Abstract
Psoriasis is chronic autoimmune hyperproliferative skin disease with a population prevalence of 1.5–3%. The cause of psoriasis is still not fully understood. It has been hypothesized to be an immune-mediated disorder in which the excessive reproduction of keratinocytes is due to cytokines such as interferon (IFN)-gamma and tumour necrosis factor (TNF)-alpha, secreted by infiltrating CD4+ and CD8+ T cells and natural killer cells. The aim of our study was to determine the serum levels of TNF-α, IL-4, IL-6 & IL-10 in psoriasis patients and compare it with healthy controls. 30 clinically diagnosed psoriasis patients and 30 age and sex matched healthy controls were included in the study. The serum cytokine levels were measured by solid phase sandwich ELISA (DIACLONE Research, France). TNF-α and IL-6 levels were significantly raised in patients and the results were statistically significant (P < 0.001). IL-4 levels were higher in patients than in controls (1.91 ± 4.7 pg/ml in cases & 0.9 ± 0.3 pg/ml in controls) but were not statistically significant. Interestingly, IL-10 levels were found to be higher in controls than in patients but again, it was not statistically significant. Pro-inflammatory cytokines play a pivotal role in the pathogenesis of psoriasis and it is the type 1(TH1) cytokine pattern, i.e., IL-6 & TNF-α, which predominate in the psoriatic T cell response. Further studies on IL-10 levels in psoriasis are recommended to establish their exact role in the pathogenesis of the disease.
Keywords: Cytokines, Psoriasis, TNF-alpha, Interleukins
Introduction
Psoriasis is a chronic, currently incurable immune-mediated skin disease defined by clinical presentation of red, heavily scaled skin plaques containing dense infiltrates of T cells, macrophages, and dendritic cells (DC) as well as hyperproliferation and incomplete differentiation of epidermal keratinocytes [1]. The disease is widespread in occurrence with a population prevalence of 2–3% [2].
Although, the pathogenesis of psoriasis has not been fully elucidated, an immunologic-genetic relationship is likely. In recent years, there is accumulating evidence that the complex interaction of structural cells, in particular keratinocytes and endothelial cells, with skin-infiltrating leukocytes is key to our understanding of psoriasis.
Activated T cells, monocytes, and pro-inflammatory cytokines, most notably TNF-α, have all been shown to play pivotal roles in the pathogenesis of psoriasis[3]. The activated T cells release TNF-α, IFN-γ, and other cytokines [4]. Collectively, TNF-α and IFN-γ induce a wide variety of responses including: signal transducer and activator of transcription 1 (STAT1) stimulation followed by expression of downstream response genes; activation of nuclear factor kappa B (NF-κB) signaling pathways; leukocyte migration to inflammatory regions; and vasodilation that results in the erythema that is characteristic of psoriatic lesions[5, 6]. It has been postulated that TNF-α and IFN-γ activate keratinocytes that produce a wide variety of cytokines, which are capable of effecting DC maturation. Chemokines, which are produced through a downstream response to IFN-γ, TNF-α and possibly other cytokines, also play a role in recruiting leukocytes to areas of inflammation. The noted cytokine, chemokine and cellular interactions may contribute to the self-perpetuation of the type 1 pathway, which can in turn be correlated with the chronic nature of psoriasis.
Several studies have revealed the elevated levels of TH1 cytokines. Interleukin-2, Interleukin-6, Interleukin-8 and tumor necrosis factor-α (TNF-α) are the hallmark cytokines in the psoriatic cytokine network [7]. IL-1β, IL-4, IL-10, IL-17 and IL-18 too have been found to play a key role in the pathogenesis of the disease [8, 9].
As far as India is concerned, to the best of our knowledge, no studies have been done to ascertain the serum cytokine profile in psoriasis. We therefore planned to undertake a study on the serum cytokine profile in Indian patients of psoriasis and compare it with healthy controls.
