Abstract
Ischemia modified albumin (IMA) and Protein Carbonyl (PC) have known as proteins that are modified on the similar basis of oxidative stress induced protein modification and may have diagnostic potential in acute myocardial infarction. This study aims to evaluate the ability of using IMA and PC content to diagnose Non-ST elevation myocardial infarction (NSTEMI) and efficiency of combining these two markers. Serum from NSTEMI and healthy control were determined for serum IMA and PC content. The results showed that both of serum IMA level and PC content in NSTEMI was significantly higher than that of healthy controls. However, the PC content showed greater diagnostic performance than IMA. Combinatorial determination of serum IMA level with PC content level was enhanced test efficiency. In conclusion, our finding demonstrated that IMA and PC content can be used as a diagnostic marker for NSTEMI.
Keywords: Acute myocardial infarction, Ischemia modified albumin, Protein carbonyl, NSTEMI
Introduction
Acute coronary syndrome (ACS) is a sequence of pathophysiologic processes in coronary arteries, myocardial ischemia and infarction [1]. Infarction resulted in loss the ability of the heart to work properly. The diagnosis of acute myocardial infarction (AMI) focus on clinical assessment such as history of chest pain associated with Electrocardiogram (ECG) changes, for instance ST-segment elevation (STEMI), and biochemical markers alterations [2]. However, lack of specificity of symptom and sensitivity of ECG becomes problematic for diagnosis and delay essential intervention. It has been suggested that the incidence of non ST-segment elevation myocardial infarction (NSTEMI) is higher than that of STEMI and that the mortality after 6 months is very similar [3]. The diagnosis of NSTEMI is more difficult than STEMI and therefore its prevalence is harder to estimate [3]. Assessment of cardiac biomarker level is the one of the most essential and effective way to detect myocardial damages. Current conventional cardiac markers e.g. Creatine Kinase-MB (CK-MB and cardiac Troponin T or I (cTnT, cTnI) are the sensitive and specific test for the detection of myocardial necrosis, but show a greater rise approximately 3–6 h after the onset of myocardial cell injury, and thus patients may wait before they are diagnosed and treated [4]. Moreover, the usual biomarkers may not rise during reversible myocardial ischemia, and other diagnostic tools (stress testing, echocardiography, etc.) are not routinely available [4]. Therefore, identification of the early cardiac biomarkers, which are actually reflect the early phase of cellular injury is extremely useful, in term of the more rapid diagnosis, as the more effectiveness of medical interventions and treatments.
It has been known that myocardial ischemia and/or ischemia–reperfusion injury claimed as an oxidative stress, which can generate Reactive Oxygen Species (ROS), subsequently resulted in biochemical modifications of some biomolecules such as protein, lipid, and DNA [5–7]. Ischemia-modified albumin (IMA) and Protein Carbonyl (PC) have known as proteins that are modified on the similar basis of oxidative stress induced protein modification [6, 8, 9]. In addition, these two proteins found to have high level in coronary artery diseases and thus having diagnostic potential for AMI [6, 10]. We aimed to evaluate the ability of using IMA and PC as cardiac markers for diagnosis of NSTEMI and capable of combining these two markers to increase test efficiency.
Materials and Methods
Study Population
We assessed 33 patients who were admitted to the Coronary Care Unit of Buddhachinaraj Hospital, Phitsanulok, Thailand, with chest pain and final diagnosis as NSTEMI according to the electrocardiogram showed non-ST elevation. All patients had high level of serum cardiac troponin T and CK-MB activity above reference value (cTnT > 0.01 ng ml and CK-MB activity > 25 U/l). Patients who had diabetes, liver diseases, renal diseases, will not be recruited in this study. Similarly, smoking behavior, taking antioxidant supplements were considered as exclusion criteria. Another thirty healthy volunteers were also recruited from healthy blood donors as control subjects. In this control group, all healthy blood donors were screened for history of any heart diseases, liver diseases, renal diseases, diabetes, smoking behavior, and taking antioxidant supplements. All subjects gave written informed consent before enrolling to this study, and the study protocol was approved by the Naresuan University ethical clearance committee on human rights related to research involving human subjects.
