Abstract
The process of epithelial lumenogenesis requires coordination of a network of signaling machinery communicated to each cell through subsequent cell divisions. Formation of a single hollow lumen has previously been shown to require Tuba, a Cdc42 GEF, for Cdc42 activation and correct spindle orientation. Using a Caco-2 model of lumenogenesis, we show that knockdown (KD) of the actin regulator N-WASP, causes a multilumen phenotype similar to Tuba KD. Defects in lumenogenesis in Tuba KD and N-WASP KD cells are observed at the two-cell stage with inappropriate marking of the pre-apical patch (PAP )—the precursor to lumen formation. Strikingly, both Tuba and N-WASP depend on each other for localization to the PAP. We conclude that N-WASP functions cooperatively with Tuba to facilitate lumenogenesis and this requires the polyproline region of N-WASP.
Key words: lumen, N-WASP, tuba, E-cadherin, pre-apical patch
Many epithelial tissues are organized as hollow tubes whose open lumina connect the body with its external environment.1,2 These tubes consist of a monolayer of polarized cells that envelope the central lumen. Lumen formation is thus a key process in epithelial morphogenesis that depends upon cell polarity to establish three cell surface domains: a basal surface adherent to the extracellular matrix, a lateral surface between cells, and an apical surface that is exposed to the luminal fluids. Of note, the apical membrane is biochemically and morphologically distinct from the baso-lateral surfaces and effectively defines the luminal surface.3,4
For a lumen to form, cells must first mark the site at which apical membrane is to be inserted, something that is achieved at the first cell division.5 Targeted trafficking of apical membrane constituents defines a pre-apical patch (PAP), the precursor to the definitive lumen.5 Such insertion of apical membrane must presumably be coordinated with the assembly of apical junctions to segregate nascent apical from lateral membrane domains.2 Subsequent cell divisions direct apical membrane and protein constituents to this point of initial apical membrane placement.6 Coordinated luminal positioning enables the initial formation of a single hollow lumen that subsequently expands through polarized fluid secretion to separate apical membranes, such as occurs in the embryonic gastrointestinal tract,7 or by apoptosis or autophagy of the central cells as is observed in mammary gland development.8,9 Failure to establish initial luminal positioning causes defective lumenogenesis, often resulting in multiple, morphologically abnormal lumina.5,6
Crucial to lumenal morphogenesis is then the mechanism(s) that mark the site where the PAP will form. Cdc42 signaling is increasingly implicated in this process,2,10 with downstream consequences that include control of mitotic spindle orientation,5 which itself influences PAP placement5 and potentially regulation of cell-cell junctions. Like other Rho family GTPases, the subcellular location of Cdc42 signaling is determined by the action of upstream proteins, notably guanine nucleotide exchange factors (GEFs).11,12 Of these, Tuba, a Cdc42-specific GEF,13 has emerged as a regulator of lumenal morphogenesis that controls PAP placement through mitotic spindle orientation.10
Tuba is also a scaffolding protein13 capable of linking the actin assembly machinery with trafficking pathways. Not only is Tuba required for Cdc42 activation to direct spindle orientation,5 it also has the potential to interact with phosphoinositides that define the PAP.14 Additionally, Tuba binds directly to the actin regulator N-WASP, a key molecule in the organization of actin and itself a Cdc42 effector.15 Further, Tuba and N-WASP cooperate in various forms of actin-driven cellular motility, such as vesicle propulsion and cell invasive behavior.16 Interestingly, in epithelial cells N-WASP is also found at cadherin-based cell-cell junctions.17 In fact it has been proposed that N-WASP functions downstream of Tuba in the maintenance of epithelial junctional homeostasis as N-WASP overexpression was capable of rescuing a Tuba KD phenotype.18 Therefore, Tuba has the potential to play a central role in coordinating the molecular complexes required for productive polarization of epithelial cells and placement of the PAP during lumenogenesis. However, whether other protein interactions contribute to the morphogenetic impact of Tuba remain to be assessed.
