Abstract
Background
In Western countries, deep vein thrombosis (DVT) and pulmonary embolism (PE), are relatively common after THA and many surgeons recommend routine pharmacologic thromboprophylaxis. There is some suggestion in the literature that the incidences of DVT and PE may be lower in East Asian patients. Therefore, it would be important to establish the incidences in a large number of East Asian patients who did not receive pharmacologic thromboprophylaxis.
Purpose
We therefore determined the incidence of DVT and PE and evaluated the associated risk factors in a series of East Asian patients who underwent primary THA without pharmacologic prophylaxis.
Methods
We retrospectively evaluated all 861 patients who underwent 992 elective primary THAs from May 2003 to December 2009. We identified patients with symptomatic DVT, symptomatic PE, and fatal PE. For potential risk factors we considered age, gender, body mass index (BMI), administration of aspirin, type of anesthesia, operation time, approach, simultaneous bilateral THAs, and duration of immobilization between symptomatic and asymptomatic patients.
Results
We identified eight patients with symptomatic DVT, one of whom also had a symptomatic PE; there were no cases of fatal PE. The incidences of fatal PE, symptomatic PE, and symptomatic DVT were 0 %, 0.1 %, and 0.8 %, respectively. Longer duration of immobilization predicted symptomatic DVT or PE.
Conclusions
East Asian patients have a low incidence of symptomatic DVT and PE and virtually no fatal PEs after primary THA. The incidences and risk factors should be taken into consideration when deciding whether to prophylactically treat these patients with pharmacologic agents.
Level of Evidence
Level IV, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
DVT is relatively common, and PE, although uncommon, is a serious and potentially life-threatening complication after THA. In Western countries, approximately 3% of patients have symptomatic DVT or PE after THA without thromboprophylaxis [32, 33]. Moreover, the incidence of imaging-confirmed asymptomatic DVT and PE after THA without thromboprophylaxis reportedly ranges from 40% to 79% and from 7% to 30%, respectively [6, 8, 10, 20]. Owing to such high incidences in western countries, the American Academy of Orthopaedic Surgeons (AAOS) [11], the American College of Chest Physicians (ACCP) [10], and the National Institute for Health and Clinical Excellence (NICE) [12], recommended routine use of thromboprophylactic agents including aspirin, low-molecular-weight heparin, and warfarin for patients undergoing THA. However, these agents expose patients to an 1.5-fold to threefold increased risk of bleeding complications including substantial bleeding or hematomas at the operative site and fatal bleeding at a nonoperative site such as the intestine [4, 29].
For East Asian patients, the reported rates of imaging-confirmed asymptomatic DVT and PE after THA range from 10% to 16% and from 2% to 6%, respectively, and routine use of pharmacologic prophylaxis is controversial [13, 14, 36]. However, we are not aware of such a study on symptomatic DVT and PE after THA with East Asian patients, although the objectives of prophylaxis are to prevent fatal PE and reduce the symptomatic morbidity associated with PE and DVT [12]. To determine whether to use a thromboprophylactic agent, a study of symptomatic DVT and PE in a large number of patients not receiving any pharmacologic thromboprophylaxis is necessary.
We therefore determined the incidence of symptomatic DVT, PE, and fatal PE in East Asian patients and evaluated the risk factors associated with DVT and PE in patients undergoing primary cementless THA.
Patients and Methods
We retrospectively reviewed the medical records of all 869 patients who underwent 1025 elective primary THAs from May 2003 to December 2009. Eight patients were treated with heparin and warfarin for thromboprophylaxis and were excluded from the study: two patients (two hips) with a history of a thromboembolic event and six patients (six hips) with cardiac disease before surgery. This left 861 patients (1017 hips) for the study. Simultaneous bilateral THAs were performed in 25 patients, who were considered to have had one procedure. Therefore, the total number of procedures was 992 in 861 patients. Of the 861 patients, 65 (74 hips) took aspirin-containing compounds or other antiplatelet agents before surgery. Because their medications were discontinued 5 to 7 days before surgery, these patients were not excluded [7, 26]. There were 414 women and 447 men with a mean age of 51.1 years (range, 18–83 years) at the time of the operation. Their mean BMI was 24.2 kg/m2 (range, 14.6–38.8 kg/m2), and the most common diagnosis for THA was osteonecrosis of the femoral head (564 hips, 55.5 %) (Table 1). In 857 patients who survived longer than 6 months postoperatively, the mean followup was 42.8 months (range, 6–91 months).
