Abstract
The amyloid-beta (Aβ) peptide is the derivative of amyloid precursor protein (APP) generated through sequential proteolytic processing by β- and γ-secretases. Excessive accumulation of Aβ, the main constituent of amyloid plaques, has been implicated in the etiology of Alzheimer disease (AD). It was found recently that the impairments of neurogenesis in brain were associated with the pathogenesis of AD. Furthermore recent findings implicated that APP could function to influence proliferation of neural progenitor cells (NPC) and might regulate transcriptional activity of various genes. Studies demonstrated that influence of neurogenesis by APP is conferred differently via its two separate domains, soluble secreted APPs (sAPPs, mainly sAPPα) and APP intracellular domain (AICD). The sAPPα was shown to be neuroprotective and important to neurogenesis, whereas AICD was found to negatively modulate neurogenesis. Furthermore, it was demonstrated recently that microRNA could function to regulate APP expression, APP processing, Aβ accumulation and subsequently influence neurotoxicity and neurogenesis related to APP, which was implicated to AD pathogenesis, especially for sporadic AD. Based on data accumulated, secretase balances were proposed. These secretase balances could influence the downstream balance related to regulation of neurogenesis by AICD and sAPPα as well as balance related to influence of neuron viability by Aβ and sAPPα. Disruption of these secretase balances could be culprits to AD onset.
Key words: Alzheimer disease, amyloid-beta, amyloid precursor protein, neural progenitor cells, neurodegeneration, neurogenesis
Neurodegenerative diseases such as Alzheimer disease (AD) are characterized by the progressive loss of neurons which are regionspecific in the brain. Accumulative evidences support the amyloid hypothesis for AD pathogenesis that amyloid-beta (Aβ), derived from amyloid precursor protein (APP), plays a crucial initial role that triggers a complex pathological cascade which leads to the neurodegenerative conditions observed in the disorder.1 Recently, the presence of adult neurogenesis has been demonstrated, which impact our understanding of physiology and pathology of brain significantly. Furthermore, it was also demonstrated recently that APP could play a role in influencing neurogenesis via its two separate domains, the soluble secreted APPs (sAPPs, mainly sAPPα) and the APP intracellular domain (AICD). The sAPPα was shown to protect neuron cells and promote neurogenesis, whereas AICD was found to negatively modulate neurogenesis. Therefore, questions were raised on whether APP could contribute to AD pathogenesis via influence of adult neurogenesis by APP processing fragments, besides via Aβ-induced toxicity. Furthermore it was demonstrated recently that microRNA (miRNAs) could function to regulate APP expression, APP processing, Aβ accumulation and subsequently lead to altered Aβ toxicity or influence neurogenesis, which was implicated to AD pathogenesis, especially for sporadic AD. Therefore dysregulation of miRNAs could be the causes for alteration of APP expression and APP processing, leading to subsequent changes in neuron viability and neurogenesis, which could be implicated to AD. Based on data accumulated so far, secretase balances related to APP processing were proposed. These secretase balances could influence the downstream balances related to AICD-induced inhibition of neurogenesis, sAPPα-induced neuroprotection and promotion of neurogenesis as well as Aβ-induced neurotoxicity. Disruption of these secretase balances could disrupt downstream balances and finally contribute to AD. This review highlights and discusses recent new findings focusing on roles of APP in neurogenesis, which would be significant to pathogenesis and therapeutic applications of AD and even other neurodegenerative diseases.
Etiology of AD Related to APP
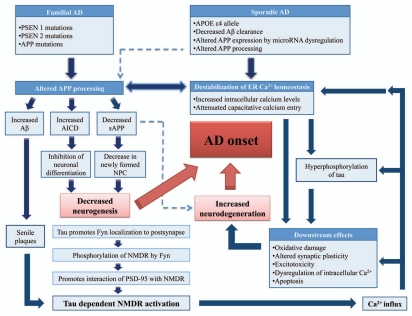
AD is the most common form of senile dementia that affects more than 30 million individuals worldwide. It is a degenerative neurological disorder characterized by gradual memory loss, cognitive impairments and deterioration of language skills.2 The disorder is characterized by neuropathological hallmarks which include the development of neuritic plaques constituting cores of aggregated Aβ derived from the APP and neurofibrillary tangles (NFT) composed of abnormally hyperphosphorylated tau (τ) proteins.3–5 Such features indicative of AD are further accompanied by gliosis, synaptic loss and neuronal death.6 Although age and environmental factors might increase the risk of the disorder, significant genetic background is implicated in AD. Based on symptomatology, the rare autosomal dominant inherited forms of early-onset AD has been linked with mutations in APP (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) genes.7,8 In contrast, the sporadic late-onset form accounting for the majority of all AD cases has been consistently associated with the presence of the apolipoprotein E (APOE) ε4 allele.9 Other susceptibility genetic factors include α2-macroglobulin, the dihydrolipoyl succinyltransferase, which is a component of α-ketoglutarate dehydrogenase, the K-variant of butyryl-cholinesterase and multiple mitochondrial genes.10–12 Epidemiological studies also demonstrated several tentative associations which can be linked to a decreased reserve capacity of the brain, previous head injury and cardiovascular disease.13 To date, the cause of both forms of AD is not well established as a conclusive molecular mechanism remains obscure.
However, a major pathogenic mechanism widely accepted to be relevant for the etiology of AD is the “amyloid cascade hypothesis.” 1 Previous studies had implicated that aggregated Aβ proteins could induce neurotoxicity via increased reactive oxygen species (ROS). The detailed mechanisms on APP induced ROS production and related to neurodegeneration in AD could be found in recent published review papers in references 14 and 15. On the other hand, dysregulation of intracellular calcium is significantly involved in the toxicity of APP related to pathogenesis of neurodegeneration in AD.16 It has been known that aggregated Aβ proteins could induce calcium influx into neurons and increase intracellular calcium concentration.15,17 Furthermore presenilins, γ-secretases responsible for APP cleavage, was found to be involved in the regulation of intracellular calcium stores.18 The detailed informations related to APP, presenilins, calcium and AD onset could be found in some well written recent reviews in reference 19. Therefore the aggregated Aβ proteins could contribute to neurodegeneration via increased ROS and dysregulated intracellular calcium as converging steps for “amyloid cascade hypothesis” related to pathogenesis of AD.