Three-dimensional cell culture systems are being utilized to identify critical components in lumen formation. In particular, Madin-Darby canine kidney cells (MDCK) and Caco-2 gastrointestinal cells are commonly used to study cyst and/or tubule formation. MDCK cells undergo both cyst and tubule growth, apoptosis being primarily responsible for the final step in lumen formation,19 while Caco-2 cells primarily utilize fluid influx to expand cysts.5 Cyst culture systems replicate aspects of in vivo organogenesis20 providing tangible, powerful models to analyze and dissect the coordinated cellular mechanisms and processes that occur during epithelial morphogenesis.
In this study we examined the relationship between Tuba and N-WASP in early epithelial lumenogenesis using Caco-2 three dimensional cyst cultures. Both Tuba and N-WASP RNAi cell lines result in mature cysts with multiple lumina, and at the two-cell stage, formed multiple PAPs. Interestingly, N-WASP KD perturbed Tuba localization at the PAP, however, N-WASP localization to the PAP was not affected to the same extent by Tuba KD. Taken together, these results suggest a complex interrelationship between Tuba and N-WASP for the coordinated formation of a single hollow lumen.
Results
Multilumen Caco-2 cysts form in the absence of N-WASP or Tuba.
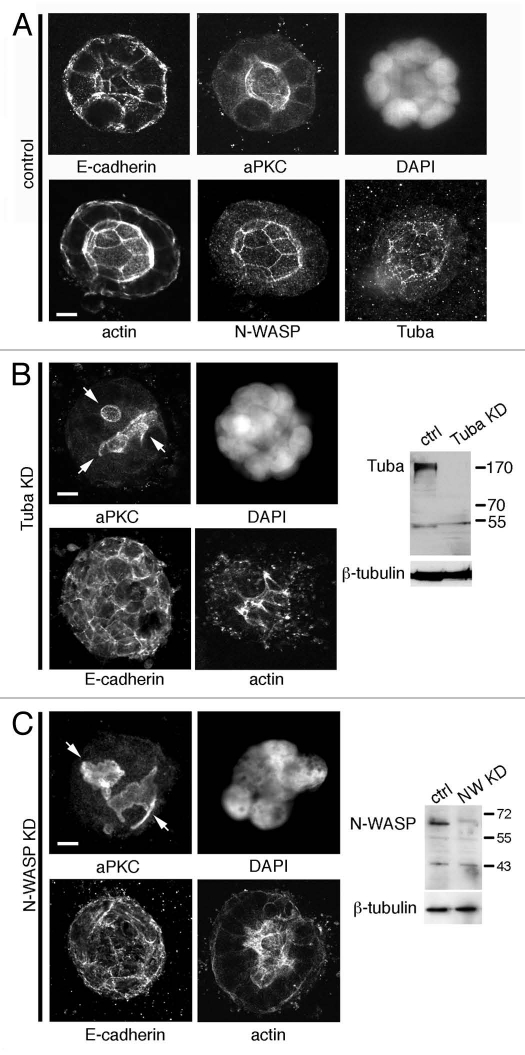
As previously reported in reference 5, Caco-2 cells grown embedded in Matrigel proliferate, forming multi-cellular aggregates from single cells. After eight days' culture in the absence of cholera toxin Caco-2 aggregates had formed cysts, each containing a single clearly defined lumen (Fig. 1A). These cysts were apically polarized, with E-cadherin immunostaining observed baso-laterally and the apical membrane marked by localization of atypical Protein Kinase C (aPKC, Fig. 1A). We found that Tuba stained prominently at the apical membrane, in contrast to previous observations in MDCK cells where Tuba was observed predominantly subapically.10 Similarly, both N-WASP and actin localized to the apical membrane of eight-day cysts (Fig. 1A). A major criticism of the Caco-2 system has been the use of cholera toxin to swell the lumen.6 However, in our hands we observe swollen lumen without the addition of agents that may affect trafficking processes and, as a result, this system provides a reliable tool for dissecting protein localization and PAP placement.
Figure 1.