Table 1.
Diagnoses
| Diagnosis | Number of hips (N = 1017) |
|---|---|
| Femoral head osteonecrosis | 564 |
| Osteoarthritis | 187 |
| Sequelae of hip infection | 87 |
| Sequelae of Legg-Calve-Perthes disease | 62 |
| Miscellaneous condition | 117 |
Cementless fixation was used for the acetabular cup and femoral stem in all patients. Regional anesthesia was used in 876 procedures, and general anesthesia in 116 procedures. All procedures were performed with the patients in a lateral position. The posterolateral approach was used in 723 procedures, and the anterolateral approach was used in 269 procedures. In 93 procedures, wider exposure was necessary and the approach was extended to a triradiate approach, combined anterior and posterior approach, or transtrochanteric approach during the operation. The median operating time was 125 minutes (range, 50–535 minutes).
No pharmacologic or mechanical prophylaxis was used postoperatively in any of the 861 patients. However, thigh-length antiembolic stockings were applied and the patients were encouraged to use an ankle pump while in bed during the hospitalization. On postoperative Days 1 to 3, closed suction drainage was removed and patients were mobilized to a wheelchair. On postoperative Days 3 to 10, patients walked with restricted weightbearing and use of assistive devices (wheelchair, walker, crutches, or cane). As the walking ability improved, the assistive devices were changed as determined appropriate by a physical therapist. The mean length of hospital stay was 15.0 days (range, 6–28 days).
After the operation, we routinely monitored patients for clinical signs of DVT including pain and tenderness in the calf or thigh, swelling or erythema of the surgically treated limb, and a positive Homans’ sign. We suspected DVT or PE in 32 patients and consulted the cardiovascular physicians. A diagnosis of DVT was confirmed by duplex ultrasonography or lower extremity CT angiography. A PE was confirmed by a ventilation/perfusion scan or pulmonary CT angiography. Patients who were diagnosed as having a DVT or PE were treated with warfarin. Patients were monitored for 1 to 3 weeks in the ward.
Most deaths attributable to PE related to surgery reportedly occur within 3 months and any death of unknown cause that occurs within 3 months of surgery is considered to be the result of PE [5, 11, 33, 35]. We confirmed the fatal PE, if present, from the death certificate.
After discharge, patients were followed routinely at 6 weeks, 3 months, and 6 months postoperatively with specific attention given to the development of DVT or PE, although no patients were recalled specifically for this study.
Four patients died of causes unrelated to the operation within 6 months after surgery. Eight hundred thirty-eight patients visited the outpatient clinic for followups once or more after 6 months postoperative. Nineteen patients, who were unable to return, were visited or contacted by telephone by two nurses and one private locator.
We determined the incidence of symptomatic DVT, PE, and fatal PE. To determine confounding factors, univariate comparisons between the VTE group and the non-VTE group were made based on the demographic data and operative parameters, including age, gender, BMI, administration of aspirin, type of anesthesia, operation time, approach, simultaneous bilateral THAs, and duration of postoperative immobilization in bed. We used Fisher’s exact test for categorized data and the Mann-Whitney U test for continuous data. For the variables with a p value less than 0.1 in the univariate analyses, multivariate logistic regression analyses using the enter method were performed. The independent variables tested for the multivariate logistic regression analyses included age, gender, anesthesia, and duration of immobilization, as confounding factors; the dependent variable was whether the DVT occurred postoperatively. From the multivariate regression analyses, it was assessed which variables were the risk factors for occurrence of DVT. Statistical analyses were conducted using SPSS for Windows statistical package (version 12.0; SPSS, Chicago, IL, USA).
The design and protocol of this study were approved by the institutional review board in our hospital, who waived informed consent.
Results
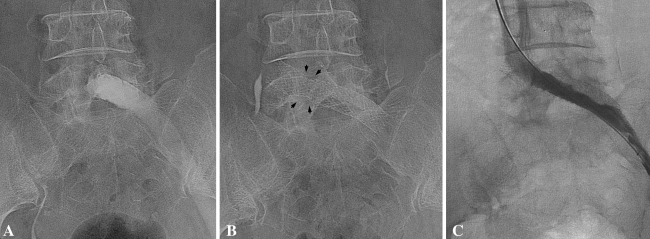
No patient had a fatal PE within 6 months after the operation. Symptomatic DVT occurred in eight patients and one of these eight had a symptomatic PE (Table 2). Incidences of fatal PE, symptomatic PE, and symptomatic DVT in our patients were 0%, 0.1%, and 0.8%, respectively. Seven of the eight patients were females and their mean age was 62.7 years. Symptomatic DVT developed 10 to 47 days after the operation (mean, 21 days). One of these patients had May-Thurner syndrome, a rare condition in which DVT occurs in the iliofemoral vein owing to compression of the left common iliac vein by the overlying right common iliac artery (Fig. 1) (Table 2) [18]. The eight patients were treated with intravenous heparin followed by oral warfarin for 2 to 12 months.
