Abstract
Background
The treatment for an early postoperative periprosthetic infection after cementless THA that results in the highest quality of life after the control of infection is unknown. Although common treatments include irrigation and débridement with component retention, a one-stage exchange, or a two-stage exchange, it is unclear whether any of these provides a higher quality of life after the control of infection.
Questions/purposes
We projected, through decision-analysis modeling, the possible estimated final health states defined as health-related quality of life based on quality-of-life studies of an early postoperative periprosthetic infection after cementless THA treated by irrigation and débridement, one-stage exchange, or two-stage exchange.
Methods
Publications addressing early postoperative infections after THA were analyzed for the estimated rate of infection control and quality-of-life measures after a specific treatment. Decision analysis was used to model the different treatments and describe which, if any, treatment results in the greatest quality of life after early THA infection.
Results
In the model, a one-stage exchange was the treatment for early THA infection that maximized quality-of-life outcomes if the probability of controlling the infection exceeded 66% with this procedure. If the probability of infection control of a one-stage exchange was less than 66% or that of irrigation and débridement was greater than 60%, then irrigation and débridement appeared to result in the greatest quality-of-life outcome.
Conclusions
A decision analysis using estimates of infection control rate and quality-of-life outcomes after different treatments for an early postoperative infection after THA showed possible outcomes for each treatment.
Level of Evidence
Level II, economic and decision analyses. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA is commonly performed to alleviate pain and restore function in patients with end-stage degenerative arthritis of the hip [18]. Despite the documented improvements in function and quality of life after THA, periprosthetic infection occurs in 0.5% to 2% of patients [1, 12, 14], and remains difficult to control and one of the most devastating complications after THA [17, 20]. Although many protocols and techniques currently are used that reduce the incidence of periprosthetic infection, from early reports of 9% [6, 7] to current levels of 0.5% to 2% [4, 12, 19, 23, 27], a finite risk of acute postoperative infection remains.
The three commonly used surgical treatment options for controlling infection include (1) open irrigation and débridement (I & D) with component retention and exchange of the modular femoral head and acetabular liner (if present) [8, 14, 21, 34]; (2) a one-stage exchange in which, along with débridement of the hip, the prosthetic components are all removed and then replaced [5, 15, 25, 26, 35, 36]; and (3) a two-stage exchange (considered by many to be the gold standard treatment for an infected THA [10, 12–14, 16, 33, 37]) whereby the prosthetic components are all removed, an antibiotic-loaded cement spacer is placed, and then at a later date, prosthetic components are reinserted once the infection has been judged to be controlled [8, 12, 13, 34]. The rates of infection control for each of these procedures varies from 14% to 74% for I & D, 60% to 83% for one-stage exchange, and 82% to 93% for two-stage exchange [14]. However, there might be a trade-off between control of infection and substantial morbidity, mortality, and disruption of the lives of patients. Two-stage exchange might have a high rate of success in controlling a periprosthetic infection, but the cost to the patient and the healthcare system is great because two operative procedures are required [10, 13]. Open I & D might be the least morbid treatment option, but this procedure is less likely to control the infection [34]. The rate of infection control for a cemented one-stage exchange might approach those of two-stage exchange [5, 25, 26, 36], but the rate of infection control of a cementless one-stage exchange has been reported in only one study [35]. That study suggested a 92% rate of infection control at a minimum of 2 years followup. Determining the treatment that best controls infection and at the same time minimizes patient morbidity and mortality might produce the highest quality of life for patients being treated for infection.
Decision-analysis modeling offers a powerful tool traditionally used in the business world [2, 3, 31], but now used to address many medical and orthopaedic decisions [31], including the cost-effectiveness of antibiotic-loaded bone cement for THA [9], surgical treatment of the contralateral hip in a patient with a slipped capital femoral epiphysis [29], and the cost-effectiveness of unicompartmental arthroplasty versus TKA [30]. Surgical treatment options are particularly well suited for this type of modeling because the surgical choice represents a discrete decision. This decision leads to one or more outcomes (eg, level of function or quality-of-life measure) at a specific probability. The desirability of each outcome can be quantified (eg, death might be the least desirable outcome, whereas perfect health might be the most desirable outcome). These two parameters, the probability of achieving an outcome and the desirability of that outcome, can be placed in a mathematical model that calculates which decision will lead to the most desirable outcome with the highest probability.