Furthermore, recent findings implicated that the APP induced neurotoxicity was tau-dependent.20–26 Tau protein is known for its role in the stabilization of microtubules, which is important for the generation and maintenance of neurite.20–23 In AD, tau loses its microtubule-binding and stabilizing function and form neurofibrillary tangles leading to the degeneration of neurons, which was implicated to AD pathogenesis.24 It was reported that tau inhibits transport of APP into axons and dendrites, causing its accumulation in the cell body.26 Furthermore recent new studies provided strong evidence for tau-dependent Aβ toxicity.25 They found that tau, which was known to be axonal protein, has a dendritic function in postsynaptic targeting of Fyn, the Src kinase. One of Fyn substrates is the NMDA receptor (NR). Missorting of tau in transgenic mice expressing truncated tau or absence of tau in tau knockout mice both disturb postsynaptic targeting of Fyn. Hereby alleviated NR-mediated excitotoxicity and abrogated Aβ toxicity. A model was proposed that Fyn localized to the postsynapse in a tau-dependent manner and phosphorylated the NR subunit NR2b at Y1472. This phosphorylation promotes the interaction of NRs with PSD–95, a scaffolding protein. The interaction enhances the stability of NRs within the postsynaptic density and facilitates NRs to excitotoxic downstream signaling, which could be the downstream pathway of Aβ induced toxicity. Hence, decrease of tau level or targeting of tau-dependent toxic mechanisms, such as the Fyn-mediated interaction of NRs and PSD-95, could be suitable strategies for therapy of AD and related disorders.
Molecular Structure of APP
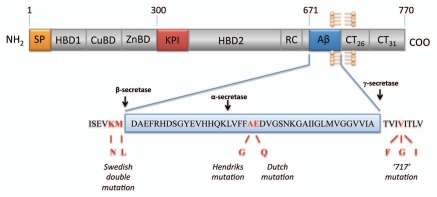
Human APP belongs to a highly conserved family of type 1 transmembrane glycoproteins which constitutes APP and the mammalian homologs APLP-1 and APLP-2, both homologs lacking the Aβ sequence.27–30 The evolutionary conservation of APP gene family also extends to invertebrate species with its orthologs APPL in Drosophila and APL-1 in Caenorhabditis elegans respectively.31,32 These proteins all share several conserved motifs within the large extracellular domain and a short cytoplasmic region which exhibits the highest sequence homology.33 The human APP gene contains 18 exons spanning more than 170 kbp.34 The region encoding the Aβ sequence comprises part of exons 16 and 17 and is composed of 40 to 43 amino acid residues that extend from the ectodomain into the transmembrane domain of this protein (Fig. 1). The presence of multiple distinct domains located within the extracellular portion includes a signal peptide (SP), a heparin-binding/growth-factor-like domain 1 (HPBD1), a copper-binding domain (CuBD), a zinc-binding domain (ZnBD), a Kunitz-type protease inhibitor domain (KPI), a second heparin-binding domain 2 (HPBD2), a random coil region (RC) and the Aβ sequence (Fig. 1). The remaining region consists of the cytoplasmic tail of APP, including AICD. Several isoforms of APP that arises from alternative splicing have been identified and the most common forms differ mainly by the absence (APP-695) or presence (alternatively spliced APP-751 and APP-770) of a KPI domain.35,36
Figure 1.
Schematic diagram of APP consisting of a large extracellular domain, a hydrophobic transmembrane domain and a short cytoplasmic carboxyl terminus. The protein is proteolytically processed by different secretases via amyloidgenic and non-amyloidgenic proceeding pathways which either releases the Aβ peptide (cleaved by β- and γ-secretase) or precludes Aβ formation (cleaved by α-secretase).
Trafficking and Proteolytic Processing of APP
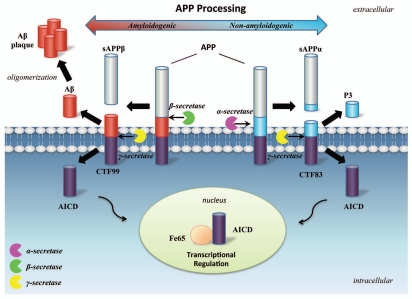
APP can undergo amyloidogenic or non-amyloidogenic processing via cleavage by different secretases.37 The amyloidogenic processing of APP, cleaved initially by β-secretase, produces a long soluble secreted form of APP (sAPPβ) and a carboxy-terminal fragment (CTF99) containing the Aβ sequence and AICD. In the brain, β-site APP cleaving enzyme (BACE1) has been found to be the major β-secretase.38 In the alternative non-amyloidogenic pathway, APP could also be proteolytically processed by a presenilin-containing α-secretase complex, which cleaves at a site within Aβ sequence and consequently abrogates Aβ formation.37 The non-amyloidogenic cleavage releases a carboxy-terminal fragment (CTF83) and another soluble fragment (sAPPα) which, in contrast to Aβ, may be neuroprotective.39–41 Both CTF99 and CTF83 fragments are then sequentially cleaved within the transmembrane domain by γ-secretase to generate AICD and Aβ or p3 respectively.42 Elevated β-secretase levels were found to induce the increase of CTF99 and Aβ generation as well as the decrease of CTF83 and AICD generation in vitro. On the contrary, elevated levels of α-secretase was found to induce an increase in AICD levels.43 Therefore α-secretase and β-secretase cleavage of APP might influence subsequent AICD release differently. The details of amyloidogenic and non-amyloidogenic processing of APP are illustrated in (Fig. 2).
Figure 2.
APP processing procedure and cleavage products. The non-amyloidogenic APP processing pathway (right) involves proteolytic cleavages by α- and γ-secretases resulting in the generation of sAPPα and carboxyl terminal fragments including P3, CTF83 and AICD. The alternative amyloidogenic APP processing pathway (left) involves proteolytic cleavages by β- and γ-secretases resulting in the generation of sAPPβ and carboxyl terminal fragments including Aβ, CTF99 and and AICD. Aβ peptides could oligomerize and fibrillize leading to AD pathology. sAPPα could function to promote neurogenesis and survival, while AICD could have effects to inhibit neurogenesis possibly via forming complex with Fe65 and leading to transcriptional regulation.
Potential Neuronal Functions of APP
While Aβ is central to AD pathogenesis, the evolutionary conservation of APP and the presence of APP isoforms lacking Aβ sequence indicates that amyloidogenesis is unlikely the main physiological function of this protein family.44 Recent accumulative evidence demonstrated that APP is important for neuron generation, neuron differentiation and neural migration. In nematode Caenorhabditis elegans, loss of APL-1 by genetic inactivation resulted in postnatal lethality due to abnormalities in multiple developmental processes such as molting defects. This phenotype could be successfully rescued by expressing the extracellular domain of APL-1 in neurons.45 Furthermore APP was found to be important in Drosophila melanogaster, as deletion of the APPL gene leads to behavioral defects in phototaxis that could be partially rescued by human APP.46 Interestingly, highly elevated APPL levels were observed in regenerating neurons of a Drosophila brain injury model.47 In contrast, lack of this stress response in APPL mutant flies increased mortality.38 As an upregulation of APPL correlated with an increase in neurite arborization, a potential role in axonal outgrowth after traumatic brain damage was attributed to APP.47 Another study showed that APPL overexpression promoted synapse differentiation, while APPL mutants resulted in decreased synaptic bouton numbers at the neuromuscular junction in Drosophila.48
APP is also ubiquitously expressed in mammalian cells and was found to have complicated physiological roles in cell adhesion, neuronal differentiation, neuronal migration, neurite outgrowth and synapse formation.49–61 The immunoreactivity of APP was found to increase after brain injury of mice, which correlated well with traumatic brain injury.62 APP knockout mice showed reductions in weight, deficits in balance and strength, impairments in behavior and long-term potentiation.63–65 The evidence from other APP knockout in vivo animal model systems demonstrated potential roles of APP in neuron generation, differentiation as well as neural migration.66
Taken together, these findings corroborate a potential crucial role for APP as part of a complex mechanism involved in a wide variety of neuronal functions, including normal neural development or response to traumatic brain injuries. Cumulative evidence suggests that the soluble sAPPα is neuroprotective and is associated with growth factor-like functions, while the interaction of AICD with a myriad of proteins links it with diverse processes such as axonal transport and transcriptional regulation. The different neuronal roles of various APP fragments will be further discussed in details below.