Sample Collection and Preparation
The whole blood from healthy donors and patients were collected and spin down for serum collection. All specimen collection processes were performed within 2 h after blood collection. The serum samples were frozen at −20°C or lower until the experiments were performed.
Spectrophotometric Determination of Serum Ischemia Modified Albumin
A rapid, spectrophotometric Albumin Cobalt Binding (ACB) test for determining serum IMA was developed from Bar-Or et al. [11]. Two hundred microliter of human serum were incubated with 50 μl of 0.1% cobalt chloride (Sigma, CoCl2·6H2O) in H2O for 10 min at room temperature for adequate cobalt-albumin binding. Fifty microliters of dithiothreitol (DTT) (Sigma, 1.5 mg/ml H2O) was added for colorizing the reaction for 2 min before quenching with 1.0 ml of 0.9% NaCl. Then, the absorbance were measured at 470 nm (Shimadzu, model UV160U), color development with DTT was compared to a serum-cobalt blank without DTT and reported in absorbance units (ABSU). The within-run coefficient of variation (CV) was 1.00% and between-day coefficient of variation (CV) was 4.75%. The linearity regression of the test in both standard albumin, and pooled serum were 0.9274 and 0.9524, respectively.
Spectrophotometric Determination of Serum Protein Carbonyl
Serum Protein Carbonyl (PC) content was spectrophotometrically determined by colorimetric DNPH assay. Basically, protein carbonyl (PC) react with 2,4-dinitrophenylhydrazone (DNPH) producing a Schiff base that subsequently produce a corresponding hydrazone, which can be measured spectrophotometrically [12, 13]. The serum was diluted in 1:10 with phosphate-buffered saline (PBS) to the final concentration of total protein less than 10 mg/ml. Two hundred microliter of diluted serum were mixed with 800 μl of 10 mM DNPH in 2.5 M HCl. Sample control were also prepared by adding equal volume of diluted serum to 800 μl of 2.5 M HCl. Then, the reactions were incubated at room temperature for 1 h, the dark environment with 15 min interval of vortexing. One milliliter of 20% (w/v) Trichloroacetic acid (TCA) was added before incubating on ice for 5 min. After centrifugation at 10,000×g for 10 min at 4°C, the supernatants were collected and mixed with 1 ml of 10% (w/v) TCA before spinning down at 10,000×g for 10 min at 4°C. The protein pellet was washed for 3 times with 1 ml of 1:1 (v/v) ethanol: ethyl acetate and centrifuged at 10,000×g for 10 min at 4°C. After final washing, the protein pellet was resuspended in 500 μl of 6 M guanidine hydrochloride and centrifuged at 10,000×g for 5 min at 4°C. The supernatant were collected and measured the absorbance at 370 nm using control as a blank. The protein carbonyl concentration (nmol/ml) was calculated from the Eq. 1. The total corrected protein amount (mg), in the final step of reaction, was calculated from a bovine serum albumin standard curve dissolved in 6 M guanidine hydrochloride and read at 280 nm. The protein amount was calculated using Eq. 2. Then the carbonyl content (nmol/mg) was calculated from protein carbonyl concentration per amount of corrected protein. The within-run coefficient of variation (CV) was 7.93% and between-day coefficient of variation (CV) was 7.47%. The linearity regression of the test using standard albumin was 0.9670.
