Abstract
Background
Locking-plate technology has renewed interest in plate fixation for treating proximal humerus fractures. Complications associated with these devices, including loss of reduction, screw cutout, and intra-articular penetration, are frequent. Establishing a second column of support may reduce complications and improve clinical outcome scores.
Questions/purposes
We asked whether addition of an endosteal cortical allograft strut, used as an augment to locking-plate fixation for displaced proximal humerus fractures, would reduce complications and improve clinical outcome scores.
Patients and Methods
We retrospectively reviewed the charts and radiographs of 38 patients treated by this method. All patients were evaluated with serial radiographs, as well as the Disabilities of the Arm, Shoulder, and Hand and Constant-Murley scores. There were seven two-part, 19 three-part, and 12 four-part fractures. The minimum followup was 49 weeks (average, 75 weeks; range, 49–155 weeks).
Results
No patient had intra-articular screw penetration or cutout. No patient had complete osteonecrosis, but one had partial osteonecrosis. The reduction was lost in one patient. The mean Disabilities of the Arm, Shoulder, and Hand score was 15 (range, 0–66.4). The mean Constant-Murley score was 87 (range, 51–95).
Conclusions
Low rates of complication and high clinical outcome scores can be achieved when treating complex proximal humerus fractures with locking-plate fixation and an endosteal strut augment.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Proximal humerus fractures (PHFs) account for 5% of all fractures and are increasing in frequency as the population ages [3]. More than 80% of these can be treated nonoperatively [22], but displaced three- and four-part fractures remain a clinical challenge [22]. Nonoperative treatment results in minimal pain but with reduced function reportedly comparable to that of shoulder fusion [7]. Each of the surgical options, such as plate fixation, intramedullary nailing, percutaneous pinning, and tension band wiring, is associated with unique complications, and there is no consensus as to which treatment is best. Some have advocated for hemiarthroplasty because of the high risk of osteonecrosis (ON) and loss of fixation with traditional plating techniques [21]. Arthroplasty procedures provide reliable pain relief but inconsistent motion [2, 14]. Open reduction and internal fixation (ORIF) reportedly restores shoulder function to 96% of an uninjured extremity when an anatomic or near-anatomic reduction is maintained until healing, and ON is avoided [13, 26].
The introduction of locking-plate technology [8] has renewed interest in plate fixation for treating PHFs because these implants provide a theoretical advantage toward maintaining stable fixation in osteoporotic bone [10]. However, complication rates for the treatment of PHFs have ranged from 9% to 36% and are reportedly highest in elderly patients [1, 4, 23, 27]. The most frequent are screw cutout with intra-articular penetration and varus collapse [23]. Establishing medial column support appears to reduce these complications and enhance functional results [11, 18]. Comminution of the surgical neck prohibits establishing support via cortical abutment, however. Use of an allograft strut has been suggested as a means of establishing a second column of support when neck comminution is present [9].
Our purposes were to (1) determine function (Disabilities of the Arm, Shoulder, and Hand [DASH], SF-36, and Constant-Murley scores) of patients with displaced PHFs treated with a lateral locking plate and endosteal strut; (2) determine whether these scores vary based on Neer fracture type; and (3) report the incidence of complications associated with this technique.
Patients and Methods
We retrospectively reviewed 48 patients with displaced PHFs treated with a locking plate and endosteal strut augment between November 2006 and June 2009. All patients with an acute, displaced PHF having cortical comminution in the region of the surgical neck were considered for this procedure. Patients who were unwilling or unable to participate in the postoperative rehabilitation program due to physical or mental illness were contraindicated. Any patient meeting the above criteria who underwent this procedure during the study period was considered for inclusion. Nine patients who did not meet the inclusion criteria underwent hemiarthroplasty. Ten patients were lost to followup before 1 year: three died within 6 months of surgery, one was a psychiatric patient who had originally fractured his humerus during a suicide attempt and could not be located, one developed dementia postoperatively and was unable to answer the questionnaires, one moved out of the country, one had an infection and sought care elsewhere, and three could not be contacted despite an exhaustive search. These 10 exclusions left 38 patients for review, all of whom had isolated injuries. The mean (± SD) age of the remaining 38 patients was 65.5 ± 9.4 years (range, 44.1–82.7 years). A total of 82.1% of these fractures were the result of low-energy trauma, typically a fall from a standing height. The minimum followup was 49 weeks (average, 75.4 weeks; range, 49–155 weeks). Data were obtained from medical records and radiographs. Those patients who had not returned for the regularly scheduled followup or had not completed the postoperative assessments were recalled for a complete evaluation. Internal review board approval was obtained.