Multilumen Caco-2 cysts form in the absence of N-WASP and Tuba. (A) Eight day Caco-2 cysts show presence of a single hollow lumen, defined by the apical marker aPKC. Tuba and N-WASP localize to the apical junctions where actin is observed to concetrate. E-cadherin localizes basolaterally. (B) A stable Tuba KD cell line created by lentiviral transduction of an shRNA directed against Tuba, forms multiple lumen at the eight-day stage of cystogenesis. (C) A stable N-WASP KD cell line created by lentiviral transduction of an shRNA directed against N-WASP, forms multiple lumen at the eight-day stage of cystogenesis. Arrows indicate multiple lumina. Scale bars = 5 µm.
A role for Tuba in lumenogenesis in Caco-2 cysts has been recently documented.5,10 To define a role for N-WASP in this system and to examine the cooperativity of Tuba and N-WASP in localization of the PAP, we utilized a lentivirus KD and rescue system which has been described previously in reference 21. Stable Caco-2 cell lines were made in which endogenous Tuba or N-WASP were depleted to similar extents (Fig. 1B and C; >90% decrease in expression by protein gel blot). When Tuba KD cells were allowed to grow for eight days embedded in Matrigel, these aggregates displayed a multilumen phenotype that has been observed previously in three-dimensional culture of MDCK cells using siRNAs to KD Tuba (Fig. 1B; reviewed in refs. 6 and 10). Instead of the single dominant lumen found in control cultures, KD cells showed multiple small, irregular lumina. Interestingly, eight-day culture of N-WASP KD cells replicated this multilumen phenotype (Fig. 1C). This result suggests that both Tuba and N-WASP activity are necessary components in the regulation of lumenogenesis.
N-WASP and Tuba KD cells display multiple PAPs at the two-cell stage.
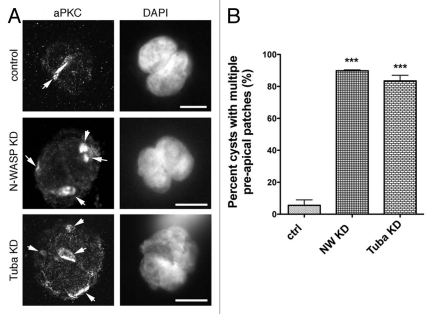
Previous observations suggested that the PAP is laid down during the first cell division and is necessary to direct subsequent apical membrane targeting.5,6 To test PAP localization we examined cysts at the twocell stage, the initial marking of the PAP being determined by aPKC immunofluorescence. aPKC showed diffuse cortical staining in isolated cells (not shown) but is observed to concentrate at juxtaposed membranes in two-cell control cysts (Fig. 2A, top). However, a significant number of two-cell cysts cultured from either N-WASP KD or Tuba KD cells show the presence of multiple aPKC patches (Fig. 2A, middle and bottom). Multiple aPKC patches occurred significantly more frequently in either KD cell system, than in control cell lines (Fig. 2B, p < 0.0001; 89.8% multiple PAP patches in N-WASP KD and 83.5% in Tuba KD compared to 5.5% for control cultures). These patches appear to be located on the cell membranes, as opposed to intracellular vacuolar apical compartments (VACs) that traffic membrane to the apical surface.19 Consistent with this, the patches were circumscribed by the tight junction protein, ZO-1 (Fig. 3D and F). This significant incidence of multiple PAPs in both N-WASP and Tuba KD cells suggested that N-WASP and Tuba may cooperate to ensure correct placement of a single PAP at the first cell division.
Figure 2.
Two-cell cyst cultures of N-WASP and Tuba KD cells display multiple PAP s. (A) A single PAP forms at the membrane between control cells following the first cell division as defined by localization of aPKC. Two cell cysts from both N-WASP KD and Tuba KD cells show the presence of multiple PAP s indicated by arrowheads. (B) 89.8 ± 0.53% of N-WASP KD cells and 83.5 ± 3.5% of Tuba KD cells form multiple PAP s compared to 5.5% for control cultures. (Data are means ± SEM of data pooled from 3 individual experiments; p < 0.0001; Student's t-test). Scale bars = 10 µm.
