Abstract
Background
The current standard of care for cervical myopathy is anterior discectomy and fusion (ACDF). Although well tolerated in the short term, this treatment might ultimately result in progressive degeneration of adjacent motion segments. Artificial disc arthroplasty offers the theoretical advantage of preservation of motion at the operative level with consequent stress reduction at adjacent levels.
Questions/purposes
We compared function, radiographic measures, and incidence of complications at 3-year followup after cervical disc arthroplasty with the Bryan® prosthesis and ACDF in patients with cervical myelopathy.
Patients and Methods
Eighty-three patients with cervical myelopathy were randomized to undergo arthroplasty with implantation of a Bryan® cervical disc prosthesis (n = 41) or ACDF (n = 42). Patients were assessed preoperatively to 3 years postoperatively using the modified Odom’s criteria, Japanese Orthopaedic Association scale, SF-36, and Neck Disability Index. ROM, stability, and subsidence of the prostheses were evaluated radiographically.
Results
Patients who received the Bryan® prosthesis scored significantly better in three of the four functional assessment methods used (Japanese Orthopaedic Association scale, SF-36, and Neck Disability Index). ROM was retained by the patients in the Bryan® group but not in the patients in the ACDF group. Patients in the Bryan® group had fewer complications, primarily because dysphagia occurred in only one patient in the Bryan® group but in seven patients in the ACDF group. Other complications included pseudarthrosis in three patients in the ACDF group and one patient had spontaneous fusion, one had deep vein thrombosis, and one had heterotopic ossification in the Bryan® group.
Conclusions
Bryan® cervical disc arthroplasty appears reliable and effective in the treatment of cervical myelopathy.
Level of Evidence
Level II, therapeutic study. See the Guidelines for Authors for a complete description of the levels of evidence.
Introduction
Cervical myelopathy is a condition in which the spinal cord becomes compressed as the result a herniated disc, sometimes accompanied by bone spurs in the spinal canal. It commonly develops in patients older than 50 years, and its symptoms are attributable to impaired motor and sensory function. Indications for intervention in cervical myelopathy are usually spinal cord compression causing progressive neurologic deficits [13]. The current standard of care for cervical myelopathy is ACDF [3]. Although well tolerated in the short term, ACDF might ultimately result in progressive degeneration of the adjacent motion segments [4]. In vivo kinematic analysis has revealed, after ACDF, the superior adjacent level exhibits increased angular motion compared with the preoperative ROM [18].
Compared with cervical fusion, artificial disc arthroplasty offers the theoretical biomechanical advantage of preservation of motion at the operative level with consequent stress reduction at the adjacent levels [5]. The Bryan® cervical disc prosthesis (Medtronic Sofamor Danek, Memphis, TN, USA) is a one-piece, biarticulating, metal-on-polymer (titanium alloy on polyurethane), unconstrained device with a fully variable instantaneous axis of rotation that is not dependent on supplemental fixation [1]. A unique polyurethane sheath surrounds the nucleus forming a barrier to contain any wear debris and prevent soft tissue ingrowth that may reduce the ROM of the device. Initial stability is achieved by precision milling of the vertebral endplates, and long-term stability is provided by bone growth into the porous-coated titanium alloy endplates, which are attached to the vertebral endplates [1]. The device has been used in the treatment of cervical radiculopathy and has yielded acceptable clinical results [10, 11, 21]. The Bryan® prosthesis might delay adjacent-level degeneration by preserving preoperative kinematics at the adjacent levels [20].
Previous studies have shown arthroplasty with implantation of the Bryan® disc prosthesis to compare favorably with discectomy and fusion, but studies have been small [8, 19] or had only a 2-year or shorter followup [10, 19, 21]. One of the most important considerations in disc surgery, however, is the possibility of long-term complications. We therefore compared function outcome, radiographic measures (ROM, stability, and subsidence), and incidence of complications of arthroplasty with the Bryan® cervical disc prosthesis and ACDF in cervical myelopathy at 3 years of followup.
