Abstract
The existence of multiple VEGF-A isoforms raised the possibility that they may have distinct functions in tumor growth. We have previously published that VEGF189 and VEGF165 contribute to breast cancer progression and angiogenesis, but VEGF165 induced the most rapid tumor uptake. Since VEGF165 has been described as a survival factor for breast tumor cells, we questioned here the effects of VEGF189 on the survival/apoptosis of MDA-MB-231 cells. We used clones that overexpress VEGF189 (V189) or VEGF165 (V165) isoforms and compared them to a control one (cV). Overexpression of VEGF189 resulted in increased cell apoptosis, as determined by Annexin-V apoptosis assay, under serum starvation and doxorubicin treatment, while VEGF 165 was confirmed to be a survival factor. Since MDA-MB-231 highly express NRP1 (a co-receptor for VEGF-A), we used short hairpin RNA (shRNA) to knock down NRP1 expression. V189shNRP1 clones were characterized by reduced apoptosis and higher necrosis, as compared with V189shCtl, under stress conditions. Unexpectedly, NRP1 knockdown had no effect on the survival or apoptosis of V165 cells. VEGF189 showed greater affinity toward NRP1 than VEGF165 using a BIAcore binding assay. Finally, since endogenously produced urokinase-type plasminogen (uPA) has been found to prevent apoptosis in breast cancers, we analyzed the level of uPA activity in our clones. An inhibition of uPA activity was observed in V189shNRP1 clones. Altogether, these results suggest a major role of NRP1 in apoptosis induced by VEGF189 in stress conditions and confirm VEGF165 as a survival factor.
Key words: VEGF isoforms, survival, apoptosis, NRP-1, breast cancer cells
Introduction
Vascular Endothelial Growth Factor (VEGF-A) is a key regulator of developmental, physiological and pathological neovascularization, including tumor growth. This growth factor consists of a family of proteins generated from a single gene (VEGF-A) by alternative splicing of the primary transcript.1,2 Processing results in VEGF isoforms consisting in proteins 121, 165, 189 and 206 amino acids in length in humans. VEGF121 and VEGF165 are usually considered as the most abundant. Depending on the presence of exons 6 and 7, these isoforms are either secreted as soluble forms (VEGF121 and VEGF165) or remain associated with the cell or extracellular matrix (VEGF189, VEGF206 and partly VEGF165). The existence of multiple isoforms exhibiting different binding affinities to heparin and heparan sulfate proteoglycans and different binding to VEGF receptors VEGF-R1 and VEGF-R2,3–7 raised the possibility that individual isoforms have distinct functions in different aspects of tumor growth.
VEGF121 and VEGF165 have been shown to accelerate breast tumor development,8,9 while the role of VEGF189 in breast cancer currently remained elusive. Recently, we have examined the role of this isoform, as compared with VEGF165, in breast cancer progression and angiogenesis, using a xenograft model of VEGF-overexpressing MDA-MB-231 cells (referred to as V165 and V189).10 Our findings showed that both VEGF189 and VEGF165 contribute to breast cancer progression and angiogenesis. However, V165 induced the most rapid tumor uptake, as compared with V189, suggesting different effects of these two isoforms through different molecular mechanisms.10 The reason for the delayed VEGF189 tumor uptake in breast cancer stays unknown.
VEGF165 contributes to the two major characteristics of invasive breast carcinoma: survival and migration of breast cancer cells,11–16 independently of angiogenesis. Several findings supported the hypothesis that carcinoma progression selects for cells that depend on VEGF165 as an autocrine survival factor (11, 13 and 16). The implication of neuropilin-1 (NRP1), a VEGF coreceptor, has been described among the mechanisms by which VEGF165 mediates the survival of tumor cells.11 The role of VEGF189 as a survival factor has not been studied yet.
NRP1, originally described in the developing nervous system,17,18 is expressed in endothelial and tumor cells.19 It is a transmembrane protein that is considered as a co-receptor of VEGF-R2 in endothelial cells.20,21 NRP1 is expressed at high levels in several tumor cells, where it has been implicated in cell migration and survival.11,12,22–27 Recently, our group showed that VEGF189 binds to NRP1,10 in addition to VEGF165. However, the role of VEGF189-NRP1 association in survival has not been elucidated. We hypothesize that differences in cell behavior, especially in survival/apoptosis, could be involved in the delay in tumor uptake observed in our in vivo experiments.10
In the present study, we analyzed the effects of VEGF189 on the survival of MDA-MB-231 breast cancer cells. We used previously generated clones which overexpress VEGF189 (V189) or VEGF165 (V165) from breast cancer cells (MDA-MB-231).10 We report that overexpression of VEGF189 results in increased apoptosis of MDA-MB-231 under two different stress conditions: serum starvation or doxorubicin treatment. Parental MDA-MB-231 cells and derived V165 and V189 clones express high levels of NRP1 protein, while VEGF-R2 expression is low and VEGF-R1 is undetectable.10 A plasmid-based system that stably expresses short hairpin RNA (shRNA) molecules, which silences NRP1 gene expression by RNA interference, has been generated using a protocol previously described for a long-term gene silencing.28–30 In V189 and V165 clones this shRNA specifically decreases NRP1 expression, and this knockdown leads to increased cell death in V189 clones under stress conditions bypassing apoptosis.
Results
Overexpression of VEGF189 induces apoptosis under nutrient deprivation stress, in contrast to VEGF165.
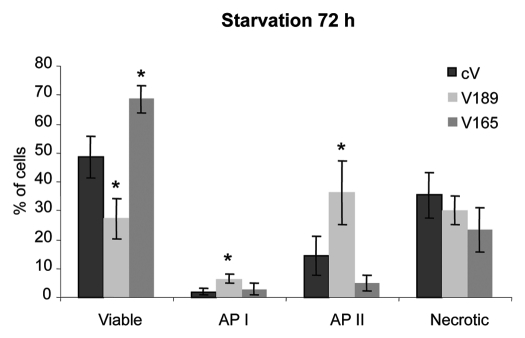
MDA-MB-231clones that overexpress VEGF189 or VEGF165 isoform (referred as V189 and V165 clones, respectively10; Table 2A) were subjected to serum deprivation (0.5% FBS) for 24–96 h. The extent of viability, apoptosis and cell death was analyzed at each time using (FITC)-labeled Annexin-V/propidium iodide (PI) flow cytometric assay (data not shown). Differences in viability/apoptosis of cells overexpressing different VEGF isoforms were observed after 72 h starvation when compared with control cV cells. V165 clones proved significant better resistance to serum starvation, when compared with cV clones (1.4-fold increased number of viable cells, p < 0.05). Unexpectedly, V189 cells were significantly less viable than V165 or cV cells (2.5- and 1.7-fold, respectively; p < 0.05) (Fig. 1). The overexpression of VEGF189 resulted in higher apoptosis as compared with cV or V165 clones [3- and 2-fold increase in the early (API) and 2.5- and 7-fold increase in the late (APII) apoptosis states, respectively, p < 0.05] (Fig. 1). Similar results were also observed with three independent V189 clones (V189-13, V189-25 and V189-26 clones; reviewed in ref. 10) in serum starved conditions. The pro-apoptotic effect of V189 was dependent on the expression level of VEGF189 in these clones (Fig. S1). These results suggested different effects of VEGF isoforms on cell survival/apoptosis.
Table 2.