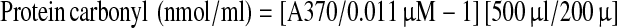 |
1 |
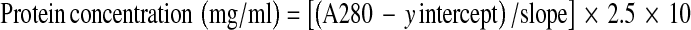 |
2 |
Statistical Analysis
Data were analyzed by the GraphPad Prism version 5.00. Differences of IMA and PC level between groups were analyzed by unpaired t test or Mann–Whitney U test. A P value < 0.05 was considered to be statistically significant. The receiver operating characteristic (ROC) curve analysis and the area under the curve was performed for determination of serum IMA and PC in the all NSTEMI patients included in the study population. The optimum cutoff values for determination of serum IMA and PC were selected from the ROC analysis. This optimum cutoff was used to dichotomously classify positive or negative serum IMA and PC level, and used for calculating of diagnostic sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic efficiency of the test.
Results
Baseline Characteristic
The baseline characteristics of all subjects in the study were summarized as shown in Table 1. The average age of subjects in NSTEMI were approximately 66 years old and was significantly higher than the average age of healthy control (P < 0.05).
Table 1.
Baseline characteristic of healthy controls and NSTEMI patients
| Variable | Controls (n = 33) | NSTEMI (n = 33) | P value |
|---|---|---|---|
| Age (years) | 35.61 ± 11.02 | 66.30 ± 10.50 | <0.0001 |
| Gender (men/women) | 14/16 | 19/14 | – |
| CK-MB (U/l) | NA | 60.28 ± 10.09 | – |
| cTnT (ng/ml) | NA | 0.92 ± 1.67 | – |
| IMA (ABSU) | 0.4713 ± 0.08 | 0.5377 ± 0.09 | 0.0045 |
| Protein carbonyl content (nmol/mg) | 0.3895 ± 0.07 | 0.526 ± 0.14 | <0.0001 |
Diagnostic Efficiency of Using IMA for Diagnosis of Myocardial Infarction
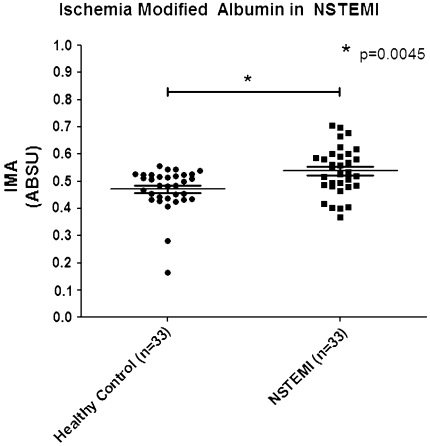
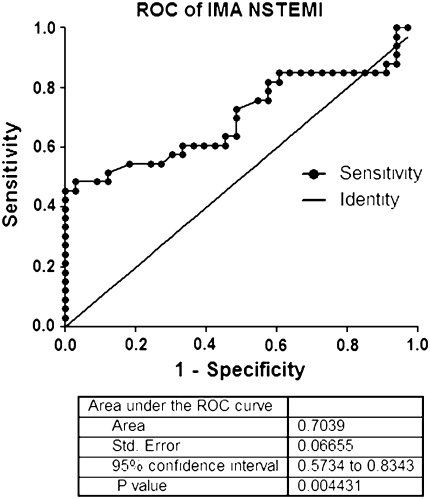
Serum IMA level in NSTEMI showed significantly higher than that of healthy controls (mean serum IMA = 0.5377 ± 0.99 vs. 0.4713 ± 0.08) (Table 1; Fig. 1). The area under ROC curve of ABC test for determining serum IMA level in NSTEMI patients was 0.7039 (95% CI, 0.5734–0.8343) (Fig. 2). The optimum decision level for IMA was determined to be 0.5330 ABSU and this decision limit was used in subsequent analyses for diagnostic performance, which was calculated for diagnostic sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and test efficiency. The performance of ABC test for diagnosis NSTEMI showed yield of sensitivity of 51.52% (95% CI, 33.54–69.20), a specificity of 87.88% (95% CI, 71.80–96.60) (Table 2), a PPV of 80.95%, a NPV of 64.44%, and a test efficiency of 69.70% (Table 3).
Fig. 1.
