in problematic cases, because they were also seen in 95.2% of GOC mimickers. Likewise, because mucous cells were seen in 71.7% of GOCs and 61.9% of GOC mimickers, they also are not helpful in discrimination. However, the presence of microcysts, epithelial spheres, clear cells, variable thickness of the cyst lining, and multiple compartments do appear to be of value in separating GOCs from GOC mimickers. The stratified analysis demonstrated that clear cells were marginally significant and multiple compartments were significant only when microcysts were present, while epithelial spheres were significant regardless of whether microcysts were present or not. When comparing only cases of GOCs and non-GOCs that were associated with unerupted teeth (dentigerous relationship), the presence of microcysts, clear cells, and epithelial spheres appears to be helpful in distinguishing GOCs from GOC mimickers (Table 3). Table 5 demonstrates that the presence of 7 or more microscopic parameters was highly predictive of a diagnosis of GOC in our series as only 3 of 46 cases we diagnosed as “GOC” contained less than 7 parameters. Similarly, the presence of 5 or less microscopic parameters was highly predictive of a non-GOC diagnosis in our series as only 3 of 21 cases we diagnosed as “non-GOC” contained more than 5 parameters.
As indicated in Table 5, two cases that displayed 8 of the 10 microscopic parameters were not diagnosed as GOC. One of these cases was a large expansile multilocular radiolucent lesion in the left anterior mandible that recurred 2½ years after initial treatment. The original cyst did not display microcysts or variable thickness of the lining. The recurrent cyst did not demonstrate microcysts, epithelial spheres, mucous cells, or variable thickness. The second case was a 4.5 cm expansile multilocular radiolucency in the anterior maxilla that by history possibly represented recurrence of a GOC removed 10 years previously (original material not available). This cyst did not contain microcysts or epithelial spheres and was significantly inflamed. While the clinical and microscopic findings raise the possibility that these cysts were actually GOCs, none of the reviewers classified them as such on initial or subsequent review. It could be argued that these two cases should not have been included in the study; however, doing so would not significantly change the statistical comparison, and we wanted to test our diagnostic criteria on all cases submitted by the study participants. It should also be emphasized that recurrent GOCs sometimes do not exhibit as many of the microscopic features as the original cyst. This may sometimes hinder the interpretation of recurrent GOCs if the original material is not available for review.
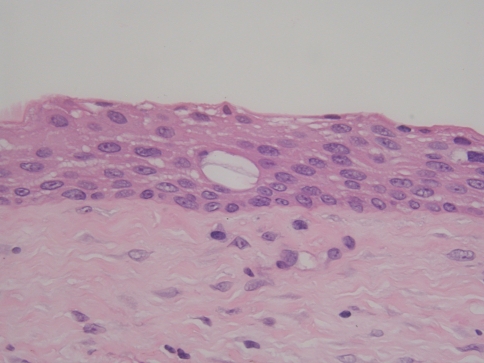
Table 3 indicates that 8 cases of GOC were associated with an unerupted tooth (dentigerous relationship). This was an unexpected finding because the majority of reported cases of GOCs are not associated with unerupted teeth, as only 6 such cases have been previously reported [6, 21, 22, 29, 30]. Moreover, because some of the microscopic features of GOC are similar if not identical to metaplastic changes in dentigerous cysts, rendering a diagnosis of GOC for a cyst in a dentigerous relationship should be done with caution. It is well-known that eosinophilic cuboidal cells, cilia, and mucous cells are occasionally seen in the lining of dentigerous cysts. Moreover, dentigerous cysts sometimes contain vacuolated areas in the cyst lining (“pseudomicrocysts”), as shown in Fig. 8. The cells lining these pseudomicrocysts tend to have a more flattened appearance rather than the cuboidal to columnar cells lining true microcysts. We do not believe such structures are true microcysts, and they do not satisfy the criteria for microcysts as defined previously. Within the context of these diagnostic pitfalls, we were strict in our interpretation of these 8 cases as evidenced by the fact that 3 of the cysts contained 8 of 10 parameters, 4 cysts contained 7 of 10 parameters, and 1 cyst contained 6 parameters. Also, all of the microscopic features identified in these cases were more than just focal findings. Unfortunately, no follow-up was available on 6 of the 8 cases. The remaining 2 cases showed no evidence of disease 18 months and 5½ years after initial treatment.
