Abstract
To investigate the stepwise autophagic-lysosomal processing of hepatocellular proteins, the abundant cytosolic enzyme, betaine:homocysteine methyltransferase (BHMT) was used as a probe. Full-length (45 kDa) endogenous BHMT was found to be cleaved in an autophagy-dependent (3-methyladenine-sensitive) manner in isolated rat hepatocytes to generate a novel N-terminal 10-kDa fragment (p10) identified and characterized by mass spectrometry. The cleavage site was consistent with cleavage by the asparaginyl proteinase, legumain and indeed a specific inhibitor of this enzyme (AJN-230) was able to completely suppress p10 formation in intact cells, causing instead accumulation of a 42-kDa intermediate. To prevent further degradation of p10 or p42 by the cysteine proteinases present in autophagic vacuoles, the proteinase inhibitor leupeptin had to be present. Asparagine, an inhibitor of amphisome-lysosome fusion, did not detectably impede either p42 or p10 formation, indicating that BHMT processing primarily takes place in amphisomes rather than in lysosomes. Lactate dehydrogenase (LDH) was similarly degraded primarily in amphisomes by leupeptin-sensitive proteolysis, but some additional leupeptin-resistant LDH degradation in lysosomes was also indicated. The autophagic sequestration of BHMT appeared to be nonselective, as the accumulation of p10 (in the presence of leupeptin) or of its precursors (in the additional presence of AJN-230) proceeded at approximately the same rate as the model autophagic cargo, LDH. The complete lack of a cytosolic background makes p10 suitable for use in a “fragment assay” of autophagic activity in whole cells.
Incubation of hepatocytes with ammonium chloride, which neutralizes amphisomes as well as lysosomes, caused rapid, irreversible inhibition of legumain activity and stopped all p10 formation. The availability of several methods for selective targeting of legumain in intact cells may facilitate functional studies of this enigmatic enzyme, and perhaps suggest novel ways to reduce its contribution to cancer cell metastasis or autoimmune disease.
Keywords: AJN-230, ammonia, amphisome, autophagy, betaine:homocysteine methyltransferase, liver, hepatocyte, legumain, leupeptin, lysosome, proteinase, proteolytic processing, proteomics, rat
Introduction
The autophagic-lysosomal pathway plays a major role in the turnover of intracellular proteins. It functions primarily as a general mechanism for the wholesale degradation of cytoplasm by macroautophagy1 (or just autophagy), a process in which compressed-cisternal organelles known as phagophores2 envelop parts of the cytoplasm to form closed vacuolar autophagosomes.3 Subsequently, these early autophagic vacuoles become acidified by fusion with endosomes, turning them into amphisomes.4–6 The amphisomes harbor proteolytic enzymes at low levels (but with significant catalytic activity), brought in by the endosomal fusion partners,7 allowing, e.g., cells engaged in immune responses to process endogenous as well as exogenous proteins to antigenic peptides that can be exocytosed and presented by MHC class II receptors on the cell surface.8,9 Additional amounts of acid hydrolases are acquired by fusion with lysosomes, transforming the amphisomes into digestive vacuoles (autolysosomes) in which sequestered cytoplasm is completely degraded to small molecules that serve as energy substrates and building materials for the cell and the organism.10,11 The autophagic-lysosomal pathway is an integral part of the cellular defense against metabolic and environmental stress,12 responding, e.g., to nitrogen/amino acid starvation at several levels: (1) at the level of autophagy (Atg) gene expression;13 (2) at phagophore assembly sites (PAS; omegasomes) where a large number of Atgs and other proteins are organized in several functional complexes to cooperate in the production of phagophores,14–18 and (3) at the level of autophagic activity, where the sequestration of cytoplasm (by the phagophores) can be switched on and off very rapidly in response to the availability of amino acids and various regulatory signals.19,20
In addition to its role in the bulk degradation of cytoplasm, it has been shown that autophagy exerts an important protective function by selectively removing aberrant, toxic proteins that may otherwise accumulate with time in the form of protein aggregates (aggresomes; amyloid bodies) to cause neurodegenerative diseases and other pathologies.21,22 Apparently nascent phagophores forming at the PAS are directed to ubiquitylated aggregates by adaptor proteins (Alfy; p62) capable of binding to the aggregates as well as to the phagophoric receptor LC3/Atg8.23 Although the vast majority of abnormal proteins are ubiquitylated and degraded by the proteasomal pathway,24 several examples of selective autophagic sequestration of specific organelles25–32 or individual proteins33–37 have been reported, in many cases aided by the ubiquitous autophagic recognition adaptor, p62.32,34,38 The observation that the phagophore-derived autophagosomal delimiting membranes are enriched in a number of proteins potentially capable of recognizing aberrant protein substrates39 would be consistent with phagophores possessing a certain selective scavenging capability on top of their ability to perform nonselective bulk sequestration. Alternatively, some of the autophagosomally enriched proteins could themselves represent selectively sequestered autophagic cargo.
In an attempt to address these questions, we have initiated studies on the sequestration and processing of the most highly enriched autophagosomal membrane proteins. Some of these exhibit a phenotypic heterogeneity that is particularly challenging. For example, the enzyme catechol-O-methyltransferase, which presents as one phosphorylated and one nonphosphorylated variant in autophagosomal membranes,39 was recently shown by immunoblotting and proteomic analysis to number no less than seven forms in rat hepatocytes.40 Betaine:homocysteine methyltransferase (BHMT), represented in autophagosomes as five membrane-associated variants according to our previous proteomic analysis,39 was found in the present study to display several additional forms detectable by immunoblotting. One of these is a novel N-terminal BHMT fragment of ∼10 kDa, generated by autophagic-lysosomal processing in intact hepatocytes through the action of the asparaginyl endopeptidase, legumain.41 The BHMT-processing activity of legumain could be effectively blocked in intact cells by specific enzyme inhibitors42,43 or by neutralization of acidic autophagic vacuoles with ammonia,44 thus offering new ways of investigating and perturbing the autophagic-lysosomal pathway for experimental and possibly therapeutic purposes. Furthermore, the confinement of the p10 BHMT fragment to autophagic-lysosomal vacuoles (no cytosolic background) may make it suitable as an autophagic activity marker, uniquely detectable in whole (unfractionated) cells.45
Results
Novel hepatocellular BHMT fragments.
In our previous study39 we identified five BHMT species that were enriched in the membrane fraction from purified rat liver autophagosomes relative to membranes from whole cytoplasm: two full-length forms (45 kDa), one C-truncated form (28 kDa) and two forms (16 and 18 kDa) that appeared to be truncated both C-terminally and N-terminally (Fig. 1A). Since the shorter forms lack the C-terminus and thus important parts of the catalytic site, an autophagic function for the enzymatic activity of BHMT would seem unlikely. The fragments could conceivably act as binding proteins, but the possibility would also have to be considered that some of them might represent selectively sequestered cargo proteins rather than functional components of the autophagic sequestration apparatus.
Figure 1.
Old and new BHMT forms. (A) Amino acid sequences (red) from BHMT forms associated with autophagosomal membranes, previously mapped by MALDI-TOF tryptic fingerprinting.39 Blue lines indicate amino acids thought to be involved in binding of the homocysteine substrate; yellow lines indicate an involvement in betaine binding and green lines indicate participation in catalysis.86 Grey lines indicate sites involved in dimerization (316–349) and tetramerization (381–407).87 (B) A frozen-thawed cytoplasmic extract (postnuclear supernatant) from rat hepatocytes was solubilized in an SDS-containing lysis buffer, fractionated by SDS-PAGE and immunoblotted with an N-terminal BHMT antibody. In addition to full-length BHMT (p45), several BHMT fragments (10–33 kDa) are detected. (C) Separation of BHMT forms by liquid-phase isoelectric focusing. A frozen-thawed cytoplasmic extract (postnuclear supernatant) from rat hepatocytes was fractionated by liquid-phase isoelectric focusing (LP-IEF) on a mini-Rotofor into 20 fractions of different pH values as indicated. Each Rotofor fraction was further fractionated by gel electrophoresis and immunoblotted with the N-terminal BHMT antibody. The figure is a composite of three separately stained blots from two different gels.
To further investigate hepatocellular BHMT forms, including a search for the “missing” N-terminal peptide from the N-truncated 16 and 18 kDa forms shown in Figure 1A, we performed an immunoblotting study using an antibody directed against the N-terminal part of BHMT.46 As shown in Figure 1B, a number of BHMT forms were observed in immunoblots of cytoplasmic extracts (frozen-thawed postnuclear supernatants). In addition to the 45-kDa full-length form (actually banding at 44 kDa, but for clarity subsequently referred to as p45), prominent bands at 30, 27 and 10 kDa and a weak band at 17 kDa were regularly seen and, less consistently, a weak band at 33 kDa (sometimes concealed by excessive p45 staining). It should be noted that the immunodetected p17 form observed here is different from the previously described autophagosomal 16-kDa and 18-kDa BHMT forms, the latter two being N-truncated and thus undetectable by the presently used N-terminal antibody. The immunostained p27 species, on the other hand, could be identical to the 28-kDa BHMT form previously described in reference 39.
Purification of the small N-terminal p10 BHMT fragment.
For further characterization of the various BHMT forms, the proteins were separated and partially purified by a two-dimensional fractionation, using liquid-phase isoelectric focusing (LP-IEF) in the first dimension and polyacrylamide gel electrophoresis (SDS-PAGE) in the second dimension. LP-IEF separates proteins in a pH gradient according to their isoelectric points (pI values) and although the Rotofor (TM) apparatus used here did not produce very sharp focusing, some degree of purification was achieved, particularly of the smallest BHMT species, p10 (Fig. 1C). The size and focusing of this small BHMT fragment in the most basic regions of the pH gradient would suggest that it might represent the N-terminal fragment excised during formation of the previously identified N-truncated 16-kDa and 18-kDa species,39 since such a short fragment would be expected to be a very basic protein, with a theoretical pI of ∼9.7.
