Abstract
The Snail family of zinc finger transcription factors plays an important role in epithelial to mesenchymal transition (EMT) in a variety of tissues and systems. Slug (SNAI2) expression has been shown to directly contribute to a subset of events required for EMT in events such as re-epithelialization during wound healing and neural crest cell migration. In addition, slug expression was shown to correlate with disease recurrence in head and neck squamous cell carcinoma (HNSCC) patients. Based on this association we chose to specifically examine the effects of exogenous slug expression in HNSCC cells and specifically assess adhesive junction assembly and the motility characteristics in these cells. Slug expression led to changes in adherens junction and desmosome assembly characterized by a classical cadherin switch and loss of desmosome assembly. Additionally, we performed gene expression profiling to identify novel Slug-dependent gene expression changes in a HNSCC cell line. In addition to genes known to be altered during EMT, we identified a novel set of Slug responsive genes that will provide a better understanding of slug overexpression during EMT and HNSCC progression.
Key words: Slug (SNAI2), cell-cell adhesion, cell motility, microarray, cadherin
Introduction
Members of the Snail superfamily of transcription factors play important roles in normal tissue development and in the initiation of epithelial to mesenchymal transition (EMT).1,2 Snail was the first member of the superfamily identified in Drosophila and subsequently other family members have been identified and characterized.3,4 Snail homolog 2 (SNAI2), also known as Slug, has been shown to function in a manner similar to that of Snail however Slug displays a distinct tissue distribution.5,6 During EMT, normally immobile epithelial cells in a tissue detach from neighboring cells and acquire the ability to remodel the extracellular environment and become migratory. This process is required for efficient re-epithelialization of the epidermis in response to wounding and for the migration of neural crest cells during development.7 Tumor cell migration and invasion are reminiscent of the EMT process and it is believed that tumor cells employ an EMT phase to acquire invasive characteristics.8 Therefore, EMT is a useful framework in which to study tumor cell migration and disease progression.
Hallmarks of EMT include decreased expression of the adherens junction component, E-cadherin, and loss of the normal epithelial morphology. Additionally, cells become spindle shaped and mesenchymal markers such as N-cadherin, vimentin and extracellular proteases are increased.9 Expression of Snail family transcription factors can induce many of these morphologic and gene expression changes in cultured cells. Cell culture studies provide a unique opportunity to identify additional factors that affect the morphological behaviors observed during EMT. Since expression of Snail transcription factors are known to participate in the EMT process identification and characterization of Snail and Slug-dependent changes in cell behavior is important to gain a comprehensive view of the EMT process.
Expression of Snail family transcription factors, including Slug, has been shown to be increased in a variety of solid tumors and Slug expression correlates with increased metastatic behavior of tumor cells and this behavior is similar to the EMT phenotype.10 Additionally, Slug expression was shown to correlate with local recurrence in HNSCC in a microarray analysis of primary tumors.11 Based on these findings we sought to investigate the effects of Slug overexpression in oral SCC cells devoid of endogenous Slug expression. In the present study, we examined the effect of Slug expression on cell motility and on expression and assembly of cell-cell adhesive junctions in this cell line. Finally, we performed a microarray analysis to catalog the global gene expression changes in cells stably expressing Slug.
Results
Epithelial-to-mesenchymal transition (EMT) is a well characterized process defined morphologically as the conversion of epithelial cells to a fibroblast or mesenchymal morphology and is known to be dependent on the Snail family of transcription factors. UM-SCC-38 cells were derived from a tumor of the tongue12 and in culture these cells display an epithelial morphology and grow as epithelial colonies in culture (Fig. 1A). In addition, we have previously shown parental UM-SCC-38 cells are relatively immobile in culture.13 We retrovirally expressed human Slug in UM-SCC-38 cells to generate a population of UM-SCC-38 cells expressing Slug. UM-SCC-38/slug cells display a distinct change in morphology compared to UM-SCC-38/neo cells infected with virus conferring G418 resistance (Fig. 1). UM-SCC-38/slug cells were more fibroblastic in appearance and were much flatter than UM-SCC-38/neo cells. The observed change in morphology was homogeneous throughout the culture of cells and was maintained over 15–20 passages. We never observed UM-SCC-38/neo cells spontaneously adopt the fibroblastic morphology observed in UM-SCC-38/slug cells. Immunoblot analysis of whole cell lysate prepared from UM-SCC38/neo and UM-SCC-38/slug cells failed to detect Slug expression using an anti SNAI2 antibody. Snail and Slug proteins are highly unstable proteins and have been shown to be degraded by the proteasome.14,15 In order to detect Slug protein in cell lysates we chose to inhibit proteosomal degradation using MG132. Immunoblot analysis of whole cell lysate prepared from UM-SCC38/neo and UM-SCC-38/slug cells treated with MG132 (5 µM) for 1 h revealed an anti-SNAI2 immunoreactive band migrating at approximately 30 kDa in the UM-SCC-38/slug cell lysate that was absent in control cell lysate (Fig. 1C).