Characterization of MDA-MB-231 breast cancer cells overexpressing VEGF isoforms used in this study
| (A) | Clones number | VEGF165 | VEGF189 | NRP1 |
| cV | 1 | 1 | 1 | |
| Clones | V189 | 1.5 | 30.5# | 1 |
| V165 | 48# | 1.4 | 1.4 | |
| (B) | Clones number | VEGF165 | VEGF189 | NRP1 |
| shCtl | 1 | 1 | 1 | |
| cV clones | shNRP1–9 | 0.9 | 0.8 | 0.6* |
| shNRP1–19 | 0.5 | 0.5 | 0.4* | |
| shCtl | 1 | 1 | 1 | |
| V189 clones | shNRP1–10 | 0.4* | 9.7* | 0.6* |
| shNRP1–11 | 0.48* | 5.8* | 0.57* | |
| shCtl | 1 | 1 | 1 | |
| V165 clones | shNRP1–3 | 1 | 0.5 | 0.5* |
| shNRP1–5 | 0.7 | 0.6 | 0.4* |
(A) VEGF overexpressing clones. Expression of VEGFmRNA was assessed using real-time PCR V189 clones (V189–13), V165 (V165–42) clones, and cV (cV-14) clones (for VEGF189 and VEGF165 overexpressing, or control clones, respectively), were initially described in reference 10; these clones were maintained in 10% SVF condition Data obtained using real-time PCR were expressed as mean values, as compared with cV clones set to 1. (B) ShNRP1 and shCtl transfected clones. Data, obtained in 10% FBS containing medium, were expressed as mean values of 4 independent experiments performed in duplicate, as compared with corresponding shCtl clone set to 1. Statistical significance (p < 0.05):
VEGF overexpressing vs. cV clone;
shNRP1 clone vs. shCtl clone.
Figure 1.
Overexpression of VEGF189 in MDA-MB-231 cells induces apoptosis under serum starvation condition, while VEGF 165 improves survival. Cells were incubated for 72 h in 0.5% FBS-containing medium. Adherent cells were harvested and percentage of viable cells, cells in early apoptosis (AP I), late apoptosis (AP II) and necrosis was determined by flow cytometry using Annexin V apoptosis/cell death assay. Each column represents a mean (±SE M) of four independent experiments for viable (Annexin V−, PI−), AP I (Annexin V+, PI−), AP II (Annexin V+, PI+), and necrotic (Annexin V−, PI+) cells. *p ≤ 0.05 vs. corresponding cV clone.
Blocking endogenous NRP1 expression using short hairpin RNA.
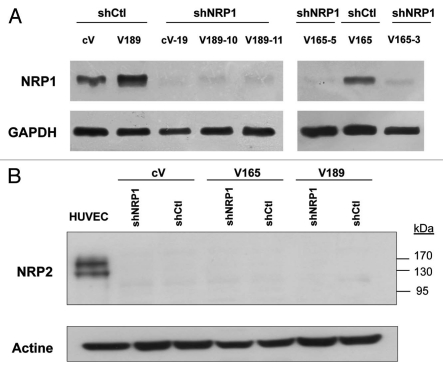
V165 and V189 clones express high levels of NRP1, a transmembrane protein which binds VEGF, and low or undetectable levels of other VEGF receptors.10 In order to analyze the implication of NRP1 in VEGF isoform-induced cell survival/apoptosis under stress conditions, we first knocked-down NRP1 expression using short hairpin RNA (shRNA). V189, V165 and cV cells were stably transfected with vectors expressing shRNA for NRP1 (shNRP1) or irrelevant shRNA (shCtl). Two clones showing a >40% reduction in NRP1 mRNA levels compared with clones containing the irrelevant vector (shCtl), were selected for each transfected clone of origin (V189 shNRP1-10 and V189 shNRP1-11, V165 shNRP1-3 and V165 shNRP1-5, and cV shNRP1-9 and cV shNRP1-19; Table 2B). Inhibition of NRP1 was confirmed at the protein level (Fig. 2A). The stability of NRP1 silencing was monitored and confirmed by qRT-PCR and western blotting along 10 passages for all the clones. NRP2 protein was not detected in these clones using western blotting (Fig. 2B).
Figure 2.
Analysis of neuropilins expression in the different MDA-MB-231 clones used in the study. cV, V189 and V165 clones were stably transfected with the control irrelevant shRNA (sh control) or NRP1 shRNA expression plasmid (shNRP1). Protein extracts (A: 70 µg; B: 100 µg) obtained from these clones were immunoblotted with a NRP1 (A) or NRP2 (B) antibodies; anti-GAPDH or actine antibodies (in A and B, respectively) were used to assess protein loading.
Overexpression of VEGF189 induces apoptosis in two different stress conditions.
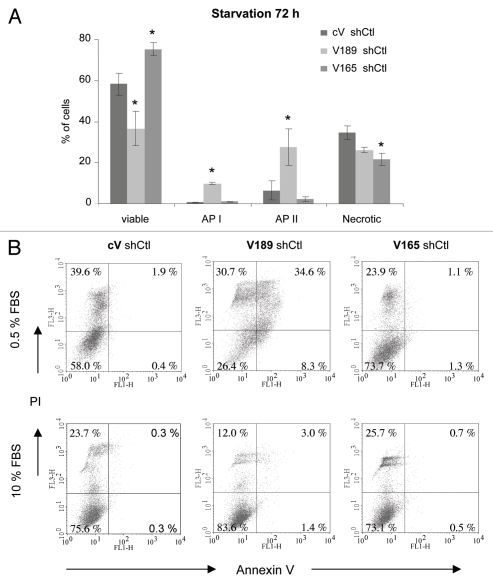
Serum starvation. In order to confirm the characteristics of clones after transfection with shRNA, we then subjected V189 shCtl, V165 shCtl and cV Ctl clones to serum deprivation (0.5% FBS for 24–96 h), as described above. Differences in viability/apoptosis between V165, V189 and control cV clones transfected with shCtl were observed after 72 h starvation (Fig. 3), as also observed in parental V189, V165 and cV clones (Fig. 1). The extent of apoptosis determined by Annexin-V staining in V189 shCtl clones was also significantly higher compared with cV shCtl or V165 shCtl clones (10-fold increase in the early apoptosis state (API) for both comparisons, p < 0.05; 4.2- and 12-fold increases in the late apoptosis state (APII) vs. cV shCtl and V165 shCtl respectively, p < 0.05) (Fig. 3A). This effect was accompanied by a significantly lower number of V189 shCtl viable cells (1.6-fold vs. cV shCtl and 2-fold vs. V165 shCtl clones, p < 0.05). It is interesting to note the tendency of V189 shCtl necrotic cell population to be smaller than that of control cells, since cV shCtl cells seemed to process directly into necrosis state in contrast to V189 shCtl (Fig. 3B).
Figure 3.
VEGF189 overexpression in shCtl-transfected clones induces apoptosis under serum starvation condition, while VEGF165 improves survival. Cells were incubated for 72 h in 0.5% FBS-containing medium. Adherent cells were harvested and percentage of viable cells, cells in early apoptosis (AP I), late apoptosis (AP II) and necrosis was determined by flow cytometry using Annexin V apoptosis/cell death assay. (A) Each column represents a mean (±SE M) of four independent experiments for viable, API, AP II and necrotic cells. *p ≤ 0.05 vs. corresponding cV shCtl clone. (B) Representative flow cytometry profiles under 0.5% FBS (upper part) and 10% FBS (lower part) conditions.