Mass spectrometric identification and characterization of BHMT fragments.
Partially purified BHMT fragments were excised from SDS-PAGE gels following LP-IEF fractionation (using fraction no. 20 from a separation like the one shown in Figure 1C; bands being cut directly from primary SDS-PAGE gels were not pure enough for identification) and trypsinized. Final identification was obtained by fingerprinting or sequencing of the tryptic peptides, using both MALDI-TOF MS (matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; Fig. 2C) and ESI-IT-MS/MS (electrospray ionization and ion trap tandem mass spectrometry; Fig. 2D), which complemented each other to achieve a high degree of sequence coverage. For full-length p45 BHMT, the coverage was 89% (Fig. 2A), but neither the N-terminus nor the C-terminus were represented, perhaps reflecting the presence of terminal modifications that precluded mass spectrometric identification.
Figure 2.
Sequencing of long and short BHMT forms by mass spectrometry. Sections corresponding to the BHMT bands p45 and p10 (from LP-IEF fraction no. 20; cf. Fig. 2) were excised from SDS-PAGE gels, minced and trypsinized, and the peptide mixtures were analyzed by ESI-IT-MS/MS or MALDI-TOF MS. (A) p45 sequence deduced from combined data; (B) p10 sequence deduced from combined data. Modified lysine residues marked by asterisk. No N-terminal peptide was recovered from p45 and no C-terminal peptide from p10. (C) ESI-IT spectrum of the peptide underlined in (B); (D), MALDI-TOF spectrum of p10. The masses of the four identifying peptides are indicated; the data provided by the SwissProt database, using the Protein Prospector search software, are shown in facsimile below.
Mass spectrometric analysis of p10 confirmed the assumption that it might represent the cleaved-off N-terminus of BHMT (Fig. 2B). In this fragment, the original N-terminal methionine had been removed, and the new N-terminal alanine was acetylated; in addition three of its lysine residues were modified either by acetylation or trimethylation (these modifications have masses of 42,016 and 42,048 Da, respectively, which could not be reliably distinguished with our present methodology). Two of the lysine modifications were also detected in p45; the third resided in an N-terminal tryptic peptide that could not be recovered from full-length BHMT.
Since no C-terminal peptide was obtained from p10, the cleavage site responsible for generation of this small BHMT fragment could not be identified. Surprisingly, the first “missing” peptide, QLHREFLR, was not found as a tryptic peptide in the p45 trypsinate either, although it is obviously generated; both of the flanking peptides being present (Fig. 2A). Presumably, this fragment is either rapidly degraded or modified in such a way as to escape detection.
In addition to p45 and p10, the identities of p17, p27 and p30 as bona fide BHMT fragments were confirmed by ESI-IT-MS/MS (results not shown), but no structural characterization of these fragments was undertaken.
Secondary BHMT proteolysis and fragment formation in vitro.
BHMT has previously been shown to be prone to proteolytic fragmentation during the preparation of subcellular liver fractions;46 it would, therefore, have to be considered that N-terminal fragments might be secondarily generated or degraded in vitro following disruption and lysis of the cells. One would perhaps expect that lysis in 0.4% SDS would preclude proteolytic activity, but as shown in Figure 3A, leaving SDS-containing hepatocyte lysates to stand for a couple of hours at 0°C resulted in a gradual loss of the larger BHMT fragments p30 and p27 (and, to a lesser extent, p33), and an accumulation of smaller fragments at 10, 15, 17 and 23 kDa. Formation of the p15, p17 and p23 fragments was fully suppressed by inclusion of leupeptin in the lysate, indicating that they were generated by a leupeptin-sensitive cysteine proteinase, probably a cathepsin extracted from the lysosomes. In contrast, the accumulation of p10 was completely leupeptin-resistant, implicating its formation by a different type of enzyme. All of the small fragments could thus be proteolytic in vitro artifacts, formed secondarily after disruption and lysis of the hepatocytes. The responsible proteinases were probably released from lysed lysosomes, since little or no fragment formation could be seen in cell disruptates (where the lysosomes are intact) kept at 0°C for a few hours (results not shown).
Figure 3.
Secondary formation of BHMT fragments by in vitro proteolysis. (A) Hepatocytes were solubilized directly (as whole cells) in an SDS-containing lysis buffer with or without leupeptin (0.3 mM) as indicated. (B–D) Frozen/thawed hepatocyte homogenates were incubated at 0°C for up to 2 h (B) without additions; (C) with a commercial proteinase inhibitor mixture or (D) with the individual components of the mixture. In a separate experiment (last two lanes), the concentration of AEBSF was trebled (to 3 mM) relative to the mixture and the legumain inhibitor, AJN-230, was tested. All samples were finally solubilized in an SDS-containing lysis buffer for 1 h at 0°C in the presence of leupeptin (0.3 mM), subjected to SDS-PAGE and immunoblotted with the N-terminal BHMT antibody. (E) Effect of legumain and legumain inhibitors on in vitro BHMT cleavage. Frozen/thawed hepatocyte homogenates were acidified to pH 5.6 (by the addition of HCl) and incubated for 16 h at 0°C with the addition of partially purified bovine kidney legumain (100 ng/ml), AEBSF (3 mM), MV-026630 (45 µM) or AJN-230 (75 µM) as indicated. The homogenates were then solubilized in SDS and electrophoresed as described above and immunoblotted with the N-terminal BHMT antibody (upper part) as well as with a C-terminal antibody (lower part) to detect both the N-terminal p10 fragment and its C-terminal cleavage partner, p32.
An accumulation of p10 could also be observed in frozen and thawed homogenates maintained at 0°C, whereas little or no formation of p15, p17 or p23 formation took place under these conditions (Fig. 3B). With the inclusion of a commercial proteinase inhibitor cocktail (Calbiochem) in the homogenate, p10 formation was suppressed (Fig. 3C). To identify the mixture component responsible for this inhibition, each component was tested individually. As shown in Figure 3D, neither pepstatin, aprotinin, bestatin or E-64 had any significant effect on the accumulation of p10 BHMT during a 2 h incubation. Elevated p10 levels were seen with leupeptin, suggesting the involvement of a cysteine proteinase in degradation of this fragment. The only indication of a reduced p10 level was seen with the serine proteinase inhibitor, AEBSF and by increasing the concentration of this inhibitor 3-fold relative to the original mixture, a consistent inhibition of p10 formation was obtained (Fig. 3D, 7th and 8th entry).
Several serine proteinase inhibitors have been reported to reduce the catalytic activity of the lysosomal asparaginyl endopeptidase, legumain.41 This prompted us to test the effect of a legumain inhibitor, AJN-230,42 on p10 formation in hepatocyte homogenates. As shown in Figure 3D (bottom entry), AJN-230 suppressed p10 formation effectively. With the addition of partially purified bovine legumain47,48 to the homogenate (and acidifying it to pH 5.6 to obtain optimal catalytic conditions for the enzyme), the accumulation of p10 was stimulated well above control levels (Fig. 3E), showing that legumain was capable of processing hepatocellular BHMT to a p10 fragment. This legumain-dependent p10 formation was completely suppressed by AJN-230; AEBSF and another legumain inhibitor, MV-026630 43 being somewhat less effective.
To further assess the possibility of BHMT cleavage by legumain, we incubated hepatocytic cell corpses49 for 2 h at 37°C with purified recombinant human BHMT. Subsequent mass spectrometric analysis (results not shown) of BHMT-derived tryptic peptides identified the peptide YVLEK (corresponding to YVAEK in the rat), apparently generated by nontryptic cleavage between Asn88 and Tyr89, consistent with the site specificity of legumain.41 This peptide could not be detected if AJN-230 was present during the incubation. An N-terminal YVAEK sequence was previously reported by Ueno et al.46 in a 35-kDa BHMT fragment from autophagic vacuoles, whereas their 32-kDa fragment, shown to accumulate in intact hepatocytes treated with leupeptin50 had the N-terminal sequence KISGQK,46 corresponding to the removal of another four amino acids (compare the sequence in Fig. 2A). Thus, BHMT cleavage by legumain may not generate p32 directly, additional processing apparently being required for its generation (which may conceivably be the case for p10 as well).
Immunoblotting with a C-terminal antibody showed that a 32-kDa BHMT fragment was indeed generated in our acidified homogenates, its formation being promoted by added legumain and suppressed by legumain inhibitors (Fig. 3E, bottom row).
Accumulation of the p10 BHMT fragment in leupeptin-treated hepatocytes.
Although the above results demonstrate that p10 as well as other BHMT fragments may be generated secondarily in hepatocyte lysates by legumain and other proteinases released from lysosomes, these artifacts could be largely eliminated by including a proteinase inhibitor cocktail, fortified by elevating the concentrations of leupeptin (to 0.3 mM) and AEBSF (to 3 mM), in all buffers used following cell disruption. This precaution allowed us to investigate how BHMT is processed in intact hepatocytes, with emphasis on the possible role of legumain. The study of Furuya et al.50 indicated that the C-terminal 32-kDa BHMT fragment accumulated in the lysosomes of leupeptin-treated hepatocytes as a result of autophagy; if legumain is responsible for the formation of both p32 and p10, it would seem likely that p10 might accumulate in intact cells under similar conditions.
