Abstract
Photodynamic therapy (PDT) is a process that can induce apoptosis, autophagy or both depending on the cell phenotype. Apoptosis is a pathway to cell death while autophagy can protect from photokilling or act as a death pathway. In a previous study, we reported a cytoprotective effect of autophagy in murine leukemia cell lines where both autophagy and apoptosis occur within minutes after irradiation of photosensitized cells. In this study, we examined the effects of mitochondrial photodamage catalyzed by low (≤1 µM) concentrations of the photosensitizing agent termed benzoporphyrin derivative (BPD, Verteporfin) on murine hepatoma 1c1c7 cells. Apoptosis was not observed until several hours after irradiation of photosensitized cells. Autophagy was clearly cytoprotective since PDT efficacy was significantly enhanced in a knockdown sub-line (KD) in which the level of a critical autophagy protein (Atg7) was markedly reduced. This result indicates that autophagy can protect from phototoxicity even when apoptosis is substantially delayed. Much higher concentrations (≥10 µM) of BPD had previously been shown to inhibit autophagosome formation. Phototoxicity studies performed with 10 µM BPD and a proportionally reduced light dose were consistent with the absence of an autophagic process in wild-type (WT) cells under these conditions.
Key words: apoptosis, autophagy, benzoporphyrin, photodynamic
Introduction
Photodynamic therapy (PDT) involves the use of photosensitizing agents that localize somewhat selectively in neoplastic tissues and their vasculature.1 Irradiation at an appropriate wavelength leads to an interaction between the photosensitizing agent and oxygen in tissues, resulting in formation of reactive oxygen species (ROS) that can initiate cell death processes. Oleinick's group was the first to demonstrate that PDT could lead to the initiation of the apoptotic death program.2 The initial target of many photosensitizing agents is the anti-apoptotic protein Bcl-2.3–5 Inactivation of Bcl-2 function can have additional consequences. The protein Beclin 1 forms a complex with Bcl-2 and related antiapoptotic proteins. Release of Beclin 1 from this complex results in interactions with additional proteins, leading to the initiation of macroautophagy.6 In this report, we use the term ‘autophagy’ to indicate this process. Autophagy involves formation of vacuoles that engulf a portion of the cytosol, often including subcellular organelles. This was initially identified as a response to starvation, permitting cells to recycle degraded, damaged or aging components, but can also lead to cell death.7–9 A review on the subject of autophagic responses to PDT was recently published in reference 10.
We have reported that PDT leads to both autophagy and apoptosis in murine leukemia cells,11,12 with autophagy protecting cells from photodamage in apoptosis-competent cells. Apoptosis occurs very rapidly in these cell types: caspase activation and DNA cleavage can be detected within minutes after irradiation13 and autophagic vacuoles are observed within 15 min.11 In contrast, in the MCF-7 cell line, Oleinick's group found that inhibition of autophagy protected from cell death after PDT.14 These data indicated that both apoptosis and autophagy represent death pathways in MCF-7 cells. It was postulated that the observed differences between leukemia vs. MCF-7 cells might relate to the apoptotic response rate. A more rapid onset of apoptosis in the leukemias was proposed to be associated with a cytoprotective role for autophagy. Where apoptosis occurred more slowly, i.e., in MCF-7 cells, autophagy clearly contributed to cell death.14
In this report, we describe effects of photodamage on another cell line, the murine hepatoma designated 1c1c7, where apoptosis occurs several hours after the irradiation of photosensitized cells. The photosensitizer used in these studies was the benzoporphyrin derivative termed BPD (Verteporfin), an agent that can be used both for cancer control and treatment of macular degeneration.1,15 BPD is known to target mitochondria for photodamage.16 This is a potentially sensitive target for PDT for several reasons, one of which involves the presence of cytochrome c in mitochondrial membranes. Migration of this protein into the cytoplasm, i.e., from photodamaged mitochondria would result in a known pathway to apoptosis.17
Results and Discussion
Cell lines.