In contrast to V189 shCtl, V165 shCtl clones survived better in serum starvation when compared with cV shCtl clones (1.3-fold increased number of viable cells, accompanied by a 1.6-fold decrease in number of necrotic AnnexinV−/PI+ cells, p < 0.05) (Fig. 3A). Altogether, these data indicate that transfection with control shRNA did not change the characteristics of the clones overexpressing different VEGF isoforms.
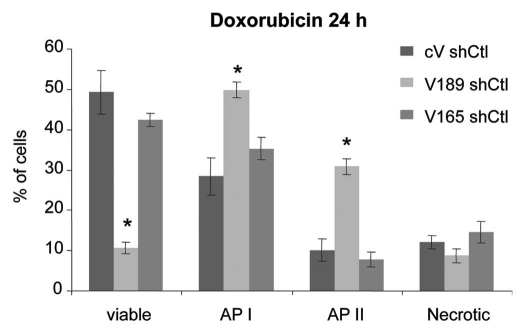
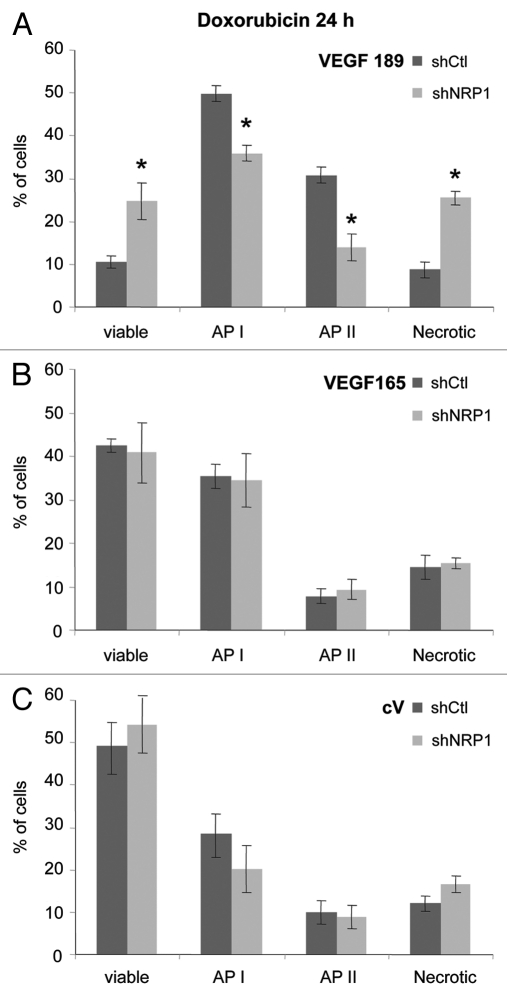
Doxorubicin treatment. To analyze cell behavior in another stress condition, shCtl-transfected V189, V165 or cV clones were treated with 2.5 µM of doxorubicin, a chemotherapeutic apoptosis-inducing agent, in 10% FBS-containing medium for 24–48 h. Doxorubicin treatment for 24 h conferred superior apoptotic behavior to V189 shCtl clones (Fig. 4). A higher number of cells in early (API; 1.6-fold) and late (APII; 3.5-fold) apoptosis states (p < 0.05), accompanied by 4-fold lower number of viable cells (p < 0.05) was observed when compared with V165 shCtl and cV shCtl clones (Fig. 4). In contrast, overexpression of VEGF165 did not result in any significant changes in viability and apoptotic profiles with respect to cV shCtl cells under 24 h doxorubicin treatment (Fig. 4). After 48 h doxorubicin treatment, the population of necrotic V189 cells was about 2-fold lower than that of cV clones while V165 clones tended (not significant) to resist to cell death in term of a lower number of necrotic V165 cells (not shown).
Figure 4.
Overexpression of VEGF189 increases doxorubicin-induced apoptosis. ShCtl transfected clones were treated with 2.5 µM doxorubicin in serum-containing medium for 24 h. Adherent cells were harvested and subjected to Annexin V apoptosis/cell death assay. Each column represents a mean (±SE M) of six independent experiments for viable, AP I, AP II and necrotic cells. *p ≤ 0.05 vs. corresponding cV shCtl clone.
Altogether, these findings indicated that the increase of VEGF189 expression in MDA-MB-231 cells leaded to a higher degree of cell apoptosis in two different stress conditions.
Downregulation of NRP1 expression decreases apoptosis under stress conditions in MDA-MB-231 overexpressing VEGF189 isoform.
We then assessed the effects of reduced NRP1 expression after stable transfection with shNRP1 (see Table 2B) on the survival of VEGF-overexpressing clones, using Annexin V apoptosis assay.
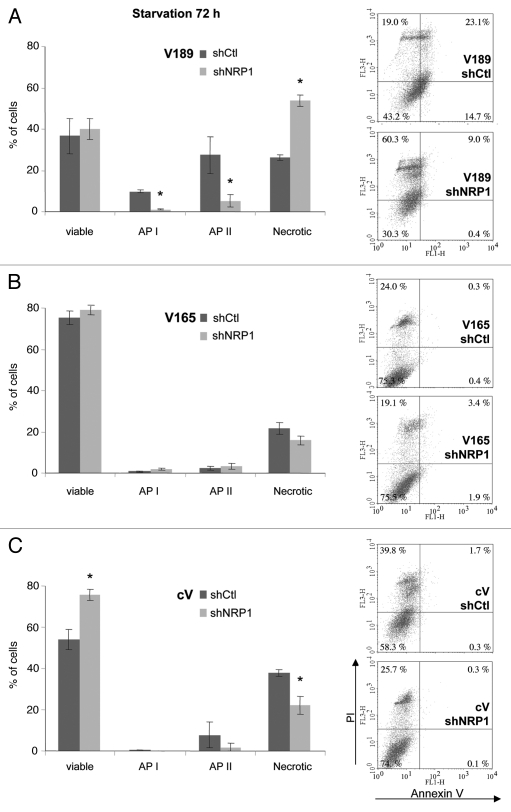
Serum starvation. Cells were subjected to serum deprivation (0.5% FBS) for 24–96 h. After 72 h starvation, V189 clones with decreased expression of NRP1 shifted significantly toward necrosis, as compared with V189 shCtl cells (2-fold increase of necrotic cells, p < 0.05) (Fig. 5A). This effect was accompanied by the decrease of early (API) and late apoptotic (APII) cell numbers (9- and 5-fold decrease, respectively) (Fig. 5A). In contrast, the number of viable cells did not change significantly (Fig. 5A). Similar results were observed with two independent clones (V189 shNRP1-10 and V189 shNRP1-11, as described in Table 2B). These results suggest that NRP1 is involved in apoptotic cell death of V189 clones, as its knockdown leaded to evasion from apoptosis toward necrosis. These observations were confirmed using a specific LDH necrosis assay (Fig. S2).
Figure 5.
NRP1 knockdown abrogates apoptosis induced by VEGF189 under serum starvation conditions. Annexin V/PI staining was performed on adherent cells harvested after 72 h incubation in 0.5% FBS-containing medium. Histograms of means (±SE M) (left) and representative flow cytometry profiles (right) stand for transfected with shNRP-1 vs. irrelevant shCtl clones overexpressing VEGF189 (A), n = 3; VEGF 165 (B), n = 4; and control cV (C), n = 3. Two clones were tested for each shRNA transfection (see Table 2B) giving similar profiles; shown results stand for cVshNRP1–19, V189shNRP1–10 and V165shNRP1–5. *p ≤ 0.05 vs. corresponding shCtl clone.