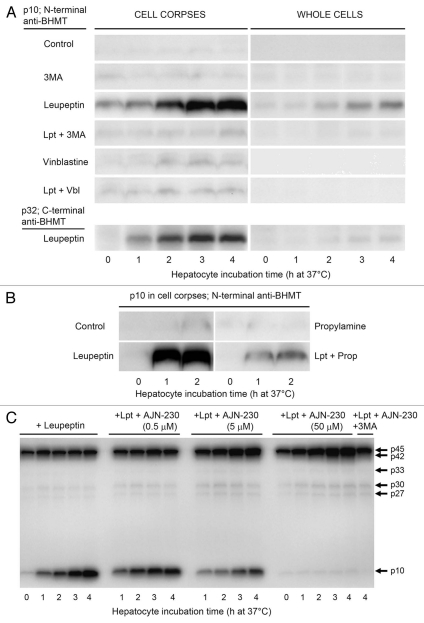
As shown in Figure 4A, hepatocytes incubated with leupeptin (0.3 mM) for up to 4 h at 37°C indeed accumulated massive amounts of the p10 BHMT fragment in the sedimentable cell fraction (cell corpses; third row), whereas virtually no p10 was detectable in control cells (incubated without leupeptin; first row). A parallel leupeptin-induced accumulation of p10s C-terminal sister fragment, p32, could also be observed (Fig. 4A, bottom left part). The accumulation of p10 was largely prevented by the autophagy inhibitor, 3-methyladenine,51 (fourth row), indicating that its formation in intact cells was the result of autophagic-lysosomal BHMT processing. Vinblastine, which blocks autophagic flux at the level of autophagosomes,52,53 did not cause any p10 accumulation on its own (fifth row) and suppressed the leupeptin-induced accumulation (sixth row), consistent with BHMT cleavages taking place after the autophagosome stage, i.e., in proteolytically active autophagic vacuoles.
Figure 4.
Time-dependent accumulation of the p10 BHMT fragment in leupeptin-treated live hepatocytes. (A) Isolated rat hepatocytes were incubated at 37°C for up to 4 h in a suspension buffer containing an energy substrate (pyruvate) only (control) or with the addition of 3-methyladenine (3MA, 10 mM), leupeptin (0.3 mM) or vinblastine (50 µM) alone or in combinations as indicated. The cells were then electrodisrupted and cytosol-free, sedimentable cell corpses prepared. Disruptates (representing whole cells) and cell corpses (containing organelles and the cytoskeleton) were solubilized and immunoblotted with the N-terminal BHMT antibody. Only the p10 fragment is shown. In a separate experiment (bottom parts), immmunoblotting with a C-terminal BHMT antibody was used to demonstrate the leupeptin-induced accumulation of p10s C-terminal sister fragment, p32. (B) Hepatocytes were incubated for up to 2 h at 37°C with leupeptin (Lpt, 0.3 mM) and/or propylamine (Prop, 10 mM) as indicated and cell corpse extracts were immunoblotted with the N-terminal BHMT antibody. Only the p10 fragment is shown. (C) Hepatocytes were incubated for up to 4 h at 37°C with leupeptin (0.3 mM) and various concentrations of AJN-230 as indicated; in the last lane with leupeptin, AJN-230 (50 µM) and 3MA (10 mM). Cell corpse extracts were immunoblotted with the N-terminal BHMT antibody. The whole gel is shown to illustrate the weak bands at 27, 30 and 33 kDa; the last of these will probably not be visible in print.
The weak base propylamine,54 which effectively suppresses autophagic-lysosomal protein degradation by neutralizing acidic proteolytic vacuoles,55 might have been expected to cause accumulation of p10 by preventing its degradation, like leupeptin. However, no p10 accumulation occurred in the presence of propylamine (Fig. 4B, upper right part), probably because this drug abolishes the acidic conditions required for proteolytic generation of p10, as indicated by its leupeptin-antagonistic effect (Fig. 4B, lower right part). If legumain is responsible for p10 generation in intact cells, it would thus seem to exert its effect in an acidic, presumably vacuolar environment.
Although BHMT is primarily a cytosolic enzyme,56 it can also associate with cellular structures,57,58 rendering the “background” of sedimentable large BHMT forms in cell corpses too high to allow reliable measurements of autophagic sequestration rates (transfer from cytosol to sedimentable structures) for BHMT. However, since the p10 background was negligible, a time-dependent accumulation of this small BHMT fragment could be readily detected even in whole cells following leupeptin treatment (Fig. 4A, whole cells, third row). It should be noted that the whole-cell extract in this experiment was diluted 10× relative to the cell corpse extract (to allow immunoblotting of the much more abundant p45 BHMT, not shown), i.e., the sensitivities of the two assays are actually quite similar. Thus, as has been suggested for p32,50 and for a fragment of transfected, genetically engineered BHMT,59 p10 can be used to monitor autophagy in intact hepatocytes.45
Involvement of legumain in BHMT processing in intact cells.
To test the possible involvement of legumain in the autophagic-lysosomal generation of p10 BHMT in intact hepatocytes, the cells were incubated for up to 4 h with various concentrations of the most effective of the tested legumain inhibitors, AJN-230. As shown in Figure 4C (bottom), the time-dependent accumulation of p10 in cell corpses was clearly diminished at 5 µM AJN-230 and completely abolished at 50 µM. At the latter inhibitor concentration, accumulation of a BHMT form with slightly higher mobility in gels than the full-length enzyme could be observed, estimated to 42 kDa. The accumulation of this p42 BHMT was completely suppressed by 3MA (Fig. 4C, last lane), consistent with its autophagic origin. Legumain would thus seem to be responsible for the autophagic-lysosomal generation of the p10 BHMT fragment from a p42-kDa intermediate rather than directly from full-length BHMT.
An increase in the amount of cell corpse-associated full length BHMT (p45) could usually be observed during the first hour of leupeptin treatment (Fig. 4C), but apparently a steady-state level was then reached as p45 became processed. A more prolonged p45 accumulation probably took place in the presence of AJN-230, but could not be clearly distinguished from the accumulating p42. No net accumulation of the large N-terminal BHMT fragments, p27, p30 and p33 could be detected in the presence of leupeptin alone, but at 50 µM AJN-230 some accumulation of p27 and p30 was indicated, suggesting that all of these cytoplasmic BHMT fragments were autophagocytosed and then processed by legumain.
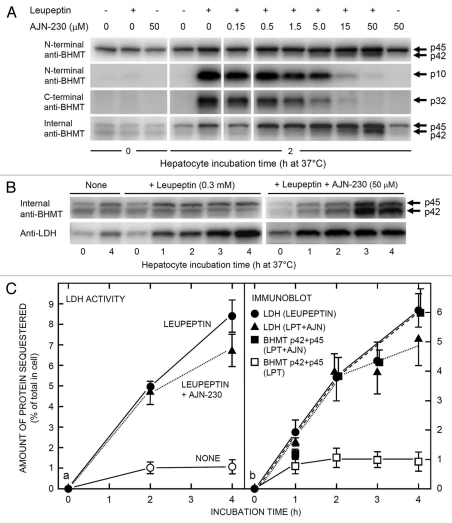
Immunoblotting with the C-terminal BHMT antibody revealed that accumulation of the C-terminal p32 fragment (Fig. 5A, third row) was gradually inhibited by increasing concentrations of AJN-230, in parallel with the decreasing accumulation of p10 (Fig. 5A, second row) and inversely correlated with the increasing p42 accumulation (first row). It is thus likely that p10 and p32 50 are complementary products formed after p42 cleavage by legumain.
Figure 5.
Effect of AJN-230 on autophagic BHMT processing and LDH accumulation in live cells. Rat hepatocytes were incubated at 37°C for up to 4 h in the presence of leupeptin (0.3 mM) and/or AJN-230 at the concentration indicated. The cells were then electrodisrupted and the cell corpses were isolated and solubilized to measure their contents of sedimentable BHMT (by immunoblotting) or lactic dehydrogenase (LDH; as enzymatic activity or by immunoblotting). (A) Inhibition of leupeptin-induced p10 and p32 BHMT formation by the legumain inhibitor, AJN-230. Note the accumulation of a p42 intermediate (upper parts) in parallel with the disappearance of p10 (second part row) and p32 (third part row). In the bottom part row, immunoblotting was done with an internal BHMT antibody that gives a lower background of sedimentable p45. Parts in the same row are from the same gel. (B) Accumulation of large BHMT forms (immunoblotted with the internal BHMT antibody) and LDH in cell corpses during a 4 h incubation of hepatocytes at 37°C in the absence or presence of leupeptin and AJN-230 as indicated. (C) Quantification of LDH accumulation in corpses from cells incubated for 4 h at 37°C without additions (open circles), with leupeptin (LPT) only (0.3 mM, filled circles, whole line) or with leupeptin + AJN-230 (50 µM; filled triangles, dotted line), measured as enzymatic activity in part a or as immunoblotted protein in (b). BHMT accumulation (part b) was measured as the sum of immunoblotted p42 and p45 in cell corpses incubated with leupeptin only (open squares, whole line) or with leupeptin + AJN-230 (filled squares, broken line). Values in part a are the means ± range of two experiments; values in (b) are the means ± SE/range of two to four experiments.
The recent availability of a commercial antibody against the internal region of BHMT prompted us to see if the previously described autophagosome-associated p16 and p18 BHMT forms, which are truncated both N- and C-terminally,39 might be detectable by immunoblotting. Unfortunately, no such intermediate-length fragments could be seen (results not shown); the nature and function of these autophagosomal BHMT forms thus must remain enigmatic. However, as compensation, the novel “internal” antibody detected full-length BHMT (p45) with a much lower cellular background than did the N-terminal antibody, and thus more clearly separated from p42 (Fig. 5A, fourth row). In hepatocytes incubated with both leupeptin and AJN-230, p45 as well as p42 could, therefore, be seen to accumulate with time (Fig. 5B, upper right part). With leupeptin alone (Fig. 5A and B) or AJN-230 alone (Figs. 5A and 6B) no prolonged accumulation of these large BHMT forms occurred (in contrast to cell corpse-associated LDH, included as a control in Fig. 5B, bottom parts), suggesting the involvement of at least two p45/p42 processing mechanisms: the AJN-230-sensitive legumain pathway and a second, leupeptin-sensitive pathway (Fig. 6D).