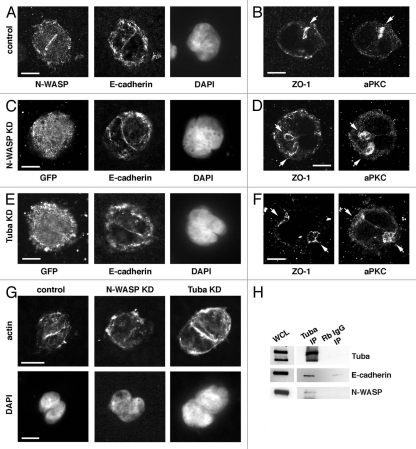
Figure 3.
Junctional integrity is maintained in N-WASP and Tuba KD two-cell cysts. (A) E-cadherin and N-WASP are observed at the juxtaposed membrane in two cell cysts of control cells. (B) A single PAP is defined in control cells circumscribed by ZO-1. (C) E-cadherin is observed at the juxtaposed membrane in two cell cysts of N-WASP KD cells. (D) Multiple PAPs are defined in N-WASP KD cells circumscribed by ZO-1. (E) E-cadherin is observed at the juxtaposed membrane in two cell cysts of Tuba KD cells. (F) Multiple PAP s are defined in Tuba KD cells circumscribed by ZO-1. (G) Actin localizes to the juxtaposed membrane in control, N-WASP KD and Tuba KD two-cell cysts, although less defined in N-WASP KD cells. (H) In Caco-2 cells, Tuba, N-WASP and E-cadherin biochemically associate. Arrows indicate PAPs. Scale bars = 10 µm.
Junctional integrity is maintained in two-cell cysts.
In Caco-2 cells, Tuba and N-WASP have been shown to act cooperatively in the formation and maintenance of E-cadherin cell-cell junctions in conventional monolayer culture,18 a critical feature of polarized cells.22 We found that Tuba and N-WASP associate biochemically in a complex with the cell-cell adhesion molecule E-cadherin (Fig. 3H) suggesting the potential for these molecules to regulate junctional homeostasis and thereby membrane segregation.
To test whether junctional integrity was affected by depletion of either N-WASP or Tuba, junctional proteins were examined in early cyst cultures of KD cells. Interestingly, E-cadherin localization in control, N-WASP KD and Tuba KD cells appeared to be unchanged, at least at the limits of light microscopy (Fig. 3A, C and E). Similarly, the tight junction protein ZO-1 was observed to localize at the periphery of the PAP as defined by staining with aPKC, adjacent to the apical membrane (Fig. 3B, D and F), consistent with its role in separating apical and lateral membrane domains. This suggests that gross disruptions in junctional integrity do not account for the impact of N-WASP or Tuba depletion on PAP placement.
N-WASP KD has been shown to affect the apical actin ring associated with the zonula adherens in monolayers of Caco-2 cells (Kovacs and Yap, unpublished observation). As such it is interesting to note that actin organization in the two-cell N-WASP KD cysts appeared to be less focused at the cell-cell junction when compared to control and Tuba KD cultures (Fig. 3G). N-WASP is a key regulator of actin assembly,23 which may explain the presence of less organized actin filaments in two-cell N-WASP KD cysts.
Tuba KD decreases N-WASP at the PAP.
As Tuba has been suggested to be upstream of N-WASP in other biological contexts16,18 we examined the effect of Tuba KD on N-WASP localization at the PAP. In control cells, 93.6% of two-cell cysts showed concentration of N-WASP to the PAP (Fig. 4A–C). In contrast, N-WASP was reduced at the PAP in Tuba KD cells, with 70.9% of two-cell cysts accumulating N-WASP at the PAP (Fig. 4A and C, p < 0.0001). This result suggests that Tuba affects the localization of N-WASP to the PAP. However, although this difference is significant, a large proportion of cysts still concentrate N-WASP at the PAP.
Figure 4.
