Abstract
Metastasis is the most deadly phase of cancer progression, during which cells detach from their original niche to invade distant tissues, yet the biological processes underlying the spread of cancer are still poorly understood. The fruit fly Drosophila melanogaster provides important insights in our understanding of how epithelial cells migrate from their original location and find their way into surrounding and distant tissues in the metastatic process. Here we review recent studies on the mechanisms of migration of embryonic hemocytes, the macrophage-like immuno-surveillance cells, during normal development and wound healing. We highlight the interesting finding that hydrogen peroxide (H2O2) has been identified as the driving force for hemocyte chemotaxis. We also give a special emphasis to studies suggesting the concept that hemocytes, together with the tumor microenvironment, act as potential inducers of the epithelial delamination required for tumor invasion. We propose that cell delamination and migration could be uncoupled from loss of cell polarity via a tumor-related inflammatory response.
Key words: Drosophila, tumor microenvironment, tumor immunology, scribble, TNF
The first description of cellular motility dates back to 1863 when Virchow reported his observation of individual leukocytes movement from canulated lymphatic ducts.1
During embryonic development movements of cell sheets shape the future body axes: cells are specified in one region of the embryo and then migrate extensively during gastrulation before they reach their final location. In adults, reactivation of cell migration has been observed during wound healing and during cancer invasion and metastasis. This underlines the clinical importance of understanding morphogenetic cell movements.
Whether occurring during development or under pathological conditions, cell migration is commonly perceived as the movement of individual cells that undergo cycles of polarized extension-contraction of their actin cytoskeleton coupled with adhesion and subsequent de-adhesion from the surrounding substrate.2–4
Single cell migration requires an initial step of cell polarization, during which intracellular actin polymerizes to form ruffles or leading pseudopodia. The Rho family small guanosine triphosphate (GTP)-binding proteins (GTPases) are pivotal regulators of actin organization and control the formation of lamellipodia and filopodia.5 At the sites where contact with the Extra Cellular Matrix (ECM) occurs, big protein complexes are assembled through the recruitment and the clustering of receptors of the integrin families. These big protein structures are known as focal adhesions or focal contacts. In order to provide space for the forward expanding cell body, pericellular matrix molecules are locally broken down by surface proteases, such as MT1-MMP.
Shortly after integrin binding with ECM, cytoplasmic actin filaments engage with contractile proteins, such as myosin II, which stabilize and shorten the membrane-tethered actin filaments. This results in local cell contraction, generally at the opposite pole respect to the leading edge. This Integrin/MMP dependent mode of cell migration is known as ‘mesenchymal.’ However, cells may be able to migrate across connective tissue by simply squeezing themselves within pre-existing ECM pores. This mode of migration is Integrin/MMP-independent and known as ‘ameboid.’6
Another less familiar notion is that cells can migrate as cohorts, chains or sheets. In collective migration the traction force arises from the cytoskeletal activity of cells at the leading edges that pull the other cells of the cluster forward. During this movement, cell-cell junctions ensure the bulk holds together and is cohesively dragged around.7 One of the best-understood example of collective migration in Drosophila is represented by border cell migration.8
Indeed Drosophila melanogaster provides elegantly characterized in vivo models that closely resemble aspects of cell motility observed in higher organisms, for both individual and collective cell migration. Drosophila researchers count on powerful genetic tools and the relative ease of live imaging in this system. Therefore they have contributed: (1) to the delineation of how a cell or a group of cells detaches from its original niche and becomes motile, (2) to the discovery of some of the guidance cues that draw cells to the target sites, during development and inflammation and (3) to explain how cells finally stop migrating once they have reached the location where they are required for their specific biological function.
During Drosophila embryonic development a wide number of well-characterized processes require either collective or single cell migration: mesoderm invagination during gastrulation, primordial germ cells transepithelial migration, branching morphogenesis and tube formation during tracheal development are just a few examples. All these processes have been extensively reviewed in references 8–10.
Box 1. Definitions.
Pseudopodia: transient projections of eukaryotic cells extending by the assembly of actin subunits into microfilaments and contracting by interaction of the actin filaments with myosin.