Figure 6.
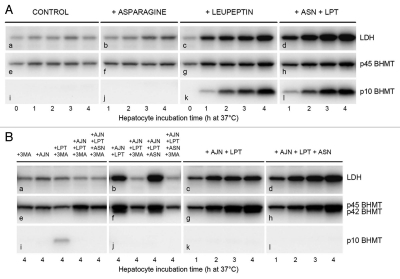
Effects of AJN-230 and autophagic-lysosomal pathway inhibitors on autophagic sequestration and processing of BHMT and LDH in live cells. Hepatocytes were incubated at 37°C for up to 4 h (A and B) or for 2 × 90 min (C) in the presence of asparagine (ASN; 20 mM), leupeptin (LPT; 0.3 mM), 3-methyladenine (3MA; 10 mM), AJN-230 (AJN; 50 µM) or ammonium chloride (NH3; 20 mM) in various combinations. Cell corpse-associated LDH (top rows), BHMT forms (N-terminal antibody; second and third rows), legumain (fourth row) or cathepsin B (bottom row) were measured by immunoblotting. (A) Effect of asparagine on LDH accumulation and p10 fragment generation. Parts in the same row are from the same gel. (B) Effects of AJN-230 and asparagine on LDH accumulation and p42 accumulation. Parts b,f and j represent a different experiment than the other parts. (C) Inhibition of legumain activity by ammonia. Hepatocytes were first incubated for 90 min at 37°C with ammonium chloride, asparagine or leupeptin as indicated, then washed and reincubated for another 90 min at 37°C with ammonium chloride, leupeptin or 3MA as indicated. Samples from two different experiments (0–90 and 90–180, respectively) were applied to the same gel. (D) Schematic summary of autophagic BHMT and LDH processing. The results of the present study indicate that BHMT is processed in amphisomes: An initial modification (e.g., a C-terminal truncation of full-length p45 BHMT by an unknown, leupeptin-resistant proteinase produces a 42 kDa intermediate that is cleaved by legumain to generate an N-terminal 10-kDa and a C-terminal 32-kDa fragment, both being degraded by leupeptin-sensitive cysteine cathepsins. In addition, p45 and p42 can (sequentially or independently) be degraded by a legumain-independent, leupeptin-sensitive pathway. LDH is degraded in amphisomes by leupeptin-sensitive proteolysis and in lysosomes by leupeptin-resistant proteolysis. Both leupeptin and asparagine suppress amphisome-lysosome fusion, whereas vinblastine (VBL) inhibits the fusion of autophagosomes with later autophagic vacuoles. Ammonia (NH3) reversibly inhibits acid proteolysis in general by neutralizing amphisomes and lysosomes; in addition, the neutralization apparently causes irreversible inhibition of legumain.
The use of protein fragments for assaying autophagic activity may be problematic with respect to quantification, because there is no guarantee that a given antibody detects an autophagic fragment and its full-length cytoplasmic precursor (the reference base for quantification) with the same sensitivity. With BHMT, the problem is compounded by the presence of both cytosolic and structure-associated enzyme variants,56–58 generating a high background as well as uncertainty about the identity of the relevant autophagic precursor. With the internal BHMT antibody, the low structure-associated background allowed ready quantification of both p42 and p45 in immunoblots from cell corpses, and their similarity in size would presumably minimize the possibility of differential sensitivity toward the antibody. The quantification (Fig. 5C, part b), performed by summation of the p42 and p45 bands from cell corpses and expressing them as per cent of the corresponding whole-cell p45 value, indicated that BHMT accumulated in cell corpses from hepatocytes treated with leupeptin + AJN-230 at a similar rate as lactate dehydrogenase (LDH), a standard autophagic cargo marker,49 measured by an activity assay (Fig. 5C, part a) or by immunoblotting (Fig. 5C, part b). Full-length BHMT would thus seem to be autophagically sequestered in a nonselective manner by isolated rat hepatocytes. A moderate inhibition of LDH accumulation by AJN-230 may be indicated (Fig. 5C), but the variability, especially in the blot quantification, is too great to warrant any discussion of its possible mechanisms.
Does the processing of BHMT take place in amphisomes or lysosomes?
High concentrations of the amino acid asparagine have been shown to interfere with amphisome-lysosome fusion,10 thus providing an experimental opportunity to address the question of where in the autophagic-lysosomal pathway BHMT processing by legumain may take place. As shown in Figure 6A, treatment of hepatocytes with 20 mM asparagine (ASN) caused a moderate accumulation of autophagically sequestered LDH in cell corpses, detectable after 3–4 h (Fig. 6A, part b). Asparagine acted in concert with leupeptin to achieve a maximal LDH accumulation (Fig. 6A, part d), consistent with an impaired delivery of LDH from amphisomes to lysosomes. However, asparagine had no effect on the leupeptin-induced accumulation of p10 (Fig. 6A, part l vs. part k), indicating that the processing of BHMT by legumain was independent of the rate of amphisome-lysosome fusion, thus probably occurring intra-amphisomally. The presence of legumain in amphisomes would be in accordance with its participation in the proteolytic processing required for class II MHC antigen presentation,60,61 known to take place in immunoamphisomes.8,9,62 It should be noted that p10 accumulation is absolutely dependent on the presence of leupeptin (to prevent its degradation), cf. the total lack of accumulation with asparagine alone (Fig. 6A, part j).
To investigate the subcellular site of the initial cleavage of p45 BHMT (to produce the p42 intermediate), further processing of p42 was blocked by treating hepatocytes with the legumain inhibitor, AJN-230, in addition to asparagine. Figure 6B shows that LDH (Fig. 6B, parts a–d) accumulated well in cell corpses from hepatocytes treated with AJN-230 and leupeptin, in a 3MA-sensitive manner (Fig. 6B, parts a and b) reflecting its autophagic origin. Again, more LDH accumulation (i.e., less degradation) was seen with asparagine (Fig. 6B, part d vs. c; lane 3 vs. lane 1 in b), corroborating its interference with the delivery of LDH to lysosomes. In contrast, the autophagic (3MA-sensitive) accumulation of p42 BHMT observed in the presence of leupeptin + AJN-230 was not affected by asparagine (Fig. 6B, part h vs. g; lane 3 vs. lane 1 in f). These data would be consistent with the initial cleavage of p45 to p42 taking place in amphisomes rather than in lysosomes.
Inhibition of p10 formation by ammonia.
To see if some BHMT processing might also take place in lysosomes, provided amphisomal processing could be bypassed, hepatocytes were pretreated with the acid-neutralizing weak base, ammonia (in the form of 20 mM ammonium chloride), to suppress all proteolytic activity in acidic autophagic-lysosomal compartments.44 Since intravacuolar neutralization has little effect on the autophagic flux to lysosomes,10 an intralysosomal accumulation of BHMT and other autophagocytosed substrates would be expected. By subsequent removal of ammonia (the neutralizing effect of which is rapidly reversible),63 and suppression of new autophagic influx (by 3MA), intralysosomal BHMT processing, if occurring, should be observable.
The effect of ammonia on BHMT processing differed from the effect of leupeptin in that no p10 was generated (Fig. 6C, third row, lane 5 vs. lane 6). Moreover, ammonia prevented the leupeptin-induced p10 accumulation (Fig. 6C, lane 7). Neutralization of pH in the BHMT-processing compartment(s) would thus seem to abolish legumain activity. Strikingly, ammonia-pretreated hepatocytes also failed to generate p10 during a second incubation in the absence of ammonia (but with leupeptin present; Fig. 6C, lane 12), indicating that pretreatment with ammonia inactivated legumain in an irreversible manner. Indeed, immunoblotting with an anti-legumain antibody (giving a single band at ∼36 kDa),41,47 demonstrated a complete disappearance of legumain immunoreactivity in ammonia-treated cells (Fig. 6C, fourth row, lanes 5 and 7), persisting after ammonia withdrawal (Fig. 6C, lanes 10 and 12–14). The rapid loss of enzymatic activity at neutral pH previously observed with purified legumain41 can thus be enforced in intact cells. Long-term (24 h) treatment of cells with the proton pump inhibitor, bafilomycin A1, has been shown to induce a decline (ascribed to protein degradation) in the activities of several lysosomal proteinases, including cathepsin B and legumain.64 However, the ammonia-induced rapid loss of legumain immunoreactivity observed here was not paralleled by reduced cathepsin B immunoreactivity at least for the first 90 min (Fig. 6C, lane 5, last two rows). Whether the selective disappearance of immunoreactive legumain reflects extensive denaturation, proteolytic cleavage (if so, leupeptin-resistant; cf. Fig. 6C, lane 7) or some other mechanism remains to be investigated.
Ammonia did not detectably compromise the cells' autophagic capacity, since LDH accumulated 3MA-sensitively during the second incubation (Fig. 6C, lanes 12 and 13). Preincubation per se (for 90 min without ammonia) was also without any adverse effect on autophagy, as indicated by the formation of p10 during reincubation with leupeptin (Fig. 6C, lanes 11 and 16); completely suppressible by 3MA (Fig. 6C, lane 17). The rapid inactivation of legumain by ammonia-induced vacuole neutralization would thus appear to be a specific effect on this enzyme rather than an indirect consequence of autophagic-lysosomal pathway disturbance.
LDH degradation also seems to occur predominantly in amphisomes.