Fig. 8.
Vacuolated area (“pseudomicrocyst”) in lining of a dentigerous cyst (H&E stain, orig. mag × 400)
Because all of the cysts in this study were treated with conservative excision, no comparisons could be made relating type of treatment to recurrence. However, with a recurrence rate of 50%, one could argue that more aggressive treatment may be indicated for these cysts. Most cases reported in the literature have also been treated conservatively and a recurrence rate of 30% has been reported [11]. Eighteen reported cases have been treated with more aggressive initial surgery to include en bloc and segmental resection [6, 8, 13, 15, 20–22, 25, 31–36], and recurrence has been documented in only one case after such treatment [8]. Radiographic evidence of possible recurrence was also reported in one case [31].
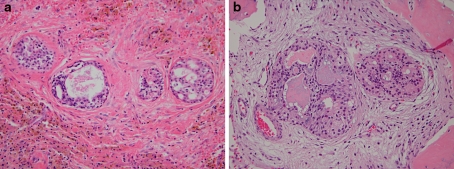
In 3 of our cases, islands resembling central mucoepidermoid carcinoma (CMEC) were noted in the cyst wall (Fig. 9). In two of these cases, the MEC-like islands invaded bone. Otherwise, the cases were “classic” GOCs microscopically. Follow-up was available on only 1 of these 3 cases, indicating no evidence of disease 6 years and 2 months after initial conservative treatment. Although it appears, based on current knowledge and experience, that these MEC-like islands in the cyst wall likely have no clinical significance, this finding suggests at least the possibility that GOC and CMEC may be related or that CMEC may arise in GOC. The possible relationship of GOC and CMEC has been previously discussed by several investigators [21, 37–40]. However, definitive evidence in this regard will have to await further study. Of interest is the discovery within the last few years that most MECs exhibit a t(11:19)(q21:p13) translocation resulting in the MECT1:MAML2 fusion protein [41–44]. Recently, this translocation has also been reported in CMEC [45, 46]. Therefore, molecular assays specifically targeting the MEC-like islands in the cyst wall of some GOCs may aid in answering the question of whether these islands represent true malignant transformation or not.
Fig. 9.
a Mucoepidermoid carcinoma-like islands in cyst wall of glandular odontogenic cyst. (Hemosiderin laden macrophages are also noted) (H&E stain, orig. mag × 200). b Mucoepidermoid carcinoma-like islands in cyst wall of glandular odontogenic cyst showing invasion of bone (H&E stain, orig. mag × 200)
Conclusions
GOC is a locally aggressive odontogenic cyst with a propensity for recurrence. In our study, GOCs exhibited a 50% recurrence rate, and multiple recurrences appear to be common if the length of follow-up is adequate.
The average age at diagnosis is 51 years with peak occurrence in the 5th–7th decades. There is no gender predilection.
The mandible is the most common location (80% of our cases), with 55.9% occurring in the anterior mandible. The canine area is a common location in maxillary cases.
Radiographically, GOCs are typically well-defined unilocular or multilocular radiolucencies involving the roots and periapical area of several teeth, but may sometimes mimic dentigerous cysts, lateral periodontal cysts, and “globulomaxillary cysts”.
Eosinophilic cuboidal cells (hobnail cells) are necessary for diagnosis, but are not diagnostic of GOC in the absence of other microscopic parameters.
When considered individually, the presence of microcysts, clear cells, epithelial spheres, variable thickness, and multiple compartments appears to be most helpful in distinguishing GOCs from GOC mimickers.
Based on the stratified analysis, when microcysts are present, epithelial spheres, multiple compartments, and clear cells appear to be most helpful in distinguishing GOCs from GOC mimickers.
The presence of microcysts, clear cells, and epithelial spheres appears to be most helpful in distinguishing GOCs associated with an unerupted tooth from dentigerous cysts with metaplastic changes.
The presence of 7 or more microscopic parameters was highly predictive of a diagnosis of “GOC” in this series.