Role of sAPPα in the Positive Regulation of Neurogenesis
The physiological functions of sAPPα have been implicated in the enhancement of synaptogenesis, neurite outgrowth, cell survival and cell adhesion.41,67 In separate reports, sAPPα has been observed to exert proliferative effects on NPC isolated from the embryonic brains.50,53 In 2005, Caille et al. first acquired evidence suggesting the in vivo role of sAPPα in adult neurogenesis.50 The authors found that sAPPα binds prominently to cells of the subventricular zone (SVZ), one of the two adult central nervous system sites harboring NPC that are capable of regeneration in the adult brain.50 Their findings suggested that sAPPα were likely to participate in the EGF-induced proliferation of type A cells, although sAPPα alone fails to induce proliferation of these cells.50 The authors also observed that infusion of sAPPα into the lateral ventricle of mice led to an increase in number of progenitor cells.50 Conversely, blocking sAPPα secretion by α-secretase inhibitor or downregulating APP synthesis by antisense oligonucleotide against APP decreases the proliferation of EGF responsive cells, which leads to a reduction of the pool of progenitors.50 Their results also showed that sAPPα activity may be delivered in an autocrine/paracrine manner.50 The crystal structure analysis at 1.8 Å resolution of APP further demonstrated that its cysteine-rich N-terminal heparin-binding domain is similar to other cysteine-rich growth factors, which is conceived to be responsible for its function to stimulate neurite outgrowth.68 These growth-promoting properties of sAPPs and its structural similarities with cysteine-rich growth factors suggest that sAPPs may function as a growth factor in vivo.68 It was reported that these growth-promoting properties of sAPPα are possibly mediated by the ability of sAPPα to downregulate CDK5 and inhibit tau hyperphosphorylation.40 Early in vitro studies have also demonstrated that sAPPα protects cultured neurons against hypoglycemia damage and glutamate neurotoxicity through the activation of potassium channels, which in turn mediates the ability of sAPPα to inhibit calcium influx and thus modulates neuronal excitability.69,70
Taken together, these results suggest that sAPPα might function as specific growth factors or as a mediator for adult NPC proliferation. However, to date, no sAPPα receptors have been identified yet and the signaling pathways triggered have not been thoroughly investigated. To this extent, it is of interest to note that two in vitro studies have reported a stimulation of MAP kinase activity by sAPPα and it would thus be of importance to dissect this signaling pathway triggered by sAPPα in detail.71,72 Intriguingly, sAPPα levels were shown to decrease in the cerebrospinal fluid (CSF) of AD individuals, while infusion of sAPPα into the brain increased synaptic density and improved memory retention.73 Therefore, these findings raised the possibility that sAPPα may contribute to neurogenesis in adult brain and sAPPα might be used for AD patients clinically, while decrease of sAPPα levels in brain may be an indispensable precondition for AD pathogenesis.
Role of AICD in the Negative Modulation of Neurogenesis
AICD was termed by analogy to NICD (Notch intracellular domain) formed by the regulated intramembrane proteolysis (RIP) of another type I transmembrane glycoprotein Notch. Both AICD and NICD were produced via cleavage of APP or Notch by the same γ-secretase complex respectively. Extracellular binding of Notch to its ligand is one of the mechanisms responsible for this regulation of cleavage, stimulating release of NICD in cells.74 The NICD translocates into the nucleus and leads to a series of downstream signaling cascades.74 Although multiple proteins have been reported to interact with AICD including Fe65 that may be necessary for AICD-dependent signaling, no functional ligands for APP have been characterized so far.75 Recently Ma et al. discovered that transient axonal glycoprotein 1 (TAG1), a neural cell adhesion molecule of the F3 family, acts as an extracellular binding partner for APP through the immunoglobulin (Ig) and fibronectin repeat (FNIII) domains of TAG1.75 The authors found that TAG1 and APP co-expressed in NPC in the neurogenic niche of the ventricular zone in developing mouse brains.75 It was also found that the extracellular interaction between APP and TAG1 was essential for initiating the release of AICD, which could be abrogated by the presence of specific γ-secretase inhibitors.75 It was further confirmed in knockout in vivo studies that the interaction between TAG1 and APP negatively modulates neurogenesis through release of AICD and triggers a Fe65-dependent molecular event.75 These findings provided valuable insights that APP could function as a transmembrane receptor protein which negatively mediates neurogenesis through recognition of its specific cell surface-associated ligands.75
However, the detailed mechanism by which AICD suppresses neurogenesis still remains to be elucidated. Several questions regarding TAG1-APP signaling pathway including its potential contributory roles in adult brain development and AD pathogenesis also remain unanswered. What physiological functions does this signaling pathway eventually mediate? As yet, few immediate downstream target genes have been identified for AICD and Fe65. Based on the resemblance of molecular structure and processing procedure between Notch and APP as well as known knowledge of the Notch cascade, it would be tempting to speculate that the AICD generated by γ-secretase cleavage may be capable of inducing an intracellular signaling pathway via modulation of gene expression after interaction with its adaptor protein Fe65. The interaction between AICD and Fe65 may promote the translocation of AICD directly to the nucleus or may initiate a Fe65-mediated nuclear signal independently of AICD translocation. However, the hypothesis that AICD could function to modulate transcriptional activity in cells appears highly controversial so far. Although numerous studies have suggested that AICD can regulate expression of various endogenous genes including KAI1, GSK-3b, APP and neprilysin, other groups were unable to replicate these findings.76,77 Therefore it would thus be necessary to delineate the downstream components of the TAG1-APP signaling cascade to clarify the precise mechanisms of negatively modulation of neurogenesis through this pathway. Knowledge of these mechanisms would be important in the context of AD as abnormal processing of APP may also result in aberrant AICD levels, which may be linked to abnormal intracellular signaling and thus consequently lead to AD pathogenesis.