The degree of the Atg7 knockdown in the KD subline is shown in Figure 1. There was a >90% decrease in protein expression, as indicated by densitometric measurements.
Figure 1.
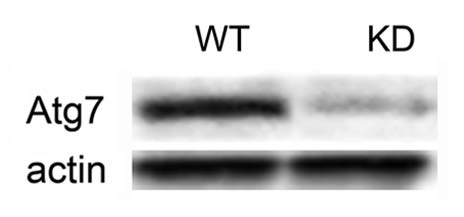
Protein gel blot showing the expression of the Atg7 protein in wild-type (WT) vs. Atg7 knockdown (KD) 1c1c7 cells. The bottom part shows actin blots.
Phototoxicity.
Varying the BPD concentration while keeping the light dose at a constant 90 mJ/cm2 revealed that a 0.5 µM drug concentration resulted in death of ∼30% of the WT line, but >60% of the KD cells (Fig. 2). When the drug concentration was increased to 1 µM, approx. 25% of the WT and 15% of the KD line survived. The shoulder on the dose-response curve seen in Figure 2 indicates the extent of the protective effect of autophagy in WT cells.
Figure 2.
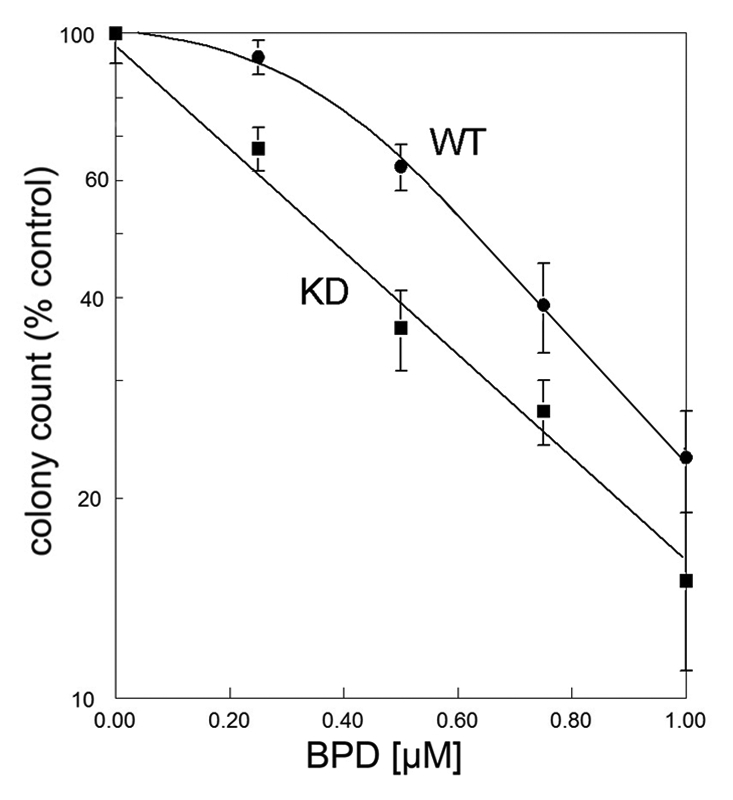
Dose-response curves for 1c1c7 WT vs. KD cells photosensitized with varying concentrations of BPD. After irradiation (90 mJ/cm2, 690 nm), viability was assessed by clonogenic assays.
Effects of PDT on cellular morphology.