Lamellipodia: characteristic features at the front, leading edge, of motile cells resulting from actin nucleation at the plasma membrane that promote the formation of a flat protrusion required to propel the cell across a substrate.
Focal Adhesions: large, dynamic protein complexes through which the cytoskeleton of a cell contacts the extracellular matrix (ECM).
Epithelial-Mesenchimal Transition (EMT): developmental program of eukaryotic cells characterized by loss of cell adhesion, repression of E-cadherin expression and increased cell motility.
Mesenchimal-Epithelial Transition (MET): can be considered as the opposite process of EMT. It is a reversible transition from motile, multipolar or spindle-shaped mesenchymal cells to stationary, planar and polarized epithelial cells.
Here we will focus on the most recent findings on cell migration coming from the in vivo studies of Drosophila macrophages and epithelial cells which in many aspects recapitulate the most intriguing features of vertebrate cell migration during development, inflammation and tumorigenesis.
Hemocyte Dispersal and Chemotaxis during Development and Tissue Repair
Embryonic hemocytes are the cellular arm of the innate immune system in flies.11,12 They share many characteristics with the mammalian blood cells development and function and it was hypothesized that have evolved from a common ancestor.13,14
Drosophila hemocytes originate in the procephalic mesoderm and can be categorized into three main classes. Plasmatocytes, small rounded cells with phagocytic capacity, represent the most abundant subpopulation of hemocytes. This particularly motile population migrates as single cells, following precise and invariant routes that allow them to distribute evenly within the organism by the end of embryogenesis. A second class, the crystal cells, distinguished by pronounced crystal-like inclusions in the cytoplasm, are involved in melanin deposition at wounds and around foreign objects. Finally, a class of large flat cells, the lamellocytes, appears when parasitoid wasps infect the larvae and participate in the encapsulation of the parasite.
Embryonic hemocytes can be specifically labeled with fluorescent proteins and visualized by live confocal microscopy and their movements can be followed over the time through timelapse imaging. By these means they appear as highly polarized, large cells with dynamic filopodial and lamellipodial protrusions continuously extending and retracting while they explore their environment.
Plasmatocytes strongly express the PDGF/VEGF receptor (PVR).15 The three PVR ligands, Pvf1, 2 and 3, are expressed along the embryonic ventral midline by the developing nerve cord16 and represent the guidance cue for the hemocyte developmental migration.
Once plasmatocytes have completed their migration along the anterior-posterior axis, a group of them starts engaging in lateral migration, in such a way that by the end of embryogenesis, they are distributed along three main parallel axes along the ventral midline and flanking the nerve cord.17
Besides being an amenable model for the study of developmental migration, hemocytes closely resemble leukocytes in their ability to become active and migrate toward wounds in a process similar to vertebrate inflammation. It is indeed a well-established notion that tissue-derived alarm signals (“damaged self ”) can as well as pathogen-associated molecules, initiate immune responses. It has been postulated that the ability of blood cells to adhere to damaged-self tissues represents an ancient function of the immune system.18
The active recognition of damaged-self tissue by the blood cell began to be addressed in vivo only very recently, and particularly in organisms that benefit from only an innate immune response and possess an open circulatory systems in which blood directly bathes the organs (i.e., the Drosophila larvae).
Using a combination of live imaging and Transmission Electron Microscopy (TEM), Stramer and coworkers were able to capture hemocytes actively migrating toward epithelial wounds and in the act of engulfment of cell debris, as observed by the appearance of large “vacuoles” within their cytoplasm and by the extension of processes to wrap around and draw a cell corpse into them.19 Moreover, they were able to demonstrate using both embryos mutant in Rho, Rac or Cdc42 and embryos expressing dominant-negative forms of these proteins—specifically in their hemocytes—that these small GTPases play different roles in plasmatocyte migration. In particular, Rac seems to be required for lamellipodial formation, while Rho signaling is necessary for hemocytes retraction from sites of cell-cell or cell-matrix adhesion and CDC42 is required to maintain cell polarity during wound chemotaxis.19
Interestingly, hemocytes do not seem to utilize a unique mode of migration. In fact, CDC42 and Rho are mostly dispensable during developmental migration,20 and wound chemotaxis does not require Pvr expression in the immune cells, since it appears to be driven by PI3K signaling instead.16
While the guidance cues required during developmental migration of hemocytes are well established, the chemotactic stimuli driving migration toward wounds had remained mysterious for a long time. The Drosophila genome does not encode any chemokines (chemotactic cytokines), which are the main known drivers of leukocyte chemotaxis in mammals.