In contrast, downregulation of NRP1 expression had no significant effect on the survival or apoptosis of cells overexpressing VEGF165 (Fig. 5B). Surprisingly, the knockdown of NRP1 expression slightly improved the survival and decrease the number of necrotic control cV cells (1.4-fold more viable cells and 1.7-fold decrease of necrosis, p < 0.05) after 72 h starvation (Fig. 5C), as also observed using the specific LDH necrosis assay (Fig. S2).
Doxorubicin treatment. A significant (3-fold, p < 0.05) increase of the number of necrotic cells was also observed when NRP1-silenced V189 clones were subjected to 2.5 µM doxorubicin treatment in 10% FBS containing medium after 24 h incubation (Fig. 6A). This effect was accompanied by a lower degree of apoptosis induced by doxorubicin, as illustrated by the significant decrease of API and APII apoptotic stages (1.4- and 2.2-fold, respectively; p < 0.05) in V189 shNRP1 clones, as compared with V189 shCtl ones (Fig. 6A). The increase in viable cells population (2.3-fold, p < 0.05) was observed after 24 h doxorubicin treatment (Fig. 6A). The differences observed for NRP1-depleted V189 cells compared with V189 shCtl cells remained at 48 h, although not statistically significant (not shown). In contrast, knockdown of NRP1 did not result in any alterations in survival behavior of either V165 or cV clones after 24 h (Fig. 6B and C) or 48 h (not shown) doxorubicin treatment.
Figure 6.
NRP1 knockdown reduces apoptosis induced by doxorubicin in VEGF189 overexpressing clones. V189 (A), n = 6, V165 (B), n = 5 and cV clones (C), n = 7 have been transfected with shNRP-1 vs. irrelevant control shRNA clones. Apoptosis assay was performed on cells harvested after 24 h treatment with 2.5 µM doxorubicin in serum-containing medium. Histograms of means (±SE M) stand obtained with the same clones, as shown in Figure 5, are presented. *p ≤ 0.05 vs. corresponding shCtl clone.
Taken together, these results suggest the involvement of NRP1 in apoptosis reinforced by VEGF189 under serum starvation and doxorubicin treatment.
Since soluble forms of NRP1 (sNRP1) have been described in the conditioned media of tumor cells,31 we asked whether the increase of apoptosis of V189 clones under stress conditions could be affected by sNRP1. sNRPs were also detected in the starved-conditioned medium of our clones. However, no increase of soluble NRP1 was observed in V189 shCtl cells compared with cV shCtl (Fig. S3), suggesting that the pro-apoptotic effect of V189 was not due to neutralization of VEGF189 by sNRP1.
VEGF189 has greater affinity toward NRP1 than VEGF165.
VEGF189 has been previously shown to bind to NRP1, as VEGF165.10 We then analyzed whether distinct effects of NRP1 on survival of VEGF165- or VEGF189-overexpressing cells could be associated with differences in binding capacities of the two isoforms toward this receptor. We used a BIAcore sensor approach10 and immobilization of NRP1 via direct coupling on the sensor chip surfaces, as previously described for VEGF165 by Fuh et al.32 and VEGF189 by our group.10 VEGF189 bound to NRP1 with higher affinity, as compared with VEGF165, and this was mainly due to a lower dissociation rate (Table 3). Since BIAcore may result in the denaturation of proteins when the protein is immobilized to sensor chip surfaces via direct covalent amine coupling, we also performed immobilization for the recombinant NRP1-Fc fusion protein via covalently coupled anti-human Fc (excluding NRP1-Fc denaturation). Both approaches produced similar results.
Table 3.
VEGF189 bound to NRP1 with higher affinity, as compared with VEGF165
| VEGF | Kon | Koff | Ka | Kd |
| VEGF189 | 14 × 105 | 1,7 × 10−4 | 82 × 108 | 1,2 × 10−10 |
| VEGF165 | 5 × 105 | 6,0 × 10−4 | 8 × 108 | 1,2 × 10−9 |
The association and dissociation constants were measured using a BIAcore sensor approach, as described in Materials and Methods. The experiments were repeated at least twice for each concentration of protein. The affinity of NRP1 binding to VEGF165 or VEGF189 was calculated from the on and off rates. We used the steady-state analysis to estimate the affinity of NRP1 for VEGF.
Decrease of NRP1 expression is associated with upregulation of VEGF189 expression.
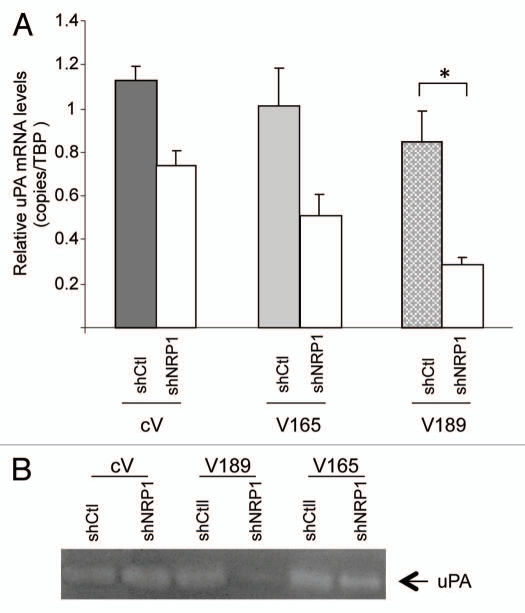
To further understand the results obtained in NRP1 knockdown experiments, we analyzed the levels of VEGF transcripts in V189, V165, cV and their corresponding NRP1-silenced clones. Surprisingly, VEGF189 mRNA expression was increased (6- to 10-fold) in NRP1-silenced V189 clones (both V189 shNRP1-10 and V189 shNRP1-11), as compared with V189 shCtl (Table 2B). In addition, the VEGF189 mRNA increase in shNRP1 V189 clones was observed in the different conditions tested: 10% FBS, serum deprivation (0.5% FBS) and hypoxia (Table 4). In contrast, no significant change in VEGF165 mRNA expression was observed in NRP1-silenced V165, as compared with V165 shCtl (Table 2B). Moreover, decrease of NRP1 expression had no significant effect on the low expression of VEGF189 mRNA in cV and V165 clones.
Table 4.
Comparison of VEGF189 mRNA levels in different conditions
| Clones number | 10% FBS | 0.5% FBS | Hypoxia | |
| cV | shCtl | 1 | 1 | 1 |
| clones | shNRP1–19 | 0.5 | 0.8 | 0.58 |
| V189 | shCtl | 30.5 | 36 | 33.4 |
| clones | shNRP1–10 | 297* | 263* | 136* |
| V165 | shCtl | 1.4 | 0.5 | 3.6 |
| clones | shNRP1–3 | 0.7 | 0.6 | 1.1 |
Sh-transfected V189, V165 or cV clones, as described in Materials and Methods, were maintained in medium containing 10% FBS, 0.5% FBS (72 h serum starvation conditions), or under hypoxia (for 24 h in 1% O2, 4.5 g/L glucose and 10% FBS). Analysis of transcripts was performed using qRT-PCR. Results are expressed as fold induction, as compared with cV shCtl clone set to 1 in corresponding condition. Note that NRP1 knockdown resulted in 10-fold increased VEGF189 expression (297 vs. 30) in 10% FBS (*p < 0.05).