A complex interrelationship exists between Tuba and N-WASP at the PAP and requires the polyproline region of N-WASP. (A) N-WASP localizes to the cell-cell junction in control and Tuba KD cells. (B) Tuba is observed to localize to the cell-cell junction in control cells and cells reconstituted with an N-WASP mutant in which the VCA domain has been deleted. Tuba does not localize to the cell-cell membrane in N-WASP KD cells or cells reconstituted with an N-WASP mutant in which the polyproline region has been deleted. (C) N-WASP localization in two cell cysts. 93.6% of control cells show accumulation of N-WASP at the cell-cell junction in two cell cysts compared to 70.9 ± 5.2% of Tuba KD cysts (p < 0.0001; Student's t-test), 6.4 ± 3.3% of N-WASP KD cysts (p < 0.0001; Student's t-test), 98.8 ± 1.2% of ΔVCA N-WASP cysts (p = 0.0071; Student's t-test) and 80.4 ± 8.8% of ΔPP N-WASP cysts (p = 0.0167; Student's t-test). (D) Tuba localization in two cell cysts. 89.3% of control cells show accumulation of Tuba at the cell-cell junction in two cell cysts compared to 21.9 ± 3.2% of N-WASP KD cysts (p < 0.0001; Student's t-test), 99.3 ± 0.7% of ΔVCA N-WASP cysts (p = 0.0149; Student's t-test) and 37.7 ± 2.6% of DPP N-WASP cysts (p < 0.0001; Student's t-test). (Data are means ± SEM of data pooled from at least 3 individual experiments). Scale bars = 10 µm.
N-WASP regulates Tuba recruitment to the PAP.
We then examined the effect of N-WASP KD on Tuba localization at the PAP. Compared to control cysts, Tuba failed to localize to the PAP in N-WASP KD cysts (Fig. 4B and D; p < 0.0001, 29.6% of N-WASP KD cysts versus 89.3% in controls cysts). Thus the impact of N-WASP KD on Tuba localization was greater than that of Tuba KD on N-WASP localization. This suggests not only that a complex interaction exists between these to proteins at the PAP but also that N-WASP assists in the recruitment and localization of Tuba to the cell junction.
The C-terminal SH3 domain of Tuba can bind directly to the polyproline region of N-WASP.13 To further assess the molecular requirements for N-WASP to control Tuba localization at the PAP, N-WASP KD cells were stably reconstituted with an N-WASP mutant lacking the polyproline region, preventing Tuba binding (ΔPP N-WASP), or with a mutant lacking the C-terminal VCA domain, essential for N-WASP to activate the Arp2/3 complex (ΔVCA N-WASP; reviewed in ref. 24).
Both N-WASP mutants localized to the PAP when expressed in N-WASP KD cells (Fig. 4B and D; 98.8% of ΔVCA N-WASP cysts; 80.4% of ΔPP N-WASP. Compared with 6.4% in N-WASP KD cysts, p < 0.0001). However, cells expressing the ΔPP mutant showed significantly reduced Tuba at the PAP than did control cells (Fig. 4B and D; 37.7% of ΔPP N-WASP cysts compared with 89.3% of control cysts; p < 0.001). In contrast, 99.3% of ΔVCA N-WASP two-cell cysts concentrated Tuba at the PAP (Fig. 4B and D). Together, these data indicate that the polyproline region of N-WASP is required for the localization of Tuba at the PAP.
Discussion
An orchestrated series of processes need to occur to create a multicellular organism, with cell behaviors coordinated both spatially and temporally. The architecture of epithelial cysts and tubules provide the basic building blocks for elaborate networks in many epithelial tissues including the kidney, lungs and mammary tissue, all performing unique functions in the mature organism. The relative tangibility of three-dimensional cyst cultures provides an in vitro cell model to examine the coordination of individual cell behaviors to create a higher order structure.
In this paper we have examined the formation of the PAP, the first step in the formation of a lumen. Incorrect placement of the PAP can have drastic results as cells are no longer able to coordinate apical membrane insertion in subsequent cell divisions, forming cell aggregates with multiple lumina.5,6,10,25 Previously, the scaffolding protein Tuba has been shown to be required for lumenogenesis, being necessary for correct spindle orientation via the activation of Cdc42.5 We examined, N-WASP a known binding partner of Tuba, actin regulator and effector of Cdc42, for its involvement in lumenogenesis. Mature cysts grown from N-WASP KD cells replicated the Tuba KD multilumen phenotype and analysis of two cell cysts from either cell line showed the distribution of multiple PAPs over the cell surfaces. This suggests that, as in other functional contexts,16 Tuba and N-WASP may cooperate during epithelial luminal morphogenesis by defining PAP placement.