We used decision analysis to estimate the potential quality-of-life outcomes that might occur when choosing from one of three surgical treatments for a periprosthetic infection of a primary cementless THA.
Materials and Methods
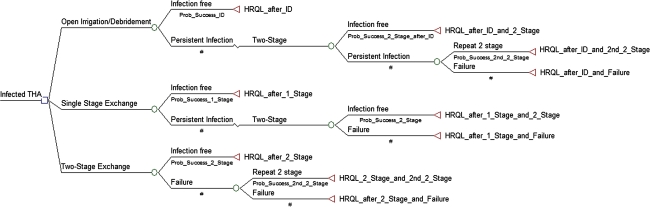
The decision tree and sensitivity analysis were constructed and performed using TreeAge Pro (Williamstown, MA, USA). The decision tree modeled a hypothetical clinical scenario of a patient who presents 3 weeks after a cementless primary THA with a deep periprosthetic joint infection. From the root node (diagnosis of periprosthetic infection), three treatment options were available: open I & D with modular femoral head and acetabular liner exchange, a one-stage revision arthroplasty with removal and immediate reimplantation of all components, or a two-stage exchange revision arthroplasty (Fig. 1).
Fig. 1.
The decision tree is shown. The initial decision occurs at the root node (first decision point) at the left side of the tree where one of three treatment options is selected. Based on the chance (probability [prob]) of this treatment resulting in a specific outcome (utility), the decision tree then describes sequentially (from left to right) possible further outcomes. The terminal node (right side of each pathway) describes the utility (health-related outcome measure [HRQL]) of the final result of that treatment path. ID = irrigation and débridement.
The model assumed that success of a given procedure was a period greater than 2 years without additional surgery for treating the infection, with or without long-term suppressive antibiotics. If the patient initially was treated with an open débridement and this failed, the model assumed the patient would undergo a two-stage exchange [12, 14, 28, 34]. If the two-stage exchange failed, the patient would undergo a second two-stage exchange. If the initial treatment is a one-stage exchange and this treatment failed, then the patient would undergo one subsequent two-stage exchange. If the initial treatment was a two-stage exchange and this failed, the patient would undergo one subsequent two-stage exchange. In this algorithm, the patient would have the possibility of undergoing a maximum of two resections and reimplantations; two two-stage exchanges after an initial open débridement, one two-stage exchange after a failed one-stage exchange, or a second two-stage exchange after a failed initial two-stage exchange. If the two subsequent two-stage exchange arthroplasties failed, the treatment was defined as a failure and we assumed the patient would undergo a resection arthroplasty without reimplantation of any components [22].
The probability of obtaining an infection-free, functioning THA after any of the specified procedures was based on previously published studies (Table 1). Crockarell et al. [8] found débridement and prosthesis retention after an infected THA controlled infection 18% of the time. They further identified patients who had the procedure performed within 2 weeks of the primary THA and found the rate of infection control was slightly greater, at 24%, the rate we used in our model.
Table 1.