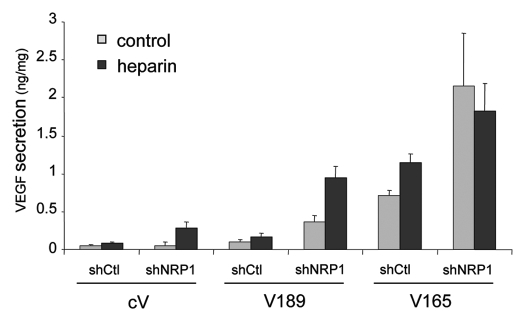
To confirm these observations at the protein level, the secretion of VEGF was analyzed in the culture supernatants from these clones, using ELISA assay. Since VEGF189 is sequestered on heparan sulfate proteoglycans on the cell surface, we used heparin treatment (50 µg/ml) to detect the total VEGF (free and cell-associated VEGF). As shown in Figure 7, heparin treatment resulted in a significant increase of VEGF secretion into the conditioned medium by V189 shCtl and to a lesser extent V165-shCtl clones (2.9- and 1.3-fold induction), corresponding to the release of cell-associated VEGF, i.e., mainly VEGF189 and to a lesser extent VEGF165, respectively. Knockdown of NRP1 expression in the V189 clone resulted in a 4–5 fold higher secretion of VEGF189 (Fig. 7), compared with V189 shCtl in similar heparin treatment. These results correlated with the increase of VEGF 189 expression at the transcript level observed in NRP1-silenced V189 clones (Table 2B). Altogether, these data suggest a correlation between increased death of NRP1-silenced V189 cells in stress conditions (see Figs. 5A and 6A) with even higher level of VEGF189 production compared with V189 shCtl clones.
Figure 7.
Analysis of VEGF expression in the different MDA-MB-231 clones used in the study. V189, V165 and cV clones, transfected with shCtl or shNRP1, were grown in serum free medium supplemented or not with heparin (50 µg/ml) for 24 h. VEGF proteins from the conditioned media were measured using an ELISA kit. Results are expressed as ng VEGF/mg protein extract. Values represent means ± SD of a representative experiment out of two independent experiments performed in triplicate.
Changes in the expression and activity of uPA in V189 clones.
It has been published that endogenously produced urokinase-type plasminogen (uPA) prevents apoptosis in MDA-MB-231 cancer cells.33 We then further analyzed whether overexpression of VEGF isoforms could modulate uPA expression and activity in breast cancer cells. As shown in Figure 8A, the expression of uPA was decreased in V189 clones, especially when NRP1 was silenced (associated with an increase of VEGF189), as compared with cV and V165 clones. The decrease of uPA activity observed in NRP1-depleted V189 cells under serum starvation (Fig. 8B) was correlated with the shift toward necrosis observed in the same clones, as compared with V189 shCtl cells (see Figs. 5A and 6A). These results indicate that VEGF189 exerts inhibition on uPA expression and activity, in agreement with VEGF189 effects on apoptosis.
Figure 8.
Analysis of the expression and activity of uPA in the different clones. (A) NRP1 knockdown decreases uPA expression in VEGF189 overexpressing clones. Cells were maintained in 10% FBS containing medium. Transcripts were analyzed by qRT-PCR. Results are normalized to their corresponding shCtl clones. Values represent means ± SE M of three independent experiments. *p < 0.05 vs. corresponding shCtl clone; (B) NRP1 knockdown results in decreased uPA activity in VEGF189 overexpressing clones. Conditioned medium from cells cultured for 24 h in serum-free DMEM was analyzed using zymography, as described in Materials and Methods. The figure shows a representative from two independent experiments.
Discussion
The mechanisms that facilitate the survival of invasive carcinoma cells are of obvious importance for understanding tumor progression and metastasis. VEGF165 has been described to be a survival factor for breast tumor cells,11,12 as well as being a key angiogenic factor. Most studies addressing the autocrine functions of VEGF in breast cancer cells used the MDA-MB-231 cell line, which expresses mainly the VEGF165 and VEGF121 isoforms. VEGF blockade (anti-VEGF antibodies or use of anti-sense VEGF RNA) in breast cancer cells results in direct apoptosis of tumor cells under normoxia11,12,15,16 or hypoxia.34 However, these experiments cannot discriminate between VEGF isoforms. Our results indicate that, unexpectedly, overexpressed VEGF189 in breast cancer (V189) cells is pro-apoptotic under stress conditions, unlike VEGF165 (V165 cells). The overexpression of VEGF165 resulted in more viable and fewer necrotic cells than cV clones under serum starvation. Changes in V189 cell survival under nutrient deprivation unlike those in control and V165 cells contrast with previous observations of their similar proliferation properties in vitro and in vivo (10% serum conditions).10 The present findings could explain, at least partially, our previous observations in a mouse xenograft model indicating that breast tumors which overexpress VEGF189 are characterized by a delay in tumor uptake as compared with tumors overexpressing VEGF165;10 similar findings were also observed in colon cancer.
Neuropilin 1 is a transmembrane protein that is expressed at high levels in several tumor cells, where it has been implicated in cell migration and survival.11,12,14,22–26 As NRP1 is widely expressed in our derived MDA-MB-231 clones, the effects of VEGF isoforms on survival/apoptosis may involve NRP1. The silencing of NRP1 decreased the apoptosis of clones overexpressing VEGF189 under serum starvation or doxorubicin treatment, and resulted in more necrosis in both conditions than observed for shCtl-transfected V189 clones. In agreement with this result, serum starvation increased LDH activity in shNRP1-V189 clones. NRP1 was thus essential for apoptotic behavior of clones overexpressing VEGF189 in stress conditions. The pro-apoptotic effect of V189 was not due to neutralization of VEGF189 by soluble NRP1 (sNRP1) described in several papers,31,35 since there was no more sNRP1 in V189 cells than in cV cells under serum starvation. We did not observe stress-induced decrease of NRP1 expression in V189 and V165 cells under serum starvation, in contrast to NRP1 degradation induced by low glucose on MDA-MB-231 cells.36 Moreover, we did not detect NRP2 protein in these clones, consistent with previous reports in MDA-MB-231.37 Altogether, these results suggest that NRP1 is required for VEGF189-induced apoptosis and not for cell survival.
In this study, we also demonstrated the “atypical” increased expression of VEGF189 in shNRP1-transfected V189 cells as compared with V189 cells. This finding was observed in three different conditions (serum deprivation, normoxia and hypoxia), and was clone-independent. The mechanism of this upregulation of VEGF189 is unknown. Changes in VEGF-A splicing have been described in embryonic development and cancer progression, but little is known about the mechanisms by which specific VEGF isoforms are selected,38 especially the critical cell-specific factors that dictate VEGF189 exon 6A recognition. We are currently using a transcriptomic approach with our cell lines to investigate the mechanisms of this VEGF intracellular feed-back loop. The increased necrotic cell death of V189 shNRP cells may thus be related to the partial inhibition of NRP1 alone, or to both the decrease of NRP1 and increase of VEGF189.