Table 2.
Patients with DVT and PE after THA
| Patient | Gender/ Age (years) | Diagnosis | Type of anesthesia | Approach | Operation time (minutes) | Interval between operation and DVT or PE | Location | Predisposing factors |
|---|---|---|---|---|---|---|---|---|
| 1 | F/71 | Osteoarthritis | Spinal | PL | 110 | 38 days | Bilateral DVT, PE | Delayed ambulation owing to pneumonia |
| 2 | M/69 | Osteoarthritis | Spinal | PL | 105 | 13 days | Bilateral DVT | Delayed ambulation owing to severe osteoarthritis of the knee |
| 3 | F/66 | Osteoarthritis | Spinal | PL | 100 | 25 days | Bilateral DVT | May-Thurner syndrome |
| 4 | F/59 | Sequelae of infection | Spinal | Combined | 160 | 16 days | Bilateral DVT | Delayed ambulation owing to tingling sensation associated with limb lengthening |
| 5 | F/62 | Sequelae of LCP | Spinal | AL | 123 | 10 days | Ipsilateral DVT | Delayed ambulation owing to sciatic and femoral nerve palsy |
| 6 | F/51 | Sequelae of infection | General | AL | 285 | 47 days | Ipsilateral DVT | Prolonged bed rest attributable to periprosthetic acetabular fracture and sciatic nerve palsy |
| 7 | F/69 | Osteonecrosis | General | AL | 127 | 22 days | Ipsilateral DVT | Prolonged bed rest attributable to periprosthetic femoral fracture |
| 8 | F/57 | Osteoarthritis | General | AL | 310 | 18 days | Unilateral DVT | Simultaneous bilateral THAs |
PL = posterolateral approach; AL = anterolateral approach; DVT = deep vein thrombosis; PE = pulmonary embolism; LCP = Legg-Calve-Perthes disease; THA = total hip arthroplasty.
Fig. 1A–C.
(A) The left common iliac vein is occluded at the level of the overlying right common iliac artery. (B) After balloon angioplasty and stent insertion, the compressed portion of left common iliac is seen (black arrows). (C) The postangioplasty angiogram shows a patent left iliac vein
In the univariate comparisons, age (p = 0.017), gender (p = 0.032), anesthesia (p = 0.056), and duration of immobilization (p = 0.016) had p values less than 0.1 (Table 3). However, the multivariate logistic regression analyses showed that only duration of immobilization was associated with the DVT (OR = 2.327; 95% CI, 1.030–5.262; p = 0.042). The value of R2 coefficient for this multivariate regression model was 0.352, suggesting that this multivariate model would explain the variation of the outcome variable to the extent of 35.2%.
Table 3.
Potential risk factors for DVT and PE
| Factor | DVT | Without DVT | p Value |
|---|---|---|---|
| Age (years) | 62.7 ± 6.8 | 51.0 ± 14.8 | 0.017 |
| Gender | 0.032 | ||
| Male | 1 | 446 | |
| Female | 7 | 407 | |
| BMI (kg/m2) | 25.8 ± 2.1 | 24.2 ± 3.4 | 0.1 |
| Aspirin or antiplatelet medication | 0.463 | ||
| No | 7 | 911 | |
| Yes | 1 | 73 | |
| Anesthesia | 0.056 | ||
| Regional (spinal/epidural) | 5 | 871 | |
| General | 3 | 113 | |
| Operation time (minutes) | 165.0 ± 84.1 | 138.7 ± 53.6 | 0.569 |
| Approach | 0.223 | ||
| Posterolateral | 4 | 719 | |
| Anterolateral | 4 | 265 | |
| Simultaneous bilateral THAs | 1 | 24 | 0.185 |
| Unilateral THA | 7 | 960 | |
| Duration of postoperative immobilization (days) | 16.9 ± 23.0 | 3.0 ± 1.0 | 0.016 |
DVT = deep vein thrombosis; BMI = body mass index; THA = total hip arthroplasty.