Interestingly, although Tuba has been implicated as an upstream regulator of N-WASP,18 Tuba KD did not abolish N-WASP from the PAP. Instead, N-WASP KD drastically reduced the presence of Tuba at the PAP. Being a scaffolding protein, it has been proposed that Tuba functions to link actin regulatory and trafficking pathways.13 Moreover, Tuba is, in fact, necessary for some N-WASP-dependent functions.16 The C-terminal domain of Tuba is required for its localization at cellular junctions18 and this domain binds directly to N-WASP.13 Further, we find that the N-WASP polyproline domain, which is known to bind directly to Tuba, is necessary for N-WASP to localize Tuba at the PAP. We are unable to rule out the possibility that N-WASP recruits additional proteins and/or GEFs to the membrane for correct PAP placement and lumen formation. But collectively our data suggest that, instead of a simple linear relationship, Tuba and N-WASP exert cooperative effects to influence each other's localization at the PAP.
Epithelial junctions play central roles in polarity and luminal morphogenesis. Both Tuba and N-WASP have been implicated in the assembly and maintenance of junctional integrity.18 Thus it was possible that N-WASP and/or Tuba might influence the nascent apical domain by controlling the integrity of apical junctions needed to segregate the membrane domains. However, E-cadherin and ZO-1 staining remained intact in relation to PAPs in both N-WASP and Tuba KD cysts. This has been observed previously in MDCK cysts25 and is consistent with a zebrafish model of lumenogenesis where E-cadherin and ZO-1 localization is unaffected by multiple lumen phenotypes.7 This implies that while cooperative activity of N-WASP and Tuba must be required for apical membrane positioning, it has limited effects on targeting of TJ proteins required for membrane segregation.
It was interesting to note that F-actin staining was less concentrated at the PAP of N-WASP KD cysts compared with controls or Tuba KD cells. We recently identified a novel mode of action for N-WASP at the ZA, where it acts at a post-nucleation stage to promote organization of nascent filaments into junctional actin bundles (Kovacs et al. In press). This mechanism worked independently of the capacity of N-WASP to stimulate Arp2/3. Given the relative severity of the N-WASP KD phenotype and the observation that the ΔVCA-mutant of N-WASP could substantially restore Tuba localization at PAPs, it is attractive to speculate that this post-nucleation pathway may contribute to PAP placement by N-WASP. Thus, association between E-cadherin and N-WASP may serve to support actin organization at the luminal surface, thereby contributing to form a scaffolding platform required for correct PAP placement.
It is important to note that Tuba contains a BAR domain capable of binding to membrane lipids13 and epithelial polarity is also influenced by asymmetric distribution of membrane lipids, notably phosphoinositides.19 The lipid phosphatase, phosphatase and tensin homolog on chromosome 10 (PTEN), has been shown to localize to the apical membrane of cysts to facilitate the apical enrichment of PtdIns(4,5)P2 for correct epithelial polarization and lumen formation.25 Loss of function of PTEN prevented apical membrane segregation and resulted in the formation of multiple lumina. More recently, Tuba and Cdc42 were shown to function in the apical transport of Rab8a/Rab11a-positive vesicles to direct lumenogenesis.6 This suggests that recruitment of Tuba to the apical membrane is likely to facilitate many functions including vesicle targeting and spindle orientation.