Materials and Methods
The study protocol was approved by Lady Hardinge Medical College’s Ethical Committee. The study population consisted of 30 randomly selected cases of clinically diagnosed psoriasis patients attending the dermatology OPD of Smt. Sucheta Kriplani Hospital and Kalawati Saran Children’s Hospital, New Delhi. 30 age and sex matched individuals attending the skin OPD for cosmetic reasons like keloids and scars were included in the control group. 5 ml of venous blood was collected from patients and controls in vials without anticoagulant. Sera was separated & immediately stored at −70°C until they were batch analyzed.
Serum cytokine levels of TNF-α, IL-6, IL-4 and IL-10 were determined by solid phase sandwich Enzyme linked immuno-sorbent assay (ELISA; DIACLONE Research, France). All assays were conducted according to manufacturer’s protocols. These experiments were performed in duplicate, and the amount of cytokine in each sample was determined by extrapolating absorbance values to cytokine concentrations using the standard curve.
Statistical Analysis
Data is presented as mean, median, 25 percentile and 75 percentile, standard deviation (SD). The results were then analyzed for significant correlation using SPSS version 12. A P value < 0.05 was considered statistically significant.
Results
A total of 30 patients (17 males, 13 females), of mean age 34.5 ± 13.3 (range: 15–65) years, and 30 age and sex matched healthy controls were enrolled in this study. The mean duration of illness for the patients group was 6.98 ± 5.94 years. Psoriasis vulgaris was the dominant form with 90% of the cases falling into this category. Out of the 30 patients included in the study group, only 4 patients (13.3%) had joint complaints in the form of pain and stiffness. Of these, only 1 patient showed radiological evidence of psoriatic arthritis.
The minimum, maximum and mean values of the studied cytokines are presented in Tables 1 and 2. Graphical representation of the data is shown in Figs. 1, 2, 3 and 4.
Table 1.
Serum TNF-α & IL-6 levels in cases and controls
| Subject group | Min | Max | Mean | SD | P value |
|---|---|---|---|---|---|
| Serum TNF-α (pg/ml) | |||||
| Cases | 12 | 1600 | 362.67 | 456.9 | <0.001 |
| Controls | 7 | 25 | 14.64 | 5.78 | |
| Serum IL-6 (pg/ml) | |||||
| Cases | 7 | 183 | 61.26 | 57.40 | <0.001 |
| Controls | 1.19 | 6.07 | 2.38 | 1.94 | |
SD Standard deviation
P < 0.05 significant, P < 0.01 highly significant, P < 0.001 very highly significant
Table 2.
Serum IL-4 & IL-10 levels in cases and controls
| Subject group | Min | Max | Mean | SD | P value |
|---|---|---|---|---|---|
| Serum IL-4 (pg/ml) | |||||
| Cases | 0.41 | 26.6 | 1.91 | 4.7 | 0.245 |
| Controls | 0.45 | 1.85 | 0.9 | 0.3 | |
| Serum IL-10 (pg/ml) | |||||
| Cases | 3.72 | 103 | 11.75 | 17.68 | 0.469 |
| Controls | 5.5 | 199 | 17.01 | 35.32 | |
SD Standard deviation
P < 0.05 significant, P < 0.01 highly significant, P < 0.001 very highly significant
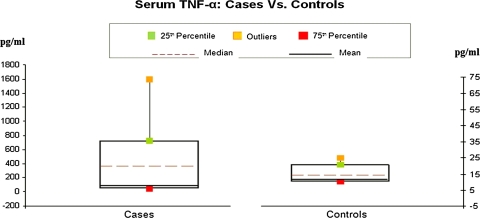
Fig. 1.
The serum TNF-alpha levels in cases and controls on x-axis as mean, median and percentile on y-axis
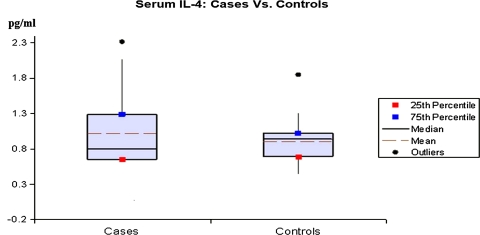
Fig. 2.