After multivariate logistic regression analyses, longer duration of immobilization after THA was a risk factor.
Discussion
Unlike in Western populations in whom the high incidence of DVT and PE require establishing guidelines for routine thromboprophylaxis after THA, baseline data were necessary before developing guidelines for East Asian patients undergoing THA. We questioned (1) the incidence of symptomatic DVT, PE, and fatal PE, in East Asian patients and (2) the risk factors associated with DVT and PE in patients undergoing primary cementless THA.
Our study has some limitations. First, our study was retrospective, not prospective. However, we recognized DVT as one of the most serious complications after THA, and being concerned about the incidence and risk factors of DVT, during the study period we routinely monitored our patients for clinical signs of DVT after surgery. In addition, the validity of medical records can influence reliability of a retrospective study. Because our institute has used a fully integrated electronic medical record system (EMR) since May 2003, there was little possibility of loss of the medical records. Second, we had a low number of patients with DVT or PE and no patients with a fatal PE, so we can draw few definitive conclusions regarding risk factors. Third, we did not perform confirmatory studies in asymptomatic patients and could not determine the incidence of asymptomatic DVT or PE. However, considering the objectives of prophylaxis in DVT are to prevent fatal PE and to reduce the symptomatic morbidity associated with DVT, we believe studies of symptomatic DVT are most relevant. Further, imaging studies to confirm asymptomatic DVT are associated with procedure-related complications and high medical costs [1, 3], and routine use of these studies in asymptomatic East Asian patients is difficult to justify.
We found low incidences of fatal PE, symptomatic PE, and symptomatic DVT even without routine thromboprophylaxis after primary cementless THA. The incidences were much lower than those after THA in Western patients who received thromboprophylaxis [4, 23, 28]. In previous epidemiologic studies of Asian populations without thromboprophylaxis, the rate of DVT varied considerably, ranging from 1.0% to 64.3%, higher than our rate (Table 4) [2, 9, 16, 17, 25]. However, these studies have several limits. Most of them used data collected from various areas of Asia and included multiple ethnicities other than just East Asian patients. The majority of these studies included other procedures such as total knee arthroplasty and included a small number of patients having THA. The outcome variable included asymptomatic DVT. In the SMART study [16], which evaluated symptomatic DVT and PE in 2420 Asian patients undergoing orthopaedic surgery in 39 centers in 11 Asian countries, the rate of symptomatic DVT in 408 THAs was 1.0%, which was similar to our rate (Table 4). One explanation for the low incidences of fatal PE, symptomatic PE, and symptomatic DVT in our patients might be the low prothrombotic risk factors and absence of some genetic factors involved with DVT in East Asian patients [13, 15, 34]. Previous studies showed that several genetic polymorphisms are associated with lower incidences of DVT and PE in East Asian patients than in Western populations [13, 24, 27, 31]. Unlike Western populations who undergo THA mainly for primary osteoarthritis, the most common diagnosis of osteonecrosis, younger age with a mean of 51.1 years, and lower mean BMI of 24.2 (kg/m2) might be reasons for the lower incidence.
Table 4.
Published studies of the incidence of DVT and PE after THA
| Study | Number of THAs | Number of DVTs (%) | Number of PEs (%) |
|---|---|---|---|
| Dhillon et al. [9] | 14 | 9 (64.3)* | 0 |
| Leizorovicz et al. [17] | 408 | 4 (1.0) | 0 (0) |
| Piovella et al. [25] | 121 | 31 (25.6)* | UI |
| Bagaria et al. [2] | 23 | 2 (8.6)* | UI |
| Leizorovicz [16] | 134 | 1 (0.7)/22 (16.4)* | 0 (0) |
| Current study | 992 | 8 (0.8) | 1 (0.1) |
* Including asymptomatic DVT; THA = total hip arthroplasty; DVT = deep vein thrombosis; PE = pulmonary embolism; UI = unidentified.
Older age, female gender, obesity, underlying disease, type of anesthesia, simultaneous bilateral THAs, surgical approach, and prolonged immobilization in bed are known risk factors for DVT and PE in studies of patients from Western countries [2, 19, 21, 30]. After multivariate analysis in this study, prolonged immobilization was identified as a risk factor for DVT. The fact that the other factors studied did not predict DVT could be related to ethnic differences between our patients and Western patients or to relatively small numbers of patients with inadequate power to discern differences.