Patients and Methods
In this prospective, randomized, controlled study, 83 patients with cervical myelopathy who sought treatment from December 2004 to September 2006 at our institution were randomly assigned in a 1:1 ratio using the random number table method to receive an arthroplasty with implantation of a Bryan® prosthesis or ACDF with an iliac crest autograft and plate (Table 1). The inclusion criteria were intractable cervical myelopathy attributable to disc herniation or stenosis at one, two, or three levels from C3–C4 to C6–C7 and failed nonoperative management for 12 weeks. Patients requiring immediate treatment were not required to meet the inclusion criteria. Immediate treatment was provided if the patients had (1) significant clinical symptoms and unbearable pain, (2) a definite sign of cervical disc prolapse discovered by radiographic examination, and (3) progressive neural damage. Patients were excluded if they had significant anatomic deformity (eg, ankylosing spondylitis); received a previous cervical procedure; had a spinal infection; or had severe osteoporosis, cervical kyphosis, ankylosing spondylitis, ossification of the posterior longitudinal ligament of the spine, or severe spondylosis (defined as bridging osteophytes) based on preoperative radiographs. Patients also were excluded if they had substantial facet disease or showed no preserved motion on preoperative flexion-extension radiographs. The study protocol was approved by our institutional review board, and all patients provided written informed consent for participation in the study.
Table 1.
Patient demographic and disease characteristics
| Variable | Bryan® group (n = 41) |
ACDF group (n = 42) |
p Value |
|---|---|---|---|
| Age (years)* | 47.2 ± 5.7 | 47.7 ± 5.8 | 0.656† |
| Gender | 1.000 | ||
| Male | 21 (51.2) | 23 (54.8) | |
| Female | 20 (48.8) | 19 (45.2) | |
| Smokers | 6 (14.6) | 8 (17.6) | 0.775 |
| Preoperative NDI* | 50.6 ± 6.0 | 50.1 ± 5.8 | 0.666† |
| Preoperative SF-36 (physical component)* | 35.7 ± 4.3 | 35.3 ± 4.3 | 0.653† |
| Preoperative JOA* | 9.0 ± 1.4 | 8.9 ± 1.4 | 0.942† |
* Data are displayed as mean ± SD; all other data are displayed as number of patients, with percentage in parentheses; †p values are based on independent two-sample t test; all other p values are based on chi square test; ACDF = anterior cervical discectomy and fusion; NDI = neck disability index; JOA = Japanese Orthopaedic Association scale.
After confirming with the patients that they would receive the operation, they were randomized using a random numbers table and notified regarding which group they had been classified. Blinding was impossible because the patients needed to know which surgery they would receive. There were 21 men and 20 women in the Bryan® group (n = 41) and 23 men and 19 women in the ACDF group (n = 42). No differences in demographic characteristics such as age, smoking status, and gender were present between the two groups (p > 0.05). One-level replacements were performed in 24 patients in the Bryan® group and 21 in the ACDF group. Two-level replacements were performed in 14 patients in the Bryan® group and 17 in the ACDF group. Three-level replacements were performed in three patients in the Bryan® group and four in the ACDF group. All patients were followed for 3 years, except for two patients in the ACDF group who were lost to followup.
For patients who received the Bryan® prosthesis, a transverse incision was made in a skin crease at the appropriate disc level on the right side of the neck. After exposure of the disc space, table-mounted retractors were used to provide stability and observation for insertion of the milling guide. Discectomy then was performed and the disc space distracted. The guide was fixed to the vertebral bodies above and below the distracted disc at the appropriate sagittal angle (perfectly parallel to the disc space), as measured by preoperative fluoroscopy. After a final check of the correct size, the endplates were smoothened with a burr and then machined with a milling tool to match the size and contour of the prosthesis. The guide was removed while the distractor pins were left in place. The spinal canal and neuroforamen then were decompressed. Substantial hypertrophic uncovertebral joint spurs, if present, were removed. The posterior longitudinal ligament was resected as needed for decompression. The Bryan® disc prosthesis was filled with normal saline and a seal plug was screwed into the center port of the prosthesis shells. The prosthesis then was placed gently into the milled interspace. The distractor was removed and final fluoroscopic images were obtained. A second prosthesis was placed using the same procedure when correction of two or three levels was required. The goals of the surgery were to achieve adequate decompression and to place as large a Bryan® disc prosthesis as possible in the proper orientation. Patients used braces for 1 week and were allowed to gently move their heads during normal daily activities but were told to avoid further physical efforts for 3 months.