Model variables
| Procedure | Probability of success (range of sensitivity analysis) | Study |
|---|---|---|
| Probabilities | ||
| Open débridement/retention of prosthesis | 0.24(0 to 0.99) | Crockarell et al. [8] |
| One-stage exchange | 0.93(0 to 0.99) | Tsukayama et al. [34] |
| Two-stage exchange | 0.92(0 to 0.99) | Haddad et al. [13] |
| Repeat two-stage exchange | 0.60(0 to 0.99) | Pagnano et al. [22] |
| Two-stage exchange following failed débridement | 0.92(0 to 0.99) | *Haddad et al. [13] |
| Repeat two-stage exchange following failed débridement | 0.60(0 to 0.99) | *Pagnano et al. [22] |
| Two-stage exchange following failed one-stage exchange | 0.92(0 to 0.99) | *Haddad et al. [13] |
| *Estimates based on initial treatment | ||
| Utilities | ||
| Quality of life | ||
| Open débridement/retention of prosthesis | 0.86 | CEVR [24, 32] |
| One-stage exchange | 0.82 | CEVR [24, 32] |
| Two-stage exchange | 0.82 | CEVR [24, 32] |
| Resection arthroplasty | 0.60 | Fisman et al. [11] |
| Disutility of revision | ||
| Toll | ||
| Open débridement/retention of prosthesis | −0.1 | |
| One-stage exchange | −0.15 | |
| Two-stage exchange | −0.2 |
Some studies [5, 25, 26, 29, 36] report the ability of a one-stage exchange to control infection using a cemented THA and antibiotic cement, where it appears infection control rates are predicated on the use of antibiotic cement and thus local delivery of antibiotics. In the scenario modeled in our study, the revisions were performed with cementless implants and thus local antibiotic delivery via bone cement would not be available. We identified two studies in which this scenario was reported. First, Tsukayama et al. [34] reviewed their patients with infected THAs and identified a cohort that had revision THAs with a cementless component for presumed aseptic loosening. Some of these patients were later determined to have septic loosening based on intraoperative cultures taken at the time of the revision surgery and were treated with intravenous antibiotics and no additional surgery. These patients effectively had a cementless revision THA in the face of infection without the need for additional treatment for infection in 93% of cases. Winkler et al. [35] reported a novel technique of a one-stage exchange for infection combined with the use antibiotic-impregnated allograft bone. They reported a 92% infection control rate at a minimum 2-year followup. Based on these two studies, we assumed a 93% success rate for a one-stage exchange with cementless components. Cementless revision THA after two-stage exchange for an infected THA with an intervening antibiotic cement spacer (a two-stage exchange) has been investigated by several groups with similar infection control rates of 92% [10, 13, 16], the value used in this study.
Pagnano et al. [22] reported infection control was successful in three of five patients undergoing a second two-stage exchange, therefore we assumed the success rate of a second two-stage exchange was 60% when performed after a failed two-stage exchange. We identified no series reporting the effectiveness of a two-stage exchange after a failed open débridement or failed one-stage exchange. For these two scenarios, we assumed the effectiveness of a two-stage exchange after an open débridement or one-stage exchange to control the infection was the same as that of an initial treatment of two-stage exchange.
A utility in a decision-tree analysis is a number between 0 and 1 used to describe the preference of the final outcome of any given pathway through the tree. In our model, the utilities after treatment of an infected THA reflected the estimated final health state defined as health-related quality of life and were based on a quality-of-life database compiled by the Institute for Clinical Research and Health Policy Studies [12]. Disutility refers to a number, often deducted from the final utility value (termed disutility toll), that estimates the negative impact an undesirable event may have on the final utility, in this case quality-adjusted life years. We defined this undesirable event as repeat surgery for treating the infection. In our model, a disutility toll was one deduction from the final quality-adjusted life year value after any reoperation (and more than one deduction for more than one reoperation). Failure of one of the three initial treatments and subsequent reoperation resulted in a second deduction (disutility toll) that further reduced the final utility value. By deducting from the final utility of any given path in the model we attempted to account for disability incurred from reoperation and generally lower health-related quality of life after multiple surgeries on the same hip. The disutility after an open débridement was assumed to be −0.1, a value similar to that used after undergoing a primary THA [6]. The disutility at the time of planned reimplantation during a two-stage exchange was estimated to be −0.2, twice the value of primary THA to account for two operations and an intervening period of an antibiotic spacer. The value assigned to a one-stage exchange was assumed to be between these two values and set in this model to be −0.15 (Table 1).