Several studies used siRNA to investigate the mechanisms by which autocrine VEGF165 sustains breast carcinoma cells survival.11,13,16 Depending on the studies, internally expressed VEGF-R1/Flt1,16 or NRP1,11 were reported to mediate VEGF-induced survival activity, suggesting more complex mechanisms than initially thought. We were unable to detect VEGF-R1 expression in V165 and V189 clones (Ct value >38 in qRT-PCR experiments). The amount of VEGF-R2 transcripts appeared to be low when detected by qRT-PCR (Ct ∼33–35), and the protein level was undetectable by protein gel blotting using total cell lysate or after protein immunoprecipitation (not shown). Native VEGF189 does not bind to VEGF-R2.6 The absence of the effect of a selective Flk-1/VEGF-R2 kinase inhibitor (SU1498) in starvation medium in control cV cells (not shown) also agrees with previous observations in MDA-MB-231 cells.16 Our findings support the complexity of NRP-VEGF interactions for cell survival. This complexity makes it difficult to propose a plausible/or unique explanation of how it might work. One explanation for the differential VEGF-mediated cellular response of cancer cells to serum starvation might be the result of a balance between VEGF isoforms and the affinities to their receptors, VEGF189 being a ‘preferred’ ligand for NRP1. Our experiments using the BIAcore assay show, for the first time, that the affinity of VEGF189 to NRP1 is stronger than that of VEGF165, extending our previous results on the binding of VEGF189 to NRP1.10 The silencing of NRP1, which is accompanied by even higher levels of VEGF189, may decrease stress-induced apoptosis mediated by this isoform, which could competitively block VEGF165 survival pathway leading to cell death.
The complexity of NRP-VEGF interactions is further underlined by the fact that NRP1 binds members of two different ligand families: the VEGF-A family of angiogenesis factors and the semaphorin family (SEMA3A and SEMA3B) of axonal guidance regulators.18 Increasing VEGF165 could modulate class3 semaphorin signaling pathway, leading to suppress apoptosis.39,40 Moreover, there is also evidence that the neuropilins may function independently of tyrosine kinase VEGF receptors. NRP1 possesses an active PDZ cytoplasmic motif which may interact with the adaptor molecule GIPC.41 This interaction was first shown to mediate NRP1 signaling in angiogenesis and to participate in the vesicular trafficking of integrin α5β1.41,42 The β1 integrin has previously been involved in VEGF189 signaling.10,43,44 The inhibition of β1 integrin in several breast cancer cell lines results in higher mortality of cancer cells and is associated with increase in apoptosis.45,46 The interaction of NRP1 with β1 also modulates cancer cell survival.25 VEGF189 might thus modulate survival/apoptosis of breast cancer cells through NRP1, and possibly β1 integrin. Moreover, recent studies have described the binding and activation of NRP1 to other transmembrane receptors such as Met and TGFβ.47 Altogether, these findings support the complexity of NRP-VEGF interactions for cell survival. Understanding the mechanisms by which VEGF189-NRP1 complexes may be pro-apoptotic under serum starvation in breast carcinoma cells is beyond the scope of this study. However, we are currently using a genomic approach to analyze the balance between pro- and anti-apoptotic proteins in our various cell lines.
Urokinase-type plasminogen activator is an important factor for breast cancer progression through intra-signaling events that affect cell adhesion, migration, proliferation and survival. Inhibition of uPA using anti-uPA blocking antibodies33 and/or knockdown of both uPA/uPAR expression in MDA-MB-231 induce apoptosis of cancer cells,48 associated with changes in pro-apoptotic gene expression.49 Our study shows that silencing NRP1 expression downregulates uPA activity in V189 clones. uPA has been shown to cleave VEGF189 into shorter active forms,6 so the lower abundance of uPA activity in V189 clones than control cells may favor native VEGF189. The correlation between uPA expression/activity and VEGF189 expression that we observed in our study suggests that the VEGF 189 isoform may induce apoptosis under stress conditions by mechanisms which could include the decrease of uPA expression.
An important issue is whether our data obtained in vitro are relevant to conditions in vivo. The stress conditions we chose (low glucose concentration, deprivation of growth factors) have been observed in vivo in rapidly growing tumors, and it has been suggested that they are found during pro-angiogenic stage (shortage of growth factors).50,51 Many studies suggest that the stress conditions encountered in vivo by tumor cells differ according to the angiogenic status.52 Before the angiogenic switch, growth factors and nutrients can access tumor cells only by diffusion and cells located at the center of the microtumor are stressed by this shortage. A delay in the tumor uptake was observed when VEGF189-expressing cells were xenografted10 that we explained by a delay in the angiogenic switch. We therefore suggest that the proapoptotic effect of VEGF189 is predominant during the preangiogenic stage, and that its proangiogenic effect on endothelial cells takes over at the moment of the angiogenic switch. We are currently testing whether the mechanisms of VEGF 189 action in vivo differ between MDA-MB-231 and endothelial cells, in view of the different VEGFR-1 and -2 expression between the two types of cells and the possible different types of cellular signaling (paracrine vs. autocrine/intracrine) (reviewed in refs. 43 and 53).
In summary, the present results indicate that an overexpression of VEGF189 induce apoptosis of MDA-MB-231 under conditions of nutrient deprivation and doxorubicin-treatment, whereas overexpression of VEGF165 increases cell survival. These findings lead to the conclusion that opposing loops involving VEGF165 or VEGF189 regulate the survival of breast carcinoma cells in starved conditions, extending previous data showing that different isoforms may have different biochemical properties (i.e., a cell surface- and extracellular matrix anchorage) and/or be associated with different cell behaviors. Our data also further confirm the previously described complexity of the NRP system. Blocking NRP1 function has an additive effect with anti-VEGF to inhibit tumor growth.54 Therefore, it cannot be excluded that neuropilin differentially modulates the effects of autocrine VEGF isoforms in vivo.
Materials and Methods
Cell culture and reagents.
Human breast cancer cells MDA-MB-231 (ATCC, Molsheim, France) were routinely maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 µg/µl sodium pyruvate and antibiotics (100 units/ml penicillin, 100 µg/ml streptomycin) at 37°C in a 5% CO2-humidified atmosphere. Stable transfected clones overexpressing VEGF165 or VEGF189 (referred as V165 and V189, respectively, and previously described in ref. 10, were cultured in DMEM-10% FBS in the presence of 500 µg/ml G418 (Life Technologies). PC3 cells (a gift from A. Chauchereau, Villejuif, France) were cultured in RPMI-10% FBS medium.
Generation of stable clones MDA-MB-231 defective for NRP1 expression.
Stable transfections of NRP1 shRNA (Sigma) were performed in V165, V189 and cV clones (control clones), using the Fugene 6 reagent and according to manufacturer's instructions (Roche Diagnostics, Meylan, France). V165, V189 and cV clones were also transfected with Mission non target sh control vector (control plasmid). The transfected cells were selected with puromycin (0.8 µg/ml; Sigma-Aldrich) in DMEM-10% FBS. Forty-two clones (20 for V189, 16 for V165 and 6 for cV) were analyzed for the expression of NRP1 transcripts. Stable transfected clones were isolated and maintained in DMEM-10% FBS in the presence of 500 µg/ml G418 and puromycin (0.4 µg/ml).
RNA extraction and RT-PCR analysis.