For patients who received ACDF with an iliac crest autograft and plate, a transverse incision was made and the anterior aspect of the cervical spine was reached using optical magnification and by passing medially from the carotid sheath and laterally from the esophagus and trachea. After the correct level was located, the anterior longitudinal ligament was cut and the intervertebral disc was excised. The spinal canal and neuroforamen were decompressed. Finally, cartilage was removed from the endplates and an appropriately sized iliac crest autograft was placed in the interspace. Patients underwent anterior cervical plating with the Orion® cervical plate system (Medtronic Sofamor Danek) and wore a hard collar postoperatively for 3 months. In both groups, NSAIDs were not routinely administered postoperatively.
Demographic and outcome data were collected. Clinical assessments including physical and neurologic examinations, functional outcome assessments, and self-evaluation of neck pain were made preoperatively and at 1 and 6 weeks, 3, 6, and 12 months, and 2 and 3 years postoperatively. These evaluations were performed by physicians who were not involved in the surgery, but they were not blinded regarding which type of surgery had been performed. The patients were evaluated using the modified Odom’s criteria [17], the SF-36, and the Japanese Orthopaedic Association (JOA) scale. The patients evaluated their neck pain using the Chinese version of the Neck Disability Index (NDI), which has a Cronbach α coefficient of 0.89 [26]. Radiographic evaluation by an independent radiologist included AP and static and dynamic flexion-extension lateral images with the patient in the standing position, performed preoperatively and postoperatively at 1 week, 3, 6, and 12 months, and 2 and 3 years. Preoperative and postoperative angular motion at the target level was evaluated. Radiographic measurements were made by three independent observers. Each of them performed each radiographic measurement twice, and the mean of a total of six measurements was used for analysis. Device stability also was evaluated. Preoperative and postoperative lateral radiographs in the neutral position were evaluated for sagittal alignment in all patients. Preoperative MRI and CT were performed in all patients to determine the cause of cervical myelopathy.
Continuous data are expressed as mean ± SD, whereas categorical data are represented by numbers and percentages. Repeated-measures ANOVA was used to assess the significance of changes from preoperative to postoperative in the NDI, JOA, and SF-36 scores. The change in motion scores at the target level was tested using the paired t test. Statistical comparisons between the two groups were performed using chi square tests/Fisher’s exact tests for categorical variables and independent two-sample t tests for continuous variables. A p value of 0.05 was used to define statistical significance. All statistical analyses were performed using SPSS® 11.5 software (SPSS Inc, Chicago, IL, USA).
Results
The mean surgical blood loss was lower (p < 0.001) in patients in the Bryan® group (100.2 ± 29.4 mL) than for patients in the ACDF group (150.2 ± 41.2 mL) (Table 2). The mean surgical time was higher (p < 0.001) for patients in the Bryan® group (132.4 ± 16.8 minutes) than for patients in the ACDF group (115.1 ± 19.1 minutes). Patients in the Bryan® group returned to work 2 months earlier (p < 0.01) than patients in the ACDF group (median time for return to work, 20 days for patients in the Bryan® group versus 84 days for patients in the ACDF group).
Table 2.
Operative and postoperative characteristics of patients
| Variable | Bryan® group (n = 41) | ACDF group (n = 42) | p Value |
|---|---|---|---|
| Surgical blood loss (mL)* | 100.2 ± 29.4 | 150.2 ± 41.2 | < 0.001† |
| Surgical time (minutes)* | 132.4 ± 16.8 | 115.1 ± 19.1 | < 0.001† |
| Fusion rate | 1 (2.4) | 38 (90.5) | < 0.001‡ |
| Dysphagia | 1 (2.4) | 7 (16.7) | 0.057§ |
| Fusion level | 0.989§ | ||
| C3/4 | 4 (9.8) | 3 (7.1) | |
| C4/5 | 6 (14.6) | 5 (11.9) | |
| C5/6 | 10 (24.4) | 9 (21.5) | |
| C6/7 | 4 (9.8) | 4 (9.5) | |
| 2 levels | 14 (34.1) | 17 (40.5) | |
| 3 levels | 3 (7.3) | 4 (9.5) |
* Data are displayed as mean ± SD; all other data are displayed as number of patients, with percentage in parentheses; †p values are based on independent two-sample t test; ‡p values are based on chi square test; §p values are based on Fisher’s exact test; ACDF = anterior cervical discectomy and fusion.