For comparison with BHMT, the proteolytic processing of LDH, known to accumulate in hepatocytes treated with ammonia or propylamine,10 was measured. As shown in Figure 6C, incubation of hepatocytes with ammonia for 90 min caused an accumulation of cell corpse-associated LDH (Fig. 6C, upper row, lane 5) as expected from an inhibition of intralysosomal protein degradation. With 3MA present during the second 90 min incubation to stop new autophagic influx, the pre-accumulated LDH largely disappeared (Fig. 6C, lane 14). Surprisingly, leupeptin failed to prevent this disappearance (Fig. 6C, lane 13), indicating that in the lysosomes, LDH was degraded by leupeptin-resistant proteinase(s). Since LDH nevertheless accumulates continuously in the presence of leupeptin (Fig. 5A, part c), the latter accumulation must take place in amphisomes, the proteinase inhibitor apparently preventing the delivery of LDH from amphisomes to lysosomes as previously demonstrated.5 Such fusion blockade alone is not sufficient to cause extensive LDH accumulation—cf. the moderate effect of asparagine (Fig. 6A, part b)—implicating that leupeptin also prevents intra-amphisomal LDH degradation. The accumulation of LDH with leupeptin alone during the second incubation after ammonia treatment (Fig. 6A, lanes 11, 12 and 16) would seem to represent new autophagic sequestration, as indicated by its 3MA-sensitivity (Fig. 6A, lanes 13 and 17).
Discussion
A large number of methods are currently available for measuring “autophagy” in a general sense,65,66 mostly based on the detection and quantification (by confocal fluorescence microscopy or immunoblotting) of proteins functionally involved in autophagic processes; often without proper distinction between the three major levels of autophagy (expression of autophagy genes, phagophore assembly and autophagic sequestration activity) or between general macroautophagy and the various modes of selective autophagy (aggrephagy, mitophagy, pexophagy, reticulophagy, ribophagy, etc.).67 However, for a quantitative assay of macroautophagic activity (i.e., bulk sequestration of cytoplasm) there is really no alternative to a cargo assay, using a cytoplasmic probe that is sequestered by phagophores and enclosed in autophagosomes in a nonselective manner.45,65
The standard autophagy assay used in the authors' laboratory is based on the transfer of a soluble cargo marker (routinely, endogenous LDH) from the cytosol to the sedimentable autophagic vacuoles (AVs, i.e., autophagosomes, amphisomes and lysosomes) of rat hepatocytes incubated in the presence of leupeptin to block intravacuolar LDH degradation.49 The AVs are conveniently separated from the cytosol by sedimentation as cell corpses, prepared by a single-step electrodisruption of the plasma membrane. Although only a small fraction of the cellular LDH becomes sequestered even under maximally autophagic conditions (typically 2–3%/h), it can readily be detected in the cytosol-free cell corpses, but not in cytosol-filled whole cells.
The p10 BHMT fragment discovered in the present study, on the other hand, is generated inside amphisomes and will accumulate there in the presence of leupeptin. Since the fragment is absent from the cytosol, its accumulation can be detected (by immunoblotting) even in whole cells. For p10 to serve as a quantitative cargo marker of macroautophagy, its cytosolic precursor (full-length p45 BHMT) should be autophagically sequestered in a nonselective manner, which appears to be the case according to the results presented above. Although the background of non-cytosolic (structure-associated) p45 in cell corpses was too high to allow reliable sequestration measurements with the N-terminal BHMT antibody routinely used for p10 staining, an “internal” BHMT antibody gave (for reasons unknown) a much lower background, allowing quantification of the autophagically sequestered fraction of p45. Both the legumain inhibitor, AJN-230 and the cysteine proteinase inhibitor, leupeptin, were required to minimize autophagic-lysosomal p45 degradation, but they could not prevent a certain degree of processing to a p42 intermediate. It was, however, assumed that p45 and p42 were sufficiently similar to react equally well with the antibody, and that the sum of these two quantified BHMT species would provide a reasonable measure of the autophagic sequestration rate (as percent of the total cellular amount of p45). The fact that the calculated rate of BHMT sequestration (∼2%/h) during 2 h of hepatocyte incubation was identical to that found for immunoblotted LDH (which is representative of several cytosolic enzymes),49 would suggest that BHMT was autophagically sequestered in a nonselective manner.
The sequestration rate calculated for the p10 fragment (as percent of total cellular p45) was also comparable to the rate of LDH sequestration,45 indicating that the immunoreactivity of the fragment was sufficiently similar to that of full-length BHMT to make it acceptable as a quantitative marker of macroautophagic activity. Although natural BHMT expression is confined to liver and kidney tissue in most species,68,69 the possibilities of ectopic expression offered by current molecular-genetic methods59 may give the p10 BHMT whole-cell immunoblotting assay more general applicability. It should also be pointed out that since autophagocytosed proteins are, in general, processed by autophagic-lysosomal endoproteinases through a series of proteolytic fragments, it should not be difficult to find suitable peptide candidates for a background-free “fragment assay” of autophagy in any cell type.
The autophagic-lysosomal processing of BHMT is apparently complex. Although formation of the sister fragments p10 and p32 following legumain cleavage is clearly the major processing pathway, p45 is still degraded by leupeptin-sensitive proteolysis even if legumain is completely inhibited by AJN-230 (Fig. 6D). Furthermore, the intermediate species p42 is generated when leupeptin and AJN-230 are both present, presumably by a third proteolytic mechanism. Fourthly, p10 as well as p32 are further processed by unidentified leupeptin-sensitive proteinases. Remarkably, all of these proteolytic events appear to take place in amphisomes, as indicated by their refractoriness toward the amphisome-lysosome fusion inhibitor, asparagine. It is known that “late endosomes,” which would include the amphisomes, are well equipped with proteinases presumably to some extent en route to the lysosomes,7,70 but both legumain and leupeptin-sensitive cathepsins also perform important late endosomal/amphisomal functions in the processing of antigens and lysosomal enzymes.60–62,64,71,72 The functional division of labor between amphisomes and lysosomes has not been clarified, but it would not seem unreasonable to assume that limited proteolytic cleavages in the amphisomes generate peptides that may subsequently become more completely degraded in the lysosomes.
Neutralization of hepatocytic amphisomes and lysosomes by ammonia (administered to cells as ammonium chloride) stops essentially all of their proteolytic activity73 while allowing autophagic flux to proceed all the way to the lysosome.10 Lysosomes can thus be preloaded with autophagocytosed material, the degradation of which can subsequently be followed after withdrawal of ammonia (whose effect on intravacuolar pH is rapidly reversible), in the presence of 3MA to stop additional autophagic influx.10 The intralysosomal degradation of preaccumulated LDH that could be observed under these conditions was, remarkably, not inhibited by leupeptin (Fig. 6D). This means that like the degradation of BHMT, all leupeptin-sensitive LDH degradation (and hence leupeptin-induced LDH accumulation), takes place in amphisomes rather than, as previously assumed,49 in lysosomes. Unlike BHMT, some LDH apparently survives the traverse through the amphisome to reach the lysosome, as indicated by the additional protection offered by blocking amphisome-lysosome fusion with asparagine.
The emerging role of the amphisome as a major site of autophagic-lysosomal proteolytic processing is surprising, considering that the amphisome was originally discovered as a prelysosomal compartment unable to cleave the autophagocytosed disaccharide, lactose.4 Presumably, activity of the β-galactosidase responsible for lactose hydrolysis is confined to the lysosome, which would be in accordance with its strongly acidic pH optimum.10,74 The major leupeptin-sensitive cathepsins B, H and L, on the other hand, exhibit high activity under slightly acidic conditions,75,76 and are thus well suited for a predominantly amphisomal function.
The lysosome-preloading experiment was primarily designed to see if legumain-dependent BHMT processing could take place in lysosomes, but the rapid, irreversible inhibition of legumain upon vacuole neutralization precluded an answer to this question, instead pointing in a different direction. Legumain has previously been shown to inactivate gradually under neutral conditions in vitro,41 and the rapid disappearance of legumain immunoreactivity in ammonia-treated hepatocytes would be consistent with an irreversible conformational alteration. No change was seen in the immunoreactivity of cathepsin B after 90 min of ammonia exposure, but a small decline could be detected after another 90 min. Since this decline required the continuous presence of ammonia, it is unlikely to reflect a secondary loss of legumain-dependent cathepsin B processing.
The availability of several methods for the selective inactivation of legumain may be useful not only experimentally, but possibly also in a therapeutic context. Legumain is known to be involved in the proteolytic generation of antigenic peptides in immunoamphisomes,8,9,60–62 suggesting that a specific inhibition of this enzyme by AJN-230 or a vacuole-neutralizing, “lysosomotropic” drug might offer protection against certain autoimmune disorders. Both ammonia and chloroquine have in fact been shown to interfere with antigen presentation,77,78 and chloroquine has even found some use in the treatment of rheumatic disease.79 The ability of chloroquine to suppress the metastatic spread of cancer cells80,81 may also conceivably be due to legumain inhibition, cancer metastasis having been shown to be promoted by exocytosed active legumain.48 It would obviously be of interest to investigate how a more specific legumain inhibitor such as AJN-230 might affect these and other pathological processes.
Materials and Methods
Antibodies and reagents.