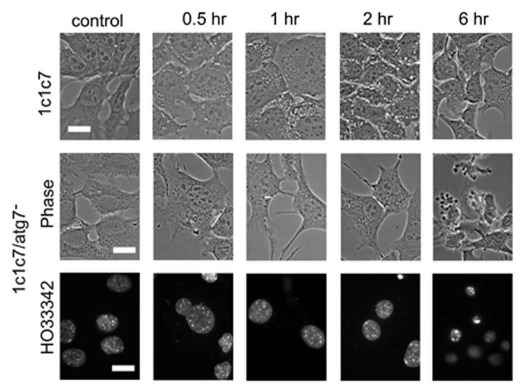
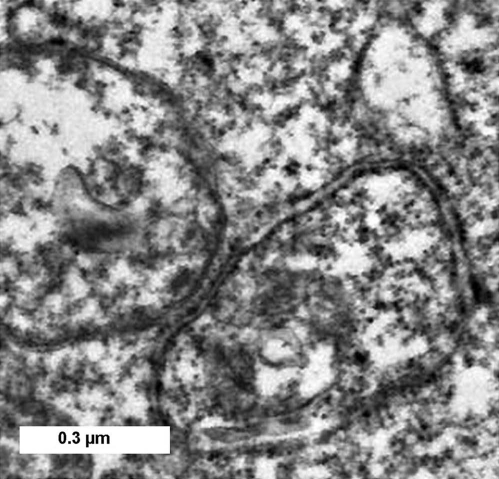
Effects of irradiation of WT and KD 1c1c7 cells using a 0.5 µM BPD concentration are shown in Figure 3. For these studies, a 90 mJ/cm2 light dose was used. This was shown (Fig. 2) to have a greater phototoxic effect on the KD sub-line than on WT cells. Images in Figure 3 demonstrate that irradiation of WT cells photosensitized with 0.5 µM BPD resulted in formation of a few vacuoles after 30 min, and substantially more after 1 h (upper part). These vacuoles persisted for an additional hour, but then began to disappear so that few were visible 6 h after irradiation. HO33342 labeling studies indicated the presence of no cells with the condensed chromatin typical of apoptotic cells (not shown). In contrast, the KD line exhibited no vacuolization after PDT (center part), along with some cells with condensed chromatin 6 h after irradiation (bottom part). Electron microscopy revealed that vacuoles formed 2 h after irradiation contained the double-membrane structure typical of autophagy (Fig. 4). This PDT dose results in a ∼30% loss of viability for WT cells and a 60% loss for the LD line. In a separate study, we also examined the effect of an LD30 PDT dose on the KD cells and, as expected, found no morphologic evidence of autophagy. A slight increase in DEVDase activity was observed in WT cells at the 0.5 µM BPD dose level, but this did not become significant until a higher drug concentration was employed (Table 1). The DEVDase measurement reflects activation of caspases 3 and 7, an element of the apoptotic response to PDT that was monitored in our prior studies.4 A similar experiment performed with the Atg7-deficient KD line (Fig. 3 and lower 2 parts) produced no vacuoles at any time point. We did, however, observe an apoptotic morphology 6 h after irradiation, along with the presence of condensed nuclei labeled by HO33342. A comparison of approximately equitoxic PDT doses (0.5 µM BPD for KD cells and 0.75 µM for WT cells) resulted in similar increases in DEVDase activity (Table 1). The level of this activity therefore is closely correlated with phototoxicity.
Figure 3.
Morphology of 1c1c7 WT and KD photosensitized with 0.5 µM BPD and then irradiated (90 mJ/cm2). Top row: WT controls vs. cells 0.5, 1, 2 or 6 h after irradiation. Center row: results of a similar study with the Atg7 KD line. Bottom row: HO33342-labeling of the KD cells incubated with this probe for the final 5 min of each incubation interval. White bars in figures = 20 µm.
Figure 4.
Electron microscopic examination of vacuoles formed in 1c1c7 cells after PDT. WT cells were photosensitized with 0.5 µM BPD and sampled 2 h after irradiation (90 mJ/cm2).
Table 1.