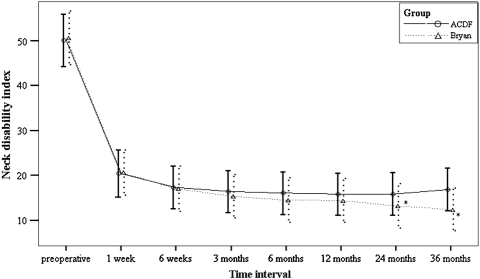
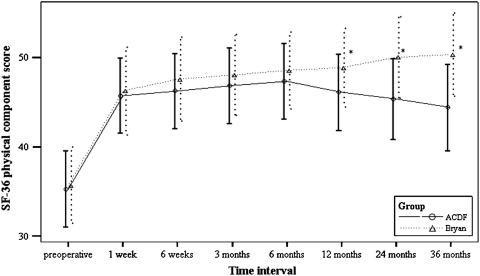
At 3-year followup, functional outcome assessments suggested better overall function for patients in the Bryan® group. Patients in the Bryan® group scored substantially better than the ACDF group in three of the four functional assessment methods used. Using the modified Odom’s criteria, patients in the Bryan® group had better results than patients in the ACDF group (58.5% excellent, 34.1% good, 7.3% fair versus 58.5% excellent, 25% good, 15% fair, 5% poor), but this difference did not reach statistical significance. Patients in the Bryan® group did have significantly better scores for the other three measures of functional outcome: NDI (p < 0.001) (Fig. 1), SF-36 (p < 0.05) (Fig. 2), and JOA (p = 0.016) (Fig. 3).
Fig. 1.
A graph shows NDI scores preoperatively and at 1 and 6 weeks, 3, 6, and 12 months, and 2 and 3 years postoperatively. * p < 0.05.
Fig. 2.
A graph shows SF-36 physical component scores preoperatively and at 1 and 6 weeks, 3, 6, and 12 months, and 2 and 3 years postoperatively. * p < 0.05.
Fig. 3.
A graph shows JOA scores preoperatively and at 1 and 6 weeks, 3, 6, and 12 months, and 2 and 3 years postoperatively. * p < 0.05.
ROM was retained by patients in the Bryan® group but not by patients in the ACDF group at 3-year followup. The Bryan® group retained an average (average of all operative segments) flexion-extension of 7.4° ± 0.5° at 3 years, which was not different from the preoperative measurements (p > 0.05). In contrast, the average ROM of the fused segments in the ACDF group was 0.6° ± 0.2° at 3 years, significantly different from the preoperative measurements (p < 0.01), which were similar in both groups. In addition, there was a difference in flexion-extension between the ACDF and Bryan® groups (p < 0.01). Radiographic analysis found no evidence of prosthesis subsidence or excursion in the Bryan® group.
The Bryan® group had fewer complications than the ACDF group (Table 2). This was primarily because dysphagia occurred in only one patient in the Bryan® group but in seven patients in the ACDF group (p < 0.001). The other complication seen in the ACDF group was pseudarthrosis (three of 42; 7.1%). Other complications in the Bryan® group were spontaneous fusion (one), deep vein thrombosis (one), and heterotopic ossification (one). No cases of device failure or explantations occurred in the Bryan® group. No intraoperative complications, including vascular or neurologic complications, were observed in either group. In addition, there was no leakage of cerebrospinal fluid or wound hematomas. No patients in either group required a second surgical procedure for the condition.