The α-p44-10R polyclonal antibody against the 10 N-terminal amino acids of BHMT was a kind gift from Drs. T. Ueno and E. Kominami (Tokyo, Japan),46 and the antibody against cathepsin B was a gift from Dr. D. Buttle (Sheffield, UK). The C-terminal BHMT antibody (EB07943) was purchased from Everest Biotech; the “internal” BHMT antibody (AV41474) from Sigma-Aldrich and a goat antibody (ab2101) against LDH (lactate dehydrogenase) from Abcam. The legumain antibody (SC67163) and HRP-conjugated anti-goat IgG (SC2020) were from Santa Cruz Biotechnology; anti-rabbit IgG (cst7074) from Cell Signaling Technologies and human recombinant BHMT (ENZ-292) from ProSpec. Modified sequence-grade trypsin (V511A) was obtained from Promega; PVDF membranes (0.2 µm) for immunoblotting and the Immobilon protein gel blotting detection kit were from Millipore. Molecular weight markers were either RPN 756 from Amersham Biosciences or Novex Sharp LC5800 from Invitrogen; dry milk powder was from Tine (Oslo, Norway). Partially purified rat kidney legumain (asparaginyl endopeptidase) was prepared as described in reference 47 and 48. The legumain inhibitors AJN-230 (CBZ-Ala-Ala-AzaAsn-CMK; 465 Da),42 and MV-026630 (benzyloxycarbonylamino-4-carbamoyl-2-oxo-butyl ester; 412.5 Da),43 were kindly donated by Andre J. Niestroj (Probiodrug AG, Halle, Germany) and Colin Watts (Dundee, UK), respectively.
Acetic acid (glacial; 1.00063), acetone (1.00014), acetonitrile (1.00030), dipotassium hydrogen phosphate trihydrate (1.05099), disodium hydrogen orthophosphate dihydrate (1.06580), formic acid (1.00264), hydrochloric acid (1.09911), methanol (1.06009), silver nitrate (1.01512), sodium acetate (1.06268), sodium carbonate (1.06392), sodium chloride (1.06404), sodium dihydrogen phosphate monohydrate (1.06346), sodium hydroxide (1.09913), sodium thiosulfate pentahydrate (1.06509) and urea (1.08488) were purchased from Merck KGaA. Bio-Lyt (163-1112), sodium dodecyl sulfate (SDS; 161-0302), acrylamide and bisacrylamide (161-0158) were from BioRad; calcium chloride dehydrate (31307), magnesium chloride hexahydrate (31413) and sodium phosphate dibasic (30427) from Riedel-de-Haën; perchloric acid (20589.247), potassium chloride (26764.298), sodium dihydrogen phosphate dihydrate (28014.291) and sucrose (27480.294) from Prolabo; dimethylfluoride (D4451), formic acid and sodium pyruvate from Fluka, ammonium persulfate (13375) from Serva and Nycodenz (1002424) from Axis-Shield. Leupeptin (4041) was obtained from the Peptide Institute; aprotinin (A2132), bestatin-HCl (A2137), E-64 (A2157) and pepstatin A (A2205) from AppliChem; okadaic acid (ALX-350-010) from Alexis and the protease inhibitor cocktail set III (539134) from Calbiochem. 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF; Pefabloc; 76307), ammonium bicarbonate (A6141), ammonium chloride (A4514), l-asparagine monohydrate (A8381), bromophenol blue (B5525), CAPS (C2632), CHAPS (C9426), α-cyano-4-hydroxycinnamic acid (C2020), dimethylsulfoxide (41640), DL-dithiothreitol (D9163), EDTA (ED3SS), formaldehyde (F1635), glycerol (G5150), glycine (G7126), HEPES (H3375), 3-methyladenine (M9281), β-nicotinamide adenine dinucleotide (NAD; N8129), potassium phosphate monobasic (P0662), propylamine (240958), sodium deoxycholate monohydrate (D5670), sodium fluoride (S6521), sodium orthovanadate (S6508), sodium pyrophosphate (S6422), sodium sulfate (S9627), TEMED (T9281), TES (T1375), thiourea (T8656), trifluoroacetic acid (T6508), tricine (T0377), Trizma base (T1503), Tween 20 (P1379) and vinblastine (V1377) were from Sigma-Aldrich.
Animals and cells.
Hepatocytes were isolated from 18 h starved male Wistar rats (250–300 g; Harlan UK Ltd.) by 2-step collagenase perfusion and purified as previously described in reference 82. Hepatocyte suspensions were routinely maintained at 0°C in suspension buffer82 containing extra Mg2+ (2 mM) and 15 mM pyruvate. For incubation as intact cells, 2-ml volumes of cell suspension (∼20 mg wet mass) were incubated in 6-cm stationary suspension dishes (Sarstedt) at 37°C, with gentle shaking each hour to maintain adequate oxygenation. Incubations were ended by rapid cooling to 0°C.
Cell disruption, homogenization and lysis.
For whole-cell lysis, hepatocytes were washed twice with ice-cold, isotonic phosphate-buffered NaCl solution (150 mM NaCl, 9.7 mM Na2HPO4, 1.4 mM NaH2PO4, pH 7.4) and lysed in 0.7 ml SDS-containing lysis buffer (1% SDS, 5 mM EDTA, 5 mM EGTA, 10 mM sodium pyrophosphate, 20 mM Tris, pH 7.2) by incubation for 1 h at 0°C, followed by heating at 95°C for 5 min. As a result of the present studies, proteinase inhibitors are now routinely included in the lysis buffer: 0.3 mM leupeptin, 10 µM pepstatin A, 15 µM E-64, 50 µM bestatin, 0.8 µM aprotinin and 3 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF). For electrodisruption, hepatocytes were washed twice in electrolyte-free, isotonic (10%) sucrose and 2.4 ml of suspended cells (six pooled 400-µl samples) were electrodisrupted83 at 0°C by a single high-voltage pulse (2 kV; 0.6 µF). The disruptate was either used as such after dilution and buffering, homogenized,53 or separated into a cell corpse fraction (containing all cellular organelles and cytoskeletal elements) and a cell sap preparation (essentially cytosol, but prepared without prior homogenization) by centrifugation (10,000 g for 15 min at 4°C) above a dense cushion as previously described in reference 49, except that Metrizamide was replaced by Nycodenz.53 The various preparations were frozen (in liquid nitrogen at −190°C) and thawed (to 0°C on a 37°C-water bath) and subsequently incubated at 0°C.
All preparations were adjusted to equivalent volumes (the volume before fractionation) and buffer composition (10% sucrose, 10 mM HEPES, 1 mM EDTA, pH 7.3). Incubations at 0°C were stopped at a given time by mixing 400 µl of the incubate with 133 µl of a 4× concentrated SDS-containing lysis buffer with leupeptin (0.3 mM final conc.). The standard lysis period of 1 h at 0°C was terminated by heating the samples for 5 min at 95°C. In some experiments, cells were dissolved directly in lysis buffer and incubated for various periods of time at 0°C.
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting was performed essentially as previously outlined.39,84 Primary antibodies were diluted in Tris-buffered saline (25 mM Tris, 0.8% NaCl, pH 7.4) with 0.1% Tween 20. For blotting with the N-terminal BHMT antibody α-p44-10R,46 we initially used a 1:40,000 dilution for blotting in the range 10–37 kDa and 1:500,000 for blotting above 37 kDa due to the large amounts of full-length BHMT in whole-cell extracts; in later studies, the whole-cell extracts have routinely been diluted 10× (to 2 µg protein/lane) relative to cell corpse extracts (20 µg protein/lane) and a uniform 1:40,000 dilution has been applied to the whole blot. The C-terminal and internal BHMT antibodies were diluted 1:5,000 and 1:750, respectively, and the LDH antibody was diluted 1:1,000 in the presence of 1% dry milk.
Image analysis and quantification.
Immunoblots were recorded with a Syngene ChemiGenius camera and presented by the GeneSnap software tool (Syngene); the resulting SGD files were converted to TIF files for image presentation. Images from different gels (adjusted to comparable contrast/brightness) or from non-adjacent areas of the same gel have been separated by lines or clear spaces when presented in the same figure. Quantifications were always performed on the primary SGD files to preserve maximal information, using the GeneTools 4.01 program (Syngene) in “lowest slope” mode.
Protein measurement.
Total protein was measured by the bicinchoninic acid (BCA) method,85 using a kit (23225) from Pierce. Absorbance was read on an iEMS Reader MF spectrophotometer (Labsystems Ltd.) at 540 nm wavelength.
Liquid-phase isoelectric focusing (LP-IEF).
A frozen-thawed cytoplasmic hepatocellular extract (postnuclear supernatant),51 was solubilized in 0.2% sodium deoxycholate (D°C) for 30 min at 0°C, then precipitated with 6% perchloric acid (PCA) at 0°C overnight. After centrifugation for 10 min at 5,000 g, the pellet was washed twice with ice-cold 25% acetone and dried at room temperature. The dried pellet was lysed in a buffer containing 7 M urea, 2 M thiourea, 3% CHAPS, 20 mM dithiothreitol, 5% ampholytes (Bio-Lyt 3-10) and inhibitors of proteinases (20 µM leupeptin, 10 µM pepstatin A, 15 µM E-64, 50 µM bestatin, 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.8 µM aprotinin) and of protein phosphatases (50 nM okadaic acid, 1 mM sodium orthovanadate, 2 mM sodium fluoride). The lysate (19 ml; ∼100 mg protein) was loaded into a mini-Rotofor apparatus (Bio-Rad) and electrofocused at 20°C for 5 h at 12 W. Twenty fractions (∼0.45 ml each) were collected, pH was measured in each fraction and a 50-µl aliquot was neutralized (with NaOH or HCl) and analyzed by immunoblotting with the N-terminal BHMT antibody.
Ion trap mass spectrometry.
Slices corresponding to the position of immunoblotted BHMT fragment bands were excised from silver-stained, blotted or (parallel) unblotted SDS-PAGE gels with a sterile scalpel. The minced gel pieces were subjected to tryptic proteolysis as previously described in reference 39. Lyophilised peptides were dissolved in 10 µl 5% acetonitrile (with 0.1% formic acid) and sequenced, identified and characterized by electrospray ionization and ion trap tandem mass spectrometry (ESI-IT-MS/MS). Duplicate samples were analyzed using a sample injection volume of 2 µl and a gradient increasing from 5% to 80% acetonitrile (with 0.1% formic acid) in 15 min. The ion trap was an Agilent MSD XCT instrument equipped with an HPLC Chip electrospray interface to an Agilent 1100-series nano-LC chromatograph. A Sorbax ZX Agilent Chip with a 40 nL C18 column (75 µm × 43 mm) was used for peptide separation. The ion trap was used in standard enhanced (8,100 m/z/s) positive mode with a scanning range of 300–1,800 m/z for MS1 and 100–2,200 m/z for MS2, performing 5 precursor scans/s with active exclusion after two spectra. The counter-electrode voltage was set at 1,850 V and the ion current count at 400,000 with no rolling average.