Preoperatively, all patients had AP and lateral radiographs of the shoulder, as well as CT scans, on admission. Fractures were classified by three reviewers (ASN, CMH, DGL) according to the Neer [22] and AO [20] systems using all preoperative imaging (Table 1). Classification was confirmed intraoperatively by the senior author (DGL). If there was a discrepancy between reviewer classifications, the intraoperative classification was used. There were seven two-part, 19 three-part, and 12 four-part fractures according to the Neer classification. There were eight AO Type A, 11 Type B, and 19 Type C fractures.
Table 1.
Fractures classified according to the AO system
| AO type | Number of fractures | ||
|---|---|---|---|
| .1 | .2 | .3 | |
| Type A | |||
| 1 | 0 | 0 | 0 |
| 2 | 0 | 1 | 3 |
| 3 | 0 | 1 | 3 |
| Type B | |||
| 1 | 3 | 0 | 0 |
| 2 | 1 | 0 | 7 |
| 3 | 0 | 0 | 0 |
| Type C | |||
| 1 | 4 | 1 | 0 |
| 2 | 5 | 3 | 4 |
| 3 | 0 | 0 | 2 |
All procedures were performed by a single surgeon (DGL). A complete description of this technique has been published previously [9]. Patients were placed in a semilateral position with the affected extremity draped free and the image intensifier included in the sterile surgical field. An anterior lateral deltoid splitting approach was used. The axillary nerve was identified and protected in all cases. Anterior and posterior dissection was limited to only that necessary for passing sutures into the anterior and posterior-superior rotator cuff. The fibular allograft was inserted into the medullary canal through the lateral fracture lines after being cut to an appropriate length, typically 6 to 8 cm. Reduction, with emphasis on recreating the natural arch of the medial column, was established and confirmed with fluoroscopy. Placement of the fibula strut graft and reduction method were dictated by the fracture pattern, specifically the angulation of the head piece. When the head piece was in varus relative to the shaft, the fibula was inserted into the canal through the lateral fracture lines and pushed distally beyond the level of the head. It was then medialized maximally to the calcar region and advanced retrograde into the subchondral bone of the head. This lifted the head superiorly, out of the varus position, and reduced the inferior margin of the head to the proximal medial shaft (Fig. 1). A Kirschner wire was used as a joystick to achieve graft position or a tamp was used to directly push on the graft. This maneuver reduced the head to the shaft and established support of the medial column.
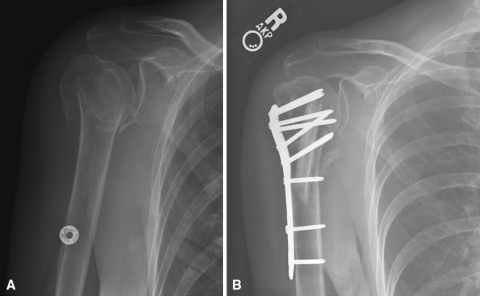
Fig. 1A–B.
(A) An injury AP radiograph shows a four-part fracture with comminution of the medial cortex. (B) The fibula allograft is used as a strut augment and placed along the medial arch.
When the head was in a position of valgus, the fibula was inserted antegrade through the bed of the displaced tuberosity. It was advanced distally until the proximal edge of the graft was just beneath the subchondral bone of the lateral head. Under fluoroscopic visualization, the graft was then pushed medially. As the graft moved, the head was gradually tipped into an anatomic position as its lateral portion was elevated by the graft and the medial portion hinged on the medial cortex (Fig. 2). The locking plate (Synthes, Inc, Paoli, PA) was then slid under the axillary nerve and screws were placed, transfixing the endosteal fibula and the humeral head. Sutures placed in the rotator cuff were tied to the plate for fixation of the tuberosities.