We now provide a link between Tuba and the actin regulator N-WASP in lumenogenesis. We demonstrate a requirement for N-WASP for the recruitment of Tuba to the apical membrane in two-cell cysts, dependent upon the polyproline region of N-WASP. One may, therefore, propose a scenario whereby Tuba and N-WASP are recruited to the cell-cell junction prior to PAP formation, and by signals independent of PAP formation. These may include ligation of E-cadherin molecules on juxtaposed membranes and/or lipid sensing. Once cellular polarization is triggered to stimulate PAP formation and membrane segregation, N-WASP then concentrates cooperatively with Tuba at the PAP to potentially facilitate spatial activation of Cdc42 for correct spindle orientation and the formation of a single lumen. This implies that N-WASP and Tuba may be interdependent. To enable efficient formation of a single lumen, a feedback mechanism must exist to regulate the activities of N-WASP and Tuba during lumenogenesis. Further investigation is required to address this interdependence and its importance in PAP placement and morphogenesis.
Materials and Methods
Cell and cyst culture.
Caco-2 cells were cultured in RPMI complete growth medium and grown at 37°C in 5% CO2 atmosphere. Caco-2 cyst cultures were prepared as described previously in reference 5, with minor alterations. Briefly, Caco-2 cells were trypsinised to produce a single cell suspension. On ice, 5.8 × 104 cells for eight day cultures and 12 × 104 cells for one day cultures were added to a solution containing 0.02 M HEPES pH 7.4, 1 mg/ml Collagen I (5 mg/ml; BD, Australia) and 40% Matrigel (BD, Australia), in a volume of 1 ml. 100 ul of solution was added to wells of a 12 well plate and allowed to solidify at 37°C for 45 mins. One milliliter of complete growth medium was added to each well and the cultures returned to the incubator for 1–8 d, with media changes every 4 d. Cysts were stained as per the previously described protocol for the staining of mammary acini9 with some minor alterations. Prior to fixation and staining collagen was digested by incubating the cultures in a solution of collagenase VII (Sigma-Aldrich) for 15 mins. After incubation with secondary antibody, nuclei were stained by the addition of a solution of DAPI 1:500 in PBS and mounted in 1% N-propyl gallate (Sigma-Aldrich).
Confocal microscopy.
Confocal images were acquired using a spinning disk confocal system (Ultra-View; Perkin Elmer) mounted on an IX81 Olympus microscope with 60x and 100x 1.40NA plan Apochromat objectives and an Hamamatsu Orca-1 ER camera and driven by Metamorph imaging software (Version 7, Universal Imaging, West Chester, PA). Background correction and contrast adjustment of raw data images were performed with ImageJ (National Institutes of Health) or Photoshop (Adobe).
Antibodies and immunoprecipitation.
Primary antibodies used in this study were: (1) mouse mAb HECD-1 against the ectodomain of E-cadherin (a kind gift from Dr. Peggy Wheelock, University of Nebraska, Omaha, NE; with the permission of Dr. M. Takeichi); (2) rabbit pAb against GFP (Molecular Probes/Invitrogen); (3) rabbit mAb 30D10 against N-WASP (Cell Signaling Technologies/NEB); (4) rabbit pAb against N-WASP (made in-house by Dr. Steve Thomas); (5) rabbit pAb against Tuba (a kind gift of Prof. Frank Gertler, MIT, Massachusetts); (5) rabbit pAb against atypical PKC? (Santa-Cruz). F-actin was visualised by staining with AlexaFluor 488- or 594-phalloidin (Invitrogen, 1:300). Secondary antibodies were species-specific antibodies conjugated with AlexaFluor488 or 594 (Invitrogen) for immunofluorescence, or where conjugated with HRP (Bio-Rad Laboratories) for immunoblotting. 5 mg of antibody was used for immunoprecipitations.
shRNA design and N-WASP mutant cloning.
shRNA sequences used to target N-WASP and Tuba were 5′-GGT GTT GCT TGT CTT GTT A-3′ and 5′-GAA GAG CCT ATA CAA CGA A-3′ respectively. For hairpin cloning see below.
The ΔPP N-WASP mutant was made by nested PCR to create a clone in which amino acids 275 to 391 were deleted. The ΔVCA N-WASP mutant was made by amplifying amino acids 1–392 of the full length murine N-WASP sequence (a kind gift of Dr. Silvia Lommel). N-WASP mutants were cloned with an N-terminal EGFP into EcorRI and SbfI-digested pLL5.0 expressing the N-WASP shRNA.