The serum IL-4 levels in cases and controls on x-axis as mean, median and percentile on y-axis
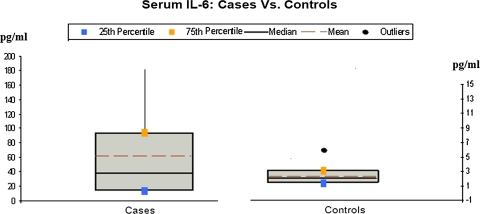
Fig. 3.
The serum IL-6 levels in cases and controls on x-axis as mean, median and percentile on y-axis
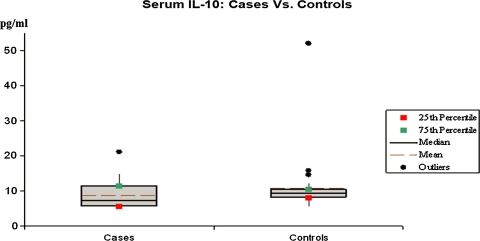
Fig. 4.
The serum IL-10 levels in cases and controls on x-axis as mean, median and percentile on y-axis
Serum TNF-α level in psoriatic patients was markedly elevated. The difference between patients and controls was found to be very highly significant (P < 0.001).
The serum level of IL-6 in patients ranged from 7 to 183 pg/ml with a mean of 61.26 ± 57.40 pg/ml. The mean value in controls was 2.38 pg/ml. The difference between the two groups was again highly significant. Significant positive correlation was also seen between TNF-alpha and IL-6 levels.
The mean value of IL-4 in cases was 1.91 pg/ml while in controls it was 0.9 pg/ml and the overall difference was not found to be statistically significant (P > 0.05). Similarly, the IL-10 level in controls was higher (17.01 pg/ml) as compared to cases (11.75 pg/ml). However, the difference in the serum values of IL-10 was not significant (P > 0.05).
Discussion
In our study, the TNF-α level in controls ranged from 7 to 25 pg/ml with a mean of 14.64 pg/ml. Serum TNF-α level in psoriatic patients was markedly elevated ranging from a minimum of 12 pg/ml to a maximum of 1600 pg/ml. The difference in serum TNF-α level between patients and controls was found to be very highly significant (P < 0.001) thereby confirming the pathogenic role of this cytokine in psoriasis.
There is conflicting data available on the serum levels of TNF-α. Abanmi et al. [10] and Ozer Arican et al. [11] reported elevated levels of TNF-α as compared to healthy controls in their cohort which is in agreement with our results. However, Jacob E et al. [12] found no difference in TNF-α level in patients and controls in their study [12]. Numerous physiological stimuli exist for the synthesis of TNF-α, like: IL-1, PDGF, IFN-β, Oncostatin M, bacterial endotoxins and viral infections [13]. TNF-α can also stimulate or inhibits its own synthesis; depending upon the cell type. Thus, this cytokine has a complex interaction with numerous cell types and mediators. Any subtle change in the micro-environment may stimulate its release. All these factors could be responsible for higher serum values in our patients but the fall in these values with anti-psoriatic treatment indicate that the rise was disease induced.
The difference between IL-6 levels in cases and controls was highly significant (P < 0.001). This was in agreement with other studies by Abanmi et al., [10] Koliadenko et al. [14] who found increased levels of IL-6 in their patients (range: 37–368 pg/ml). But contrary results were reported by Jacob et al. [12] who found no difference in serum IL-6 levels [12].
IL-6 mediates T-cell activation, stimulates proliferation of keratinocytes and, at the beginning of acute inflammation, mediates the acute phase responses [15]. Our finding of raised IL-6 levels in psoriatics are therefore consistent with the significant role it plays in the pathogenesis of this disease.
As far as IL-4 is concerned, we found no difference between the patients and healthy controls. Even though the maximum value in the control group was only 1.85 pg/ml, while in the patient group it was as high as 26.62 pg/ml, the overall difference was not found to be statistically significant (P > 0.05).