Although no studies evaluate the utilities for the specific final health states in this model, the value reported from the largest series in the Clinical Research and Health Policy Studies database for an uncomplicated primary THA was 0.86 [24]. Because repeat surgery to treat infection leads to a lower quality of life than an uncomplicated THA (ie, because of a repeat procedure, time of treatment, loss of income), a disutility toll was applied to the final quality-of-life estimate to account for this additional treatment. We assumed the final utility after a successful open débridement was approximately the same as that for an uncomplicated THA (0.86) but because of the repeat surgery, a disutility toll of 0.1 was subtracted from the quality-of-life estimate of an uncomplicated THA (to account for the morbidity of a second procedure). Thus, the estimate of utility (quality of life) after a débridement for THA infection was 0.86 (utility of an uncomplicated THA) minus 0.1 (disutility toll for débridement procedure), or 0.76. The quality-of-life value estimate for a patient undergoing a revision THA is reportedly 0.82 [32]. We found no studies that specifically reported utility values of one-stage exchanges as compared with two-stage exchanges. In our model, the utility after a one-stage or two-stage revision THA for sepsis was assigned the value of 0.82. We assumed a two-stage exchange would lead to an ultimate lower quality of life than a one-stage exchange owing to a second operation and interval without a formal hip prosthesis. We accounted for this difference in the final quality-of-life outcome estimates between one-stage and two-stage exchanges in the disutility toll subtracted from the final quality-adjusted life year estimate after each procedure. The utility of a resection arthroplasty was estimated to be 0.60 [11] (Table 1).
The initial analysis for a decision tree is to determine the pathway that leads to the greatest expected outcome (utility) based on the initial estimates of each parameter (utilities, probabilities, disutilities) in the tree. This is accomplished by folding back the decision tree, where, working from right to left along the branches of the tree, the utility at each branch is multiplied by its respective probability. The sum of these products along each branch that yields the highest number then will predict the pathway with the highest chance of achieving the most desirable outcome.
Because uncertainty exists in many estimates used in decision analysis, a technique termed sensitivity analysis uses a numerical calculation to evaluate the effect that these uncertainties might have on the decision-analysis outcome. First, a plausible clinical range of each of the parameters used in the model is determined. The probability rates of success of each of the initial and subsequent treatment options were analyzed over a large range (0%–99%). The wide range of probabilities for these parameters was used in an attempt to mitigate any potential bias from the variance reported in the small number of studies reporting these rates. Sensitivity analysis is used repeatedly to reevaluate the decision tree as each model parameter is varied over the plausible range to determine if a change in that parameter could change the product of the analysis. In a one-way sensitivity analysis, one variable is changed over the estimated range while all other parameters are held constant. The value of the parameter that leads to a change (if one exists in the plausible range of values) of the optimal pathway through the decision tree is recorded as a threshold value. If varying more than one parameter leads to a different optimal pathway, a multiple-way sensitivity analysis is performed where those variables are compared to estimate which variable might have the most influence on the product of the model.
Results
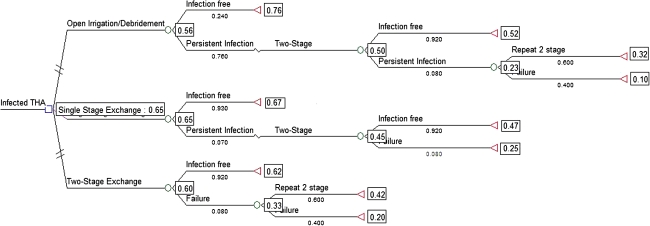
Based on the initial analysis of this decision tree, where the tree is folded back with the estimated values of utility (health-related quality of life), disutility, and probabilities of each treatment being effective, a one-stage exchange led to the highest chance of obtaining the maximum final health-related quality of life (Fig. 2).
Fig. 2.
The initial decision tree analysis shows the initial choice of one-stage exchange appears to result in the greatest expected benefit. Working from right to left along the branches of the tree, the utility at each branch is multiplied by its respective probability. The sum of these products along each branch that yields the highest number then will predict the pathway with the highest expected outcome.