DEVDase activity and viability after PDT
| BPD (µM) | 1c1c7 | 1c1c7/Atg7− | ||||
| viability | DEVDase | viability | DEVDase | |||
| 2 hr | 6 hr | 2 hr | 6 hr | |||
| 0 | 100 ± 8.5 | 0.10 ± 0.03 | 0.13 ± 0.04 | 100 ± 9.4 | 0.16 ± 0.06 | 0.16 ± 0.06 |
| 0.25 | 92 ± 5.5 | 0.16 ± 0.05 | 0.19 ± 0.07 | 67 ± 5.5 | 0.19 ± 0.09 | 2.8 ± 0.42* |
| 0.5 | 63 ± 5.1 | 0.32 ± 0.09 | 1.9 ± 0.24* | 36 ± 4.8 | 0.98 ± 0.17* | 6.8 ± 0.96* |
| 0.75 | 39 ± 4.9 | 0.45 ± 0.11* | 7.2 ± 0.56* | 27 ± 6.0 | 3.2 ± 0.69* | 7.6 ± 1.3* |
| 1.00 | 23 ± 4.2 | 0.42 ± 0.16* | 8.8 ± 0.64* | 15 ± 4.1 | 4.65 ± 0.93* | 8.1 ± 1.5* |
| ANOVA F ratio | 4.8 | 31.6 | 45.5 | 35.6 | ||
| ANOVA p value | 0.02 | <0.01 | <0.01 | <0.01 | ||
Viability is expressed as % control using conditions outlined in the text. DEVDase levels (nmol/mg protein) were measured 2 and 6 hr after irradiation using a 90 mJ/cm2 light dose (690 ± 10 nm). ANOVA analysis indicates the probability that DEVDase values in each group are significantly different.
Significantly different from control value (p < 0.02).
An estimate of the autophagic flux can be provided by measuring the rate of conversion of the protein LC3 into the lipidated form.18 We observed an increase in LC3-II formation in the WT 1c1c7 after an LD50 PDT dose, but a similar effect was also seen in the Atg7 KD cell line (Fig. 5). Such an effect of an Atg7 knockdown has been observed before and was interpreted to indicate that processing of LC3 can occur even in a knockdown line, or that there may be alternative routes for such processing in cells with a markedly reduced level of Atg7.14
Figure 5.
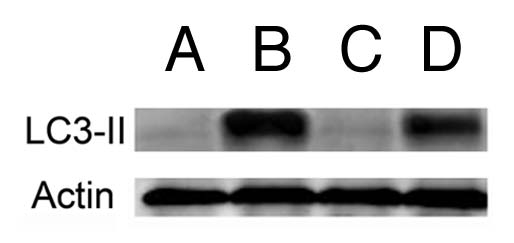
Protein gel blot showing formation of the lipidated form (LC3-II) of the LC3 protein. (A and B) = wild-type 1c1c7 cells; A = controls, B = cells 30 min after an LD70 PDT dose using BPD. (C and D) the same sequence in a similar study using the Atg7 KD line.
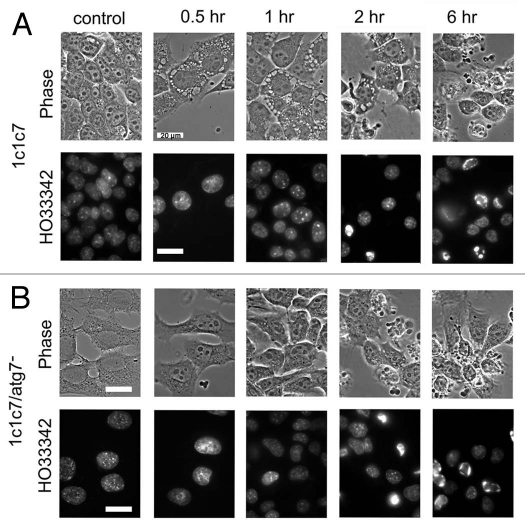
Figure 6 shows the effects of photosensitization with a 1.0 µM BPD concentration and a 90 mJ/cm2 light dose. Vacuole formation was observed in the WT cell line (part A) within 30 min after irradiation, with a few apoptotic cells appearing after 2 h. A greater apoptotic response and level of DEVDase activity was observed 6 h after irradiation. The KD line showed an even greater degree of photokilling at the higher drug dose (Fig. 2). This was also evident from DEVDase measurements (Table 1) along with the appearance of more cells with an apoptotic morphology and condensed chromatin (Fig. 6B). No cytoplasmic vacuoles were observed in the KD line at any time point examined.
Figure 6.