Figure 1.
Slug expression in UM-SCC-38 cells induces a mesenchymal phenotype. UM-SCC-38/neo (A) and UM-SCC-38/slug (B) cells were plated and allowed to reach 70% confluency before being photographed. Note the epithelial morphology and close apposition of UM-SCC-38/neo cells compared to the fibroblastic appearance of UM-SCC-38/slug cells. (C) Immunoblot analysis of cell lysates prepared from UM-SCC-38/neo cells and UM-SCC-38/slug cells using an anti-Slug antibody revealed an immunoreactive band at approximately 30 kDa. The band in both lanes at the bottom of the cropped figure is a non-specific band recognized by the antibody.
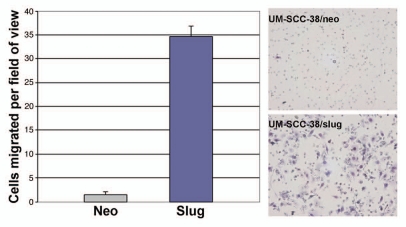
Since Slug is a factor known to participate in EMT and UM-SCC-38 cells expressing Slug undergo this phenotypic transition in culture we tested the migratory ability of UM-SCC-38/slug cells. We performed in vitro motility assays comparing UM-SCC-38/slug cells to UM-SCC-38/neo cells. In this assay the UM-SCC-38/slug cells exhibited a 15-fold increase in motility compared to control cells (Fig. 2). Collectively, these data suggest that overexpression of Slug in UM-SCC-38 cells results in an EMT phenotype.
Figure 2.
Slug expression results in increased motility of UM-SCC-38 cells. UM-SCC-38/neo and UM-SCC-38/slug cells were plated on top of a membrane possessing 8 mm pores. Non-migratory cells were removed 24 h later and cells on the bottom of the membrane were fixed, stained and counted. Slug-expressing cells were found to be significantly more migratory. Representative membranes are shown on the right.
Slug-dependent changes in cell-cell adhesion junctions.
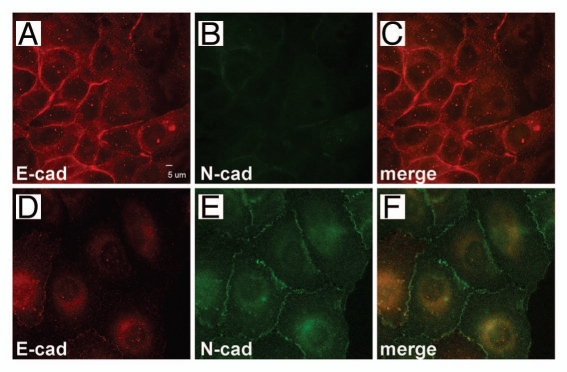
Expression of cell adhesion proteins has been shown to be altered during EMT. For example, a hallmark of EMT is the reduced expression of the classical cadherin, E-cadherin. We examined cadherin localization and expression in UM-SCC-38/neo- and Slug-expressing cells. As expected, E-cadherin localization was observed at cell-cell borders in UM-SCC-38/neo cells; however E-cadherin localization in UM-SCC-38/slug cells was diffuse and was rarely observed at sites of cell-cell contact in these cells (Fig. 3A and D). Additionally, we examined the localization of the classical cadherin N-cadherin in these cells as well. UM-SCC-38/neo cells had no detectable N-cadherin while this classical cadherin was strongly expressed and localized at cell borders in UM-SCC-38/slug cells (Fig. 3B and E). Induction of N-cadherin during EMT has previously been reported in other systems.16
Figure 3.
Slug expression induces a cadherin switch in UM-SCC-38 cells. UM-SCC-38/neo (A–C) and UM-SCC-38/slug (D–F) cells were grown on glass cover slips, fixed and co-immunostained using anti E-cadherin (A and D) and anti Ncadherin (B and E). UM-SCC-38/neo cells express E-cadherin and no detectable N-cadherin while Slug-expressing cells express lower levels of E-cadherin that is not localized at cell borders. Slug-expressing cells express N-cadherin and the N-cadherin is localized at cell borders.
In addition to E-cadherin, UM-SCC-38 cells express P-cadherin and incorporate this cadherin into adherens junctions in these cells. Unlike E-cadherin, a significant amount of P-cadherin remains in UM-SCC-38/slug cells however the protein is not expressed in a homogeneous manner as was observed in control cells or as N-cadherin is expressed in UM-SCC-38/slug cells (Fig. 4A and B). In addition to the adherens junction we examined desmosome assembly by immunostaining with antibodies specific for the desmosomal plaque protein desmoplakin. Desmoplakin was robustly expressed and localized in punctate structures in UM-SCC-38/neo cells as expected and as previously reported in reference 17. Desmoplakin expression is lost in UM-SCC-38/slug cells (Fig. 4C and D) indicating that desmosome assembly is severely reduced or lost in Slug-expressing cells.18
Figure 4.