The one-way sensitivity analysis showed that when the probability of treatment success of an initial open débridement and retention of the prosthesis was varied from 0% to 99% success, and with all other variables held constant, the success rate of open débridement would need to be at least greater than 60% effective to shift the model to favor open débridement over one- or two-stage exchange to maximize the health-related quality of life. Moreover, when the treatment success of a one-stage exchange was varied over the same range (0%–99% success), the success of this procedure would have to decrease to less than 66% effective for a decision of a treatment other than one-stage exchange; in this case, the model would favor an initial two-stage exchange over open débridement or one-stage exchange (Table 2). When the success rate of two-stage exchange was varied from 0% to 99% success, there was no threshold above which a two-stage exchange would lead to the greatest quality of life. The two variables that appeared to have the most substantial effect on the model was if the probability of success of an open débridement improved to greater than 60% or if that of a one-stage exchange decreased to less than 66%.
Table 2.
One-way sensitivity analysis
| Variable | Threshold at which treatment dominates |
|---|---|
| Open débridement/retention of prosthesis | > 60% |
| One-stage exchange | > 66% |
| Two-stage exchange | None |
| Repeat two-stage exchange | None |
| Two-stage exchange after failed débridement | None |
| Repeat two-stage exchange after failed débridement | None |
| Two-stage exchange after failed one-stage exchange | None |
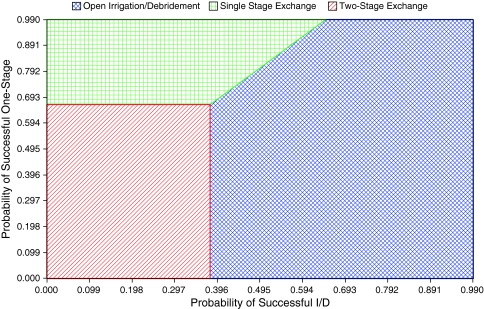
The multi-way sensitivity analysis performed showed that two variables, the probability of success of I & D and probability of success of a one-stage exchange, had any influence over the model. These two variables were compared in a two-way sensitivity analysis. Only when the success of an open débridement approached a value less than 38% and the success of a one-stage exchange approached a value less than 66% did an initial two-stage exchange lead to the maximum final health-related quality of life (Fig. 3).
Fig. 3.
A two-way sensitivity analysis comparing the probability of success of an I & D (x-axis) with that of a one-stage exchange (y-axis) shows the treatment that potentially results in the greatest utility. The position of any coordinate on the graph corresponding to a value of success of I & D (x-axis) and success of one-stage (y-axis) predicts the treatment with the greatest outcome. With less than approximately 38% success of I & D and less than approximately 69% success of one-stage exchange, the model predicted a two-stage exchange might result in the greatest health-related quality of life.
Discussion
Decision analysis is a powerful tool that can be applied to orthopaedic decision making by evaluating multiple treatment options with multiple potential outcomes. For a given condition, the analysis numerically determines the potential treatment choice that might lead to the most favorable outcome with the greatest probability. The diagnosis of an acute postoperative infection after a primary THA is a devastating complication and can have substantial consequences on patient outcome. This decision analysis attempts to pool all relevant data regarding treatment of early infections after THA and to mathematically derive the initial treatment choice that could lead to the highest quality of life with the greatest probability.
This approach is subject to some limitations. First, the decision tree is a static model that attempts to broadly address a clinical problem, however in reality, each clinical scenario poses a unique challenge with multiple factors and many permutations. Thus, the decision analysis most likely will not be able to estimate the outcomes of a specific scenario, but rather provide a potential framework by which to evaluate treatments and design future studies. Second, our approach represented an early attempt to synthesize data for different treatments for an infected THA in the early postoperative period. There is a lack of specific data regarding success rates of different procedures and outcomes after those procedures. Specifically, we could identify no references in the peer-reviewed literature to estimate the probability of success with a single-stage exchange for acute postoperative infection. Several of the variables used in this study were best estimates from the available literature. However, the strength of the decision analysis is that the uncertainty of the values can be abated through a sensitivity analysis. Third, the data used in this decision analysis are based on studies that were not randomized or controlled. This introduces inherent problems with the reliability of the data as those results are likely biased by the choice of treatment at the time, based on a priori clinical decisions to optimize outcome (ie, perhaps a one-stage exchange would not have been performed if it was known the patient had an infection). The choice of sequential surgical care for failed treatments in this algorithm is based on the authors’ consensus on the current standard of care for an infected THA. This algorithm could have been designed differently; for example, a patient might undergo an I & D after a failed two-stage exchange or a one-stage exchange after a failed two-stage exchange, and thus have changed the model. Ultimately these current pathways were chosen because they most closely reflect the current understanding of standard of care. Finally, the gold standard for determining the best treatment for a clinical problem is a randomized control trial. Decision analysis in general and this study in particular are not designed to make recommendations for treatment in place of a randomized controlled trial. The goals are to evaluate and analyze currently available data to attempt to understand which treatments might lead to the highest health-related quality of life and to assist in generating future, more focused studies.