Discussion
The current standard of care for cervical myopathy is ACDF. Although well tolerated in the short term, this treatment might ultimately result in progressive degeneration of adjacent motion segments. Artificial disc arthroplasty offers the theoretical advantage of preservation of motion at the operative level with consequent stress reduction at adjacent levels. We therefore compared functional outcome, ROM, and incidence of complications at 3-year followup after the use of either artificial disc arthroplasty or ACDF in the repair of cervical myopathy. Our results showed arthroplasty (the Bryan® group) resulted in better functional outcome, patients retained the presurgery ROM, and had fewer side effects than patients who underwent ACDF.
Limitations of this study are the relatively small number of patients, the fact that blinding could not be performed because bone grafting was used, and that we did not use Nurick numbers [16]. At the time of the study, interbody structural cages were not yet used in China, so we could not use them. Also, outcome data 5 to 10 years after prosthesis implantation will be necessary for definitive observation of the advantages of disc arthroplasty in cervical myelopathy.
The 3-year followup results showed significantly better function for the Bryan® group in three tests of functional outcome, NDI, SF-36, and JOA. Marked improvement was seen in all three tests in both study groups 1 week after surgery. A difference in favor of the Bryan® group did not appear until 1 year postsurgery. Bryan® disc arthroplasty preserves the motion of the target segment, and this preserved motion could contribute to the better functional performance of this procedure over ACDF. However, Emery et al. reported overall cervical motion was increased on late followup evaluations [7]. This could also be a reason we did not observe the difference earlier.
A previous study [9] on disc arthroplasty for cervical spondylotic disease reported success rates of 86% and 90% at 6 months and 1 year, respectively, which included patients with excellent, good, or fair outcomes according to the modified Odom’s criteria. Our 3-year results, using the same criteria, were 100% for arthroplasty with implantation of the Bryan® disc prosthesis and 95% for ACDF. Sekhon [22], treating spondylotic myelopathy by arthroplasty with the Bryan® disc prosthesis, reported NDI scores improved by 41.5 points at a mean 18-month followup. Our results were similar, showing a 40-point decrease at 3 years. One of the potential criticisms of artificial discs is that, in the absence of fusion, there could be further pain. The postoperative NDI scores of our patients suggest this is not the case.
The NDI scores of our cohort and those of Heller et al. [11] were similar before surgery and up to 12 months after surgery. Although neck pain was more severe in patients in the study by Heller et al. than in ours, both studies used the NDI to assess neck pain, and pain perception is subject to cultural and ethnic differences [23, 24]. Therefore, our cohort might have a different threshold for pain perception and differences in pain toleration. However, to our knowledge, there has been no study on differences in pain perception between Chinese and other ethnic groups.
Our radiographic results showed 92.6% of the patients in the Bryan® group retained motion at the target segment at the 3-year followup, which compares favorably with the results of Lafuente et al. [15] where 91% of 42 patients had evidence of movement in the prosthesis at 1 year. Duggal et al. [6] studied 26 patients with radiculopathy and/or myelopathy treated with single-level or bilevel implantation of the Bryan® disc prosthesis with a mean followup of 12.3 months and found the Bryan® prosthesis provided in vivo functional spinal motion at the treated levels without compromising overall cervical spinal motion. Wigfield et al. [25] found, in all 15 patients with cervical radiculopathy and/or myelopathy who underwent arthroplasty with implantation of the Frenchay artificial disc prosthesis, the procedure maintained motion at the operative levels at 2-year followup.
Patients in the Bryan® group experienced fewer complications than patients in the ACDF group, largely owing to the dysphagia experienced by some patients in the ACDF group. In our patients, dysphagia was present in 16.5% of patients who received ACDF. Swallowing difficulties are the most common postoperative problem after ACDF, with a reported incidence ranging from 11% [27] to 24.2% [14]. Complications relating to bone grafting and plating might also occur. Emery et al. [7] reported a 6.9% rate of pseudarthrosis in 108 patients treated with anterior cervical decompression and arthrodesis for cervical spondylotic myelopathy. In our study, pseudarthrosis was present in 7.2% (three of 42) of the patients who received ACDF. However, ACDF can result in progressive degeneration of the adjacent segments [12], and long-term studies have shown a 25% prevalence of symptoms of adjacent segments within 10 years [2].