Morphology of 1c1c7 WT and KD photosensitized with 1.0 µM BPD and then irradiated (90 mJ/cm2). (A) WT cells (controls vs. cells 0.5, 1, 2 or 6 h after irradiation). (B) HO33342 labeling patterns of cells shown in (A). (C) Results of a similar study with the Atg7 KD line. (D) HO33342-labeling of the cells shown in (C). The fluorescent probe was added during the final 10 min of each incubation. White bars in figures = 20 µm.
Effects of high-dose BPD.
A recent report described the unexpected ability of BPD (in the dark) to antagonize autophagosome formation after starvation or other stimuli.19 This inhibitory effect was not observed until the BPD concentration was ≥10 µM. In studies reported above, the concentration of BPD never exceeded 1 µM. When we incubated WT 1c1c7 cells with 10 µM BPD, no cytoplasmic vacuoles were observed 60 min after an LD70 PDT dose (Fig. 7A). In contrast, using 1 µM BPD and a light dose corresponding to ∼LD70 conditions, substantial numbers of vacuoles were seen (Fig. 6). When the light dose was varied, using a 1 or 10 µM BPD concentration, we observed the shoulder on the dose-response curve only at the lower drug dose (Fig. 7B). These results are consistent with a report that a 10 µM BPD concentration inhibits autophagosome formation.19
Figure 7.
(A) Images (phase contrast and HO33342 labeling) of WT cells treated with 10 µM BPD. These were acquired 60 min after a 5 mJ/cm2 light dose. (B) Dose-response curves for 1c1c7 WT cells photosensitized with 1 µM (●) or 10 µM (○) BPD for 60 min, then irradiated with the specified light doses. The upper series of numbers on the X axis (0–120 mJ/cm2) corresponds to the lower BPD dose; the lower series to the higher drug dose. Viability was assessed by clonogenic assays. White bars in figures = 20 µm.
Comparison with murine leukemias.
In a study involving the L1210 murine leukemia and an ER photosensitizer termed CPO, we observed formation of autophagic vacuoles within minutes after irradiation of photosensitized cells.11,12 A subline lacking Atg7 was found to be more responsive to PDT than were wild-type cells.11
Oleinick's group reported that loss of the Atg7, thereby inhibiting an autophagic response, protected from photokilling in MCF-7 cells. The same result was obtained in a subline that was apoptosis competent, using an Atg8 knockdown.14 It was suggested that the rate of initiation of apoptosis might be a factor with regard to effects of autophagy of photokilling, i.e., that cells that had a rapid apoptotic response to PDT might use autophagy as a protective pathway while in cells exhibiting a slow apoptotic response, autophagy could also be a death pathway. Apoptosis was observed in the MCF-7 line after 22 h had elapsed after PDT. Intermediate time points between 2 and 22 h were not evaluated.
The 1c1c7 cell line appears to have an intermediate response rate, with the first appearance of apoptosis a function of the concentration of photosensitizer. Using 1.0 µM BPD, we found that significant DEVDase activation was not observed until 6 h after irradiation and autophagy was clearly cytoprotective. This cell line may be typical of non-leukemic cells capable of undergoing apoptosis, but not as rapidly as do the leukemias. Solid tumors with a defective apoptotic program will likely be more dependent on other death pathways, e.g., autophagy, after photodamage.
Materials and Methods
Chemicals and biological.
Benzoporphyrin derivative (Verteporfin, BPD) was purchased from VWR (1711461). Stock solutions were prepared in DMSO. Tissue culture media MEM-Eagle α modification, (M0894) and HO33342 (B2261) were provided by Sigma-Aldrich. A rabbit polyclonal antibody to a peptide mapping to the carboxy terminus of human Atg7 was purchased from Prosci Inc., (3615). Proteintech provided a purified custom antibody to the murine LC3 protein.
Cells and cell culture.