Level of serum ischemia modified albumin in NSTEMI compared with healthy control. Inside the scatter plot represent the mean and standard deviation. Comparison of serum IMA level is performed using Mann–Whitney test. The mean of serum IMA level in NSTEMI was 0.5377 ± 0.09 ABSU, which is significantly higher than that of healthy controls (P = 0.0045)
Fig. 2.
Receiver Operator Characteristic curve (ROC) of ischemia modified albumin (IMA) for detecting NSTEMI. The area under the ROC = 0.7039 (95% Confidence Intervals: 0.5734–0.8343). Best IMA cut-off point: 0.5300 ABSU (sensitivity: 51.52%; specificity: 87.88%)
Table 2.
Diagnostic sensitivity and specificity of measuring serum IMA and PC content in NSTEMI
| Markers | Cutoff | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI |
|---|---|---|---|---|---|
| IMA (ABSU) | 0.5300 | 51.52 | 33.54–69.20 | 87.88 | 71.80–96.60 |
| PC (nmol/mg) | 0.4930 | 57.58 | 39.22–74.52 | 93.94 | 79.77–99.26 |
Table 3.
Diagnostic efficiency of measuring serum IMA, PC content, and combination of IMA and PC in NSTEMI
| PPV (%) | NPV (%) | Efficiency (%) | |
|---|---|---|---|
| IMA | 80.95 | 64.44 | 69.70 |
| PC | 90.00 | 67.40 | 74.24 |
| IMA + PC | 77.42 | 74.29 | 75.75 |
Diagnostic Efficiency of Using PC Content for Diagnosis of Myocardial Infarction
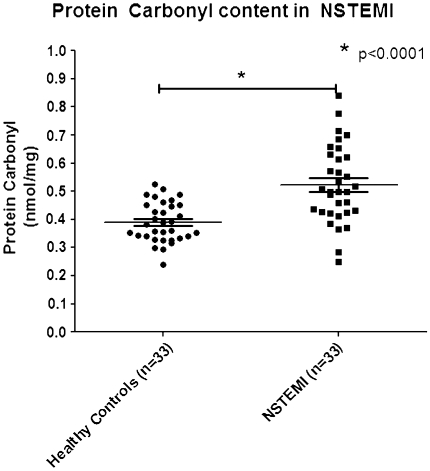
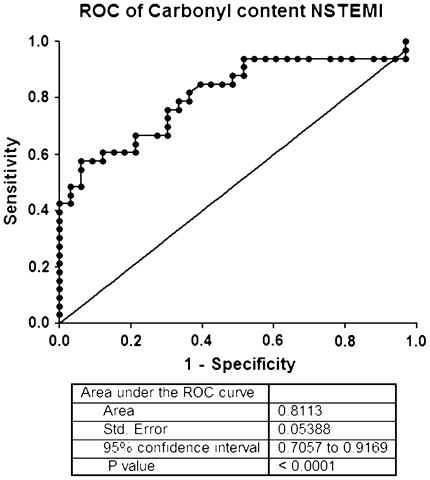
The results showed that the level of serum PC content in NSTEMI was 0.526 ± 0.14 nmol/mg, respectively. Serum PC level in NSTEMI showed significantly higher than that of healthy controls (0.3895 ± 0.078) (Table 1; Fig. 3). Analysis of ROC curve showed that the area under ROC curve of determining PC content in NSTEMI patients was 0.8113 (95% CI, 0.7057–0.9169). The optimum decision level for PC content was calculated at 0.4930 nmol/mg (Table 2). Calculation of the test performance showed the performance of spectrophotometric DNPH assay for diagnosis NSTEMI showed yield of sensitivity of 57.58% (95% CI, 39.22–74.52), a specificity of 93.94% (95% CI, 79.77–99.26) (Table 2), a PPV of 90.00%, a NPV of 67.40%, and a test efficiency of 74.24% (Table 3; Fig. 4).