To this extend, it is interesting to note that several recent studies have reported AICD-expressing transgenic mice recapitulate such AD-like pathological features as activation of GSK-3β, phosphorylation and aggregation of tau, memory deficits and aberrant neural activity and seizure susceptibility.77–79 Interestingly, these AD characteristic lesions are observed with negligible changes in APP metabolism or Aβ generation, demonstrating that AICD itself was capable of inducing the deleterious effects.78 The authors showed that overexpressed AICD impairs adult neurogenesis in transgenic mice through induction of neuroinflammation, which could be prevented by treatment with anti-inflammatory drugs that supports the potential of these drugs as prophylactic therapeutic agents.78,80 However although inflammation may play a pivotal role in impaired neurogenesis in AICD transgene mice, abnormally expressed AICD may also confer its deleterious effects via stimulation of GSK-3β activity and alteration of the activity of wnt signaling pathway, which has been shown to perturb neurogenesis in mice and in AD patients.81–83 This hypothesis ties in with previous studies suggesting that AICD is capable of regulating the expression of various endogenous genes including GSK-3b.77 In contrast, other groups were unable to replicate these findings.76,84 Therefore a conclusive understanding of the TAG1-APP signaling pathway related to regulation of neurogenesis by AICD would be necessary as it may offer unique opportunities for pharmacological intervention of AD in the future. The influence of neurogenesis by APP fragments was illustrated in Figure 3. The potential pathogenesis of AD related to APP is summarized and illustrated in Figures 4 and 5.
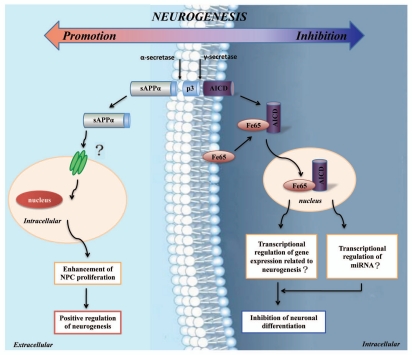
Figure 3.
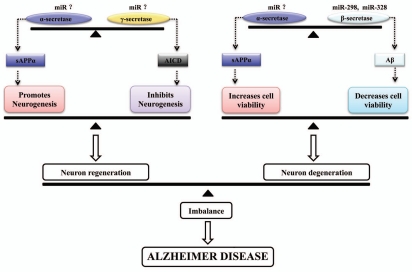
Schematic diagram for influence of neurogenesis by sAPPα and AICD. The α-secretases cleavage of APP could release sAPPα into extracellular space, which could function on cells possibly via binding with its specific receptors. The sAPPα could function to promote NPC proliferation and positively regulate neurogenesis. On the contrary, γ-secretases cleavage of APP could release AICD into cytoplasm, which could complex with Fe65 and enter nucleus. The AICD and Fe65 complex might function to transcriptionally regulate gene expressions related to neurogenesis, including miRNAs genes. Finally AICD will function to inhibit neuronal differentiation via so far unknown mechanisms, hereby negatively regulate neurogenesis.
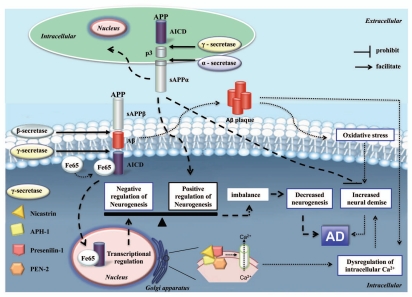
Figure 4.
The pathogenesis of AD involving Aβ-induced toxicity via increased ROS and dysregulation of intracellular calcium as well as altered neurogenesis due to imbalance between positive regulation of neurogenesis by sAPPα and negative regulation of neurogensis by AICD. β- and γ-secretases cleavage of APP could produce Aβ. The oligomerization and fibrillization of Aβ could lead to formation of Aβ plaque, leading to increased ROS and dysruption of intracellular calcium. The γ-secretases, presenilins, can function as ER calcium leak channels, which could aggravate the increased intracellular calcium due to Aβ accumulation. The ROS and dysregulation of intracellular calcium could contribute to neuron demise. On the other hand, the α-secretases cleavage of APP could lead to sAPPα formation. The sAPPα can function on secreted cell itself or nearby cells via autocrine or paracrine mechanisms. The sAPPα can promote neurogenesis via so far unknown mechanisms. Furthermore the sAPPα can function to promote neurons survival, which could counter against neuron demise induced by Aβ accumulation. The γ-secretase cleavage of APP can produce AICD fragments. The AICD can bind with Fe65 and form complex, which can enter nucleus and function to negatively regulate neurogenesis via unknown mechanisms. Pathological factors which disruption the balance between positive regulation of neurogenesis by sAPPα and negative regulation of neurogenesis by AICD will lead to decreased neurogenesis, increased neuron demise and cooperate with Aβ plaque induced toxicity to contribute to AD.
Figure 5.
Flow chart summarization of potential pathogenesis of AD related to APP. The altered APP processing could contribute to Aβ accumulation, increased AICD and decreased sAPP. The increased AICD and decreased sAPP could finally lead to decreased neurogenesis, while decreased sAPP could also facilitate neurodegeneration. The Aβ accumulation could lead to Aβ senile plaques and induce calcium flux via a tau protein-dependent manner. The dysregulated intracellular calcium and increased ROS level finally contribute to neurodegeneration. Hereby the decreased neurogenesis and increased neuron degeneration contribute to AD.
miRNAs, APP, Neurogenesis and AD
miRNAs are small non-coding RNA molecules that regulate gene expression by binding to the 3′UTR of their target mRNAs for repression of target gene expression by translation inhibition or mRNA degradation.85 It was known that miRNAs are abundantly expressed in the central nervous system and they have essential functional roles in brain development and neuronal specification.86–88 However dysfunctions or aberrant signaling of the miRNA pathway was demonstrated to result in neurodegenerative diseases.86–88 Recent accumulating evidence implicated the dysregulation of miRNAs expression in AD.89,90 It was reported that there was an upregulation of miR-9, miR-125b and miR-128 in hippocampus of AD affected post-mortem brain samples.91 Their findings also implicated that ROS might contribute to AD via pathways mediated by miRNAs. The alterations of miRNA expression profiles between AD and control brain samples have also been reported by other research groups.89,90 Importantly, all reports consistently demonstrated the consecutive upregulation of miR-125b and downregulation of miR-9 and miR-210 in AD brains. This is suggestive of important roles of these miRNAs, as miR-125b and miR-9 have also been implicated in neurodegenerative diseases such as Down Syndrome and Huntington disease.92,93 Furthermore miRNA dysregulation has also been observed in CSF of AD patients, suggesting that specific miRNAs may be used as putative biomarkers for neurodegenerative diseases.89
Studies also demonstrated that miRNAs can regulate APP expression, APP processing and Aβ accumulations.90,94–96 It was found that miRNAs hsa-mir-106a and hsa-mir-520c could bind to their predicted target sequences in the APP 3′UTR and negatively regulate APP expression.94 Another recent study showed that miR-101 is a negative regulator of APP expression and could affect the accumulation of Aβ, suggesting a possible role for miR-101 in neuropathological conditions.95 Furthermore miRNAs belonging to the miR-20a family (miR-20a, miR-17-5p and miR-106b) was found to regulate APP expression in vitro and at the endogenous level in neuronal cell lines.90 In this study a tight correlation between these miRNAs and APP was found during brain development and in differentiating neurons. Such possibility is further corroborated by the observation that a significant decrease in miR-106b expression was found in sporadic AD patients.90 On the other hand, two miRNAs (miR-298 and miR-328) was found to regulate BACE mRNA translation, while BACE was responsible for APP processing and Aβ production.96 These findings implicated the interesting internal link among miRNA, neurogenesis, APP and AD, which deserve extensive studies in the future.