The presence of 5 or less microscopic parameters was highly predictive of a diagnosis of “non-GOC” in this series.
At this time, not enough information is available to determine whether GOC and CMEC share a histopathologic spectrum, or whether MEC-like islands in GOCs are associated with more aggressive or malignant behavior.
In this study we have applied statistical methods to the microscopic diagnosis of GOC in an attempt to aid the pathologist in cases that display some but not all of the microscopic features described for this cyst. It should be noted that our criteria regarding diagnosis are based on detailed microscopic analysis of the largest collection of GOCs to date. However, because this is still a relatively small sample size with regard to statistical analysis, over time, our criteria may require modification as they are applied to a larger number of cases. Finally, it should be emphasized that during evaluation of individual cases, the pathologist should always correlate the microscopic features with clinical and radiographic findings to ensure correct diagnosis.
Acknowledgments
We wish to acknowledge Dr. Anneke Bush, Sc.D. for her assistance in preparation and interpretation of the statistical comparisons.
Conflict of interest The authors deny any financial or other conflict of interest related to this study.
Footnotes
Disclaimer: The opinions or assertions expressed herein are those of the authors and do not reflect the views of the Department of the Air Force, the Navy Department, or the Department of Defense.
References
- 1.Padayachee A, Wyk CW. Two cystic lesions with features of both the botryoid odontogenic cyst and the central mucoepidermoid tumour: sialo-odontogenic cyst? J Oral Pathol. 1987;16:499–504. doi: 10.1111/j.1600-0714.1987.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 2.Gardner DG, Kessler HP, Morency R, et al. The glandular odontogenic cyst: an apparent entity. J Oral Pathol. 1988;17:359–366. doi: 10.1111/j.1600-0714.1988.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 3.Kramer IRH, Pindborg JJ, Shear M. Histopathological typing of odontogenic tumors. In: WHO international histological classification of tumors, 2nd ed. 1992, Springer: Berlin, p. 25–6.
- 4.Semba I, Kitano M, Mimura T, et al. Glandular odontogenic cyst: analysis of cytokeratin expression and clinicopathological features. J Oral Pathol Med. 1994;23:377–382. doi: 10.1111/j.1600-0714.1994.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 5.Pires FR, Chen S-Y, da Cruz Perez DE, et al. Cytokeratin expression in central mucoepidermoid carcinoma and glandular odontogenic cyst. Oral Oncol. 2004;40:545–551. doi: 10.1016/j.oraloncology.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Shen J, Fan M, Chen X, et al. Glandular odontogenic cyst in China: report of 12 cases and immunohistochemical study. J Oral Pathol Med. 2006;35:175–182. doi: 10.1111/j.1600-0714.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 7.Koppang HS, Johannessen S, Haugen LK, et al. Glandular odontogenic cyst (sialo-odontogenic cyst): report of two cases and literature review of 45 previously reported cases. J Oral Pathol Med. 1998;27:455–462. doi: 10.1111/j.1600-0714.1998.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 8.Sousa SOM, Cabezas NT, Oliveira PT, et al. Glandular odontogenic cyst: report of a case with cytokeratin expression. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;83:478–483. doi: 10.1016/S1079-2104(97)90149-9. [DOI] [PubMed] [Google Scholar]