A recent study in zebrafish larvae reported that H2O2 originating from an epithelial wound is responsible for attracting neutrophils to the wound. Knockdown of this gradient with the drug diphenyleneiodonium (DPI), which inactivates the NADPH oxidases responsible for generating H2O2, blocks the wound inflammatory response.21
Similarly, using a combination of genetic and pharmacological approaches Moreira and collaborators identified H2O2 as the chemoattractant that guides Drosophila hemocytes toward a wound22 (Fig. 1).
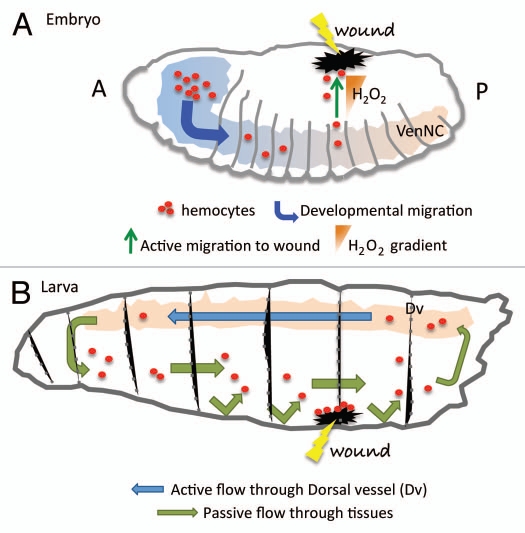
Figure 1.
Hemocytes migration during development and wound healing. (A) During the embryonic phase hemocytes (red dots) migrate following precise routes that distribute them in three parallel rows, following the ventral nerve cord (VenNC). At this stage the cue for their migration is represented by Pvr signaling (see text). In response to H2O2 production at the wounded site hemocytes divert from their conventional routes of migration to accumulate at the damaged tissue and initiate a wound healing response. A, anterior; P, posterior. (B) At the larval stage hemocytes are pumped in circulation by the dorsal vessel (Dv) that with its contractions ensures the hemolymph circulates with a posterior-to-anterior directionality (blue arrow). The opposite-oriented flow is rather a slower, passive one (green arrow). Hemocytes are thought to adhere to the damaged tissues once they randomly bump into them. The chemotactic signal at the wound at the larval stage has not been identified yet.
Embryonic hemocytes fail to migrate efficiently toward the wounded site in animals with reduced Duox—the enzyme responsible for H2O2 production23—specifically within the embryonic epidermis.22 H2O2 production in vivo was monitored in control and Duox mutant embryos through the injection of a fluorochrome (acetyl-pentafluorobenzene sulphonyl fluorescein) that is normally converted into its fluorescent form when exposed to H2O2. In the same study, Moreira and collaborators illustrated that, similar to vertebrate embryos,24 there is a refractive period where macrophages cannot be deviated from their developmental migratory routes to a site of tissue damage. This suggests a hierarchy for the interpretation of different chemotactic cues by the macrophages in vivo.22 In this period of non-responsiveness to wound signals embryonic tissues can still repair and they do so generally without producing scarring or fibrosis, suggesting the intriguing idea that scars are the result of the inflammatory process that takes place at the adults wounds.25
Beyond their roles in the embryo, Drosophila hemocytes play an important role in efficiently fighting infections26 and repairing tissue damage27–29 during the larval and adult stages of the fly.
Just before hatching into larva, the heart (dorsal vessel) begins to beat, and the hemolymph (fly blood) circulation is established. Drosophila has an open circulatory system in which the dorsal vessel (heart), with its rhythmic contraction, pumps around immune cells together with nutrients and eventually wastes. Larval hemocytes can either be found at the lymph gland, the major hematopoietic organs in the larva, in the circulation or attached to epithelial tissues (sessile population). A majority of the sessile cells are found in a banded pattern under the larval epidermis, but many are also found attached to the imaginal discs.