Alteration of Neurogenesis in AD
Several studies have suggested that the rate of neurogenesis in both SVZ and dentate gyrus (DG) declines with age, raising the possibility that reduced or misregulated neurogenesis may account, to a certain extent, for the cognitive deterioration in the elderly or contribute to learning and memory deficits in individuals with AD.97–100 Indeed, in vitro experiments have shown that excessive accumulation of Aβ affected the function of cultured human cortical NPC by suppressing their proliferation and neuronal differentiation and ultimately inducing apoptosis.101 The deteriorative effect of Aβ on neurogenesis has also been demonstrated in in vivo AD mouse models that have either been genetically mutated or intra-ventricularly infused with Aβ to cause neuritic plaque accumulation.102–104 These mice displayed impairments in neuronal generation from NPC in DG as well as reduced capacity of differentiation and survival of newly generated neurons.101,105,106 In support of these observations, it was revealed that a significant decrease in proliferation of progenitor cells was found in aged and AD brain.107 Recently, a study reported that levels of stem cell factor (SCF), a hematopoietic growth factor that supports neurogenesis in brain, was downregulated in plasma and CSF of patients with early onset AD.108 It was also reported in studies on mice harboring FAD-linked mutant APP that the proliferation of newly formed cells and neuronal differentiation were reduced in the SGL of the DG and in the SVZ in their mouse models.101,109 In these transgenic mice, the mice exhibited increased β-secretase activity, resulting in elevated levels of CTF, Aβ and sAPPβ, concomitantly with lower levels of sAPPα. Therefore the alterations of the ratio of these APP fragments may also contribute to decreased neurogenesis in AD.110,111
However, conflicting results have been achieved on whether the decline of neurogenesis happens at the early or late stages of AD.112–114 Li et al. examined the extent of cell proliferation in subgranule layer (SGL) of DG in FAD-linked APPswe/PS1ΔE9 mice (expressing human mutant APP plus a deletion of exon 9 of presenilin 1) at ages 6 and 9 months.112 Although both time points were post-amyloid deposition, significant reduction in cell proliferation was only observed in latter group, suggesting that alterations in neurogenesis are likely a function of age-specific neuropathology.112 This finding was supported by another similar study from Taniuchi et al. that the number of proliferating and newly differentiating neurons in SGL decreased significantly in 9-month-old FAD-linked APPswe/PS1ΔE9 mice but not in 5-month-old mice.113 However, a recent study by Demars et al. examined the fate of NPC in both SGL and SVZ of young APPswe/PS1ΔE9 transgenic mice.114 They showed that proliferation and differentiation of NPC were severely impaired early in mice at 2 months age, preceding onset of amyloid deposition and memory impairments.114 Furthermore, a dramatic increase in steady-state levels of Aβ and tau hyperphosphorylation in neurogenic niches was shown.114 Therefore, these results suggest that NPC are affected early in AD in both neurogenic areas of adult brain and may contribute to deficits in hippocampus- and olfaction-dependent memory in AD.
On the contrary other reports demonstrated increased neurogenesis in the context of AD.115–118 A recent report showed that transgenic mice expressing three or more FAD-linked APP mutations showed an upregulation of cell proliferation and neuronal differentiation in hippocampus and SVZ.115 In a different study, Jin et al. found an increase in numbers of newly proliferating cells in the SGL and SVZ in FAD-linked transgenic mice expressing human APP isoforms APP695, APP751 and APP770 with (V717F, K670N, M671L) mutations.116,117 Lopez-Toledana et al. also demonstrated an increase in proliferation of hippocampal cells and their neuronal differentiation in APP mice models similar to Jin.118 Furthermore one report on postmortem study of senile AD brain showed increased levels of cells with proliferative and immature neuron markers.119 On the contrary, another study on presenile AD brains demonstrated that the increased proliferation of cells in DG were non-neuronal, which could not reflect an increase in neurogenesis in AD brains.120
In summary, conflicting results were observed in seemingly similar AD mice models. However, as APP metabolites, including sAPPα, CTF, AICD and Aβ, may have unique roles that modulate neurogenesis differently, the complexities may result from the numerous FAD-linked variables that could influence APP metabolism in cells. Although it still remains to be firmly established whether impairments of neurogenesis contribute to the pathogenesis of AD, findings on molecular links between neurogenesis and AD so far implicates the disorders of neurogenesis to be an integral part of AD pathology.
Neurogenesis as a Therapeutic Strategy for AD
Currently, ongoing clinical trials are directed towards evaluating therapeutic approaches to stall the progression of AD by preventing neurons from further degeneration and providing symptom relief.121,122 Unfortunately, strategies aimed to arrest the degenerative process may fall short of cognitive recovery as they still leave the brain marred with defective neural synapses and neuronal loss. The understanding of stem cell biology and discovery of neurogenesis in adult brain thus hold the promise on the regeneration of damaged neural tissue and restoration of neuronal circuits essential for cerebral function. In the developing brain, most stem cells and microenvironments are spatially shifting and are temporally transient, as the cellular and molecular programs of neurogenesis and morphogenesis are “assembled and disassembled.”123 In contrast, the adult brain restricts such proliferative potential of NPC to special selective microenvironments.123 These specialized domains are restricted to the SVZ of the lateral ventricle and the DG subgranular zone of the hippocampus, retaining developmental potential throughout life span.123 Therefore the adult CNS may be amenable to repair and this provide the basis for new strategies for AD therapy: to stimulate endogenous NPC or stem cells of the adult brain and to transplant adult-derived NPC or stem cells into brains of AD patients.121 While the potential of stem cell regeneration in the adult brain is vast, its delivery to target areas of the brain poses a challenge. Systemic injection provides a non-invasive strategy but direct delivery of NPC to the brain faces the challenge of how to distribute cells throughout the brain as AD is characterized by a diffuse pattern of degeneration. Furthermore, as the progression of AD is not uniformly distributed, the affected regions of degenerating and degenerated neural circuits present challenges for integration of injected NPC.