Fig. 3.
Level of serum Protein Carbonyl (PC) content in NSTEMI compared with healthy controls. Inside the scatter plot represent the mean and standard deviation. Comparison of serum PC content level is performed using unpaired t test. The mean of serum PC content level in NSTEMI was 0.526 ± 0.14 nmol/mg, which is significantly higher than that of healthy controls (P < 0.0001)
Fig. 4.
Receiver Operator Characteristic curve (ROC) of Protein Carbonyl (PC) content for detecting NSTEMI. The area under the ROC = 0.8113 (95% Confidence Intervals: 0.7057–0.9169). Best PC cut-off point: 0.4930 nmol/mg (sensitivity: 57.58%; specificity: 93.94%)
Combination of IMA and PC Content Level Enhanced Test Efficiency
The efficiency of the test for measuring serum IMA level in combination with serum PC content level was calculated as showed in Table 3. Combination of determining IMA and PC content in NSTEMI showed a PPV of 77.41%, a NPV of 74.29%, and a test efficiency of 75.75% (Table 3).
Discussion and Conclusion
In the present study we demonstrated the ability of two oxidative modified proteins, ischemia modified albumin (IMA) and protein carbonyl (PC), as a potent cardiac biomarkers for diagnosis of NSTEMI and hypothesized that measuring the level of serum IMA in combination with the level of serum PC content can improve the efficiency of diagnosis NSTEMI.
Generation of reactive oxygen species resulted in modification of some biomolecules such as lipid, proteins and nucleic acid. The process of protein oxidation introduces new functional groups such as hydroxyls and carbonyls group [14, 15], which generated by many different mechanisms, which can then indicate the degree of ROS mediated protein oxidation contribute to altered function and turnover [14, 15]. The outcomes of protein oxidation are fragmentation, cross-linking and unfolding may accelerate or hinder proteolytic and proteosome-mediated turnover, according to the severity of oxidative damage. IMA and PC were found to be modified according to oxidative stress and also in many oxidative related disorders such as myocardial ischemia, renal ischemia [5, 6, 11, 16–18]. According to this, there are many studies demonstrate the ability of these two proteins as early biomarkers. For IMA, N-terminal modifications resulted in reduction to cobalt binding capacity of serum protein albumin [9]. Therefore, the albumin cobalt binding (ACB) test was developed to determine the level of serum IMA and was also approved by US Food and drug Administration (FDA) in 2003 as the diagnostic test for acute myocardial infarction [19]. However, the ACB test is not specific for myocardial ischemia. It also found that IMA can be increased in cerebral, gastrointestinal intestinal and skeletal muscular ischemia as well as myocardial ischemia [20]. Therefore, it is recommended that the interpretation of a positive IMA finding should be combined with other clinical indices [20].
Our study show that diagnosis of NSTEMI was not improved by combination of serum IMA and PC level, because of the poor sensitivity of these two biomarkers. We found that the sensitivity and negative predictive value (NPV) of IMA measurements were lower than in other studies. This lack of sensitivity and low NPV also found in PC content determination. However, it seems that individual determination of serum PC content showed a good area under ROC curve and high PPV for NSTEMI diagnosis, suggesting that the level of PC content capable of using as diagnostic biomarkers. However, the area under curve of ROC curve of IMA was not good enough and might not be a good candidate for diagnosis of myocardial infarction. The lack of diagnostic potential of IMA was also mentioned in many studies [1, 21, 22]. However, our results showed that combinatorial determination of these two biomarkers gave not good enough for NSTEMI diagnosis as it, although it slightly increased NPV and test efficiency. The disability of IMA to diagnosis patients with NSTEMI is also found in some studies. Wudkowska et al. [1] suggested that the IMA concentration does not differentiate Acute Coronary Syndrome (ACS) patients without ST segment elevation myocardial infarction from patients with unstable angina. Moreover, Charpentier et al. [4] also demonstrated in a large cohort study of patients admitted to an emergency department for chest pain that IMA did not provide valuable information for ACS diagnosis. The possible explanation is NSTEMI patients did not have major myocardial necrosis, unlike in patients with STEMI. Therefore, the minor myocardial damage possibly has less degree of ROS mediated protein oxidation.