In Western countries, routine use of pharmacologic prophylaxis has been recommended to reduce the incidence of asymptomatic DVT, on the assumption it will reduce the incidence of symptomatic PE and the overall rate of mortality [22]. However, the objectives of prophylaxis are to prevent fatal PE and reduce the symptomatic morbidity associated with PE and DVT [12]. Furthermore, pharmacologic prophylaxis is associated with a potential risk of a bleeding complication at the operative or nonoperative site [4]. We found that East Asian patients who have undergone THAs have a low incidence of symptomatic DVT and PE, and prolonged immobilization was identified as a risk factor of DVT or PE.
Acknowledgement
We thank Chong Bum Chang MD for statistical advice.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was waived by the institutional review board of their hospital.
This work was performed at the Department of Orthopaedic Surgery, Seoul National University Bundang Hospital.
References
- 1.Albrechtsson U, Olsson CG. Thrombotic side-effects of lower-limb phlebography. Lancet. 1976;1:723–724. doi: 10.1016/S0140-6736(76)93094-4. [DOI] [PubMed] [Google Scholar]
- 2.Bagaria V, Modi N, Panghate A, Vaidya S. Incidence and risk factors for development of venous thromboembolism in Indian patients undergoing major orthopaedic surgery: results of a prospective study. Postgrad Med J. 2006;82:136–139. doi: 10.1136/pgmj.2005.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettmann MA, Robbins A, Braun SD, Wetzner S, Dunnick NR, Finkelstein J. Contrast venography of the leg: diagnostic efficacy, tolerance, and complication rates with ionic and nonionic contrast media. Radiology. 1987;165:113–116. doi: 10.1148/radiology.165.1.3306781. [DOI] [PubMed] [Google Scholar]
- 4.Burnett RS, Clohisy JC, Wright RW, McDonald DJ, Shively RA, Givens SA, Barrack RL. Failure of the American College of Chest Physicians-1A protocol for lovenox in clinical outcomes for thromboembolic prophylaxis. J Arthroplasty. 2007;22:317–324. doi: 10.1016/j.arth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J, Jr, Hobbins TE, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 6.Clagett GP, Anderson FA, Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S–334S. doi: 10.1378/chest.108.4_Supplement.312S. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AT, Skinner JA, Kakkar VV. Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ. 1994;309:1213–1215. doi: 10.1136/bmj.309.6963.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coventry MB, Nolan DR, Beckenbaugh RD. “Delayed” prophylactic anticoagulation: a study of results and complications in 2, 012 total hip arthroplasties. J Bone Joint Surg Am. 1973;55:1487–1492. [PubMed] [Google Scholar]
- 9.Dhillon KS, Askander A, Doraismay S. Postoperative deep-vein thrombosis in Asian patients is not a rarity: a prospective study of 88 patients with no prophylaxis. J Bone Joint Surg Br. 1996;78:427–430. [PubMed] [Google Scholar]
- 10.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW, Ammerican College of Chest Physicians Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th Edition. Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 11.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge: an underestimated risk. Arch Surg. 1992;127:310–313. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar VV, Stringer MD. Prophylaxis of venous thromboembolism. World J Surg. 1990;14:670–678. doi: 10.1007/BF01658824. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Kim JS. The 2007 John Charnley Award Factors leading to low prevalence of DVT and pulmonary embolism after THA: analysis of genetic and prothrombotic factors. Clin Orthop Relat Res. 2007;465:33–39. doi: 10.1097/BLO.0b013e318156bfac. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, Suh JS. Low incidence of deep-vein thrombosis after cementless total hip replacement. J Bone Joint Surg Am. 1988;70:878–882. [PubMed] [Google Scholar]
- 15.Klatsky AL, Armstrong MA, Poggi J. Risk of pulmonary embolism and/or deep venous thrombosis in Asian-Americans. Am J Cardiol. 2000;85:1334–1337. doi: 10.1016/S0002-9149(00)00766-9. [DOI] [PubMed] [Google Scholar]
- 16.Leizorovicz A. Epidemiology of post-operative venous thromboembolism in Asian patients: results of the SMART venography study. Haematologica. 2007;92:1194–1200. doi: 10.3324/haematol.10819. [DOI] [PubMed] [Google Scholar]