On the other hand, although the appropriate delivery of exogenous NPC to restricted regions of the affected brain remains a challenge, ongoing neurogenesis by endogenous NPC provides an exciting avenue that has the potential to resolve cognitive deficits in individuals with AD. Endogenous stem cells exist in low abundance in the adult brain, but could be stimulated to induce proliferation under appropriate conditions.124 To this aim, a potential therapeutic approach is the delivery of growth factors to brain to promote neurogenesis. Basic scientific analyses and human trials indicated that constituents of microenvironments within the brain determine the potential of neurogenesis, differentiation of NPC and magnitude of the NPC pool.103,125–127 Multiple analyses have been documented that DG neurogenesis is regulated by FGF-2, IGF-1 and VEGF.102,104,128–131 For example, FGF-2 enhanced DG neurogenesis in both neonatal and adult brain and intra-cerebroventricular (ICV) infusions of FGF-2 upregulated DG neurogenesis in aged brain.132 Likewise, IGF-1 increased DG neurogenesis in adult and aged brain following ICV administration of IGF-1.133 VEGF can promote DG neurogenesis in both intact and injured adult brain following ICV administration.134 Recently, a report has also demonstrated that fetal NPC transplantation reduced memory deficits and amyloid plaque deposition in a mouse AD model, transgenic overexpression of K670N or M671L APP mutation. In addition, the mice model showed significant improvement in cognition after NPC transplantation.135
While NPC are currently being investigated as potential therapies for neurodegenerative diseases like AD, concerns have also been raised over the safety of this experimental therapeutic approach. This includes the possibility of tumor formation from transplanted NPC in human brain. A report on a human brain tumor from NPC complicating NPC therapy suggests that NPC may also be involved in gliomagenesis and this finding provides an example of a donor-derived human brain tumor.136 Therefore, further researches are urgently required to assess the safety of these therapies.
Potential Secretase Balances and Implications to AD
It was known that sAPPα could protect neurons and promote neurogenesis, while sAPPα could be produced via α-secretase cleavage. Furthermore the α-secretase cleavage of APP could abrogate Aβ proteins production. On the other hand, β-secretase and γ-secretase cleavage of APP could lead to Aβ proteins formation, which should be deleterious and could contribute to neurodegeneration in AD. Furthermore the γ-secretase cleavage of APP could lead to AICD formation, which could be a prohibiting factor to neurogenesis. Therefore it seemed that secretase balance between α-secretase and β-secretase as well as balance between α-secretase and γ-secretase exists, which was important to pathogenesis of AD. The balance between α-secretase and β-secretase will determine the Aβ proteins and sAPPα formation and neurons viability. The disruption of this secretase balance will lead to increased Aβ formation, decrease sAPPα and increased neurons demise. The balance between α-secretase and γ-secretase will influence neurogenesis in brain. Such secretase balance could affect the downstream balance between AICD induced inhibition of neurogenesis and sAPPα induced promotion of neurogenesis. The consequence of disruption of the secretase balance between α-secretase and γ-secretase will lead to decreased neurogenesis in brain. Finally the disturbance of these secretase balances would lead to disruption of balance between neurogenesis and neuon demise and contribute to AD. These secretase balances and relationship to AD was summarized and illustrated in (Fig. 6). Therefore factors leading to imbalance of these secretase balances might be culprits of AD onset, especially for sporadic AD. Based on above-mentioned analysis, it can also be concluded that the activity of α-secretase was a key factor related to AD onset. Therefore factors to downregulation of α-secretase activity should be paid more attentions, as they might be the original underlying cause for AD, especially sporadic AD. On the other hand, factors to control β-secretase and γ-secretase, such as miRNAs, should also be paid attention. As dysfunction of these controlling factors might lead to enhancement of activities of β-secretase and γ-secretase, which will overwhelm the activity of α-secretase and contribute to AD.
Figure 6.
The proposed secretase balances related to AD pathogenesis. The balance between α- and β-secretase will determine Aβ protein and sAPPα formation and cell viability influenced by Aβ or sAPPα. The disruption of this secretase balance could lead to decreased sAPPα production and increased Aβ accumulation and neurons degeneration. The balance between α- and γ-secretase will influence neurogenesis. The disruption of this balance might contribute to impaired neurogenesis in brain. The imbalance of these secretase balances could contribute to AD. The miR-298 and miR-328 was reported to control β-secretase expression. Therefore dysregulation of these miRNAs might lead to imbalance between α- and β-secretase and be even related to AD. So far miRNAs for α- and γ-secretase have not been identified. Studies on them should be interesting in the future.
Future Prospects and Directions
The secreted sAPPα has been demonstrated to promote neurogenesis, which could be a factor to prohibit AD onset. However, so far it is unclear how sAPPα functions to influence neurogenesis. A possibility could be that the sAPPs might bind with its specific receptor on the cell membrane and exert downstream effects. Another possibility could be that sAPPα might act as a modulating protein that regulates the signal transduction of another signal pathway, which is crucial to neurogenesis and neuron cell survival. In this context, sAPPα might function as a modulator to influence the binding affinity between ligands and receptors. However the third possibility that sAPPα could penetrate through cell membrane and confer it effects in the cytoplasma still cannot be completely excluded. One practical way to solve these questions is to utilize LC-MS-MS to identify sAPPα interacting proteomics. Progresses within this aspect in the future should help to further understand the detailed mechanism of sAPPα induced neurogenesis and anti-apoptosis effects. This will also help to develop better therapeutic strategies for AD.
On the other spectrum, further studies on the detailed molecular events of TAG1-APP signaling pathway in cells should also be vital to understanding the influence of neurogenesis by APP. Currently, it is of great interest urgent to decipher how AICD functions to negatively regulate neurogenesis following the activation of the TAG1-APP pathway. It can be hypothesized that the AICD-Fe65 complex might act as transcription factors, transcription co-activators or transcription co-repressors to regulate various gene expressions. Another possibility could be that AICD might inhibit neurogenesis via interaction with key players of other signal pathways, which are vital to neurogenesis. The LC-MS-MS analysis to search for AICD interacting partners in cells should also help to provide interesting information for the answers to these questions.
Recently, it has been demonstrated that the dysregulation of miRNAs was linked to AD pathogenesis. The dysregulation of miRNAs could also influence APP expression level, APP processing and even Aβ accumulations in cells. Therefore, identifying miRNAs that could function to regulate the expression of APP as well as miRNAs which could regulate expressions of BACE (β-secretase) and presenilins (γ-secretase), should be significant to AD pathogenesis in the near future especially for sporadic AD. Future progress on miRNAs to control the expressions of APP, secretase for APP cleavage or factors related to TAG1-APP signaling pathway should be significant to AD pathogenesis and therapy.