It is important to understand the appropriate indications of cervical disc arthroplasty for cervical myelopathy. The indications are similar to those for patients undergoing ACDF for degenerative disc disease associated with symptomatic nerve root and/or spinal cord compression. These include disc herniation, protrusion or prolapse of the nucleus pulposus, cervical foraminal stenosis, and myelopathy. Osteoporosis, severe facet joint arthrosis, ossification of the posterior longitudinal ligament, infection, cervical instability, and prior laminectomy are considered contraindications. In our view, huge osteophytes, especially lateral and posterior ones, are also a contraindication for arthroplasty.
We considered arthroplasty with implantation of the Bryan® disc prosthesis the preferred method for our patients because (1) it can achieve good anterior decompression, remove all sources of neural compression, restore lordosis, and increase disc height; (2) our patients were young, preservation of motion in the cervical spine segments is important for maintaining normal neck function, and disc arthroplasty can manage multilevel spinal cord compression and maintain motion while avoiding fusion and donor harvest-site complications; and (3) arthroplasty with implantation of the Bryan® disc prosthesis might protect the adjacent levels from late degeneration. Our patients’ clinical symptoms were caused by spinal cord compression resulting from cervical disc herniation. Thus, the patients were suitable to be treated by the anterior approach alone.
Our prospective, randomized trial compared disc arthroplasty using the Bryan® cervical disc prosthesis with ACDF in the treatment of cervical myelopathy. We showed arthroplasty with implantation of the Bryan® cervical disc prosthesis is effective and safe for the treatment of patients with cervical myelopathy and comparable to ACDF in improving the functional outcomes of patients 1 year and up to 3 years after surgery. Our data provide high-level quality of evidence supporting the safety and effectiveness of arthroplasty with implantation of the Bryan® disc prosthesis at 3 years of followup.
Acknowledgments
We thank Ben Liu and Yan Cai Yan for assistance with preparation of this manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Anderson PA, Sasso RC, Rouleau JP, Carlson CS, Goffin J. The Bryan Cervical Disc: wear properties and early clinical results. Spine J. 2004;4(6 suppl):303S–309S. doi: 10.1016/j.spinee.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Bridwell KH, Anderson PA, Boden SD, Vaccaro AR, Zigler JE. What’s new in spine surgery. J Bone Joint Surg Am. 2004;86:1587–1596. doi: 10.2106/00004623-200407000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Caspar W, Geisler FH, Pitzen T, Johnson TA. Anterior cervical plate stabilization in one- and two-level degenerative disease: overtreatment or benefit? J Spinal Disord. 1998;11:1–11. doi: 10.1097/00002517-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 4.DiAngelo DJ, Foley KT, Vossel KA, Rampersaud YR, Jansen TH. Anterior cervical plating reverses load transfer through multilevel strut-grafts. Spine (Phila Pa 1976) 2000;25:783–795. doi: 10.1097/00007632-200004010-00005. [DOI] [PubMed] [Google Scholar]
- 5.DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003;16:314–323. doi: 10.1097/00024720-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Duggal N, Pickett GE, Mitsis DK, Keller JL. Early clinical and biomechanical results following cervical arthroplasty. Neurosurg Focus. 2004;17:E9. doi: 10.3171/foc.2004.17.3.9. [DOI] [PubMed] [Google Scholar]
- 7.Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: two to seventeen-year follow-up. J Bone Joint Surg Am. 1998;80:941–951. doi: 10.1302/0301-620X.80B6.9517. [DOI] [PubMed] [Google Scholar]
- 8.Garrido BJ, Taha TA, Sasso RC. Clinical outcomes of Bryan cervical disc arthroplasty: a prospective, randomized, controlled, single site trial with 48-month follow-up. J Spinal Disord Tech. 2010;23:367–371. doi: 10.1097/BSD.0b013e3181bb8568. [DOI] [PubMed] [Google Scholar]