- 17.Leizorovicz A, Turpie AG, Cohen AT, Wong L, Yoo MC, Dans A, SMART Study Group Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis: the SMART study. J Thromb Haemost. 2005;3:28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 18.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419–427. doi: 10.1177/000331975700800505. [DOI] [PubMed] [Google Scholar]
- 19.Memtsoudis SG, Besculides MC, Gaber L, Liu S, Gonzalez Della Valle A. Risk factors for pulmonary embolism after hip and knee arthroplasty: a population-based study. Int Orthop. 2009;33:1739–1745. doi: 10.1007/s00264-008-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miric A, Lombardi P, Sculco TP. Deep vein thrombosis prophylaxis: a comprehensive approach for total hip and total knee arthroplasty patient populations. Am J Orthop (Belle Mead NJ) 2000;29:269–274. [PubMed] [Google Scholar]
- 21.Mraovic B, Hipszer BR, Epstein RH, Pequignot EC, Parvizi J, Joseph JI. Preadmission hyperglycemia is an independent risk factor for in-hospital symptomatic pulmonary embolism after major orthopedic surgery. J Arthroplasty. 2010;25:64–70. doi: 10.1016/j.arth.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Paiement GD, Wessinger SJ, Hughes R, Harris WH. Routine use of adjusted low-dose warfarin to prevent venous thromboembolism after total hip replacement. J Bone Joint Surg Am. 1993;75:893–898. doi: 10.2106/00004623-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen AB, Sorensen HT, Mehnert F, Overgaard S, Johnsen SP. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg Am. 2010;92:2156–2164. doi: 10.2106/JBJS.I.00882. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini VD, Jr, Clement D, Lush-Ehmann C, Keller GS, Evarts CM. The John Charnley Award Natural history of thromboembolic disease after total hip arthroplasty. Clin Orthop Relat Res. 1996;333:27–40. doi: 10.1097/00003086-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Piovella F, Wang CJ, Lu H, Lee K, Lee LH, Lee WC, Turpie AG, Gallus AS, Planes A, Passera R, Rouillon A, AIDA investigators Deep-vein thrombosis rates after major orthopedic surgery in Asia: an epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3:2664–2670. doi: 10.1111/j.1538-7836.2005.01621.x. [DOI] [PubMed] [Google Scholar]
- 26.Price MJ. Monitoring platelet function to reduce the risk of ischemic and bleeding complications. Am J Cardiol. 2009;103(3 suppl):35A–39A. doi: 10.1016/j.amjcard.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet. 1995;346:1133–1134. doi: 10.1016/S0140-6736(95)91803-5. [DOI] [PubMed] [Google Scholar]
- 28.Samama CM, Ravaud P, Parent F, Barre J, Mertl P, Mismetti P. Epidemiology of venous thromboembolism after lower limb arthroplasty: the FOTO study. J Thromb Haemost. 2007;5:2360–2367. doi: 10.1111/j.1538-7836.2007.02779.x. [DOI] [PubMed] [Google Scholar]
- 29.Schulman S, Beyth RJ, Kearon C, Levine MN, American College of Chest Physicians Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):257S–298S. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- 30.Sikorski JM, Hampson WG, Staddon GE. The natural history and aetiology of deep vein thrombosis after total hip replacement. J Bone Joint Surg Br. 1981;63:171–177. doi: 10.1302/0301-620X.63B2.7217137. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DP, Roberts HR. Hypercoagulability in venous and arterial thrombosis. Ann Intern Med. 1997;126:638–644. doi: 10.7326/0003-4819-126-8-199704150-00009. [DOI] [PubMed] [Google Scholar]
- 32.Warwick D. New concepts in orthopaedic thromboprophylaxis. J Bone Joint Surg Br. 2004;86:788–792. doi: 10.1302/0301-620X.86B6.15085. [DOI] [PubMed] [Google Scholar]
- 33.Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement: a series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77:6–10. [PubMed] [Google Scholar]
- 34.Westrich GH, Weksler BB, Glueck CJ, Blumenthal BF, Salvati EA. Correlation of thrombophilia and hypofibrinolysis with pulmonary embolism following total hip arthroplasty: an analysis of genetic factors. J Bone Joint Surg Am. 2002;84:2161–2167. doi: 10.2106/00004623-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Wroblewski BM, Siney PD, White R. Fatal pulmonary embolism after total hip arthroplasty: seasonal variation. Clin Orthop Relat Res. 1992;276:222–224. [PubMed] [Google Scholar]
- 36.Yoo MC, Kang CS, Kim YH, Kim SK. A prospective randomized study on the use of nadroparin calcium in the prophylaxis of thromboembolism in Korean patients undergoing elective total hip replacement. Int Orthop. 1997;21:399–402. doi: 10.1007/s002640050194. [DOI] [PMC free article] [PubMed] [Google Scholar]