The completed data sets were analyzed with the Spectrum Mill program (Agilent), using 13 as the summed spectrum peptide score threshold for proteins and 10 for individual peptides with a spectral peak intensity percentage above 70. Database searches were performed through Swissprot (build 70.2; http://expasy.org).
MALDI-TOF mass spectrometry.
MALDI-TOF mass spectrometry was performed as previously detailed in reference 39, for identification of BHMT fragments by tryptic fingerprinting, and for mass-based structural characterization of the tryptic peptides.
Acknowledgements
This work has been generously supported by The Norwegian Cancer Society and by the South-Eastern Norway Regional Health Authority. The excellent technical assistance provided by Berit Elin Nedrebø and Abir Ahmad is gratefully acknowledged.
Abbreviations
- ACN
acetonitrile
- AEBSF
4-(2-aminoethyl)benzenesulfonyl fluoride
- AJN-230
carbobenzyloxy-Ala-Ala-AzaAsnchloromethylketone
- BHMT
betaine:homocysteine methyltransferase
- CAPS
N-cyclohexyl-3-aminopropanesulfonic acid
- CHAPS
3-[(3-cholamidopropyl) dimethyl-ammonio]-1-propanesulfonate
- DOC
deoxycholate
- ESI
electrospray ionisation
- IT
ion trap
- LC3
microtubule-associated protein 1 light chain 3
- LDH
lactate dehydrogenase
- LP-IEF
liquid-phase isoelectric focusing
- MALDI-TOF
matrix-assisted laser desorption/ionisation time-of flight
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- MV-026630
benzyloxycarbonylamino-4-carbamoyl-2-oxo-butyl ester
- PAS
phagophore assembly site
- SDS
sodium dodecyl sulfate
- SDS-PAGE
SDS- polyacrylamide gel electrophoresis
- TEMED
N,N,N′,N′-tetramethylethylenediamine
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mortimore GE, Hutson NJ, Surmacz CA. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci USA. 1983;80:2179–2183. doi: 10.1073/pnas.80.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seglen PO. Regulation of autophagic protein degradation in isolated liver cells. In: Glaumann H, Ballard FJ, editors. Lysosomes: Their Role in Protein Breakdown. London: Academic Press; 1987. pp. 369–414. [Google Scholar]
- 3.de Duve C. The lysosome. Sci Am. 1963;208:64–72. doi: 10.1038/scientificamerican0563-64. [DOI] [PubMed] [Google Scholar]
- 4.Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun. 1988;151:40–47. doi: 10.1016/0006-291X(88)90556-6. [DOI] [PubMed] [Google Scholar]
- 5.Berg TO, Fengsrud M, Strømhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes: Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 6.Seglen PO. Where endocytosis and autophagy meet: The amphisome. In: Sporstøl Fønhus M, Mousavi SA, Berg T, editors. Hepatic Endocytosis. Trivandrum, India: Transworld Research Network; 2008. pp. 61–100. [Google Scholar]
- 7.Berg T, Gjøen T, Bakke O. Physiological functions of endosomal proteolysis. Biochem J. 1995;307:313–326. doi: 10.1042/bj3070313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Müller M, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid D, Münz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høyvik H, Gordon PB, Berg TO, Strømhaug PE, Seglen PO. Inhibition of autophagic-lysosomal delivery and autophagic lactolysis by asparagine. J Cell Biol. 1991;113:1305–1312. doi: 10.1083/jcb.113.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fengsrud M, Lunde Sneve M, Øverbye A, Seglen PO. Structural aspects of mammalian autophagy. In: Klionsky DJ, editor. Autophagy. Georgetown, TX: Landes Bioscience; 2004. pp. 11–25. [Google Scholar]
- 12.Chiacchiera F, Simone C. Signal-dependent control of autophagy-related gene expression. Methods Enzymol. 2009;453:305–324. doi: 10.1016/S0076-6879(08)04015-9. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, et al. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol. 2001;21:4347–4368. doi: 10.1128/MCB.21.13.4347-68.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Huang WP, Stromhaug PE, Klionsky DJ. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J Biol Chem. 2002;277:763–773. doi: 10.1074/jbc.M109134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K, Ohsumi Y. Current knowledge of the pre-autophagosomal structure (PAS) FEBS Lett. 2010;584:1280–1286. doi: 10.1016/j.febslet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polson HEJ, de Lartigue J, Rigden DJ, Reedijk M, Urbé S, Clague MJ, et al. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–522. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- 19.Kovács AL, Seglen PO. Inhibition of hepatocytic protein degradation by methylaminopurines and inhibitors of protein synthesis. Biochim Biophys Acta. 1981;676:213–220. doi: 10.1016/0304-4165(81)90189-6. [DOI] [PubMed] [Google Scholar]
- 20.Gordon PB, Tolleshaug H, Seglen PO. Use of digitonin extraction to distinguish between autophagic-lysosomal sequestration and mitochondrial uptake of [14C] sucrose in hepatocytes. Biochem J. 1985;232:773–780. doi: 10.1042/bj2320773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto A, Simonsen A. The elimination of accumulated and aggregated proteins: A role for aggrephagy in neurodegeneration. Neurobiol Dis. 2011;43:17–28. doi: 10.1016/j.nbd.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filimonenko M, Isakson P, Finley KD, Anderson M, Jeong H, Melia TJ, et al. The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Mol Cell. 2010;38:265–279. doi: 10.1016/j.molcel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 25.Luiken JJFP, van den Berg M, Heikoop JC, Meijer AJ. Autophagic degradation of peroxisomes in isolated rat hepatocytes. FEBS Lett. 1992;304:93–97. doi: 10.1016/0014-5793(92)80596-9. [DOI] [PubMed] [Google Scholar]
- 26.Tuttle DL, Lewin AS, Dunn WA. Selective autophagy of peroxisomes in methylotrophic yeasts. Eur J Cell Biol. 1993;60:283–290. [PubMed] [Google Scholar]
- 27.Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J Biol Chem. 1996;271:17621–17624. doi: 10.1074/jbc.271.30.17621. [DOI] [PubMed] [Google Scholar]
- 28.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 29.Kissová I, Deffieu M, Manon S, Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem. 2004;279:39068–39074. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 30.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:423–424. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol. 2008;10:602–610. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- 32.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onodera J, Ohsumi Y. Ald6p is a preferred target for autophagy in yeast, Saccharomyces cerevisiae. J Biol Chem. 2004;279:16071–16076. doi: 10.1074/jbc.M312706200. [DOI] [PubMed] [Google Scholar]
- 34.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan P, Qing G, Qu Z, Wu CC, Rabson A, Xiao G. Targeting autophagic regulation of NFκB in HTLV-I transformed cells by geldanamycin: Implications for therapeutic interventions. Autophagy. 2007;3:600–603. doi: 10.4161/auto.4761. [DOI] [PubMed] [Google Scholar]
- 37.Kageyama T, Suzuki K, Ohsumi Y. Lap3 is a selective target of autophagy in yeast, Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2009;378:551–557. doi: 10.1016/j.bbrc.2008.11.084. [DOI] [PubMed] [Google Scholar]
- 38.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss JC, Kahle PJ, et al. PINK I/Parkin-mediated mitophagy is dependent on VDAC and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 39.Øverbye A, Fengsrud M, Seglen PO. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy. 2007;3:300–322. doi: 10.4161/auto.3910. [DOI] [PubMed] [Google Scholar]
- 40.Øverbye A, Seglen PO. Phosphorylated and nonphosphorylated forms of catechol-O-methyltransferase in rat liver, brain and other tissues. Biochem J. 2009;417:535–545. doi: 10.1042/BJ20081284. [DOI] [PubMed] [Google Scholar]
- 41.Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, et al. Cloning, isolation and characterization of mammalian legumain, an asparginyl endopeptidase. J Biol Chem. 1997;272:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- 42.Niestroj AJ, Feussner K, Heiser U, Dando PM, Barrett A, Gerhartz B, et al. Inhibition of mammalian legumain by Michael acceptors and AzaAsn-halomethylketones. Biol Chem. 2002;383:1205–1214. doi: 10.1515/BC.2002.133. [DOI] [PubMed] [Google Scholar]
- 43.Loak K, Li DN, Manoury B, Billson J, Morton F, Hewitt E, et al. Novel cell-permeable acyloxymethylketone inhibitors of asparaginyl endopeptidase. Biol Chem. 2003;384:1239–1246. doi: 10.1515/BC.2003.136. [DOI] [PubMed] [Google Scholar]
- 44.Seglen PO, Reith A. Ammonia inhibition of protein degradation in isolated rat hepatocytes: Quantitative ultrastructural alterations in the lysosomal system. Exp Cell Res. 1976;100:276–280. doi: 10.1016/0014-4827(76)90148-8. [DOI] [PubMed] [Google Scholar]
- 45.Seglen PO, Øverbye A, Satre F. Sequestration assays for mammalian autophagy. Methods Enzymol. 2009;452:63–83. doi: 10.1016/S0076-6879(08)03605-7. [DOI] [PubMed] [Google Scholar]