- 9.Goffin J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, Pointillart V, Calenbergh F, Loon J. Preliminary clinical experience with the Bryan Cervical Disc Prosthesis. Neurosurgery. 2002;51:840–845. doi: 10.1227/00006123-200209000-00048. [DOI] [PubMed] [Google Scholar]
- 10.Goffin J, Calenbergh F, Loon J, Cassey A, Kehr P, Liebig K, Lind B, Logroscino C, Sgrambiglia R, Pointillart V. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine (Phila Pa 1976) 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 11.Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, Hacker RJ, Coric D, Cauthen JC, Riew DK. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976) 2009;34:101–107. doi: 10.1097/BRS.0b013e31818ee263. [DOI] [PubMed] [Google Scholar]
- 12.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Hsu W, Dorsi MJ, Witham TF. Surgical management of cervical spondylotic myelopathy. Neurosurg Q. 2009;19:302–307. doi: 10.1097/WNQ.0b013e3181bd5f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung A, Schramm J, Lehnerdt K, Herberhold C. Recurrent laryngeal nerve palsy during anterior cervical spine surgery: a prospective study. J Neurosurg Spine. 2005;2:123–127. doi: 10.3171/spi.2005.2.2.0123. [DOI] [PubMed] [Google Scholar]
- 15.Lafuente J, Casey AT, Petzold A, Brew S. The Bryan cervical disc prosthesis as an alternative to arthrodesis in the treatment of cervical spondylosis: 46 consecutive cases. J Bone Joint Surg Br. 2005;87:508–512. doi: 10.1302/0301-620X.87B4.15436. [DOI] [PubMed] [Google Scholar]
- 16.Nurick S. The pathogenesis of spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. doi: 10.1093/brain/95.1.87. [DOI] [PubMed] [Google Scholar]
- 17.Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc. 1958;166:23–28. doi: 10.1001/jama.1958.02990010025006. [DOI] [PubMed] [Google Scholar]
- 18.Park DK, Lin EL, Phillips FM. Index and adjacent level kinematics after cervical disc replacement and anterior fusion: in vivo quantitative radiographic analysis. Spine (Phila Pa 1976) 2011;36:721–730. doi: 10.1097/BRS.0b013e3181df10fc. [DOI] [PubMed] [Google Scholar]
- 19.Riew KD, Buchowski JM, Sasso R, Zdeblick T, Metcalf NH, Anderson PA. Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. J Bone Joint Surg Am. 2008;90:2354–2364. doi: 10.2106/JBJS.G.01608. [DOI] [PubMed] [Google Scholar]
- 20.Sasso RC, Best NM. Cervical kinematics after fusion and Bryan disc arthroplasty. J Spinal Disord Tech. 2008;21:19–22. doi: 10.1097/BSD.0b013e3180500778. [DOI] [PubMed] [Google Scholar]
- 21.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Artificial disc versus fusion: a prospective, randomized study with 2-year follow-up on 99 patients. Spine (Phila Pa 1976. 2007;2941:2942. doi: 10.1097/BRS.0b013e31815d0034. [DOI] [PubMed] [Google Scholar]
- 22.Sekhon LH. Cervical arthroplasty in the management of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;17:E8. doi: 10.3171/foc.2004.17.3.8. [DOI] [PubMed] [Google Scholar]
- 23.Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med. 2000;62:517–523. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Weisenberg M, Caspi Z. Cultural and educational influences on pain of childbirth. J Pain Symptom Manage. 1989;4:13–19. doi: 10.1016/0885-3924(89)90059-6. [DOI] [PubMed] [Google Scholar]
- 25.Wigfield CC, Gill SS, Nelson RJ, Metcalf NH, Robertson JT. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine (Phila Pa 1976) 2002;27:2446–2452. doi: 10.1097/00007632-200211150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wu S, Ma C, Mai M, Li G. Translation and validation study of Chinese versions of the Neck Disability Index and the Neck Pain and Disability Scale. Spine (Phila Pa 1976) 2010;35:1575–1579. doi: 10.1097/BRS.0b013e3181c6ea1b. [DOI] [PubMed] [Google Scholar]
- 27.Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J. 2005;14:677–682. doi: 10.1007/s00586-004-0849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]