Abstract
Autophagy and apoptosis are tightly regulated biological processes that are crucial for cell growth, development and tissue homeostasis. UVRAG (UV radiation resistance-associated gene), a mammalian homolog of yeast Vps38, activates the Beclin 1/PtdIns3KC3 (class III phosphatidylinositol-3-kinase) complex, which promotes autophagosome formation. Moreover, UVRAG promotes autophagosome maturation by recruiting class C Vps complexes (HOPS complexes) and Rab7 of the late endosome. We found that UVRAG has anti-apoptotic activity during tumor therapy through interactions with Bax. UVRAG inhibits Bax translocation from the cytosol to mitochondria during chemotherapy- or UV irradiation-induced apoptosis of human tumor cells. Moreover, deletion of the UVRAG C2 domain abolishes Bax binding and anti-apoptotic activity. These results suggest that, in addition to its previously recognized pro-autophagy activity in response to starvation, UVRAG has cytoprotective functions in the cytosol that control the localization of Bax in tumor cells exposed to apoptotic stimuli.
Key words: UVRAG, Bax, apoptosis, autophagy, mitochondria, tumor therapy
Radiation and chemotherapy are the most common nonsurgical methods used in cancer treatment. These therapies induce autophagy as well as apoptosis in tumor cells, and the role of autophagy in these cells depends on the type of tumor, the stage of tumorigenesis, and the nature and extent of the insult. Although UVRAG-mediated autophagy has been documented in mammals since 2006, the expression and function of UVRAG during apoptosis is poorly understood. In our recent study, we demonstrated that genotoxic and metabolic stress lead to apoptosis and increase the expression of UVRAG in human tumor cells. We found that UVRAG binds to Bax in the cytosol and prevents Bax translocation to mitochondria and subsequent induction of apoptosis.
Overexpression of UVRAG during Anticancer Therapy
Autophagy is a major pathway for degradation of cytosolic proteins and organelles, and has been implicated in tumor suppression and anticancer therapy resistance. UVRAG was first cloned from the myeloid leukemia cell line. UVRAG promotes autophagy through interactions with Beclin 1, a protein that forms a complex with PtdIns3KC3. UVRAG may also have tumor suppressor activity, as mutations in the UVRAG gene have been identified in various human colon cancer cells and tissues. Following genetic and metabolic stress (e.g., doxorubicin, cisplatin and UV radiation), we found an upregulation of UVRAG mRNA and protein levels. UVRAG expression was related to levels of autophagy, but not apoptosis. Cancer cells treated with autophagy inhibitors (e.g., 3-methyladenine), and autophagy-deficient Atg5-/- mouse embryonic fibroblasts (MEFs) demonstrate decreased levels of UVRAG during administration of chemotherapy and radiation, suggesting that autophagy mediates UVRAG overexpression. Thus, there may be a positive feedback loop between UVRAG expression and autophagy.
UVRAG Regulates Anticancer Therapy Resistance in vivo and in vitro
Resistance to anticancer therapy emerges due to a variety of reasons including genetic or epigenetic changes, which alter the binding site of the drug target, cellular metabolism, export mechanisms or mechanisms of cell death. To explore the potential role for overexpression of UVRAG in response to anticancer therapy, we decreased UVRAG expression by transfection with specific short hairpin RNAs. Interestingly, knockdown of UVRAG in human tumor cells (e.g., HL60 leukemia cells, HCT116 clone cancer cells and HeLa cervical cancer cells) significantly increases the population of apoptotic annexin V+ cells following doxorubicin, cisplatin and UV radiation treatment. As expected, autophagy is decreased after suppression of UVRAG following anticancer therapy. Autophagy and apoptosis can be triggered by common upstream signals. To explore whether the increase in apoptosis is a direct result of loss of UVRAG expression rather than a secondary result of autophagy inhibition causing a shift to the apoptosis pathway, we knocked down UVRAG in autophagy-deficient Atg5-/- MEFs. Indeed, knockdown of UVRAG in Atg5-/- MEFs increases doxorubicininduced apoptosis, which is inhibited by pan-caspase inhibitors (e.g., Z-VAD-FMK). These findings suggest that UVRAG has a direct role in the regulation of apoptosis, which is not dependent on its pro-autophagic activity. Furthermore, in a xenotransplantation mouse model, inhibition of UVRAG expression increases the sensitivity of leukemia cells to doxorubicin, decreases tumor growth and increases apoptosis as determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. These findings demonstrate that UVRAG plays a critical role in modulating apoptosis and autophagy in cancer cells in vitro and in vivo in response to stressful stimuli.