- 46.Ueno T, Ishidoh K, Mineki R, Tanida I, Murayama K, Kadowaki M, et al. Autolysosomal membrane-associated betaine homocysteine methyltransferase: Limited degradation fragment of a sequestered cytosolic enzyme monitoring autophagy. J Biol Chem. 1999;274:15222–15229. doi: 10.1074/jbc.274.21.15222. [DOI] [PubMed] [Google Scholar]
- 47.Yamane T, Takeuchi K, Yamamoto Y, Li YH, Fujiwara M, Nishi K, et al. Legumain from bovine kidney: Its purification, molecular cloning, immunohistochemical localization and degradation of annexin II and vitamin D-binding protein. Biochim Biophys Acta. 2002;1596:108–120. doi: 10.1016/S0167-4838(02)00209-1. [DOI] [PubMed] [Google Scholar]
- 48.Briggs JJ, Haugen MH, Johansen HT, Riker AI, Abrahamson M, Fodstad Ø, et al. Cystatin E/M suppresses legumain activity and invasion of human melanoma. BMC Cancer. 2010;10:17. doi: 10.1186/1471-2407-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopitz J, Kisen GØ, Gordon PB, Bohley P, Seglen PO. Non-selective autophagy of cytosolic enzymes in isolated rat hepatocytes. J Cell Biol. 1990;111:941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuya N, Kanazawa T, Fujimura S, Ueno T, Kominami E, Kadowaki M. Leupeptin-induced appearance of partial fragment of betaine homocysteine methyltransferase during autophagic maturation in rat hepatocytes. J Biochem. 2001;129:313–320. doi: 10.1093/oxfordjournals.jbchem.a002859. [DOI] [PubMed] [Google Scholar]
- 51.Seglen PO, Gordon PB. 3-Methyladenine: Specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovács AL, Reith A, Seglen PO. Accumulation of autophagosomes after inhibition of hepatocytic protein degradation by vinblastine, leupeptin or a lysosomotropic amine. Exp Cell Res. 1982;137:191–201. doi: 10.1016/0014-4827(82)90020-9. [DOI] [PubMed] [Google Scholar]
- 53.Strømhaug PE, Berg TO, Fengsrud M, Seglen PO. Purification and characterization of autophagosomes from rat hepatocytes. Biochem J. 1998;335:217–224. doi: 10.1042/bj3350217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seglen PO, Gordon PB. Effects of lysosomotropic monoamines, diamines, amino alcohols and other amino compounds on protein degradation and protein synthesis in isolated rat hepatocytes. Mol Pharmacol. 1980;18:468–475. [PubMed] [Google Scholar]
- 55.Seglen PO. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/S0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- 56.McKeever MP, Weir DG, Molloy A, Scott JM. Betaine-homocysteine methyltransferase: Organ distribution in man, pig and rat and subcellular distribution in the rat. Clin Sci. 1991;81:551–556. doi: 10.1042/cs0810551. [DOI] [PubMed] [Google Scholar]
- 57.Sandu C, Nick P, Hess D, Schiltz E, Garrow TA, Brandsch R. Association of betaine-homocysteine S-methyltransferase with microtubules. Biol Chem. 2000;381:619–622. doi: 10.1515/BC.2000.080. [DOI] [PubMed] [Google Scholar]
- 58.Sehayek E, Wang R, Ono JG, Zinchuk VS, Duncan EM, Shefer S, et al. Localization of the PE methylation pathway and SR-BI to the canalicular membrane: Evidence for apical PC biosynthesis that may promote biliary excretion of phospholipid and cholesterol. J Lipid Res. 2003;44:1605–1613. doi: 10.1194/jlr.M200488-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Mercer CA, Kaliappan A, Dennis PB. Macroautophagy-dependent, intralysosomal cleavage of a betaine homocysteine methyltransferase fusion protein requires stable multimerization. Autophagy. 2008;4:185–194. doi: 10.4161/auto.5275. [DOI] [PubMed] [Google Scholar]
- 60.Manoury B, Hewitt EW, Morrice N, Dand PM, Barrett AJ, Watts C. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 1998;396:695–699. doi: 10.1038/25379. [DOI] [PubMed] [Google Scholar]
- 61.Matthews SP, Werber I, Deussing J, Peters C, Reinheckel T, Watts C. Distinct protease requirements for antigen presentation in vitro and in vivo. J Immunol. 2010;184:2423–2431. doi: 10.4049/jimmunol.0901486. [DOI] [PubMed] [Google Scholar]
- 62.Blanchet FP, Moris A, Nicolic DS, Lehman M, Cardinaud S, Stalder R, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lecaille F, Muno D, Kominami E, Ishidoh K. Proteinases participating in the processing and activation of prolegumain in primary cultured rat macrophages. Biol Chem. 2004;385:511–516. doi: 10.1515/BC.2004.060. [DOI] [PubMed] [Google Scholar]
- 65.Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3:181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- 66.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klionsky DJ, Cuervo AM, Dunn WA, Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy. 2007;3:413–416. doi: 10.4161/auto.4377. [DOI] [PubMed] [Google Scholar]
- 68.McKeever MP, Weir DG, Molloy A, Scott JM. Betaine-homocysteine methyltransferase: organ distribution in man, pig and rat and subcellular distribution in the rat. Clin Sci (Lond) 1991;81:551–556. doi: 10.1042/cs0810551. [DOI] [PubMed] [Google Scholar]
- 69.Delgado-Reyes CV, Wallig MA, Garrow TA. Immunohistochemical detection of betaine-homocysteine S-methyltransferase in human, pig and rat liver and kidney. Arch Biochem Biophys. 2001;393:184–186. doi: 10.1006/abbi.2001.2474. [DOI] [PubMed] [Google Scholar]
- 70.Claus V, Jahraus A, Tjelle T, Berg T, Kirschke H, Faulstich H, et al. Lysosomal enzyme trafficking between phagosomes, endosomes and lysosomes in J774 macrophages. Enrichment of cathepsin in early endosomes. J Biol Chem. 1998;273:9842–9851. doi: 10.1074/jbc.273.16.9842. [DOI] [PubMed] [Google Scholar]
- 71.Shirahama-Noda K, Yamamoto A, Sugihara K, Hashimoto N, Asano M, Nishimura M, et al. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J Biol Chem. 2003;278:33194–33199. doi: 10.1074/jbc.M302742200. [DOI] [PubMed] [Google Scholar]
- 72.Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002;383:1827–1831. doi: 10.1515/BC.2002.206. [DOI] [PubMed] [Google Scholar]
- 73.Seglen PO, Grinde B, Solheim AE. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979;95:215–225. doi: 10.1111/j.1432-033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 74.Baccino FM, Zuretti MF, Pernigotti L. Stimulation of rat liver β-galactosidase activity by ions. Biochem J. 1975;151:567–573. doi: 10.1042/bj1510567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kirschke H, Langner J, Wiederanders B, Ansorge S, Bohley P, Broghammer U. Intrazellulärer Proteinabbau VII. Kathepsin L und H: Zwei neue Proteinasen aus Rattenleberlysosomen. Acta Biol Med Ger. 1976;35:285–299. [PubMed] [Google Scholar]
- 76.Kirschke H, Langner J, Wiederanders B, Ansorge S, Bohley P, Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977;74:293–301. doi: 10.1111/j.1432-033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- 77.Alexander MA, Bennicelli J, Guerry D. Defective antigen presentation by human melanoma cell lines cultured from advanced, but not biologically early, disease. J Immunol. 1989;142:4070–4078. [PubMed] [Google Scholar]
- 78.Lindau D, Rönnefarth V, Erbacher A, Rammensee HG, Decker P. Nucleosome-induced neutrophil activation occurs independently of TLR9 and endosomal acidification: Implications for systemic lupus erythematosus. Eur J Immunol. 2011;41:669–681. doi: 10.1002/eji.201040593. [DOI] [PubMed] [Google Scholar]
- 79.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: From malaria to autoimmunity. Clin Rev Allergy Immunol. 2011 doi: 10.1007/s12016-010-8243-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren T, Wen ZK, Liu ZM, Liang YJ, Guo ZL, Xu L. Functional expression of TLR9 is associated to the metastatic potential of human lung cancer cell. Functional active role of TLR9 on tumor metastasis. Cancer Biol Ther. 2007;6:1704–1709. doi: 10.4161/cbt.6.11.4826. [DOI] [PubMed] [Google Scholar]
- 81.Jiang PD, Zhao YL, Deng XQ, Mao YQ, Shi W, Tang QQ, et al. Antitumor and antimetastatic activities of chloroquine diphosphate in a murine model of breast cancer. Biomed Pharmacother. 2010;64:609–614. doi: 10.1016/j.biopha.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/S0091-679X(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 83.Gordon PB, Seglen PO. Autophagic sequestration of [14C]sucrose, introduced into isolated rat hepatocytes by electropermeabilization. Exp Cell Res. 1982;142:1–14. doi: 10.1016/0014-4827(82)90402-5. [DOI] [PubMed] [Google Scholar]
- 84.Samari HR, Møller MTN, Holden L, Asmyhr T, Seglen PO. Stimulation of hepatocytic AMP-activated protein kinase by okadaic acid and other autophagy-suppressive toxins. Biochem J. 2005;386:237–244. doi: 10.1042/BJ20040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 86.Evans JC, Huddler DP, Jiracek J, Castro C, Millian NS, Garrow TA, et al. Betaine-homocysteine methyltransferase: zinc in a distorted barrel. Structure. 2002;10:1159–1171. doi: 10.1016/S0969-2126(02)00796-7. [DOI] [PubMed] [Google Scholar]
- 87.González B, Pajares MA, Martinez-Ripoll M, Blundell TL, Sanz-Aparicio J. Crystal structure of rat liver betaine homocysteine S-methyltransferase reveals new oligomerization features and conformational changes upon substrate binding. J Mol Biol. 2004;338:771–782. doi: 10.1016/j.jmb.2004.03.005. [DOI] [PubMed] [Google Scholar]