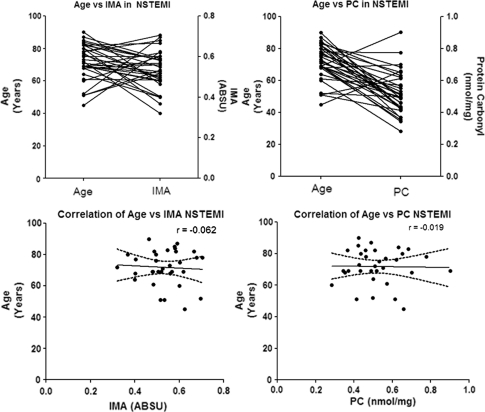
This study has some limitations. There is a significant different in patients and healthy subjects’ age. Although the age of NSTEMI patients were not statistically different, but the age of patients was significantly differed compared to healthy controls. It has been reported that oxidative modified forms of proteins accumulate during aging [15]. For example, increases in protein carbonyls occur in rat hepatocytes, drosophila, brain, and kidney of mice and in brain tissue of gerbils [23]. In humans protein carbonyls increase with age in brain, muscle, and human eye lens [23]. The carbonyl content of human fibroblasts also increases as a function of age of the donor [23]. However, in our study, age different between NSTEMI and healthy controls were not affected with the interpretation of serum IMA level and PC content level (Fig. 5). There were no correlation between age and the level of serum IMA and PC content which similar to the findings from Aparci et al. [24] that the relation of albumin cobalt binding capacity test results is independent to the advanced age.
Fig. 5.
Correlation of age and serum IMA, PC content level in NSTEMI. There are no correlation between age and serum IMA, PC content level in NSTEMI, r = −0.062 and −0.019, respectively
In conclusion, our finding demonstrated that determination of IMA is not good diagnostic marker for NSTEMI. However, measurement of PC content is benefit as a diagnostic marker for NSTEMI. In addition, determination of IMA level in combination with PC content showed slightly enhance diagnostic efficiency of NSTEMI, suggesting that measuring the serum level PC has potential as a diagnostic marker for NSTEMI.
Acknowledgment
This work was supported by Naresuan University Research Fund (R2553C076) and Faculty of Allied Health Science Postgraduate Research Studentship for Kritsanee Maneewong.
References
- 1.Wudkowska A, Goch J, Goch A. Ischemia-modified albumin in differential diagnosis of acute coronary syndrome without ST elevation and unstable angina pectoris. Kardiol Pol. 2010;68(4):431–437. [PubMed] [Google Scholar]
- 2.Johanson P, Wagner GS, Dellborg M, Krucoff MW. ST-segment monitoring in patients with acute coronary syndromes. Curr Cardiol Rep. 2003;5(4):278–283. doi: 10.1007/s11886-003-0063-7. [DOI] [PubMed] [Google Scholar]
- 3.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28(13):1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier S, Ducasse JL, Cournot M, Maupas-Schwalm F, Elbaz M, Baixas C, et al. Clinical assessment of ischemia-modified albumin and heart fatty acid-binding protein in the early diagnosis of non-ST-elevation acute coronary syndrome in the emergency department. Acad Emerg Med. 2010;17(1):27–35. doi: 10.1111/j.1553-2712.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 5.Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, Tate J, et al. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem. 2005;51(5):810–824. doi: 10.1373/clinchem.2004.046292. [DOI] [PubMed] [Google Scholar]