The circulatory dynamics of blood cells and their response to tissue injury have been only recently investigated in vivo at the larval stage.30 Free circulating cells slowly flow, in a posterior direction within an open larval body cavity while are actively pumped through the heart and run much faster in the anterior direction.
Time-lapse and real-time imaging studies have shown that, during an inflammatory response a large number of blood cells rapidly accumulate at the site of the wound. These cells belong mostly to the free circulating population, as revealed by live imaging studies using fluorescently labeled hemocytes.30 Indeed, tissue-bound cells even when in a close range to the wound remain sessile and completely unresponsive to the injury. Once recruited at the wound site, hemocytes spread across the damaged surface and assume an adhesive morphology. The appearance of ample vacuoles in their cytoplasm clearly indicates they become phagocytically active to clear the wound site before being released back into circulation.30 This process resembles the early response of blood cells to damaged tissue in vertebrates.
The small GTPases Rac or Rho, which are thought to be universally required for cell migration31 and which block blood cell recruitment to wound sites when mutated in embryonic hemocytes,19 are not necessary for migration during the larval stages. Plasmatocytes-specific expression of dominant-negative forms of these proteins has little or no effect on their accumulation at larval wound sites.30 These results suggest that the migration to wounds at the larval stage may be a rather passive event and hemocytes accumulation at the site of tissue damage is caused by preferential adhesion of the circulating cells (Fig. 1).
Given that Drosophila hemocytes arise from the head mesoderm at early stages of embryogenesis and persist throughout development until the adult, these discrepancies in their behavior are intriguing and suggest there must be a developmental change at hatching that probably occurs to meet the new needs of the organism.
Hemocytes and Tumorigenesis
Burnet Macfarlane originally formulated the idea that the immune system can recognize transformed cells as non-self tissue and react against them.32 This concept is know as “the cancer immuno-surveillance hypothesis” and it was further developed when it was postulated that the immune system may take part in a more general process of immunoediting. In the attempt to eliminate the transformed cells, the immune system selects variants of them better suited to survive in the immunologically activated environment, much like what happens with bacteria, viruses or other parasites, allowing the tumor to escape the surveillance mechanism and colonize the tissue.33
The immuno-surveillance hypothesis was the matter of a long debate that came to an end almost a century after its initial formulation.
The initial approaches to the understanding of the interactions between cancer cells and the immune system were inconclusive, mostly for technical reasons. New insights into tumor-host interactions were gained with the observation that tumors might be thought of as chronic wounds,34–36 since wound healing and tumorogenesis share many characteristics such as stromal remodeling and inflammation. In both instances the immune system must be able to target and eliminate self-tissue but the specific mechanisms by which cancer cells or damaged tissues stimulate an immune response are largely unknown.
Drosophila melanogaster represents a powerful model for immunological studies because many of the mammalian immune signaling pathways, such as the NFκB, Toll or JAK/STAT, are conserved and are fundamental for the immune responses in flies.37
Many aspects of human tumorogenesis are recapitulated in mutants of a class of Tumor Suppressor Genes (TSGs) coding for proteins involved in the establishment of apical-basal polarity of epithelial cells.38,39 These Drosophila tumor models display a loss of cellular architecture, dramatic over-proliferation, basal membrane (BM) degradation and invasive capacity.39,40 In this context Pastor-Pareja and coworkers investigated the role of the cellular immune system in cancer detection and surveillance and found hemocytes adhered to the tumor surface at the site of BM disruption.41 In tumor-bearing flies, the increase in hemocyte number—as a consequence of secretion of JAK-STAT activating cytokines by the tumor cells—was suggested to be the means to restrict tumor growth. They also reported that the same mechanisms are at work in response to aseptic wounds, further confirming the idea that tumors resemble chronic damaged tissues. More recently, using hemolymph transfusion assays, Cordero et al. demonstrated that the population of tumor-associated hemocytes (TAHs) is composed, at least in part, by cells recruited from the circulation.42
Crossing the Basement Membrane: A Small Step for a Cell, a Giant Leap for Cancer
The movement of tumor cells from their primary site and their crossing of the basal lamina constitute initial key events in invasion and metastasis. Epithelial outgrowths that do not cross such a boundary (i.e., ‘in situ’ tumors) are in most cases benign. The biology of invasion and metastasis has only recently started to be revealed. The current dogma—supported by a large body of evidence—suggests that tumor cells must rely on epithelial to mesenchymal transition (EMT) to achieve loss of cell adhesion and the acquisition of a motile phenotype.43