RNAi lentivirus system.
A lentivirus-based system26,27 was used to functionally silence N-WASP or Tuba in mammalian cells. The lentivirus expression vector pLL5.0 (backbone pLL3.7) and the third-generation packaging constructs pMDLg/pRRE, RSV-Rev and pMD.G are generous gifts from Dr. James E. Bear (UNC Chapel Hill, NC).27 In brief, the shRNA was cloned downstream of the U6 promoter (HpaI and XhoI) into pLL5.0 carrying either soluble EGFP or mCherry as reporter genes driven from an LTR promoter.
The generation and titer of lentivirus stocks has been described previously in reference 21. In brief, the expression vector pLL5.0 and packaging vectors were transfected by using CaCl2 precipitation into HEK 293T cells. Virus-like particles (VLP) were harvested from the supernatant 48 hr post-transfection and virus was concentrated with PVDF spin columns (Milipore) to obtain high titers of VLP fractions. Aliquots of virus were subsequently used for titration or stored at −80°C.
Titers were determined by infecting HEK 293T cells with serial dilutions of concentrated lentivirus. We determined expression of infected cells by flow cytometry 48–72 hrs after infection. A typical preparation yielded approximately 1–5 × 108 pfu/ml.
Lentivirus particles were used to infect Caco-2 cells. Briefly, cells were incubated at 37°C with MOI = 10 of lentivirus in RPMI complete medium supplemented with polybrene solution (8 µg/ml) and harvested 3 days post-infection by trypsinization. Single cell suspensions were sorted by flow cytometry according to a moderate to high level of expression. Sorted cell populations were replated thereafter into RPMI complete medium. Cells maintained silencing of target proteins for at least three months post-infection (data not shown).
Acknowledgments
We thank our lab colleagues for their support and encouragement. This work was supported by the National Health and Medical Council (NHMRC) of Australia; A.S.Y. is a research fellow of the NHMRC. Confocal microscopy was performed at the IMB/ACRF Cancer Biology Imaging Facility, established with the generous support of the Australian Cancer Research Foundation.
Abbreviations
- aPKC
atypical protein kinase C
- PAP
pre-apical patch
- GEF
guanine nucleotide exchange factor
- shRNA
short hairpin RNA
References
- 1.Nejsum LN, Nelson WJ. Epithelial cell surface polarity: the early steps. Front Biosci. 2009;14:1088–1098. doi: 10.2741/3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 4.Vega-Salas DE, Salas PJ, Gundersen D, Rodriguez-Boulan E. Formation of the apical pole of epithelial (Madin-Darby canine kidney) cells: polarity of an apical protein is independent of tight junctions while segregation of a basolateral marker requires cell-cell interactions. J Cell Biol. 1987;104:905–916. doi: 10.1083/jcb.104.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, et al. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–4022. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- 9.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 10.Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–669. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 12.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, et al. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278:49031–49043. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Belmonte F, Mostov K. Phosphoinositides control epithelial development. Cell Cycle. 2007;6:1957–1961. doi: 10.4161/cc.6.16.4583. [DOI] [PubMed] [Google Scholar]
- 15.Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs EM, Makar RS, Gertler FB. Tuba stimulates intracellular N-WASP-dependent actin assembly. J Cell Sci. 2006;119:2715–2726. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Hagios C, Lochter A, Bissell MJ. Tissue architecture: the ultimate regulator of epithelial function? Philos Trans R Soc Lond B Biol Sci. 1998;353:857–870. doi: 10.1098/rstb.1998.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 23.Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, et al. Hierarchical regulation of WASP/WAVE proteins. Mol Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 27.Vitriol EA, Uetrecht AC, Shen F, Jacobson K, Bear JE. Enhanced EGFP-chromophore-assisted laser inactivation using deficient cells rescued with functional EGFP-fusion proteins. Proc Natl Acad Sci USA. 2007;104:6702–6707. doi: 10.1073/pnas.0701801104. [DOI] [PMC free article] [PubMed] [Google Scholar]