The composition of the adherens junction is altered and desmosome assembly is impaired in UM-SCC-38/slug cells. UM-SCC-38/neo (A and C) and UM-SCC-38/slug (B and D) cells were immunostained with antibodies specific for P-cadherin and desmoplakin (C and D). Nuclei are visualized by DAPI counterstain (B and D). P-cadherin expression is heterogeneous and desmoplakin expression is lost in UM-SCC-38/slug cells.
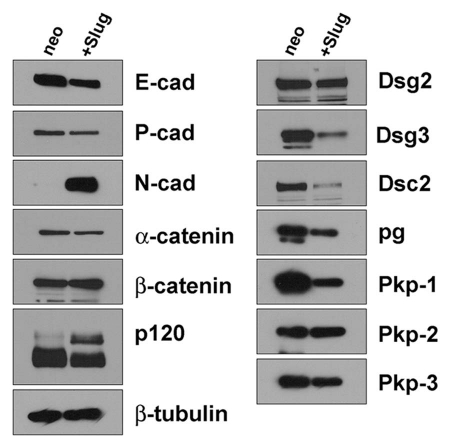
We examined the expression of other adherens junction and desmosomal components by immunoblot. In agreement with the immunofluorescence microscopy presented above, UM-SCC-38/neo cells express E-cadherin and P-cadherin as the primary classical cadherins. Upon Slug expression, a cadherin expression switch occurs and E-cadherin expression is reduced while N-cadherin expression is upregulated. Interestingly, expression of the classical cadherin P-cadherin is only slightly reduced and heterogeneously localized in UM-SCC-38/slug cells. Expression of the cytoplasmic catenins is largely unaffected by Slug expression. However p120 catenin undergoes an isoform switch in UM-SCC-38/slug cells compared to control cells. Different isoforms of p120 arise due to the use of alternative exons containing alternative start codons and expression of the longer isoform 1 is associated with increased invasiveness and increased Rho A activity.19 UM-SCC-38/neo cells predominantly express isoform 3 while Slug-expressing cells also express the slower migrating isoform 1 in addition to the faster migrating isoform 3.
Next we examined the expression of desmosomal components and found decreased expression of desmoglein-3, desmocollin-2 and plakophilin-1. Interestingly, expression of desmoglein 2, plakophilin-2 and plakophilin-3 was unaffected by Slug expression.
Identification of Slug-responsive genes.
Expression of the transcription factor Slug is expected to result in a plethora of gene expression changes. To begin to elucidate these gene expression changes we performed a gene expression profiling experiment in UM-SCC-38 cells. We isolated total RNA from UM-SCC-38/slug and compared the mRNA expression profile to UM-SCC-38/neo cells using the Affymetrix microarray platform. A complete list of raw signal intensities and fold change is presented in Supplemental Table 1. There were 657 genes increased at least two fold in UM-SCC-38/slug cells compared to UM-SCC-38/neo cells. Additionally, there were 661 genes decreased in UM-SCC-38/slug cells compared to control cells. Found within the list of gene expression changes induced by Slug overexpression are gene signatures that are characteristic of EMT, including E-cadherin (decreased), N-cadherin (increased) and vimentin (increased) expression in the UM-SCC-38/slug cells compared to control cells. It is important to note that the increase in Slug expression is not identified within the microarray data due to the fact that we expressed the Slug open reading frame and the probe set present on the Affymetrix chip was directed against the 3′UTR of the endogenous Slug mRNA.
Examination of the top 20 upregulated (Table 1) and downregulated (Table 2) genes expressed in Slug-expressing cells reveals several genes that have been previously demonstrated to participate in the EMT phenotype. Interestingly, several genes were identified that have not previously been associated with EMT progression. For example, increased expression of collagen, fibronectin, Zeb1 and DAB2 are known to occur during EMT. Epithelial makers such as keratin, connexin 30 and SPRR1B would be expected to be reduced in cells that have proceeded through EMT. Examination of the list of genes altered in response to Slug expression (Sup. Table 1) reveals an interesting list of potential Slug responsive genes and genes that contribute to the EMT process.
Table 1.