EMT is crucial phenomenon not only for tumorogenesis but also for many developmental processes. Tumor cells, in particular those isolated from the circulation, ectopically express transcription factors (initially identified in Drosophila) capable of orchestrating the EMTs during embryo development. These factors include members of the twist and snail/slug families. Their expression in tumor cells correlates with E-cadherin transcriptional downregulation and induction of N-cadherin, as well as Vimentin and Smooth muscles Actin, all typical markers of cells of mesenchymal origin.44
The acquisition of mesenchymal characteristics is thought to allow the former epithelial cells to migrate to distant sites of metastasis. Interestingly, the pathological analysis of the metastases usually indicates that these secondary tumors display epithelial characteristics, morphological and molecular differentiation markers characteristic of the primary tumors.45 This suggests that once in their new niche, the tumors cells could undergo the opposite process of mesenchymal to epithelial transition (MET) to regain the ability to proliferate and therefore colonize this new site.
These observations are also consistent with recent studies in support of the cancer stem cell (CSC) hypothesis, in which the CSCs invade and migrate to distant sites, eventually expand and differentiate into their epithelial progeny. Remarkably, recent studies link ‘stemness’ with EMT, as both seem connected.46,47
A key open question is what induces EMT in tumors. A classical view is that the accumulation of genetic lesions eventually triggers EMT in a cell-autonomous fashion. Support for this concept comes from recent work implicating mutant isoforms of the key tumor suppressor gene p53 as inducers of EMT via microRNA deregulation.48 However, other studies point to the tumor microenvironment as the cause of EMT: In fact, numerous cell-extrinsic factors, including hypoxia and inflammatory cytokines such as TGFβ49 are capable of inducing an EMT. This environmentally induced EMT would be reversible and allow for future METs at the metastatic niches.
Nevertheless, it is possible that EMT/MET are not the universal mechanisms for invasion and metastasis. First, lineage-tracing experiments required to formally demonstrate EMT and MET in metastasis are still missing from the supporting evidence. Second, tumor cells often invade as cohesive groups that retain epithelial characteristics. This is a particular feature of squamous cell carcinomas.45,49 And so, at least in SCCs the mechanisms that direct basal membrane crossing and migration may differ from EMTs.
Work in Drosophila suggests that the normal epithelial neighbors might recognize transformed cells and actively extrude them from the epithelium. For example, cells deficient for the tumor suppressor gene Csk only delaminate and invade when in close proximity with normal epithelial cells.50 Remarkably, these observations have been reproduced in a mammalian tissue culture system using co-cultures of normal and either Src- or Ras-transformed MDCK epithelial cells.51,52
In the last decade a wealth of evidence has indicated that tumor-related inflammation can stimulate invasion and metastasis. This concept is paradoxical since the inflammatory response was previously thought only to target tumors for destruction. In fact, the key pro-inflammatory TNFα was named after its ability to induce rapid necrotic death of tumor cells.
As has been recently shown in Drosophila, the results of the inflammatory response can be context-dependent; nevertheless factors influencing such contrasting outputs remain largely unknown. Importantly, the activation of the Ras oncogene may itself be a key switch for the induction of tumor-promoting inflammation.42 Previous work in flies indicated that Ras/RAF activation could prevent JNK-dependent death of cells mutant for polarity tumor suppressor genes. In this context, JNK signaling directs growth and invasion instead of promoting cell death.39,53–56 Importantly, Ras can cooperate with other oncogenic pathways that result in JNK activation without directly regulating cell polarity, such as Src50 and Rho-family GTPases.57 Remarkably, because JNK activation propagates across imaginal disc epithelia, the cooperation between JNK and Ras does not need to be cell autonomous.58
In the case of scribble-deficient ‘pre-malignant lesions,’ Eiger-expressing hemocytes associate in large numbers with the tumor lesion, specifically in presence of the Ras oncoprotein and promote invasion.42 Interestingly, recent studies demonstrate that Eiger/TNF is also produced within the epithelium by the mutant cells themselves59 and by the surrounding normal epithelial cells.60 On the other hand, in the absence of Eiger/TNF and regardless of Ras activation, scribble-deficient cells develop into ‘benign’ tumors that grow in situ without crossing the basal lamina and rarely affecting organismal viability. Therefore, in this case loss of cell polarity and adhesion are distinct from epithelial delamination, breach of the basal membrane and migration.