- 9.Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med. 2006;35:500–507. doi: 10.1111/j.1600-0714.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 10.Meningaud J-P, Oprean N, Pitak-Arnnop P, et al. Odontogenic cysts: a clinical study of 695 cases. J Oral Sci. 2006;48:59–62. doi: 10.2334/josnusd.48.59. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan I, Anavi Y, Hirshberg A. Glandular odontogenic cyst: a challenge in diagnosis and treatment. Oral Dis. 2008;14:575–581. doi: 10.1111/j.1601-0825.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan I, Anavi Y, Manor R, et al. The use of molecular markers as an aid in the diagnosis of glandular odontogenic cyst. Oral Oncol. 2005;41:895–902. doi: 10.1016/j.oraloncology.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Patron M, Colmenero C, Larrauri J. Glandular odontogenic cyst: clinicopathologic analysis of three cases. Oral Surg Oral Med Oral Pathol. 1991;72:71–74. doi: 10.1016/0030-4220(91)90192-F. [DOI] [PubMed] [Google Scholar]
- 14.Heerden WFP, Raubenheimer EJ, Turner ML. Glandular odontogenic cyst. Head Neck. 1992;14:316–320. doi: 10.1002/hed.2880140412. [DOI] [PubMed] [Google Scholar]
- 15.Hussain K, Edmondson HD, Browne RM. Glandular odontogenic cysts. Diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:593–602. doi: 10.1016/S1079-2104(05)80101-5. [DOI] [PubMed] [Google Scholar]
- 16.High AS, Main DMG, Khoo SP, et al. The polymorphous odontogenic cyst. J Oral Pathol Med. 1996;25:25–31. doi: 10.1111/j.1600-0714.1996.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 17.Magnusson B, Goransson L, Odesjo B, et al. Glandular odontogenic cyst. Report of seven cases. Dentomaxillofac Radiol. 1997;26:26–31. doi: 10.1038/sj.dmfr.4600205. [DOI] [PubMed] [Google Scholar]
- 18.Tosios KI, Kakarantza-Angelopoulou E, Kapranos N. Immunohistochemical study of bcl-2 protein, Ki-67 antigen and p53 protein in epithelium of glandular odontogenic cysts and dentigerous cysts. J Oral Pathol Med. 2000;29:139–144. doi: 10.1034/j.1600-0714.2000.290306.x. [DOI] [PubMed] [Google Scholar]
- 19.Noffke C, Raubenheimer EJ. The glandular odontogenic cyst: clinical and radiological features: review of the literature and report of nine cases. Dentomaxillofac Radiol. 2002;31:332–338. doi: 10.1038/sj.dmfr.4600730. [DOI] [PubMed] [Google Scholar]
- 20.Manor R, Anavi Y, Kaplan I, et al. Radiological features of glandular odontogenic cyst. Dentomaxillofac Radiol. 2003;32:73–79. doi: 10.1259/dmfr/22912856. [DOI] [PubMed] [Google Scholar]
- 21.Qin X-N, Li J-R, Chen X-M, et al. The glandular odontogenic cyst: clinicopathologic features and treatment of 14 cases. J Oral Maxillofac Surg. 2005;63:694–699. doi: 10.1016/j.joms.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan I, Gal G, Anavi Y, et al. Glandular odontogenic cyst: treatment and recurrence. J Oral Maxillofac Surg. 2005;63:435–441. doi: 10.1016/j.joms.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Velez I. Glandular odontogenic cyst. Report of two cases and review of the literature. N Y State Dent J. 2006;72:44–45. [PubMed] [Google Scholar]
- 24.Shear M, Speight P. Glandular odontogenic cyst (sialo-odontogenic cyst). In: Cysts of the oral and maxillofacial regions, 4th ed. Blackwell: Oxford, 2007; p. 94–9.