Acknowledgments
We thank National Medical Research Council, Singapore Millennium Foundation and Duke-NUS Graduate Medical School for their support.
References
- 1.Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer's disease. Curr Alzheimer Res. 2006;3:421–430. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 3.Chohan MO, Haque N, Alonso A, El-Akkad E, Grundke-Iqbal I, Grover A, et al. Hyperphosphorylation-induced self assembly of murine tau: a comparison with human tau. J Neural Transm. 2005;112:1035–1047. doi: 10.1007/s00702-004-0241-9. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 5.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 6.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 7.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 8.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 9.Poirier J, Minnich A, Davignon J. Apolipoprotein E, synaptic plasticity and Alzheimer's disease. Ann Med. 1995;27:663–670. doi: 10.3109/07853899509019253. [DOI] [PubMed] [Google Scholar]
- 10.Ali G, Wasco W, Cai X, Szabo P, Sheu KF, Cooper AJ, et al. Isolation, characterization and mapping of gene encoding dihydrolipoyl succinyltransferase (E2k) of human alpha-ketoglutarate dehydrogenase complex. Somat Cell Mol Genet. 1994;20:99–105. doi: 10.1007/BF02290679. [DOI] [PubMed] [Google Scholar]
- 11.Blacker D, Wilcox MA, Laird NM, Rodes L, Horvath SM, Go RC, et al. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat Genet. 1998;19:357–360. doi: 10.1038/1243. [DOI] [PubMed] [Google Scholar]
- 12.Law A, Gauthier S, Quirion R. Say NO to Alzheimer's disease: the putative links between nitric oxide and dementia of the Alzheimer's type. Brain Res Brain Res Rev. 2001;35:73–96. doi: 10.1016/s0165-0173(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 13.Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 14.Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 15.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 16.Khachaturian ZS. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol Aging. 1987;8:345–346. doi: 10.1016/0197-4580(87)90073-x. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J Neurosci. 30:8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattson MP. ER calcium and Alzheimer's disease: in a state of flux. Sci Signal. 3:10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343:461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- 22.Cleveland DW, Hwo SY, Kirschner MW. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116:227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 23.Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr Opin Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 25.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldgaber D, Lerman MI, McBride WO, Saffiotti U, Gajdusek DC. Isolation, characterization and chromosomal localization of human brain cDNA clones coding for the precursor of the amyloid of brain in Alzheimer's disease, Down's syndrome and aging. J Neural Transm Suppl. 1987;24:23–28. [PubMed] [Google Scholar]
- 28.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 29.Wasco W, Bupp K, Magendantz M, Gusella JF, Tanzi RE, Solomon F. Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta protein precursor. Proc Natl Acad Sci USA. 1992;89:10758–10762. doi: 10.1073/pnas.89.22.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasco W, Gurubhagavatula S, Paradis MD, Romano DM, Sisodia SS, Hyman BT, et al. Isolation and characterization of APLP2 encoding a homologue of the Alzheimer's associated amyloid beta protein precursor. Nat Genet. 1993;5:95–100. doi: 10.1038/ng0993-95. [DOI] [PubMed] [Google Scholar]
- 31.Daigle I, Li C. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc Natl Acad Sci USA. 1993;90:12045–12049. doi: 10.1073/pnas.90.24.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen DR, Martin-Morris L, Luo LQ, White K. A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci USA. 1989;86:2478–2482. doi: 10.1073/pnas.86.7.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikai S, Sasaki H, Doh-ura K, Furuya H, Sakaki Y. Genomic organization of the human amyloid beta-protein precursor gene. Gene. 1990;87:257–263. doi: 10.1016/0378-1119(90)90310-n. [DOI] [PubMed] [Google Scholar]
- 35.Kitaguchi N, Takahashi Y, Tokushima Y, Shiojiri S, Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988;331:530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- 36.Konig G, Monning U, Czech C, Prior R, Banati R, Schreiter-Gasser U, et al. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J Biol Chem. 1992;267:10804–10809. [PubMed] [Google Scholar]
- 37.Ling Y, Morgan K, Kalsheker N. Amyloid precursor protein (APP) and the biology of proteolytic processing: relevance to Alzheimer's disease. Int J Biochem Cell Biol. 2003;35:1505–1535. doi: 10.1016/s1357-2725(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 38.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, et al. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 40.Han P, Dou F, Li F, Zhang X, Zhang YW, Zheng H, et al. Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation. J Neurosci. 2005;25:11542–11552. doi: 10.1523/JNEUROSCI.3831-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 42.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kume H, Maruyama K, Kametani F. Intracellular domain generation of amyloid precursor protein by epsilon-cleavage depends on C-terminal fragment by alpha-secretase cleavage. Int J Mol Med. 2004;13:121–125. [PubMed] [Google Scholar]
- 44.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 45.Hornsten A, Lieberthal J, Fadia S, Malins R, Ha L, Xu X, et al. APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci USA. 2007;104:1971–1976. doi: 10.1073/pnas.0603997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo L, Tully T, White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992;9:595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- 47.Leyssen M, Ayaz D, Hebert SS, Reeve S, De Strooper B, Hassan BA. Amyloid precursor protein promotes postdevelopmental neurite arborization in the Drosophila brain. EMBO J. 2005;24:2944–2955. doi: 10.1038/sj.emboj.7600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breen KC, Bruce M, Anderton BH. Beta amyloid precursor protein mediates neuronal cell-cell and cell-surface adhesion. J Neurosci Res. 1991;28:90–100. doi: 10.1002/jnr.490280109. [DOI] [PubMed] [Google Scholar]
- 50.Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 51.Herms J, Anliker B, Heber S, Ring S, Fuhrmann M, Kretzschmar H, et al. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, et al. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 53.Ohsawa I, Takamura C, Morimoto T, Ishiguro M, Kohsaka S. Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. Eur J Neurosci. 1999;11:1907–1913. doi: 10.1046/j.1460-9568.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 54.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ. Cell-surface beta-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. J Neurosci. 1995;15:2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schubert D, Jin LW, Saitoh T, Cole G. The regulation of amyloid beta protein precursor secretion and its modulatory role in cell adhesion. Neuron. 1989;3:689–694. doi: 10.1016/0896-6273(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 57.Siemes C, Quast T, Kummer C, Wehner S, Kirfel G, Muller U, et al. Keratinocytes from APP/APLP2-deficient mice are impaired in proliferation, adhesion and migration in vitro. Exp Cell Res. 2006;312:1939–1949. doi: 10.1016/j.yexcr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Wang B, Yang L, Wang Z, Zheng H. Amyolid precursor protein mediates presynaptic localization and activity of the high-affinity choline transporter. Proc Natl Acad Sci USA. 2007;104:14140–14145. doi: 10.1073/pnas.0704070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, et al. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang G, Gong YD, Gong K, Jiang WL, Kwon E, Wang P, et al. Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (APP) and APP-like protein 2. Neurosci Lett. 2005;384:66–71. doi: 10.1016/j.neulet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 61.Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–14469. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat Rev Neurosci. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O'Dowd G, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- 64.Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, et al. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 65.Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 66.Bergmans BA, Shariati SA, Habets RL, Verstreken P, Schoonjans L, Muller U, et al. Neurons generated from APP/APLP1/APLP2 triple knockout embryonic stem cells behave normally in vitro and in vivo: lack of evidence for a cell autonomous role of the amyloid precursor protein in neuronal differentiation. Stem Cells. 28:399–406. doi: 10.1002/stem.296. [DOI] [PubMed] [Google Scholar]