Genes upregulated in Slug (SNAI2) expressing UM-SCC-38 cells
| Fold-change | Probe set | Gene symbol | Description |
| 125.764 | 215076_s_at | COL3A1 | collagen, type III, alpha1 |
| 77.033 | 212464_s_at | FN1 | fibronectin 1 |
| 34.892 | 225242_s_at | CCDC80 | coiled-coil domain containing 80 |
| 31.631 | 212764_at | ZEB1 | zinc finger E-box binding homeobox 1 |
| 31.440 | 201280_s_at | DAB2 | disabled homolog 2, mitogen-responsive phosphoprotein (Drosophila) |
| 29.900 | 202627_s_at | SERPINE1 | serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1) |
| 29.051 | 235236_at | LOC100131897 | Uncharacterized protein LOC100131897 |
| 27.903 | 202998_s_at | LOXL2 | lysyl oxidase-like 2 |
| 25.733 | 203700_s_at | DIO2 | deiodinase, iodothyronine, type II |
| 25.347 | 226218_at | IL7R | interleukin 7 receptor |
| 23.453 | 218332_at | BEX1 | brain expressed, X-linked 1 |
| 23.422 | 201426_s_at | VIM | vimentin |
| 22.666 | 215446_s_at | LOX | lysyl oxidase |
| 19.681 | 235004_at | RBM24 | RNA binding motif protein 24 |
| 16.772 | 219935_at | ADAMTS5 | ADAM metallopeptidase with thrombospondin type 1 motif, 5 |
| 16.169 | 220014_at | PRR16 | proline rich 16 |
| 16.004 | 215767_at | ZNF804A | zinc finger protein 804A |
| 15.927 | 231867_at | ODZ2 | odz, odd Oz/ten-m homolog 2 (Drosophila) |
| 15.657 | 222862_s_at | AK5 | adenylate kinase 5 |
| 15.590 | 224823_at | MYLK | myosin light chain kinase |
Table 2.
Genes downregulated in Slug (SNAI2) expressing UM-SCC-38 cells
| Fold change | Probe set | Gene symbol | Description |
| −35.7 | 213240_s_at | KRT4 | keratin 4 |
| −31.000 | 231771_at | GJB6 | gap junction protein, beta6, 30 kDa |
| −27.600 | 205413_at | MPPED2 | metallophosphoesterase domain containing 2 |
| −23.800 | 209173_at | AGR2 | anterior gradient homolog 2 (Xenopus laevis) |
| −15.900 | 217272_s_at | SERPINB13 | serpin peptidase inhibitor, clade B (ovalbumin), member 13 |
| −14.100 | 238439_at | ANKRD22 | ankyrin repeat domain 22 |
| −13.800 | 205569_at | LAMP3 | lysosomal-associated membrane protein 3 |
| −12.900 | 203453_at | SCNN1A | sodium channel, nonvoltage-gated 1alpha |
| −12.200 | 203571_s_at | C10orf116 | chromosome 10 open reading frame 116 |
| −12.100 | 205064_at | SPRR1B | small proline-rich protein 1B (cornifin) |
| −11.400 | 222830_at | GRHL1 | grainyhead-like 1 (Drosophila) |
| −11.400 | 207935_s_at | KRT13 | keratin 13 |
| −11.400 | 202718_at | IGFBP2 | insulin-like growth factor binding protein 2, 36 kDa |
| −11.000 | 218966_at | MYO5C | myosin VC |
| −10.700 | 211138_s_at | KMO | kynurenine 3-monooxygenase (kynurenine 3-hydroxylase) |
| −10.300 | 37892_at | COL11A1 | collagen, type XI, alpha1 |
| −10.100 | 239196_at | ANKRD22 | ankyrin repeat domain 22 |
| −10.100 | 212224_at | ALDH1A1 | aldehyde dehydrogenase 1 family, member A1 |
| −10.100 | 215465_at | ABCA12 | ATP-binding cassette, sub-family A (ABC1), member 12 |
| −9.900 | 204469_at | PTPRZ1 | protein tyrosine phosphatase, receptor-type, Z polypeptide 1 |
Discussion
Snail family transcription factors are known to be central players in mediating epithelial-to-mesenchymal transition (EMT) and several growth factor signaling pathways have been shown to result in upregulation of Snail and Slug individually or in combination.18,20,21 These transcription factors are in turn responsible for activation of a complex gene expression program that can vary depending on cell type and cues from the microenvironment. In this study we chose to examine Slug-dependent changes in large part due to the finding that Slug expression was shown to correlate with disease recurrence in HNSCC patients.11 We selected an HNSCC cell line that did not express detectable levels of endogenous Slug protein in order to assess the effect of Slug overexpression in these cells. In addition, we sought to characterize the changes in expression of individual adhesive junction components.