UVRAG Regulates Bax-Dependent Apoptosis
The B-cell lymphoma 2 (Bcl-2) family comprises a class of pro- and anti-apoptotic proteins whose interactions regulate the balance between cellular survival and death. Bax and Bak, the critical pro-apoptotic effector proteins of the Bcl-2 family, are responsible for mitochondrial membrane permeabilization. Notably, suppression of UVRAG increases Bax-induced apoptosis in tumor cells, whereas overexpression of UVRAG by gene transfection inhibits Bax-induced apoptosis. However, UVRAG does not suppress apoptosis induced by other pro-apoptotic Bcl-2 family effector proteins (e.g., Bad or Bid) or by ‘extrinsic’ cell death stimuli (e.g., Fas or TRAIL). Moreover, UVRAG inhibits caspase activation and mitochondrial cytochrome C release, which are downstream mitochondrial apoptotic events following Bax activation. These findings suggest that UVRAG selectively regulates Bax-dependent apoptosis in tumor cells. Interestingly, other studies have demonstrated that Bax plays a dual role in the control of apoptosis and autophagy. On the one hand, Bax-induced autophagy contributes to apoptosis. On the other hand Bax-induced apoptosis contributes to inhibition of autophagy. It is unknown whether UVRAG regulates Bax-associated autophagy.
UVRAG Interacts with Bax and Regulates Bax Mitochondrial Translocation
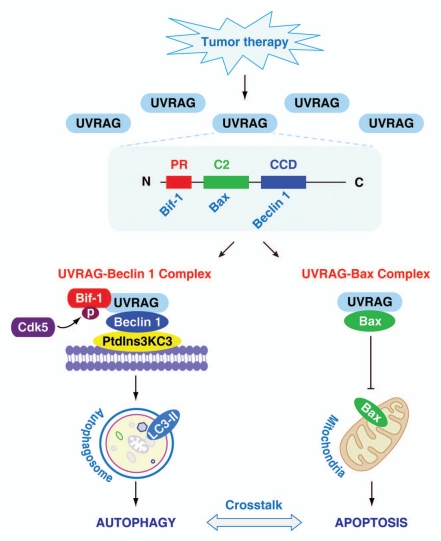
UVRAG confers anti-apoptotic activities potentially via controlling Bax translocation to mitochondria. UVRAG does not mediate this effect by regulating the expression of Bax. Rather, our experimental data suggest that UVRAG may function as a specific anchoring protein for Bax in the cytosol, because: (1) UVRAG is specifically co-immunoprecipitated with Bax, but not Bad and Bid in tumor cells; (2) GST pull-down assays confirmed an interaction between UVRAG and Bax; (3) deletion of UVRAG expression leads to a parallel increase in mitochondrial Bax during apoptosis; (4) overexpression of UVRAG decreases mitochondrial translocation of Bax in apoptosis. Structurally, UVRAG contains an N-terminal proline rich (PR) sequence followed by a potential calcium-dependent phospholipid binding C2 domain, a central Beclin 1-binding coiled-coil domain (CCD) and a C-terminal region (Fig. 1). A previous study demonstrated that UVRAG binds Beclin 1 through its CCD, and binds Bif-1 (also known as Endophilin B1) through the PR domain, which promotes autophagy. Moreover, cyclin-dependent kinase 5 (Cdk5)-mediated phosphorylation of Bif-1 is required for recruitment of UVRAG and induction of autophagy. In contrast, we demonstrated that UVRAG binds Bax through its C2 domain. Deletion of the C2 domain inhibits the interaction between UVRAG and Bax, rendering it unable to inhibit chemotherapy and radiation-induced apoptosis. Furthermore, loss of UVRAG enhances conformational changes in Bax following treatment with doxorubicin and UV radiation, suggesting that UVRAG inhibits exposure of the Bax N terminus during apoptosis.
Figure 1.
Conceptual relationships between UVRAG and apoptosis/autophagy. Tumor therapies, such as chemotherapy and radiation, increase UVRAG expression in tumor cells. Moreover, UVRAG forms two different complexes to regulate crosstalk between apoptosis and autophagy. On the one hand, UVRAG promotes autophagy through interactions with Beclin 1 via the CCD domain. Cdk5-mediated phosphorylation of Bif-1 interacts with the PR domain within UVRAG, which promotes formation of the Beclin 1/PtdIns3KC3 complex. Microtubule-associated protein 1 light chain 3 (LC3), a mammalian homolog of yeast Atg8, is the most widely-used marker of autophagosomes. During autophagy, LC3-I is converted to LC3-II. On the other hand, UVRAG binds directly to Bax in the cytosol through the C2 domain, which prevents Bax translocation to mitochondria and subsequent induction of apoptosis.
Therefore, our experimental data suggest that UVRAG forms two different complexes (UVRAG-Beclin 1 and UVRAG-Bax), which regulate the balance between apoptosis and autophagy (Fig. 1). In this complex, UVRAG is proposed to function as a positive regulator of autophagy and a negative regulator of apoptosis. Our findings provide a possible mechanism for the crosstalk between apoptosis and autophagy in determining which process will dominate. Indeed, the existence of negative and/or positive feedback loops increases the complexity of signaling pathways and may influence the predominance of autophagy and apoptosis.
Acknowledgements
This work was supported by grants from The National Natural Sciences Foundation of China (30772353, 30973234 to L.C.), Doctoral Program of Higher Education of China (20070533042 to L.C.), and a grant from University of Pittsburgh (D.T.).
Punctum to: Yin X, Cao L, Kang R, Yang M, Wang Z, Peng Y, et al. UV irradiation resistance-associated gene suppresses apoptosis by interference with Bax activation. EMBO Rep. 2011;12:727–734. doi: 10.1038/embor.2011.79.