Murine hepatoma 1c1c7 cells were maintained in α-MEM medium supplemented with 5% fetal bovine serum (Atlanta Biologicals, S11150) and antibiotics in 5% CO2 at 37°C in plastic culture dishes. A retroviral vector that encoded a short hairpin RNA construct directed against murine Atg7 was obtained from Origene (TR504956). The protocol for the construction and selection of a cell line that stably expresses shRNAs to Atg7 has been published in reference 11. The KD line was periodically monitored by protein gel blotting to insure continued significant silencing of Atg7. For studies involving microscopy, 1c1c7 cells were cultured on 1 cm glass coverslips placed at the bottom of plastic dishes.
Protein gel blots.
These studies were performed previously described in reference 21. To inhibit hydrolysis of LC3-II by lysosomal proteases, incubation buffers were supplemented with the protease inhibitors E64d (Sigma-Aldrich, E8640) and acetylpepstatin A (Sigma-Aldrich, A-4815).
PDT protocols.
Cells were incubated with specified concentrations of BPD for 60 min at 37°C, then the medium was replaced and the dishes cooled to 15°C. This low temperature prevents initiation of apoptosis during irradiation.22 Light was provided by a 600 W quartz-halogen source filtered with 10 cm of water to remove infrared. The bandwidth was further confined to 690 ± 10 nm using an interference filter (Oriel, 59455). The time of irradiation was adjusted to yield the desired loss of viability as determined by clonogenic assays. Viability was determined by colony counting using an Oxford Optronix GelCount device. An algorithm was used to confine the colony identification to groups of 30 cells or more. Although this device can detect colonies without additional staining, crystal violet was used as a colony marker for studies reported here. All experiments were performed in triplicate. After irradiation, cells were incubated at 37° as specified.
Microscopy.
Chromatin condensation (apoptosis) was assessed by fluorescence using HO33342 as described before in reference 5, using 330–380 nm excitation and measuring emission at 420–450 nm. The probe was added during the final 5 min of the incubation at 37° that followed irradiation of photosensitized cells.
Phase-contrast and fluorescence images were acquired with a Nikon E-600 microscope using a Photometrics CoolSnap HQ CCD camera. Images were processed using MetaMorph software. For electron microscopy, cells were trypsinized and the pellets fixed with glutaraldehyde and osmium tetroxide, treated with uranyl acetate + lead citrate and the samples then dehydrated in ethanol. The resulting pellets were embedded in epon resin and cut with an ultramicrotome to a 70 nm thickness before viewing.
DEVDase assays.
Cells were collected 2 and 6 h after irradiation and lysates were assayed for DEVDase activity.13 A kit provided by Invitrogen was used for this purpose, cat. no. E13184. Enzyme levels are reported in terms of nmol product/min/mg protein. Each assay was performed with triplicate. The BioRad assay (500-0006) was used to estimate protein concentrations, using bovine serum albumin as the standard.
Conclusions
Apoptosis occurs at a slower rate in 1c1c7 cells than in the murine leukemias, an effect possibly related to levels of procaspases or rate of operation of pertinent signaling pathways. Autophagy was found to be a cytoprotective process in both cell lines after PDT. This may be based on the ability of autophagy to recycle photo-damaged mitochondria before cytochrome c can be released.20 In other cell lines, the autophagic process may occur too late to be able to prevent initiation of apoptosis or, alternatively, in the absence of apoptosis, may serve as the major death pathway. The photosensitizer used in these studies was found to inhibit autophagosome formation at a 10 µM concentration in the dark.19 Consistent with studies involving the KD line, photosensitization with the higher BPD level, followed by irradiation with a reduced light flux eliminated the shoulder on the dose-response curve associated with the cytoprotective effect of autophagy. Use of high-dose BPD could potentiate PDT efficacy where autophagy offers protection from photokilling, if tumor selectivity is not thereby lost.
Acknowledgements
These studies were supported by grant CA 23378 from the National Cancer Institute. Michael Price was supported by NIH grants GM-058905-11 and T32-CA009531. Ann Marie Santiago provided excellent technical assistance during the course of this work. We thank Dr. James Hatfield, Department of Pathology at the John Dingell VA Hospital, for acquisition of images involving electron microscopy.