Upon Slug expression in UM-SCC-38 cells, we observed an expected reduction in E-cadherin expression and a striking loss of E-cadherin localization of the remaining E-cadherin from cell-cell borders. There was a concomitant increase in expression and localization of N-cadherin at cell borders. It is possible that N-cadherin could functionally replace E-cadherin at cell borders however other cell lines have been reported to co-express E-cadherin and N-cadherin and each cadherin is present at cell borders participating in junction assembly.22,23 Additionally, we observed a heterogeneous staining pattern for P-cadherin in these cells however we did not observe a reduction in the total protein levels of P-cadherin in these cells. Slug expression leads to the re-arrangement of adherens junction composition and a classical cadherin switch in these cells. Slug is known to bind E-box consensus sequences in the E-cadherin promoter and repress E-cadherin at the transcriptional level.24 The mechanisms affecting increased N-cadherin expression are currently unknown.
Expression of cytoplasmic components of the adherens junction was largely unaffected by Slug expression. However, we did observe a change in p120 isoform expression. Isoforms 1 and 3 are the primary p120 isoforms expressed in many cultured cells and each isoform is generated by alternative splicing of the p120 mRNA. Alternative splicing of the pre-mRNA results in the use of one of four potential translation start sites.25 Epithelial cells predominantly express isoforms 3 and 4 while mesenchymal cells often express isoform 1.26 UM-SCC-38/neo cells express the faster migrating form of p120 that is likely to be isoform 3. Upon expression of Slug, there is increased expression of the slower migrating isoform 1 in addition to isoform 3. Recently it has been demonstrated that two RNA binding proteins/splicing factors (ESRP1 and 2) have been shown to direct alternative splicing of the p120 mRNA. Expression of these splicing factors is associated with an epithelial phenotype and these splicing factors promote expression of p120 isoform 3.27 Interestingly, examination of the microarray data (Sup. Table 1) revealed that expression of ESRP1 and ESRP2 mRNA were decreased in UM-SCC-38/slug cells compared to control cells in agreement with the observed alternative splicing of the p120 mRNA.
Previous work has demonstrated that Slug activity, induced by growth factor activation, results in desmosome re-modeling and reduced desmoplakin localization at sites of cell-cell contact.18 In UM-SCC-38 cells, exogenous Slug expression had a strong effect on desmoplakin expression and localization at cell borders. In this study we observed additional changes in the expression of other desmosomal components that have not been previously reported. Desmoglein 3, desmocollin 2 and plakophilin-1 protein expression were also reduced in Slug-expressing cells. Interestingly expression of other desmosomal components (desmoglein-2, plakoglobin and plakophilin-2) was largely unaffected by Slug expression. Inspection of the promoter region of plakophilin-1 reveals a putative E-box sequence that can potentially serve as a Slug binding element however the functionality of this site has not been investigated (data not shown). We did not observe E-box sites in the promoter regions of the desmoglein-3 and desmocollin-2 genes sequences.
Due to its primary function as a DNA binding transcription factor, Slug expression is predicted to have an enormous effect on global gene expression in a particular cell type or cells in a tissue. Previous work identified Slug expression as a marker of recurrent disease in HNSCC.11 We reasoned that identification of the Slug-dependent gene expression signature will allow a more detailed examination of genes and processes that contribute to EMT and or tumor progression. We performed Affymetrix microarray analysis to identify the global changes in gene expression in the UM-SCC-38 cell line. In addition to adhesion junction components, the results of the microarray experiment revealed other genes that have been previously shown to be involved with the EMT process including collagen, keratin, vimentin and fibronectin. DAB2 expression was also found to be increased in Slug-expressing cells and has previously been shown to be required for TGFβ induced EMT.28 Recently LOXL2 was also reported to play a role in regulating Snail function in downregulating E-cadherin expression and promoting the EMT phenotype.29
Loss of the epithelial phenotype and downregulation of epithelial markers such as connexin 30 (GJB6), SERPINB13, cornifin (SPRR1B) and grainyhead-like1 (GRHL1) were also observed. Grainyhead-like 1 was recently shown to regulate desmoglein-3 expression in mouse epidermis30 and downregulation of this transcription factor in Slug-expressing cells may lead to the observed decrease in desmoglein-3 and desmocollin-2 (Fig. 5).
Figure 5.
Immunoblot analysis of adhesive junction components in UM-SCC-38/slug cells. Whole cell lysates were prepared from UM-SCC-38/neo and UM-SCC-38/slug cells and protein was resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membrane and immunoblotted using the antibodies indicated.
In addition to genes known to be associated with EMT we identified others that had not been previously linked to the EMT phenotype but could potentially play a role in this process. CCDC80 (coiled-coil domain 80), interleukin 7 receptor and RNA binding motif protein 24 are upregulated in Slug-expressing UM-SCC-38 cells and have not previously been associated with EMT.
In summary, we examined the effect of exogenous Slug expression in a HNSCC cell line in order to characterize the changes in cell adhesion, cell motility and global gene expression in a cell line. The data presented here reveal that Slug expression induced EMT characterized by a cadherin switch and loss of desmosomal adhesion. In addition the microarray data provide a unique examination of the Slug-dependent gene expression signature in a HNSCC cell line and provides a list of genes that potentially contribute to the EMT process.