- 67.Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, et al. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- 68.Rossjohn J, Cappai R, Feil SC, Henry A, McKinstry WJ, Galatis D, et al. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Biol. 1999;6:327–331. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- 69.Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- 70.Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 71.Yogev-Falach M, Amit T, Bar-Am O, Weinstock M, Youdim MB. Involvement of MAP kinase in the regulation of amyloid precursor protein processing by novel cholinesterase inhibitors derived from rasagiline. FASEB J. 2002;16:1674–1676. doi: 10.1096/fj.02-0198fje. [DOI] [PubMed] [Google Scholar]
- 72.Youdim MB, Amit T, Bar-Am O, Weinstock M, Yogev-Falach M. Amyloid processing and signal transduction properties of antiparkinson-antialzheimer neuroprotective drugs rasagiline and TV3326. Ann NY Acad Sci. 2003;993:378–386. doi: 10.1111/j.1749-6632.2003.tb07548.x. [DOI] [PubMed] [Google Scholar]
- 73.Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, et al. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 75.Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, et al. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol. 2008;10:283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- 76.Hebert SS, Serneels L, Tolia A, Craessaerts K, Derks C, Filippov MA, et al. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006;7:739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryan KA, Pimplikar SW. Activation of GSK-3 and phosphorylation of CRMP2 in transgenic mice expressing APP intracellular domain. J Cell Biol. 2005;171:327–335. doi: 10.1083/jcb.200505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci USA. 2009;106:18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogt DL, Thomas D, Galvan V, Bredesen DE, Lamb BT, Pimplikar SW. Abnormal neuronal networks and seizure susceptibility in mice overexpressing the APP intracellular domain. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 81.Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 83.Goodger ZV, Rajendran L, Trutzel A, Kohli BM, Nitsch RM, Konietzko U. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J Cell Sci. 2009;122:3703–3714. doi: 10.1242/jcs.048090. [DOI] [PubMed] [Google Scholar]
- 84.Aydin D, Filippov MA, Tschape JA, Gretz N, Prinz M, Eils R, et al. Comparative transcriptome profiling of amyloid precursor protein family members in the adult cortex. BMC Genomics. 2011;12:160. doi: 10.1186/1471-2164-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I, et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res. 2004;14:2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, et al. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 89.Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, et al. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 90.Hebert SS, Horre K, Nicolai L, Bergmans B, Papadopoulou AS, Delacourte A, et al. MicroRNA regulation of Alzheimer's Amyloid precursor protein expression. Neurobiol Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Lukiw WJ, Zhao Y, Cui JG. An NFkappaB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr Genomics. 2009;10:154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel N, Hoang D, Miller N, Ansaloni S, Huang Q, Rogers JT, et al. MicroRNAs can regulate human APP levels. Mol Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vilardo E, Barbato C, Ciotti M, Cogoni C, Ruberti F. MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J Biol Chem. 285:18344–18351. doi: 10.1074/jbc.M110.112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Provost P. Interpretation and applicability of microRNA data to the context of Alzheimer's and age-related diseases. Aging (Albany NY) 2:166–169. doi: 10.18632/aging.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 98.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 100.Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolecular Med. 2002;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- 102.Klassen HJ, Imfeld KL, Kirov II, Tai L, Gage FH, Young MJ, et al. Expression of cytokines by multipotent neural progenitor cells. Cytokine. 2003;22:101–106. doi: 10.1016/s1043-4666(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 103.Lindvall O, Bjorklund A. Cell replacement therapy: helping the brain to repair itself. NeuroRx. 2004;1:379–381. doi: 10.1602/neurorx.1.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Podar K, Tai YT, Cole CE, Hideshima T, Sattler M, Hamblin A, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278:5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 105.Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 107.Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3:185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- 108.Laske C, Stellos K, Stransky E, Seizer P, Akcay O, Eschweiler GW, et al. Decreased plasma and cerebrospinal fluid levels of stem cell factor in patients with early Alzheimer's disease. J Alzheimers Dis. 2008;15:451–460. doi: 10.3233/jad-2008-15311. [DOI] [PubMed] [Google Scholar]
- 109.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 110.Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the golgi apparatus. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- 111.Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13:159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 112.Li D, Tang J, Xu H, Fan X, Bai Y, Yang L. Decreased hippocampal cell proliferation correlates with increased expression of BMP4 in the APPswe/PS1DeltaE9 mouse model of Alzheimer's disease. Hippocampus. 2008;18:692–698. doi: 10.1002/hipo.20428. [DOI] [PubMed] [Google Scholar]
- 113.Taniuchi N, Niidome T, Goto Y, Akaike A, Kihara T, Sugimoto H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport. 2007;18:1801–1805. doi: 10.1097/WNR.0b013e3282f1c9e9. [DOI] [PubMed] [Google Scholar]
- 114.Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin K, Mao XO, Cottrell B, Schilling B, Xie L, Row RH, et al. Proteomic and immunochemical characterization of a role for stathmin in adult neurogenesis. FASEB J. 2004;18:287–299. doi: 10.1096/fj.03-0973com. [DOI] [PubMed] [Google Scholar]
- 117.Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, et al. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopez-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- 119.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 121.Taupin P. The therapeutic potential of adult neural stem cells. Curr Opin Mol Ther. 2006;8:225–231. [PubMed] [Google Scholar]
- 122.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 123.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 124.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 125.Horner PJ, Gage FH. Regeneration in the adult and aging brain. Arch Neurol. 2002;59:1717–1720. doi: 10.1001/archneur.59.11.1717. [DOI] [PubMed] [Google Scholar]
- 126.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 128.Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 129.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1 and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- 132.Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J Gerontol A Biol Sci Med Sci. 2007;62:117–125. doi: 10.1093/gerona/62.2.117. [DOI] [PubMed] [Google Scholar]
- 133.Sun LY. Hippocampal IGF-1 expression, neurogenesis and slowed aging: clues to longevity from mutant mice. Age (Dordr) 2006;28:181–189. doi: 10.1007/s11357-006-9009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 135.Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci USA. 2007;104:12506–12511. doi: 10.1073/pnas.0705346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]