This model provides a paradigm where EMT upon the loss of polarity and adhesion, is uncoupled from delamination and migration as a result of the inflammatory response (Fig. 2).
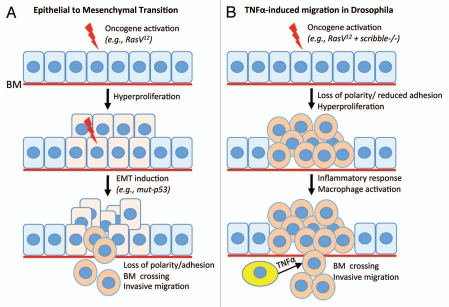
Figure 2.
Models for invasive migration in epithelial tumors. (A) The ‘classic’ epithelial to mesenchymal transition (EMT) can be induced by a variety of stimuli, both by genetic mutations within tumor epithelial cells (notably mutations in the p53 tumor suppressor), and by microenvironmental factors. When activated in epithelial cells, the EMT program orchestrates the simultaneous loss of epithelial characteristics and cell-cell adhesion and the gain of mobility and the ability to cross the basal membrane (BM). (B) TNFα-dependent alternative model to EMT. Drosophila epithelial cells with activated Ras oncogene and mutant for the scribble polarity tumor suppressor overproliferate to form tumor-like outgrowths. These mutant cells also loose epithelial polarity and display reduced cell-cell adhesion. However, in the absence of TNFα such cells do not migrate nor cross the basal membrane and proliferate in situ. Upon an inflammatory response from the host's blood cells (yellow), TNFα signaling stimulates basal membrane crossing and invasive migration. In this case, loss of polarity and adhesion are uncoupled from delamination and migration. These cells migrate as cohesive groups. Therefore, this paradigm appears independent of EMT. See the text for details.
Concluding Remarks
Cancer is a complex multistep pathology that requires the accumulation of several mutations conferring to cells an aberrant proliferative advantage, increased resistance to pro-apoptotic stimuli and loss of differentiation markers.
Tumors of epithelial origins are characterized by a loss of cellular architecture (i.e., apical-basal polarity), while the most invasive front becomes less adhesive and more prone to migration. Understanding how cell polarity is established and maintained and how it is linked to cell proliferation is extremely relevant to cancer biology.
An increasing body of evidence underlines the importance of the tumor microenvironment in the outcome of cancer cells growth. The role of the immune system in fighting cancer progression has been paradoxical since it has been shown to exert both pro- and anti-tumoral effects. Remarkably, live imaging studies in murine models for breast cancer illustrate how tumor cells can migrate guided by—and closely associated with—macrophages.61–63 This intimate connection between epithelial tumor cells and immunosurveillance cells seems highly conserved in metazoa. Therefore, due to the ease of genetic manipulations, Drosophila research can bring meaningful insights to our understanding of the mechanisms of communication between cancerous and normal cells, as well as between the tumor tissue and the immune system.
Acknowledgments
We thank Rhoda Stefanatos for comments on the manuscript, Cancer Research UK for funding and anonymous reviewers for useful comments.