Abbreviations
- BPD
benzoporphyrin derivative
- KD
Atg7 knockdown
- PDT
photodynamic therapy
- ROS
reactive oxygen species
- WT
wild-type 1c1c7 cells
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunez AR, Oleinick NL. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991;51:5993–5996. [PubMed] [Google Scholar]
- 3.Kim HR, Kessel D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999;59:3429–3432. [PMC free article] [PubMed] [Google Scholar]
- 4.Xue LY, Chiu SM, Oleinick NL. Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2001;20:3420–3427. doi: 10.1038/sj.onc.1204441. [DOI] [PubMed] [Google Scholar]
- 5.Kessel D, Castelli M. Evidence that bcl-2 is the target of three photosensitizers that induce a rapid apoptotic response. Photochem Photobiol. 2001;74:318–322. doi: 10.1562/0031-8655(2001)074<0318:etbitt>2.0.co;2. http://dx.doi.org/0.1562/0031-8655(2001)0740318ETBITT2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 6.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 7.Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- 8.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/OCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiners JJ, Jr, Agostinis P, Berg K, Oleinick NL, Kessel D. Assessing autophagy in the context of photodynamic therapy. Autophagy. 2010;6:7–18. doi: 10.4161/auto.6.1.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessel D, Arroyo AS. Apoptotic and autophagic responses to Bcl-2 inhibition and photodamage. Photochem Photobiol Sci. 2007;6:1290–1295. doi: 10.1039/b707953b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessel D, Reiners JJ., Jr Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage. Photochem Photobiol. 2007;83:1024–1028. doi: 10.1111/j.1751-097.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessel D, Luo Y. Photodynamic therapy: a mitochondrial inducer of apoptosis. Cell Death Differ. 1999;6:28–35. doi: 10.1038/sj.cdd.4400446. [DOI] [PubMed] [Google Scholar]
- 14.Xue LY, Chiu SM, Oleinick NL. Atg7 deficiency increases resistance of MCF-7 human breast cancer cells to photodynamic therapy. Autophagy. 2010;6:248–255. doi: 10.4161/auto.6.2.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter AM, Waterfield E, Jain AK, Allison B, Sternberg ED, Dolphin D, et al. Photosensitising potency of structural analogues of benzoporphyrin derivative (BPD) in a mouse tumour model. Br J Cancer. 1991;63:87–93. doi: 10.1038/bjc.1991.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng TI, Chang CJ, Guo MJ, Wang YH, Yu JS, Wu HY, et al. Mitochondrion-targeted photosensitizer enhances the photodynamic effect-induced mitochondrial dysfunction and apoptosis. Ann NY Acad Sci. 2005;1042:419–428. doi: 10.1196/annals.1338.035. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 18.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 19.Donohue E, Tovey A, Vogl AW, Arns S, Sternberg E, Young RN, et al. Inhibition of autophagosome formation by the benzoporphyrin derivative verteporfin. J Biol Chem. 2011;286:7290–7300. doi: 10.1074/jbc.M110.139915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim I, Lemasters JJ. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid Redox Signal. 2011;14:1919–1928. doi: 10.1089/ars.2010.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso JA, Mathieu PA, Joiakim A, Leeson B, Kessel D, Sloane BF, et al. Differential susceptibilities of murine hepatoma 1c1c7 and Tao cells to the lysosomal photosensitizer NPe6: influence of aryl hydrocarbon receptor on lysosomal fragility and protease contents. Mol Pharmacol. 2004;65:1016–1028. doi: 10.1124/mol.65.4.1016. [DOI] [PubMed] [Google Scholar]
- 22.Pryde JG, Walker A, Rossi AG, Hannah S, Haslett C. Temperature-dependent arrest of neutrophil apoptosis. Failure of Bax insertion into mitochondria at 15 degrees C prevents the release of cytochrome c. J Biol Chem. 2000;275:33574–33584. doi: 10.1074/jbc.M001008200. [DOI] [PubMed] [Google Scholar]