- 25.Krishnamurthy A, Sherlin HJ, Ramalingam K, et al. Glandular odontogenic cyst: report of two cases and review of literature. Head Neck Pathol. 2009;3:153–158. doi: 10.1007/s12105-009-0117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald-Jankowski DS. Glandular odontogenic cyst: systematic review. Dentomaxillofac Radiol. 2010;39:127–139. doi: 10.1259/dmfr/30943934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browne RM. Metaplasia in odontogenic cysts. J Dent Res (Suppl) 1971;50:1177. [Google Scholar]
- 28.Gorlin RJ. Potentialities of oral epithelium manifest by mandibular dentigerous cysts. Oral Surg Oral Med Oral Pathol. 1957;10:271–284. doi: 10.1016/0030-4220(57)90092-0. [DOI] [PubMed] [Google Scholar]
- 29.Ide F, Shimoyama T, Horie N. Glandular odontogenic cyst with hyaline bodies: an unusual dentigerous presentation. J Oral Pathol Med. 1996;25:401–404. doi: 10.1111/j.1600-0714.1996.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 30.Geist J, Gordon S, Wesley R. Oral pathology quiz: an unusual pericoronal cyst. J Michigan Dent Assoc. 2003;85(40):42–43. [PubMed] [Google Scholar]
- 31.Ficarra G, Chou L, Panzoni E. Glandular odontogenic cyst (sialo-odontogenic cyst). A case report. Int J OralMaxillofac Surg. 1990;19:331–333. doi: 10.1016/S0901-5027(05)80074-8. [DOI] [PubMed] [Google Scholar]
- 32.Chavez JA, Richter KJ. Glandular odontogenic cyst of the mandible. J Oral Maxillofac Surg. 1999;57:461–464. doi: 10.1016/S0278-2391(99)90291-4. [DOI] [PubMed] [Google Scholar]
- 33.Thor A, Warfvinge G, Fernandes R. The course of a long-standing glandular odontogenic cyst: marginal resection and reconstruction with particulated bone graft, platelet-rich plasma, and additional vertical alveolar distraction. J Oral Maxillofac Surg. 2006;64:1121–1128. doi: 10.1016/j.joms.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 34.Foss RD, Fielding CG. Glandular odontogenic cyst. Head Neck Pathol. 2007;1:102–103. doi: 10.1007/s12105-007-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wharton L, Saini T, Ogunleye A, et al. Glandular odontogenic cyst of the mandible: from incidental radiographic discovery to prosthetic reconstruction. Gen Dent. 2008;56:554–558. [PubMed] [Google Scholar]
- 36.Boffano P, Cassarino E, Zavattero E, et al. Surgical treatment of glandular odontogenic cysts. J Craniofac Surg. 2010;21:776–780. doi: 10.1097/SCS.0b013e3181d7a3e6. [DOI] [PubMed] [Google Scholar]
- 37.Waldron CA, Koh ML. Central mucoepidermoid carcinoma of the jaws: report of four cases with anaysis of the literature and discussion of the relationship to mucoepidermoid, sialodontogenic, and glandular odontogenic cysts. J Oral Maxillofac Surg. 1990;48:871–877. doi: 10.1016/0278-2391(90)90349-7. [DOI] [PubMed] [Google Scholar]
- 38.Toida M, Nakashima E, Okumura Y, et al. Glandular odontogenic cyst: a case report and literature review. J Oral Maxillofac Surg. 1994;52:1312–1316. doi: 10.1016/0278-2391(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 39.Manojlovic S, Grgurevic J, Knezevic G, et al. Glandular odontogenic cyst: a case report and clinicopathologic analysis of the relationship to central mucoepidermoid carcinoma. Head Neck. 1997;19:227–231. doi: 10.1002/(SICI)1097-0347(199705)19:3<227::AID-HED11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Prabhu S, Kumar RK. Glandular odontogenic cyst mimicking central mucoepidermoid carcinoma. J Oral Maxillofac Pathol. 2010;14:12–15. doi: 10.4103/0973-029X.64303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheuk W, Chan JKC. Advances in salivary gland pathology. Histopathol. 2007;51:1–20. doi: 10.1111/j.1365-2559.2007.02719.x. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama T, Miyabe S, Okabe M, et al. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Mod Pathol. 2009;22:1575–1581. doi: 10.1038/modpathol.2009.126. [DOI] [PubMed] [Google Scholar]
- 43.Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2011;34:1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 44.Seethala RR. Histologic grading and prognostic biomarkers in salivary gland carcinomas. Adv Anat Pathol. 2011;18:29–45. doi: 10.1097/PAP.0b013e318202645a. [DOI] [PubMed] [Google Scholar]
- 45.Bell D, Holsinger CF, El-Naggar AK. CRTC1/MAML2 fusion transcript in central mucoepidermoid carcinoma of mandible—diagnostic and histogenetic implications (Abstr). Mod Pathol. 2011;24(Suppl 1):274A. [DOI] [PubMed]
- 46.Garcia JJ, Barnes EL, Cieply K, et al. Utility of MAML2 rearrangement detection using fluorescent in situ hybridization to distinguish glandular odontogenic cyst from central mucoepidermoid carcinoma (Abstr). Mod Pathol 2011;24(Suppl 1):277–8A.