Total RNA was isolated from cultured MDA-MB-231 clones using the TRIzol reagent according to manufacturer's procedure (Invitrogen, France). Reverse transcription was performed using 200 units of Superscript II RNase H-reverse transcriptase. Transcript quantification of target genes (VEGF165 and VEGF189 isoforms, NRP1, uPA) was determined by qRT-PCR as previously described in reference 55, using the TATA box-binding protein (TBP) as an endogenous RNA control. Each sample was normalized on the basis of its TBP content. Results are expressed as N-fold differences in normalized target gene expression in VEGF/shNRP1 transfected clone of interest relative to its control-transfected clone set to 1. The primers used for this study are described in Table 1. The specificity of PCR amplicons was checked by agarose gel electrophoresis. PCR was performed using the SYBR Green PCR Core Reagents kit (Perkin-Elmer Applied Biosystems). Experiments were performed with duplicates for each data point. Target gene expressions were only determined for samples with a target gene Ct value lower than 35. Qualitative mRNA abundance was considered low (30 < Ct ≤ 35), moderate (25 < Ct ≤ 30), high (20 < Ct ≤ 25) or very high (Ct ≤ 20). Target gene expressions were considered to be detectable (but not quantifiable) when the Ct value was between 35 and 38, while those with Ct values above 38 were considered non expressed.
Table 1.
Sequences of primers used in this study for real-time PCR
| TBP-U | 5′TGC ACA GGA GCC AAG AGT GAA 3′ |
| TBP-L | 5′ CAC ATC ACA GCT CCC CAC CA 3′ |
| VEGF189-U1 | 5′ TAA GTC CTG GAG CGT TCC CTG T 3′ |
| VEGF189-L1 | 5′ CTT GCA ACG CGA GTC TGT GTT T 3′ |
| VEGF165-U2 | 5′ CCT CAC CAA GGC CAG CAC ATA 3′ |
| VEGF165-L2 | 5′ CAA GGC CCA CAG GGA TTT TCT 3′ |
| NRP1-U1 | 5′ GTG TCT TGC AGA GCA GTG TCT CAG A 3′ |
| NRP1-L1 | 5′ TGG TGC TAT ACT GGG AAG AAG CTG T 3′ |
| UPA-U4 | 5′ AAA ATG CTG TGT GCT GCT GAC C 3′ |
| UPA-L4 | 5′ GCC TTG GAG GGA ACA GAC GA 3′ |
VEGF quantification in conditioned media using Elisa assay.
Cells (5 × 105) were plated in 10 cm Petri dishes and grown in DMEM medium 10% FBS until sub-confluence was reached. Cells were then incubated for 24 h in DMEM medium without serum, and further supplemented or not with heparin (50 µg/ml; Sigma, Steinheim, Germany) allowing the release of cell-associated VEGF.10 Collected conditioned media (CM) were used to measure VEGF concentrations using a standard ELISA protocol (Quantikine human VEGF; R&D Systems, Minneapolis, MN). All data were normalized to total cell protein content (obtained after cell lysis), using BCA protein assay. Results were expressed as ng VEGF/mg protein.
Immunoprecipitation and concanavaline A purification analyses.
For detection of soluble NRP1, conditioned medium (CM) of cells after 72 h starvation was incubated with concanavaline A sepharose beads (Amersham Biosciences) before to be separated by SDS-PAGE, as previously described in reference 31.
Protein gel blot analysis.
Cells were lysed in RIPA lysis buffer. Protein samples (40–100 µg) in Laemli buffer were loaded onto a 12.5% (or 6% for VEGF-R2) sodium dodecyl sulfate-polyacrylamide gel for electrophoresis under reducing conditions and then transferred to PVDF membrane (Bio-Rad, Hercules, CA). Proteins of interest were revealed after overnight incubation with primary antibodies at 4°C. These antibodies were goat anti-NRP1 (C-terminal, sc-7239) or rabbit anti-NRP1 (N-terminal; sc-5541) (1:1,000 and 1:500 dilution, respectively), mouse anti-NRP2 (sc-13117, 1:200), all from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-actin or anti-GAPDH antibodies were used to control protein loading. This step was followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5,000, Dako, Glostrup, Denmark). Recombinant NRP2 (Santa Cruz), cell lysates from HUVEC and PC3 cells were used as positive controls.
Apoptosis/cell death assay.
MDA-MB-231 cells transfected with either isoform of VEGF or control vehicle, and with shNRP1 or shCtl, were starved for 24–96 h in serum depleted medium (DMEM 1 g/L D-glucose, 0.5% FBS) or treated with 2.5 µM Doxorubicin for 24–48 h in 10% FBS containing medium (DMEM 4.5 g/L D-glucose). Adherent cells were harvested, rinsed with PBS and subjected to double staining with fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (PI) following manufacturer's instructions for Annexin V apoptosis assay (Beckman Coulter, Fullerton, CA). Annexin V reveals a phosphatidylserine translocation specific to early (API) and late (APII) apoptosis stages, while APII and the first stage of necrosis are revealed by incorporation of PI, which enters into the cells when cell membrane damage has occurred. The immediate analysis in a FACScan (Becton Dickinson, Sunnyvale, CA) was performed for 10,000 cells gated on the basis of forward and side scatter. Cell debris was excluded from the analysis based on scatter signals. The position of the quadrant gate was set, as previously described in reference 56, so that main populations (>70%) of untreated relevant cells were viable (AnnexinV−/PI−).
Cytotoxicity assay.
The release of LDH (lactate dehydrogenase) from damaged/dying cells was measured in cell-free supernatants. LDH activity was quantitated using LDH Cytotoxicity Detection Kit (Roche) according to manufacturer's instructions, and the Modular PP apparatus (RocheDiagnostics, Meylan, France).
Surface plasmon resonance spectroscopy.
Binding analysis were performed at 25°C with the BIACORE 2000 system (BIAcore AB, Uppsala, Sweden) using HBS-EP buffer [10 mM HEPES, pH 7.4 containing 150 mM NaCl, 3 mM EDTA and 0.005% (v/v) Surfactant P20], as previously described in reference 10. Recombinant NRP1 (NPR1-Fc fusion protein, R&D Systems) was immobilized on a sensor chip using two different immobilization approaches, all according to the manufacturer's instructions: (1) via direct covalent coupling to a CM5 sensorchip, pre-equilibrated in 10 mM sodium acetate buffer, (pH 5.0) using an Amine Coupling Kit (BIAcore) and a flow rate of 5 µl/mn;10 (2) via an anti-human Fc covalently coupled to a CM5 sensorchip by the same procedure as in the first method. In the case of covalent coupling remaining active esters in both flow cells were then deactivated using 1M ethanolamine hydrochloride, pH 8.5, and then washed twice with HBS-EP buffer. Recombinant human VEGF189 or VEGF165 (gifts from J. Plouët), which were used as analytes, were diluted in HBS-EP buffer to the required concentration and injected using a flow rate of either 30 or 300 µl/min and a 10 min injection at 25°C for the binding association. Dissociation of the complex was performed during 20 min. To perform binding assays, samples of various concentrations were injected in PBS. The affinity of NRP1 binding to VEGF165 or VEGF189 was calculated from the on and off rates, as described in Fuh et al.32,57 The experiments were repeated at least twice for each concentration of protein. We used the steady-state analysis to estimate the affinity of NRP1 for VEGF isoform. Kinetic analysis for binding of the immobilized NRP1 to VEGF isoforms was achieved using BIAevaluation software version 3.1. Global analysis was performed employing the Langmuir 1:1 binding isotherm.
Zymographic analysis.
uPA activity was determined in conditioned medium of clones by SDS-PAGE, as previously described in reference 58. Briefly, the 10% SDS-polyacrylamide gel contained acrylamide to which purified plasminogen (10 mg/ml, Calbiochem) and casein (2 mg/ml, Sigma) were added before polymerizarion. Equal amounts of proteins in the samples were then subjected to electrophoresis, and the gel was washed and stained as previously described in reference 57.