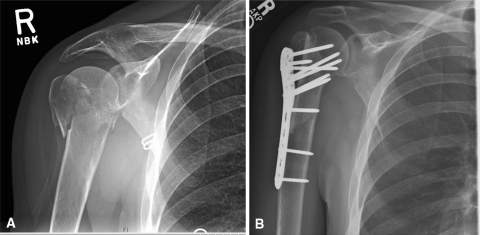
Fig. 2A–B.
(A) An injury AP radiograph shows the humeral head with valgus displacement and comminution of the lateral cortex. (B) The fibula allograft is placed laterally to function as a buttress for the reduced humeral head.
All patients began active-assisted and passive shoulder ROM under the direction of an occupational therapist beginning the morning after surgery. Therapy was performed two times per day during the hospital stay, and a continuous passive motion machine for forward flexion was used for approximately 4 to 6 hours daily. Upon discharge, therapy was continued daily with home stretching exercises (forward elevation and external rotation) and three times per week with a therapist focusing on regaining ROM in all planes. Strengthening was begun after radiographic evidence of healing appeared.
Patients were examined clinically and radiographically at 2, 6, 12, 24, and 52 weeks postoperatively and then on a yearly basis. Radiographic views routinely obtained at all visits included AP, scapula Y, and axillary views. SF-36 [30], DASH [15], and Constant-Murley [5] scores were obtained by an independent reviewer (OP) approximately 1 year after surgery or at latest followup. Due to practical matters of scheduling, the timing of the 1-year followup varied between 49 and 54 weeks. Any patient who had not returned for adequate followup at the time of the study was recalled to undergo the above assessments. All motion measurements were made with a goniometer and measurements of power were made with a dynamometer (AliMed, Dedham, MA).
We defined complications using the following standards. Maintenance of reduction was assessed using the method of Gardner et al. [11]. A change of humeral head height of greater than 3 mm was considered to be a loss of reduction. Intra-articular screw penetration was defined as screw violation of the articular surface seen on any postoperative radiographic view without an associated loss of reduction. Screw cutout, in contrast, was considered present if a screw penetrated the articular surface and was associated with a loss of reduction. ON was evaluated using postoperative radiographs only. The presence of collapse in some portion, or all, of the humeral subchondral bone was considered evidence for ON. This was classified using the method of Gerber et al. [12]. Any other complications, such as heterotopic bone formation, hardware breakage, or infection, for example, were based on the clinical or radiographic examination by the senior author and treating surgeon (DGL). Major complications were defined as those requiring reoperation. Minor complications did not.
We compared the mean values of the clinical scores for Neer fracture groups using ANOVA on Microsoft® Excel® (Microsoft Corp, Redmond, WA).
Results
The mean DASH score was 15 ± 17 (range, 0–66.4) (Table 2). The mean Constant-Murley score was 87 ± 7 (range, 51–95). The mean total SF-36 score was 80 ± 18.5. Patients had on average 155° of passive forward flexion, 148° active forward flexion, 61° external rotation, and internal rotation was within a mean 0.8 levels of the contralateral side (Table 3). Contralateral active forward flexion was 167°, and external rotation was 68°.
Table 2.
Postoperative ROM
| Variable | Mean | SD | Range |
|---|---|---|---|
| Active forward flexion (affected) (°) | 147.9 | 29.9 | 70–180 |
| Active forward flexion (contralateral) (°) | 166.6 | 18.1 | 90–180 |
| External rotation (affected) (°) | 60.7 | 14.5 | 20–80 |
| External rotation (contralateral) (°) | 68.0 | 13.6 | 20–80 |
| Internal rotation (difference in number of vertebral levels from contralateral) | 0.8 | 1.6 | −2–4 |
Table 3.