Material and Methods
Cell culture.
Head and neck squamous cell carcinoma (HNSCC) cells (UM-SCC-38) were obtained from Dr. Thomas Carey (The University of Michigan) and were grown in MEM medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT). Retrovirally infected cell populations were routinely grown in medium containing 500 µg/mL G418 (Mediatech Inc., Herndon, VA).
Generation of cDNA constructs.
A human Slug (SNAI2) cDNA was generated by RT-PCR using total RNA from UM-SCC-1 cells as a target (primers slug1 GAA TTC ATG CCG CGC TCC TTC CTG GTC AAG and slug 2 GAT ATC TCA GTG TGC TAC ACA GCA GCC). PCR products were sequenced to verify that the cDNA matched the human SNAI2 sequence in Genbank (Genbank ID: NM_003068). The full-length human SNAI2 cDNA was cloned into the retroviral expression vector LZRS-ms-neo.13,31
Retrovirus production and infection.
Retrovirus production and infection was performed as previously described in references 13 and 31. Briefly, phoenix cells (5 × 105 per 100 mm dish) were transfected with a LZRS-MS-neo/SNAI2 vector or LZRS-MS-neo empty vector using calcium phosphate (Stratagene, La Jolla, Ca). Forty-eight hours following transfection, cells were switched to medium containing 2 µg/ml puromycin (Sigma) to select for virus producing cells. A population of puromycin resistant cells was grown in DMEM supplemented with 10% FBS lacking puromycin and grown at 32°C for 24 h to produce viral particles. Conditioned medium was collected and passed through a 0.45 µm syringe filter and polybrene (Sigma) was added to 4 µg/ml. For infection of UM-SCC-38 cells, 2 × 105 cells were plated in one well of a six well dish 18 h prior to infection. Fresh viral conditioned media was incubated with target cells for 6 h at 32°C. Following infection, the media was replaced with fresh media and cells were returned to 37°C. Two days after infection selective media containing 0.5 mg/ml G418 was added and a population of retrovirally infected cells was isolated.
Detergent extraction of cells and immunoblot analysis.
Total cell lysates were prepared by washing cells three times with phosphate buffered saline containing 2 mM sodium orthovanadate and extraction in 1 mL Empigen BB extraction buffer (10 mM Tris HCl pH 7.0, 0.1% Empigen BB (Calbiochem), 5 mM EDTA, 2 mM EGTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 2 mM phenylmethylsulfonyl fluoride). In order to detect exogenously expressed Slug, cells were cultured in the presence of 5 µM MG132 (Sigma Chemical Co.x) for 1 h prior to extraction. Cells were placed on ice, scraped and triturated vigorously for 2 min. Insoluble material was pelleted by centrifugation at 14,000x g for 15 min at 4°C, and the supernatant was used immediately or stored at −70°C. Protein concentration was determined using DC protein assay (BioRad, Hercules, CA) with bovine serum albumin as a standard. Cell lysates were resolved by SDS-PAGE and proteins were electrophoretically transferred overnight to nitrocellulose membranes and blocked in 5% non-fat dry milk in TBST (10 mM Tris HCl pH 7.5, 150 mM NaCl, 0.05% Tween-20) for 45 min. Blocking solution was removed by washing for 15 min followed by 2 × 5 min in TBST. For immunoblots, hybridoma conditioned media was used at 1:100 dilution in TBST for 1 h. Antibodies specific for E-caherin (4A2), P-cadherin (6A9), N-cadherin (13A9), α-catenin (1G5), β-catenin (15B8), desmoglein-2 (6D8), desmoglein 3 (5G11), desmocollin-2 (7G6), plakophilin-1 (14B11), plakophilin-2 (8H6), plakophilin-3 (11F2) and desmoplakin (20B6) have been previously described in references 32–35 or developed as described in references 34 and 36. Anti p120 catenin (Cat# 610133; BD Biosciences) and anti SNAI2 (Slug) (clone 2B6 Cat # MAB4371; Millipore Inc.,) were purchased and used as per manufacturer's instructions. The anti-β-tubulin antibody (E7) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52,242. Membranes were washed 15 min followed by 2 × 5 min in TBST. Membranes were incubated with horseradish peroxidase conjugated anti-mouse secondary antibody (Jackson Immunoresearch, West Grove, PA) at 1:10,000 for 1 h. Secondary antibody was removed by washing 15 min followed by 4 × 5 min in TBST. Immunoreactive bands were detected using Super Signal Pico substrate (Pierce Chemical Co., Rockford, IL).
Immunoflurescence microscopy.
Cells were grown to 70–80% confluence on glass cover slips and processed for immunostaining as previously described in references 37 and 38.