Decision analysis has been applied to orthopaedic problems where no clear best treatment exists. For examples, decision analysis was used to estimate the potential benefit to a patient if an advanced (and more expensive) bearing surface was used in hip replacement surgery [2]. The investigators noted a potential benefit might be observed if there is a 19% reduction in the 20-year implant failure rate when compared with a conventional bearing. In another study in which decision analysis was applied to orthopaedics, it was noted the use of antibiotic cement appears to be cost-effective when used in primary THAs [6]. In another study, prophylactic pinning of the contralateral hip in a slipped capital femoral epiphysis appeared to be of long-term benefit for the patient [24]. In these three studies, as in the current study, decision analysis was used as a tool to determine the best potential choice for a treating physician by synthesizing disparate data from various studies that address similar issues, but not the exact situation at hand. Similar limitations exist with those studies, as those described above that limit this study.
If the parameters of this decision tree accurately reflect a specific clinical scenario, a one-stage exchange might lead to the greatest health-related quality of life for treatment of an acute postoperative infection, assuming the failure rate of attempted débridement and component retention is greater than 40%, whereas the success of a one-stage exchange is 66% or greater. In the face of an early postoperative infection, surgeons might opt to treat the patient with an open débridement, exchange of the modular head and polyethylene liner (if applicable), and a course of intravenous antibiotics. Although this treatment might be of little morbidity and cost, several studies suggest this treatment is not particularly effective at controlling infection and often leads to failure and additional procedures [5, 11, 23]. Two-stage exchange, which is considered to be the standard treatment for chronic infections [10, 12–14, 16, 33, 37], might be relatively effective, but this leaves the patient debilitated with an antibiotic cement spacer for several weeks to months and also ensures another surgical procedure for reimplantation. Direct, or single-stage, exchange might offer the best treatment for an acute postoperative infection. During the early postoperative period, osteointegration of cementless components has not yet occurred, and thus removal of the implants should not be technically challenging or compromising to the bone stock. Removal of the implants provides access to all of the bony interfaces for a thorough débridement and removes any potential foreign body nidus for infection. With well-preserved bone stock, reimplantation of new components should be routine.
Sensitivity analysis, which attempts to account for uncertainty in specific variables [5, 15, 36], revealed that with all other parameters being constant, only two variables appreciably affected the model: the success rate of one-stage exchange and the success rate of open débridement. The success rate of a one-stage exchange must decrease to less than 66% or the success rate of an open débridement must surpass 60% to favor a different treatment. Although it seems that, based on all studies that evaluated one-stage exchange, with cemented and cementless components, nearly uniformly the success rate reportedly is greater than 70% [5, 15, 36]. Most of the literature relating to one-stage exchange has been with the use of cemented components, and subsequent use of antibiotic cement for fixation of the implants in this procedure; little has been published regarding the use of cementless components [15, 35]. For patients who underwent revision surgery for presumed aseptic loosening but later were found to have infection, Tsukayama et al. [34] found the use of cementless components and postoperative antibiotics yielded a 2-year infection-free success rate of 93%. These cases potentially can be regarded as chronic infections treated with a one-stage exchange; acute postoperative infections treated early potentially can be expected to have a similar rate of success. Thus, once the decision has been made to reoperate for infection, it appears from this analysis that a one-stage exchange might lead to the highest health-related quality of life with little increase in the time or technical skill of the treating surgeon.