Clinical outcome measures stratified by Neer classification
| Neer | Number of patients | DASH score | Constant-Murley score | SF-36 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Physical health | Mental health | Physical function | Pain | General health | Vitality | ||||
| 2 | 7 | 16.6 ± 13.6 | 85.6 ± 9.6 | 76.0 ± 17.3 | 74.6 ± 18.9 | 74.7 ± 15.2 | 76.7 ± 26.4 | 78.7 ± 19.8 | 80.8 ± 17.0 | 57.5 ± 24.9 |
| 3 | 19 | 13.4 ± 17.4 | 87.8 ± 6.6 | 78.4 ± 19.1 | 78.0 ± 16.9 | 78.1 ± 20.8 | 86.1 ± 18.9 | 73.7 ± 21.9 | 83.6 ± 19.9 | 69.6 ± 17.3 |
| 4 | 12 | 17.3 ± 19.2 | 86.1 ± 6.3 | 83.2 ± 19.3 | 82.9 ± 22.3 | 85.2 ± 16.1 | 82.7 ± 20.0 | 84.6 ± 26.2 | 93.7 ± 17.6 | 80.5 ± 17.8 |
| All | 38 | 15.2 ± 17.0 | 86.8 ± 7.0 | 79.6 ± 18.5 | 79.0 ± 18.9 | 79.9 ± 18.1 | 83.1 ± 20.4 | 78.6 ± 22.9 | 86.7 ± 18.8 | 71.1 ± 20.2 |
Values are expressed as mean ± SD; there were no significant differences between the fracture groups; DASH = Disabilities of the Arm, Shoulder, and Hand.
Mean values for the DASH, Constant-Murley, and SF-36 were similar between the Neer fracture types (p = 0.81, p = 0.71, p = 0.6, respectively).
No major complications occurred. The following minor complications occurred. One patient lost reduction with varus collapse but has not had further surgery; this patient had a DASH score of 5.6 and a Constant-Murley score of 85. This patient also suffered mild atrophy of her anterior deltoid, but we did not further evaluate axillary nerve function because of her acceptable level of function. No patient had intra-articular screw penetration or screw cutout. No patient suffered complete ON. The first patient in this series had radiographic evidence of partial ON but had no further operations (DASH, 12; Constant-Murley, 87). Two patients had heterotrophic bone formation, neither of which was symptomatic, and one had a wound infection that resolved with 2 weeks of oral antibiotics.
Discussion
Displaced PHFs are common fractures in the elderly and pose a clinical challenge. High rates of ON and fracture collapse have led some authors to advocate for hemiarthroplasty as the treatment of choice for “at-risk” fractures [21]. However, motion after hemiarthroplasty is highly variable and two reports found no functional difference between hemiarthroplasty and nonoperative treatment [6, 31]. Internal fixation can produce better functional outcomes than hemiarthroplasty [28], particularly when complications are avoided [13, 26]. Several previous reports have identified reconstruction of the medial column as an important factor in minimizing these complications [11, 18]. Use of the endosteal strut allograft can re-establish medial support, even in the comminuted osteoporotic bone commonly found in these fractures. In this study, we (1) determined the function (DASH, SF-36, and Constant-Murley scores) of patients with displaced PHFs treated with a lateral locking and endosteal strut; (2) determined whether these scores vary based on Neer fracture type; and (3) reported the incidence of complications associated with this technique.
The limitations of our study are as follows. First, the study is from a single surgeon whose practice is limited exclusively to orthopaedic trauma. This may limit the general applicability of results. However, several facets of this technique, such as the approach, are popular and no more technically difficult than the traditional methods. Second, we had no control group of similar fractures treated with alternative methods and cannot make direct comparisons of treatment methods. Third, the duration of followup is also relatively short and ON may appear with longer monitoring. There is no established standard for monitoring ON in the reconstructed proximal humerus, but postfracture ON occurring in the femoral head has been reported to occur after a longer duration [25]. The retrospective nature of this study is another inherent weakness and could lead to underreporting of complications that were not well documented.
The functional scores of our patients are superior to those reported after hemiarthroplasty for fracture [2, 14, 16]. The mean Constant-Murley score in this study was almost 30 points higher than the mean reported in a recent meta-analysis of hemiarthroplasty results (57) [16]. Subjective quality-of-life measures (DASH, SF-36) also compare favorably to patients treated with hemiarthroplasty and suggest greater patient satisfaction after treatment with this comprehensive technique than after joint arthroplasty [24]. Our study demonstrates complex PHFs can be reliably treated with joint-preserving techniques.