This observation is in agreement with the findings of Zalewska et al. [16] who found no difference between stable psoriatic and controls [16]. IL-4 usually counteracts the action of IFN-γ, and it has been reported to play a role in the production of antibody and the inhibition of cell-mediated immunity, participating in pathogenesis through these and other, as yet undetermined, complex interactions.
The mean value of IL-10 in controls was higher (17.01 pg/ml) as compared to cases (11.75 pg/ml). However, the difference in the serum values of IL-10 was not significant (P > 0.05).
Mainly produced by monocytes, macrophages, B and T cells, IL-10 is a multifunctional cytokine with diverse effects on most hematopoietic cells. It inhibits the antigen-presentation capacity of macrophages and dendritic cells (DC) and the proliferation of CD4+ T cells. IL-10 strongly inhibits cytokine production such as IL-2 synthesis by T cells and the synthesis of the pro-inflammatory cytokines IL-1β, IL-6 and tumor necrosis factor α (TNF-α) [17].
Not many studies have been done on serum IL-10 in psoriasis. Jacob et al. [12] found a significant decrease in serum IL-10 level in the patients with psoriasis compared to healthy controls [12]. Low levels of IL-10 have been observed in the cutaneous lesions of psoriatic patients and with established antipsoriatic therapy higher IL-10 mRNA levels can be found in peripheral blood mononuclear cells. The fact that serum levels of IL-10 (a Th2 cytokine) in our patients were decreased is consistent with the hypothesis that psoriasis is a Th1 mediated disease. Preliminary results obtained with cytokine and anti-cytokine therapies appear promising and as such continued research is clearly indicated.
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 2.Christopher E. Psoriasis-epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 3.Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 2007;28(2):51–57. doi: 10.1016/j.it.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Chamian F, Krueger JG. Psoriasis vulgaris: interplay of T-lymphocytes, dendritic cells, and inflammatory cytokines in pathogenesis. Curr Opin Rheumatol. 2004;16(4):331–337. doi: 10.1097/01.bor.0000129715.35024.50. [DOI] [PubMed] [Google Scholar]
- 5.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25(6):295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Lowes MA, Lew W, Krueger JG. Current concepts in the immunopathogenesis of psoriasis. Dermatol Clin. 2004;22(4):349–369. doi: 10.1016/j.det.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 9.Flisiak I, Zaniewski P, Chodynicka B. Plasma TGF-beta1, TIMP-1, MMP-1 and IL-18 as a combined biomarker of psoriasis activity. Biomarkers. 2008;13(5):549–556. doi: 10.1080/13547500802033300. [DOI] [PubMed] [Google Scholar]
- 10.Abanmi A, Al Harthi F, Al Agla R, Khan HA, Mohamed T. Serum levels of pro-inflammatory cytokines in psoriasis patients from Saudi Arabia. Int J Dermatol. 2005;44(1):82–83. doi: 10.1111/j.1365-4632.2004.02082.x. [DOI] [PubMed] [Google Scholar]
- 11.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17 & IL-18 in patients with active psoriasis & correlation with disease severity. Mediat Inflamm. 2005;5:273–279. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob SE, Nassiri M, Kerdel FA, Vincek V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediat Inflamm. 2003;12(5):309–313. doi: 10.1080/09629350310001619753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7(2):93–124. [PubMed] [Google Scholar]
- 14.Koliadenko VH, Chernyshov PV. IL-6 as a marker of the activity of a pathological process in patients with psoriasis. Lik Sprava. 2005;5–6:80–82. [PubMed] [Google Scholar]
- 15.Paquet P, Pièrard GE. Interleukin-6 and the skin. Int Arch Allergy Immunol. 1996;109:308–317. doi: 10.1159/000237257. [DOI] [PubMed] [Google Scholar]
- 16.Zalewska A, Wyczółkowska J, Dziankowska-Bartkowiak B, Sysa-Jedrzejowska A. IL-4 plasma levels in psoriasis vulgaris patients. Med Sci Monit. 2004;10(4):156–162. [PubMed] [Google Scholar]
- 17.Howard M, O’Garra A. Biological properties of Interleukin-10. Immunol Today. 1992;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]