Decision analysis is a potentially powerful tool when applied to clinical decision making. By evaluating competing risks and benefits, a numerical algorithm is developed to attempt to identify the decision that leads to the most favorable possible outcome with the greatest probability, while at the same time minimizing potential risks. In the current study, decision analysis was applied to the clinical scenario of an infected cementless THA in the early postoperative period. Based on the currently available data, this decision analysis favors the choice of a one-stage exchange to treat cementless THAs that become infected during the early postoperative period, as long as the success rate of a one-stage exchange is greater than 66% or the success rate of an open débridement is less than 60%. Although this decision analysis attempts to inform a treatment decision based on the best literature currently available, more robust studies designed to answer this specific question should be performed before any definitive conclusions can be drawn.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Rush University Medical Center, Chicago, IL, and the University of California, San Francisco, San Francisco, CA.
References
- 1.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Morshed S, Silverstein MD, Rubash HE, Kahn JG. Use of cost-effectiveness analysis to evaluate new technologies in orthopaedics: the case of alternative bearing surfaces in total hip arthroplasty. J Bone Joint Surg Am. 2006;88:706–714. doi: 10.2106/JBJS.E.00614. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133–138. doi: 10.1016/j.arth.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Bratzler DW, Houck PM; Surgical Infection Prevention Guidelines Writers Workgroup; American Academy of Orthopaedic Surgeons; American Association of Critical Care Nurses; American Association of Nurse Anesthetists; American College of Surgeons; American College of Osteopathic Surgeons; American Geriatrics Society; American Society of Anesthesiologists; American Society of Colon and Rectal Surgeons; American Society of Health-System Pharmacists; American Society of PeriAnesthesia Nurses; Ascension Health; Association of periOperative Registered Nurses; Association for Professionals in Infection Control and Epidemiology; Infectious Diseases Society of America; Medical Letter; Premier; Society for Healthcare Epidemiology of America; Society of Thoracic Surgeons; Surgical Infection Society. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706–1715. [DOI] [PubMed]
- 5.Callaghan JJ, Katz RP, Johnston RC. One-stage revision surgery of the infected hip: a minimum 10-year followup study. Clin Orthop Relat Res. 1999;369:139–143. doi: 10.1097/00003086-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Charnley J. Postoperative infection after total hip replacement with special reference to air contamination in the operating room. Clin Orthop Relat Res. 1972;87:167–187. doi: 10.1097/00003086-197209000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Charnley J, Eftekhar N. Postoperative infection in total prosthetic replacement arthroplasty of the hip-joint: with special reference to the bacterial content of the air of the operating room. Br J Surg. 1969;56:641–649. doi: 10.1002/bjs.1800560902. [DOI] [PubMed] [Google Scholar]
- 8.Crockarell JR, Hanssen AD, Osmon DR, Morrey BF. Treatment of infection with debridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am. 1998;80:1306–1313. doi: 10.2106/00004623-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesaeter LB, Finlayson SR. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91:634–641. doi: 10.2106/JBJS.G.01029. [DOI] [PubMed] [Google Scholar]
- 10.Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty. 1999;14:175–181. doi: 10.1016/S0883-5403(99)90122-5. [DOI] [PubMed] [Google Scholar]
- 11.Fisman DN, Reilly DT, Karchmer AW, Goldie SJ. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419–430. doi: 10.1086/318502. [DOI] [PubMed] [Google Scholar]
- 12.Garvin KL, Hanssen AD. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am. 1995;77:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82:689–694. doi: 10.1302/0301-620X.82B5.9668. [DOI] [PubMed] [Google Scholar]