- 6.Pantke U, Volk T, Schmutzler M, Kox WJ, Sitte N, Grune T. Oxidized proteins as a marker of oxidative stress during coronary heart surgery. Free Radic Biol Med. 1999;27(9–10):1080–1086. doi: 10.1016/S0891-5849(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 7.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Or D, Rael LT, Lau EP, Rao NK, Thomas GW, Winkler JV, et al. An analog of the human albumin N-terminus (Asp-Ala-His-Lys) prevents formation of copper-induced reactive oxygen species. Biochem Biophys Res Commun. 2001;284(3):856–862. doi: 10.1006/bbrc.2001.5042. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Or D, Winkler JV, Vanbenthuysen K, Harris L, Lau E, Hetzel FW. Reduced albumin-cobalt binding with transient myocardial ischemia after elective percutaneous transluminal coronary angioplasty: a preliminary comparison to creatine kinase-MB, myoglobin, and troponin I. Am Heart J. 2001;141(6):985–991. doi: 10.1067/mhj.2001.114800. [DOI] [PubMed] [Google Scholar]
- 10.Govender R, De GJ, Delport R, Becker PJ, Vermaak WJ. Biological variation of ischaemia-modified albumin in healthy subjects. Cardiovasc J Afr. 2008;19(3):141–144. [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—a preliminary report. J Emerg Med. 2000;19(4):311–315. doi: 10.1016/S0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 12.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 13.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/S0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 14.Le-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 16.Kiyici A, Mehmetoglu I, Karaoglan H, Atalay H, Solak Y, Turk S. Ischemia-modified albumin levels in patients with end-stage renal disease patients on hemodialysis: does albumin analysis method affect albumin-adjusted ischemia-modified albumin levels? J Clin Lab Anal. 2010;24(4):273–277. doi: 10.1002/jcla.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turedi S, Cinar O, Yavuz I, Mentese A, Gunduz A, Karahan SC, et al. Differences in ischemia-modified albumin levels between end stage renal disease patients and the normal population. J Nephrol. 2010;23(3):335–340. [PubMed] [Google Scholar]
- 18.Melanson SF, Tanasijevic MJ. Laboratory diagnosis of acute myocardial injury. Cardiovasc Pathol. 2005;14(3):156–161. doi: 10.1016/j.carpath.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Van BE, Dallongeville J, Vicaut E, Degrandsart A, Baulac C, Montalescot G. Ischemia-modified albumin levels predict long-term outcome in patients with acute myocardial infarction. The French Nationwide OPERA study. Am Heart J. 2010;159(4):570–576. doi: 10.1016/j.ahj.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Shen XL, Lin CJ, Han LL, Lin L, Pan L, Pu XD. Assessment of ischemia-modified albumin levels for emergency room diagnosis of acute coronary syndrome. Int J Cardiol 2010. doi:10.1016/j.ijcard.2010.01.013. [DOI] [PubMed]
- 21.Kim JH, Choi JH, Lee HK, Bae WH, Chun KJ, Kim YS, et al. Ischemia-modified albumin (IMA) is not useful for detecting myocardial ischemia during symptom-limited exercise stress tests. Korean J Intern Med. 2008;23(3):121–126. doi: 10.3904/kjim.2008.23.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin RM, Fatovich DM, Grasko JM, Vasikaran SD. Ischaemia modified albumin cannot be used for rapid exclusion of acute coronary syndrome. Emerg Med J. 2010;27(9):668–671. doi: 10.1136/emj.2009.082693. [DOI] [PubMed] [Google Scholar]
- 23.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32(9):797–803. doi: 10.1016/S0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 24.Aparci M, Kardesoglu E, Ozmen N, Ozcan O, Cebeci BS, Cingozbay BY, et al. Prognostic significance of ischemia-modified albumin in patients with acute coronary syndrome. Coron Artery Dis. 2007;18(5):367–373. doi: 10.1097/MCA.0b013e3281689a50. [DOI] [PubMed] [Google Scholar]