Cell motility and invasion assays.
In vitro motility assays were performed as described in Nieman et al. 5 × 105 cells were plated in the top chamber of non-coated polyethylene tetraphthalate (PET) membranes (6-well insert, pore size 8 µm; BD Biosciences). Cells were incubated for 24 h and cells that did not migrate through the membrane were scraped from the membrane using a cotton swab. Cells that migrated to the bottom of the membrane were fixed and stained using Diff-Quick stain kit (Fisher Scientific). Cells in ten random fields of view were counted and expressed as the average number of cells/field of view. Three independent experiments were done in each case. The data are represented as the average of the three independent experiments and the standard deviation of the average is indicated.
Microarray analysis.
Total RNA was isolated from UM-SCC-38/neo and UM-SCC-38/slug cells using the Trizol reagent following the manufacturer instructions. Three separate RNA samples were isolated from UM-SCC-38/neo cells and UM-SCC-38/slug cells. Gene expression profiling was performed using the Affymetrix platform and the human genome U133 Plus 2.0 array at the University of Nebraska Microarray Core Facility. Phycoerythrin labeled cRNA probes were generated per the Affymetrix protocol and hybridized to individual chips. Immediately after hybridization GeneChips were processed on a Gene Chip Fluidics Station and was scanned with a Gene Chip Scanner. Raw data were collected and processed using MAS 5.0 software (Affymetrix).
Data analysis.
Analyses were conducted with BRB ArrayTools version 3.8.0 beta_2 release, developed by Dr. Richard Simon and Amy Peng (1) and R programming language with Bioconductor project (www.bioconductor.org).
Low-level analysis which converts probe level data to a gene level expression data was done using RMA. RMA was implemented using the rma function of the affy package of the Bioconductor project in the R programming language.40 The background correction method corrects the perfect match (PM) probe intensities by using a model based on the assumption that the observed intensities are the sum of signal and noise. Quantile normalization is used to normalize the PM probes and the calculation of summary expression measures was done using the median polish method, which fits a multichip linear model to the data, and gives the expression on the log2 scale.40
Filtering was done prior to analysis to reduce the number of genes considered for testing. If less than 20% (two) of the expression data values have at least a 1.5-fold change in either direction from the genes median value, the gene was excluded.
Statistical analysis.
For each gene, t-statistic was computed to determine if there was a significant difference in expression by treatment group. p-values less than 0.001 with a fold change in expression of 2 are considered to be statistically significant. The Benjamini-Hochberg41 method was used to estimate the false discovery rate (FDR) for the significant gene list.
Acknowledgments
This project was supported by NIH R01 DE016905 and NCRR P20 RR018759 (J.K.W.).
Supplementary Material
References
- 1.De Craene B, van Roy F, Berx G. Unraveling signalling cascades for the Snail family of transcription factors. Cell Signal. 2005;17:535–547. doi: 10.1016/j.cellsig.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Shirley SH, Hudson LG, He J, Kusewitt DF. The skinny on Slug. Mol Carcinog. 2010;49:851–861. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrallo-Gimeno A, Nieto MA. Evolutionary history of the Snail/Scratch superfamily. Trends Genet. 2009;25:248–252. doi: 10.1016/j.tig.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 5.Parent AE, Newkirk KM, Kusewitt DF. Slug (Snai2) expression during skin and hair follicle development. J Invest Dermatol. 2010;130:1737–1739. doi: 10.1038/jid.2010.22. [DOI] [PubMed] [Google Scholar]
- 6.Murray SA, Oram KF, Gridley T. Multiple functions of Snail family genes during palate development in mice. Development. 2007;134:1789–1797. doi: 10.1242/dev.02837. [DOI] [PubMed] [Google Scholar]
- 7.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandl M, Seidler B, Haller F, Adamski J, Schmid RM, Saur D, et al. IKK(alpha) controls canonical TGF(ss)-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells. J Cell Sci. 2010;123:4231–4239. doi: 10.1242/jcs.071100. [DOI] [PubMed] [Google Scholar]
- 10.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 11.Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64:55–63. doi: 10.1158/0008-5472.can-03-2144. [DOI] [PubMed] [Google Scholar]
- 12.Baker SR. An in vivo model for squamous cell carcinoma of the head and neck. Laryngoscope. 1985;95:43–56. doi: 10.1288/00005537-198501000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Sobolik-Delmaire T, Katafiasz D, Keim SA, Mahoney MG, Wahl JK., 3rd Decreased plakophilin-1 expression promotes increased motility in head and neck squamous cell carcinoma cells. Cell Commun Adhes. 2007;14:99–109. doi: 10.1080/15419060701463082. [DOI] [PubMed] [Google Scholar]