Addition of the strut augment appears to provide benefit beyond use of a locking plate alone, particularly in four-part fractures. In a systematic review of locking-plate treatment, Thanasas et al. [29] found a mean Constant-Murley score of 74 for all fractures, which decreased to 68 for four-part fractures. We found no decline in functional outcome for more severe fracture types. All groups had mean Constant-Murley scores of more than 80. Several elements of our treatment protocol may contribute to these improved results. The use of an endosteal fibula allograft reportedly increases the initial stiffness and load to failure in a locking-plate, PHF model by 3.84 and 1.72 times, respectively [19]. This stability permits an early and aggressive rehabilitation program, which improves function after fracture reconstruction [18].
The added stability also indirectly improves functional outcome scores by reducing complications and the functional deficits incurred from them. We found lower rates of reduction loss (2.6%), screw cutout (0%), and ON (2.6%) than are typically reported for locking plates [1, 4, 8, 27] (Table 4). One systematic review of locking-plate treatment found these complications occur in 12.2%, 11.6%, and 7.9% of patients, respectively [30]. Several reports have identified a second support column as critical to maintaining reduction and avoiding cutout [11, 18]. The addition of an endosteal strut establishes this support regardless of bone quality or comminution of the surgical neck. The endosteal implant may also minimize postoperative ON by increasing the biomechanical strength of the construct and resisting loss of reduction. Maintenance of reduction, which was achieved in all but one patient in our study, may permit revascularization of head pieces rendered ischemic at the time of injury [17].
Table 4.
Summary of complications related to the use of proximal humerus locking plates
| Study | Number of fractures | Intra-articular screw penetration | Screw cutout | Loss of reduction | Osteonecrosis |
|---|---|---|---|---|---|
| Agudelo et al. [1] | 153 | 0% | 4% | 13.6% | 4.5% |
| Brunner et al. [4] | 158 | 14% | 8% | 5.7% | 8% |
| Frankhauser et al. [8] | 28 | NR | 18% | 11% | 7% |
| Lee and Shin [18] | 45 | NR | 4% | 11% | 2.2% |
| Owsley and Gorczyca [23] | 53 | NR | 23% | 25% | 4% |
| Neviaser et al. | 38 | 0% | 0% | 2.6% | 2.6% |
NR = not clearly reported.
Our study demonstrates complex PHFs can be reliably treated with joint-preserving techniques. Consistently high functional scores can be achieved when treating even three- and four-part fractures with plate fixation. Use of an endosteal implant provides several advantages over plating alone. A second column of support is easily established, the stability of the reconstruction is improved, and reduction is maintained. This minimizes the most frequent complications reported with proximal humeral locking plates and allows early and aggressive therapy.
Acknowledgment
The authors thank Omesh Paul, MD, for his help in data collection for this study.
Footnotes
One of the authors (JSD) has received fees for consulting from BioMimetic Therapeutics (Franklin, TN), Tornier Sports Medicine (Edina, MN), and Arthrex, Inc (Naples, FL). Each of the other authors certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at The Hospital for Special Surgery.