- 14.Hanssen AD, Spangehl MJ. Treatment of the infected hip replacement. Clin Orthop Relat Res. 2004;420:63–71. doi: 10.1097/00003086-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;381:101–105. doi: 10.1097/00003086-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res. 2005;441:243–249. doi: 10.1097/01.blo.0000194312.97098.0a. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 18.Laupacis A, Bourne R, Rorabeck C, Feeny D, Wong C, Tugwell P, Leslie K, Bullas R. The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am. 1993;75:1619–1626. doi: 10.2106/00004623-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Mahomed NN, Barrett JA, Katz JN, Phillips CB, Losina E, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85:27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Malchau H, Herberts P, Ahnfelt L. Prognosis of total hip replacement in Sweden: follow-up of 92, 675 operations performed 1978–1990. Acta Orthop Scand. 1993;64:497–506. doi: 10.3109/17453679308993679. [DOI] [PubMed] [Google Scholar]
- 21.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 22.Pagnano MW, Trousdale RT, Hanssen AD. Outcome after reinfection following reimplantation hip arthroplasty. Clin Orthop Relat Res. 1997;338:192–204. doi: 10.1097/00003086-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Phillips CB, Barrett JA, Losina E, Mahomed NN, Lingard EA, Guadagnoli E, Baron JA, Harris WH, Poss R, Katz JN. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85:20–26. doi: 10.1302/0301-620X.85B3.13201. [DOI] [PubMed] [Google Scholar]
- 24.Rasanen P, Paavolainen P, Sintonen H, Koivisto AM, Blom M, Ryynanen OP, Roine RP. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop. 2007;78:108–115. doi: 10.1080/17453670610013501. [DOI] [PubMed] [Google Scholar]
- 25.Raut VV, Siney PD, Wroblewski BM. One-stage revision of total hip arthroplasty for deep infection: long-term followup. Clin Orthop Relat Res. 1995;321:202–207. [PubMed] [Google Scholar]
- 26.Raut VV, Siney PD, Wroblewski BM. One stage revision arthroplasty of the hip for deep gram negative infection. Int Orthop. 1996;20:12–14. doi: 10.1007/s002640050019. [DOI] [PubMed] [Google Scholar]
- 27.Salvati EA, Robinson RP, Zeno SM, Koslin BL, Brause BD, Wilson PD., Jr Infection rates after 3175 total hip and total knee replacements performed with and without a horizontal unidirectional filtered air-flow system. J Bone Joint Surg Am. 1982;64:525–535. [PubMed] [Google Scholar]
- 28.Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97–100. doi: 10.1016/j.arth.2006.02.090. [DOI] [PubMed] [Google Scholar]
- 29.Schultz WR, Weinstein JN, Weinstein SL, Smith BG. Prophylactic pinning of the contralateral hip in slipped capital femoral epiphysis: evaluation of long-term outcome for the contralateral hip with use of decision analysis. J Bone Joint Surg Am. 2002;84:1305–1314. doi: 10.2106/00004623-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Slover J, Espehaug B, Havelin LI, Engesaeter LB, Furnes O, Tomek I, Tosteson A. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients: a Markov decision analysis. J Bone Joint Surg Am. 2006;88:2348–2355. doi: 10.2106/JBJS.E.01033. [DOI] [PubMed] [Google Scholar]
- 31.Sporer SM, Rosenberg AG. Decision analysis in orthopaedics. Clin Orthop Relat Res. 2005;431:250–256. doi: 10.1097/01.blo.0000153074.19245.be. [DOI] [PubMed] [Google Scholar]
- 32.The Center for the Evaluation of Value and Risk in Health (CEVR). The Cost-Effectiveness Analysis Registry. Institute for Clinical Research & Health Policy Studies, Tufts Medical Center; 2009. Available at: http://www.cearegistry.org. Accessed June 6, 2011.
- 33.Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br. 2006;88:149–155. doi: 10.1302/0301-620X.88B2.17058. [DOI] [PubMed] [Google Scholar]
- 34.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Winkler H, Stoiber A, Kaudela K, Winter F, Menschik F. One stage uncemented revision of infected total hip replacement using cancellous allograft bone impregnated with antibiotics. J Bone Joint Surg Br. 2008;90:1580–1584. doi: 10.1302/0301-620X.90B12.20742. [DOI] [PubMed] [Google Scholar]
- 36.Wroblewski BM. One-stage revision of infected cemented total hip arthroplasty. Clin Orthop Relat Res. 1986;211:103–107. [PubMed] [Google Scholar]
- 37.Younger AS, Duncan CP, Masri BA, McGraw RW. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplasty. 1997;12:615–623. doi: 10.1016/S0883-5403(97)90133-9. [DOI] [PubMed] [Google Scholar]