- 14.Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:719–721. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH, et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol. 2009;11:694–704. doi: 10.1038/ncb1875. [DOI] [PubMed] [Google Scholar]
- 16.Maeda M, Johnson E, Mandal SH, Lawson KR, Keim SA, Svoboda RA, et al. Expression of inappropriate cadherins by epithelial tumor cells promotes endocytosis and degradation of E-cadherin via competition for p120(ctn) Oncogene. 2006;25:4595–4604. doi: 10.1038/sj.onc.1209396. [DOI] [PubMed] [Google Scholar]
- 17.Sobolik-Delmaire T, Katafiasz D, Keim SA, Mahoney MG, Wahl JK., 3rd Decreased plakophilin-1 expression promotes increased motility in head and neck squamous cell carcinoma cells. Cell Commun Adhes. 2007;14:99–109. doi: 10.1080/15419060701463082. [DOI] [PubMed] [Google Scholar]
- 18.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, et al. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastatic disease. J Biol Chem. 2008;283:18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusewitt DF, Choi C, Newkirk KM, Leroy P, Li Y, Chavez MG, et al. Slug/Snai2 is a downstream mediator of epidermal growth factor receptor-stimulated reepithelialization. J Invest Dermatol. 2009;129:491–495. doi: 10.1038/jid.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- 22.Bair EL, Massey CP, Tran NL, Borchers AH, Heimark RL, Cress AE, et al. Integrin- and cadherin-mediated induction of the matrix metalloprotease matrilysin in cocultures of malignant oral squamous cell carcinoma cells and dermal fibroblasts. Exp Cell Res. 2001;270:259–267. doi: 10.1006/excr.2001.5347. [DOI] [PubMed] [Google Scholar]
- 23.Jaggi M, Wheelock MJ, Johnson KR. Differential displacement of classical cadherins by VE-cadherin. Cell Commun Adhes. 2002;9:103–115. doi: 10.1080/15419060214150. [DOI] [PubMed] [Google Scholar]
- 24.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 25.Mo YY, Reynolds AB. Identification of murine p120 isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 1996;56:2633–2640. [PubMed] [Google Scholar]
- 26.Keirsebilck A, Bonne S, Staes K, van Hengel J, Nollet F, Reynolds A, et al. Molecular cloning of the human p120ctn catenin gene (CTNND1): expression of multiple alternatively spliced isoforms. Genomics. 1998;50:129–146. doi: 10.1006/geno.1998.5325. [DOI] [PubMed] [Google Scholar]
- 27.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prunier C, Howe PH. Disabled-2 (Dab2) is required for transforming growth factor beta-induced epithelial to mesenchymal transition (EMT) J Biol Chem. 2005;280:17540–17548. doi: 10.1074/jbc.M500974200. [DOI] [PubMed] [Google Scholar]
- 29.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilanowski T, Caddy J, Ting SB, Hislop NR, Cerruti L, Auden A, et al. Perturbed desmosomal cadherin expression in grainy head-like 1-null mice. EMBO J. 2008;27:886–897. doi: 10.1038/emboj.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol. 1996;135:1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacco PA, McGranahan TM, Wheelock MJ, Johnson KR. Identification of plakoglobin domains required for association with N-cadherin and alpha-catenin. J Biol Chem. 1995;270:20201–6. doi: 10.1074/jbc.270.34.20201. [DOI] [PubMed] [Google Scholar]
- 34.Wahl JK., 3rd Generation of monoclonal antibodies specific for desmoglein family members. Hybrid Hybridomics. 2002;21:37–44. doi: 10.1089/15368590252917629. [DOI] [PubMed] [Google Scholar]
- 35.Wahl JK, Sacco PA, McGranahan-Sadler TM, Sauppe LM, Wheelock MJ, Johnson KR. Plakoglobin domains that define its association with the desmosomal cadherins and the classical cadherins: identification of unique and shared domains. J Cell Sci. 1996;109:1143–1154. doi: 10.1242/jcs.109.5.1143. [DOI] [PubMed] [Google Scholar]
- 36.Johnson KR, Lewis JE, Li D, Wahl J, Soler AP, Knudsen KA, et al. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res. 1993;207:252–260. doi: 10.1006/excr.1993.1191. [DOI] [PubMed] [Google Scholar]
- 37.Narayana N, Gist J, Smith T, Tylka D, Trogdon G, Wahl JK. Desmosomal component expression in normal, dysplastic and oral squamous cell carcinoma. Dermatol Res Pract. 2010;2010:649731. doi: 10.1155/2010/649731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobolik-Delmaire T, Reddy R, Pashaj A, Roberts BJ, Wahl JKI. Plakophilin-1 localizes to the nucleus and interacts with single-stranded DNA. J Invest Dermatol. 2010;130:2638–2646. doi: 10.1038/jid.2010.191. [DOI] [PubMed] [Google Scholar]
- 39.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses. 1995;45:486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.