References
- 1.Agudelo J, Schurmann M, Stahel P, Helwig P, Morgan SJ, Zechel W, Bahrs C, Parekh A, Ziran B, Williams A, Smith W. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21:676–681. doi: 10.1097/BOT.0b013e31815bb09d. [DOI] [PubMed] [Google Scholar]
- 2.Antuna SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17:202–209. doi: 10.1016/j.jse.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52:243–249. doi: 10.1016/S0895-4356(98)00167-X. [DOI] [PubMed] [Google Scholar]
- 4.Brunner F, Sommer C, Bahrs C, Heuwinkel R, Hafner C, Rillmann P, Kohut G, Ekelund A, Muller M, Audigé L, Babst R. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23:163–172. doi: 10.1097/BOT.0b013e3181920e5b. [DOI] [PubMed] [Google Scholar]
- 5.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 6.Hartog D, Lieshout EM, Tuinebreijer WE, Polinder S, Beeck EF, Breederveld RS, Bronkhorst MW, Eerenberg JP, Rhemrev S, Roerdink WH, Schraa G, Vis HM, Thiel TP, Patka P, Nijs S, Schep NW. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97–98. doi: 10.1186/1471-2474-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelson G, Safuri H, Salami J, Vigder F, Militianu D. Natural history of complex fractures of the proximal humerus using a three-dimensional classification system. J Shoulder Elbow Surg. 2008;17:399–409. doi: 10.1016/j.jse.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop Relat Res. 2005;430:176–181. doi: 10.1097/01.blo.0000137554.91189.a9. [DOI] [PubMed] [Google Scholar]
- 9.Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22:195–200. doi: 10.1097/BOT.0b013e31815b3922. [DOI] [PubMed] [Google Scholar]
- 10.Gardner MJ, Evans JM, Dunbar RP. Failure of fracture plate fixation. J Am Acad Orthop Surg. 2009;17:647–657. doi: 10.5435/00124635-200910000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21:185–191. doi: 10.1097/BOT.0b013e3180333094. [DOI] [PubMed] [Google Scholar]
- 12.Gerber C, Hersche O, Berberat C. The clinical relevance of posttraumatic avascular necrosis of the humeral head. J Shoulder Elbow Surg. 1998;7:586–590. doi: 10.1016/S1058-2746(98)90005-2. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Werner CM, Vienne P. Internal fixation of complex fractures of the proximal humerus. J Bone Joint Surg Br. 2004;86:848–855. doi: 10.1302/0301-620X.86B6.14577. [DOI] [PubMed] [Google Scholar]
- 14.Goldman RT, Koval KJ, Cuomo F, Gallagher MA, Zuckerman JD. Functional outcome after humeral head replacement for acute three- and four-part proximal humeral fractures. J Shoulder Elbow Surg. 1995;4:81–86. doi: 10.1016/S1058-2746(05)80059-X. [DOI] [PubMed] [Google Scholar]
- 15.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: The DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Kontakis G, Koutras C, Tosounidis T, Giannoudis P. Early management of proximal humeral fractures with hemiarthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90:1407–1413. doi: 10.1302/0301-620X.90B11.21070. [DOI] [PubMed] [Google Scholar]
- 17.Lee CK, Hansen HR. Post-traumatic avascular necrosis of the humeral head in displaced proximal humeral fractures. J Trauma. 1981;21:788–791. doi: 10.1097/00005373-198109000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18:83–88. doi: 10.1016/j.jse.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech (Bristol, Avon). 2010;25:642–646. doi: 10.1016/j.clinbiomech.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Müller ME. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 21.Neer CS., 2nd Displaced proximal humeral fractures. Part II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52:1090–1103. [PubMed] [Google Scholar]
- 22.Neer CS., 2nd Displaced proximal humeral fractures. Part I. Classification and evaluation. 1970. Clin Orthop Relat Res. 2006;442:77–82. doi: 10.1097/01.blo.0000198718.91223.ca. [DOI] [PubMed] [Google Scholar]
- 23.Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected] J Bone Joint Surg Am. 2008;90:233–240. doi: 10.2106/JBJS.F.01351. [DOI] [PubMed] [Google Scholar]
- 24.Padua R, Bondi R, Ceccarelli E, Campi A, Padua L. Health-related quality of life and subjective outcome after shoulder replacement for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17:261–264. doi: 10.1016/j.jse.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Reddix RN, Russell G, Woodall J, Jackson B, Dedmond B, Webb LX. Relationship between intraoperative femoral head bleeding and development of avascular necrosis after acetabular fracture surgery. J Surg Orthop Adv. 2009;18:129–133. [PubMed] [Google Scholar]
- 26.Resch H, Beck E, Bayley I. Reconstruction of the valgus-impacted humeral head fracture. J Shoulder Elbow Surg. 1995;4:73–80. doi: 10.1016/S1058-2746(95)80073-5. [DOI] [PubMed] [Google Scholar]
- 27.Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16:202–207. doi: 10.1016/j.jse.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91:1689–1697. doi: 10.2106/JBJS.H.00133. [DOI] [PubMed] [Google Scholar]
- 29.Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18:837–844. doi: 10.1016/j.jse.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7:85–89. doi: 10.1016/S1058-2746(98)90215-4. [DOI] [PubMed] [Google Scholar]