Statistical analysis.
All data were analyzed in GraphPad InStat software (version 3.0; GraphPad Software, Inc.). Comparisons were performed by performing one-way Anova test. p values of = 0.05 were considered statistically significant.
Acknowledgments
The present paper is dedicated to the memory of Dr. Jean Plouët (Paris, France), co-discoverer of VEGF-A. We also thank G. Velasco for helping establishing silenced clones, A. Chauchereau for providing respectively PC3 cells, S. Vacher for real-time PCR quantification, A. Starzec and R. Vassy for providing materials (Bobigny, France), J. Robert-Lezennes and A. Sansoneti (INSERM U940, Hôpital Saint Louis) for critical reading of the manuscript, and E. Savariau for micrografts and H. El Sheikh Saad and N. Peyri. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and the Institut National du Cancer (INCA). A.T. is supported by a grant from Region Ile de France.
Supplementary Material
References
- 1.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Tischer E, Mitchell R, Hartman T, Silva M, ospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 3.Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992;267:6093–6098. [PubMed] [Google Scholar]
- 4.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 5.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plouët J, Moro F, Bertagnolli S, Coldeboeuf N, Mazarguil H, Clamens S, et al. Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J Biol Chem. 1997;272:13390–13396. doi: 10.1074/jbc.272.20.13390. [DOI] [PubMed] [Google Scholar]
- 7.Soker S, Fidder H, Neufeld G, Klagsbrun M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem. 1996;271:5761–5767. doi: 10.1074/jbc.271.10.5761. [DOI] [PubMed] [Google Scholar]
- 8.Schoeffner DJ, Matheny SL, Akahane T, Factor V, Berry A, Merlino G, et al. VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab Invest. 2005;85:608–623. doi: 10.1038/labinvest.3700258. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, et al. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br J Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hervé MA, Buteau-Lozano H, Vassy R, Bieche I, Velasco G, Pla M, et al. Overexpression of vascular endothelial growth factor 189 in breast cancer cells leads to delayed tumor uptake with dilated intratumoral vessels. Am J Pathol. 2008;172:167–178. doi: 10.2353/ajpath.2008.070181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilinexpressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 12.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 13.Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercurio AM, Lipscomb EA, Bachelder RE. Nonangiogenic functions of VEGF in breast cancer. J Mammary Gland Biol Neoplasia. 2005;10:283–290. doi: 10.1007/s10911-006-9001-9. [DOI] [PubMed] [Google Scholar]
- 15.Miralem T, Steinberg R, Price D, Avraham H. VEGF(165) requires extracellular matrix components to induce mitogenic effects and migratory response in breast cancer cells. Oncogene. 2001;20:5511–5524. doi: 10.1038/sj.onc.1204753. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/S0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 18.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/S0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 19.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/S0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 21.Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 22.Baba T, Kariya M, Higuchi T, Mandai M, Matsumura N, Kondoh E, et al. Neuropilin-1 promotes unlimited growth of ovarian cancer by evading contact inhibition. Gynecol Oncol. 2007;105:703–711. doi: 10.1016/j.ygyno.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation and function. Exp Cell Res. 2006;312:584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 25.Fukasawa M, Matsushita A, Korc M. Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther. 2007;6:1184–1191. doi: 10.4161/cbt.6.8.4363. [DOI] [PubMed] [Google Scholar]
- 26.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 27.Ochiumi T, Kitadai Y, Tanaka S, Akagi M, Yoshihara M, Chayama K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int J Oncol. 2006;29:105–116. [PubMed] [Google Scholar]
- 28.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 29.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyatt CA, Geoghegan JC, Brinckerhoff CE. Short hairpin RNA-mediated inhibition of matrix metalloproteinase-1 in MDA-231 cells: effects on matrix destruction and tumor growth. Cancer Res. 2005;65:11101–11108. doi: 10.1158/0008-5472.CAN-05-2446. [DOI] [PubMed] [Google Scholar]
- 31.Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 33.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–3396. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 34.Barr MP, Bouchier-Hayes DJ, Harmey JJ. Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. Int J Oncol. 2008;32:41–48. [PubMed] [Google Scholar]
- 35.Cackowski FC, Xu L, Hu B, Cheng SY. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics. 2004;84:82–94. doi: 10.1016/j.ygeno.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae D, Lu S, Taglienti CA, Mercurio AM. Metabolic stress induces the lysosomal degradation of neuropilin-1 but not neuropilin-2. J Biol Chem. 2008;283:28074–28080. doi: 10.1074/jbc.M804203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crepin M, et al. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagnard D, Sainturet N, Meyronet D, Perraut M, Miehe M, Roussel G, et al. Differential MAP kinases activation during semaphorin3A-induced repulsion or apoptosis of neural progenitor cells. Mol Cell Neurosci. 2004;25:722–731. doi: 10.1016/j.mcn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA. 2004;101:11432–11437. doi: 10.1073/pnas.0403969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 42.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration and survival through integrin ligation. FASEB J. 2003;17:1520–1522. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 45.Chung J, Yoon S, Datta K, Bachelder RE, Mercurio AM. Hypoxia-induced vascular endothelial growth factor transcription and protection from apoptosis are dependent on alpha6beta1 integrin in breast carcinoma cells. Cancer Res. 2004;64:4711–4716. doi: 10.1158/0008-5472.CAN-04-0347. [DOI] [PubMed] [Google Scholar]
- 46.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Y, Szabolcs A, Dutta SK, Yaqoob U, Jagavelu K, Wang L, et al. Neuropilin-1 mediates divergent R-Smad signaling and the myofibroblast phenotype. J Biol Chem. 2010;285:31840–31848. doi: 10.1074/jbc.M110.151696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, et al. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival and tumorigenicity in vivo. J Biol Chem. 2005;280:36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood. 2009;113:1383–1390. doi: 10.1182/blood-2008-06-164210. [DOI] [PubMed] [Google Scholar]
- 50.North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 52.Mankoff DA, Dunnwald LK, Partridge SC, Specht JM. Blood flow-metabolism mismatch: good for the tumor, bad for the patient. Clin Cancer Res. 2009;15:5294–5296. doi: 10.1158/1078-0432.CCR-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Bièche I, Lerebours F, Tozlu S, Espie M, Marty M, Lidereau R. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res. 2004;10:6789–6795. doi: 10.1158/1078-0432.CCR-04-0306. [DOI] [PubMed] [Google Scholar]
- 56.Abdelkarim M, Guenin E, Sainte-Catherine O, Vintonenko N, Peyri N, Perret GY, et al. New symmetrically esterified m-bromobenzyl non-aminobisphosphonates inhibited breast cancer growth and metastases. PLoS ONE. 2009;4:4685. doi: 10.1371/journal.pone.0004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuh G, Li B, Crowley C, Cunningham B, Wells JA. Requirements for binding and signaling of the kinase domain receptor for vascular endothelial growth factor. J Biol Chem. 1998;273:11197–11204. doi: 10.1074/jbc.273.18.11197. [DOI] [PubMed] [Google Scholar]
- 58.Quemener C, Gabison EE, Naimi B, Lescaille G, Bougatef F, Podgorniak MP, et al. Extracellular matrix metalloproteinase inducer upregulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res. 2007;67:9–15. doi: 10.1158/0008-5472.CAN-06-2448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.