References
- 1.Virchow R. Ueber bewegliche thierische Zellen. Arch Path Anat Physiol. 1863;28:237–240. doi: 10.1007/BF01930783. (Ger). [DOI] [Google Scholar]
- 2.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 4.Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–449. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 5.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 6.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 8.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 9.Aman A, Piotrowski T. Cell migration during morphogenesis. Dev Biol. 2010;341:20–33. doi: 10.1016/j.ydbio.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Jang ACC, Starz-Gaiano M, Montell DJ. Modeling migration and metastasis in Drosophila. J Mammary Gland Biol Neoplasia. 2007;12:103–114. doi: 10.1007/s10911-007-9042-8. [DOI] [PubMed] [Google Scholar]
- 11.Evans IR, Hu N, Skaer H, Wood W. Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos. Development. 2010;137:1625–1633. doi: 10.1242/dev.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–580. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/S1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartenstein V. Blood cells and blood cell development in the animal kingdom. Annu Rev Cell Dev Biol. 2006;22:677–712. doi: 10.1146/annurev.cellbio.22.010605.093317. [DOI] [PubMed] [Google Scholar]
- 15.Heino TI, Kärpänen T, Wahlström G, Pulkkinen M, Eriksson U, Alitalo K, et al. The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech Dev. 2001;109:69–77. doi: 10.1016/S0925-4773(01)00510-X. [DOI] [PubMed] [Google Scholar]
- 16.Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood W, Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol. 2007;8:542–551. doi: 10.1038/nrm2202. [DOI] [PubMed] [Google Scholar]
- 18.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 19.Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, et al. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paladi M, Tepass U. Function of Rho GTPases in embryonic blood cell migration in Drosophila. J Cell Sci. 2004;117:6313–6326. doi: 10.1242/jcs.01552. [DOI] [PubMed] [Google Scholar]
- 21.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreira S, Stramer B, Evans I, Wood W, Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 24.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107:1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- 25.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 26.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 27.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch M, Serras F, Martin-Blanco E, Baguna J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol. 2005;280:73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Mattila J, Omelyanchuk L, Kyttala S, Turunen H, Nokkala S. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int J Dev Biol. 2005;49:391–399. doi: 10.1387/ijdb.052006jm. [DOI] [PubMed] [Google Scholar]
- 30.Babcock DT, Brock AR, Fish GS, Wang Y, Perrin L, Krasnow MA, et al. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci USA. 2008;105:10017–10022. doi: 10.1073/pnas.0709951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Burnet M. Cancer; a biological approach. I. The processes of control. BMJ. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 34.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 37.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 38.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci USA. 2007;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beaucher M, Hersperger E, Page-McCaw A, Shearn A. Metastatic ability of Drosophila tumors depends on MMP activity. Dev Biol. 2007;303:625–634. doi: 10.1016/j.ydbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008:144–154. doi: 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007.. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidal M, Salavaggione L, Ylagan L, Wilkins M, Watson M, Weilbaecher K, et al. A role for the epithelial microenvironment at tumor boundaries: evidence from Drosophila and human squamous cell carcinomas. Am J Pathol. 2010;176:3007–3014. doi: 10.2353/ajpath.2010.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidal M, Warner S, Read R, Cagan RL. Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res. 2007;67:10278–10285. doi: 10.1158/0008-5472.CAN-07-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, et al. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci. 2010;123:171–180. doi: 10.1242/jcs.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogan C, Dupre-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, et al. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol. 2009;11:460–467. doi: 10.1038/ncb1853. [DOI] [PubMed] [Google Scholar]
- 53.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 55.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grzeschik NA, Parsons LM, Richardson HE. Lgl, the SWH pathway and tumorigenesis: It's a matter of context & competition! Cell Cycle. 2010;9:3202–3212. doi: 10.4161/cc.9.16.12633. [DOI] [PubMed] [Google Scholar]
- 57.Brumby AM, Goulding KR, Schlosser T, Loi S, Galea R, Khoo P, et al. Identification of novel ras-cooperating oncogenes in Drosophila melanogaster: A RhoGEF/Rho-Family/JNK pathway is a central driver of tumorigenesis. Genetics. 2011;188:105–125. doi: 10.1534/genetics.111.127910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16:458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T. Elimination of Oncogenic Neighbors by JNK-Mediated Engulfment in Drosophila. Dev Cell. 2011;20:315–328. doi: 10.1016/j.devcel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3:3285. doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inman GJ. Switching TGFβ from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev. 2011;21:93–99. doi: 10.1016/j.gde.2010.12.004. [DOI] [PubMed] [Google Scholar]