Abstract
Autophagy is often found in apoptosis-defective cancer cells and contributes to chemotherapy resistance. However, it is far from clear how the coordination of apoptosis and autophagy determines sensitivity of cancer cells to chemotherapy. Our recent study showed that Beclin 1, a key regulator of autophagy, is cleaved by caspase 8 at the execution stage of chemotherapy-induced and mitochondria-mediated apoptosis. Perturbation of Beclin 1 cleavage, by knock-in of a mutation, phenocopies the autophagy observed in apoptosis-defective cancer cells, and renders chemotherapy resistance in vitro and in vivo. These results demonstrate an important role of caspases in suppressing autophagy by cleaving autophagic machinery.
Key words: Beclin 1, caspase 8, autophagy, apoptosis, chemotherapy
Apoptosis interplays with autophagy to influence the very existence of a cell: life or death. Compared with apoptosis, the role of autophagy in cell death is complicated. Both cytoprotective and cytotoxic functions of autophagy are well documented. In cancer cells that are undergoing chemotherapy-induced apoptosis, autophagy appears to be passive and, in most circumstances, cytoprotective. Autophagy is typically difficult to detect following chemotherapeutic treatment, but becomes more obvious if apoptosis is initiated, and then disrupted, for example, by caspase inhibition. Several ATG (autophagy-related) proteins, including Beclin 1, ATG5 and ATG4D, are subject to cleavage by caspases and other proteases that are activated in apoptosis. However, a large number of proteins can be cleaved by caspases during apoptosis, and it is unclear whether cleavage of the autophagy regulators plays a functional role in cell death.
Using a genetic approach, we dissected the relationship between chemotherapy-induced apoptosis and autophagy. Stress-induced apoptosis in cancer cells often proceeds through the mitochondria-mediated intrinsic pathway, which is characterized by mitochondrial outer membrane permeabilization (MOMP) and cytosolic release of mitochondrial apoptogenic proteins such as cytochrome c (Fig. 1). To determine at which point along this pathway autophagy is suppressed, we generated colon cancer cells expressing a cytochrome c point mutant that disrupts its binding to Apaf-1 and caspase activating function. In response to chemotherapeutic treatment, cytochrome c mutant cells display markedly enhanced autophagy compared with wild-type cells, characterized by increased LC3 lipidation, p62 degradation and autophagosome formation, yet MOMP and cytochrome c release still occur. Therefore, autophagy, which is likely triggered by damaged mitochondria due to MOMP, is suppressed after cytochrome c release at the execution stage of chemotherapy-induced apoptosis.
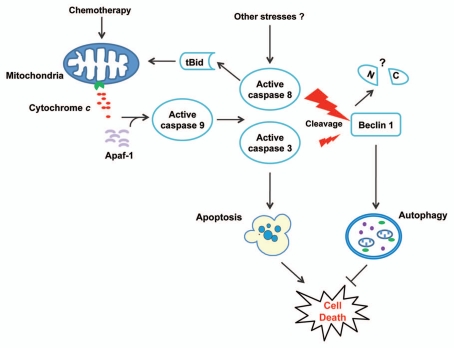
Figure 1.
A model of crosstalk between chemotherapy-induced apoptosis and autophagy in human cancer cells. Chemotherapy induces apoptosis through the mitochondrial pathway by triggering cytochrome c release and caspase activation. Active caspase 8, and to a lesser extent active caspase 3, can cleave Beclin 1 to abrogate its function in autophagy. As a result, autophagy is suppressed and the cell death program can be fully executed. Additional stresses may also activate caspases to regulate autophagy and cell survival. The functions of N- (N) and C-terminal (C) Beclin 1 fragments generated by caspase cleavage are unknown.
Autophagy in cytochrome c mutant cells is dependent on Beclin 1, which is cleaved in a caspase-dependent manner during chemotherapy-induced apoptosis in wild-type cells. Active caspase 8 accounts for most of Beclin 1 cleavage, although active caspase 3 also contributes (Fig. 1), via 2 aspartic acid residues, D133 and D146. Beclin 1 cleavage by caspases is common and occurs in different cell types in response to a variety of treatments. However, different sites and caspases may be utilized preferentially depending on cell types and apoptotic stimuli. Interestingly, cleavage of Beclin 1 is incomplete (<50%) in cell-free assays and in chemotherapy-treated cells, which might be explained by the co-existence of multiple Beclin 1 species with distinct conformation or function. Cleaved fragments of Beclin 1 remain cytosolic, and cannot induce either apoptosis or autophagy. Thus, cleavage of Beclin1 by caspases results in loss of function.
To determine the functional role of Beclin 1 cleavage, we knocked in a mutant version of Beclin 1 (D133A D146A) in colon cancer cells using homologous recombination. Expression of this mutant blocks cleavage of endogenous Beclin 1, resulting in enhanced LC3 lipidation, p62 degradation and autophagosome formation following chemotherapeutic treatment. Nonetheless, Beclin 1 mutation does not affect apoptosis induction and caspase activation. Therefore, specific blockage of Beclin 1 cleavage recapitulates the autophagy induction observed in apoptotic cells treated with caspase inhibitors and those expressing the mutant cytochrome c, indicating that Beclin 1 cleavage by caspases is sufficient to suppress autophagy. Importantly, the long-term survival of Beclin 1 mutant cells following chemotherapeutic treatment is significantly improved compared with wild-type cells. Xenograft tumors established using Beclin 1 mutant cells are also less sensitive to chemotherapy in vivo, despite unaltered apoptosis induction and caspase activation.
Our results provide unequivocal evidence for a functional role of Beclin 1 cleavage in the suppression of autophagy and killing of cancer cells, and have several important implications. MOMP and cytochrome c release are widely considered as a point of no return in programmed cell death. It is intriguing that autophagy induction by blocking Beclin 1 cleavage improves the long-term survival of cells with MOMP and caspase activation. It is possible that these cells are protected from death by autophagy due to changes in energy metabolism, as cancer cells have abnormal bioenergetics. Caspase 8 is better known for initiating the extrinsic apoptotic pathway, but also has nonapoptotic functions. Cleavage of Beclin 1 by caspase 8 may serve as a general link between autophagy and other stress response mechanisms (Fig. 1). Numerous studies have shown that inhibition of autophagy sensitizes cancer cells to chemotherapy. Our work provides new information and tools that may facilitate targeting of autophagy for chemosensitization.
Acknowledgements
Our work is supported by grants from NIH (R01CA106348, R01CA121105, R01CA129829 and U01DK085570), American Cancer Society (RSG-07-156-01-CNE and RSG-10-124-10-CCE), and the American Lung Association/CHEST Foundation.
Punctum to: Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011;71:3625–3634. doi: 10.1158/00085472.CAN-10-4475.