Abstract
MDA-7/IL-24 has noteworthy potential as an anticancer therapeutic because of its diversity of antitumor properties, its lack of toxicity toward normal cells and tissues, and its safety and efficacy as evidenced in a phase I clinical trial. In a recent study, we document that Ad.mda-7-induced ER stress and ceramide production leads to early autophagy that subsequently switches to apoptosis in human prostate cancer cells. During the apoptotic phase, the MDA-7/IL-24 protein physically interacts with Beclin 1 and this interaction might inhibit Beclin 1 function culminating in apoptosis. Conversely, Ad.mda-7 infection leads to calpain-mediated cleavage of the Atg5 protein that might also facilitate a biochemical switch from autophagy to apoptosis. Our recent paper reveals novel aspects of the interplay between autophagy and apoptosis that underlie the cytotoxic action of MDA-7/IL-24 in prostate cancer cells. These new insights into MDA-7/IL-24 action provide intriguing leads for developing innovative combinatorial approaches for prostate cancer therapy.
Key words: mda-7/IL-24, protective autophagy, apoptosis, Beclin 1, Atg5
Ad.mda-7 induces autophagy selectively in different types of human prostate cancer cells, without promoting this effect in immortal normal human prostate epithelial cells. We document that Ad.mda-7-induced ER stress and ceramide generation result in induction of autophagy in prostate cancer cells, without affecting normal human prostate epithelial cells, via the canonical autophagic pathway, involving BECN1, ATG5 and hVPS34.
MDA-7/IL-24 induces ER stress and ceramide production and these changes play a pivotal role in autophagy induction (Fig. 1). We examined the effect of the ER stress inhibitor salubrinal and the ceramide inhibitor ISP-1 on Ad.mda-7-induced autophagy by analyzing LC3-II accumulation 24 h postinfection. Co-treatment of Ad.mda-7-infected DU-145 cells with noncytotoxic doses of salubrinal (5 µM) or ISP-1 (10 µM) significantly inhibit LC3-II accumulation, and a combination of salubrinal and ISP-1 further reduce Ad.mda-7-induced LC3-II accumulation. The three major transducers of ER stress are IRE1, PERK and ATF6, which all sense the presence of unfolded proteins and transduce signals to the nucleus or cytosol. We hypothesized that one or more of these transducers must activate the signaling required for Ad.mda-7-induced autophagy and tested this possibility using siRNA targeting IRE1 and ATF6, and with a PERK dominant negative mutant. Among these treatments, IRE1 and ATF6 knockdown cells show no change in GFP-LC3-II accumulation during a 24 h period after Ad.mda-7 infection. However, Ad.mda-7 induces punctate staining of GFP-LC3 vacuoles and a dominant negative inhibitor of PERK (Dn-PERK) significantly inhibits LC3-II accumulation in DU-145 cells. These results suggest a potential involvement of PERK, an ER stress mediator, in Ad.mda-7-induced autophagy. Collectively, these observations support the hypothesis that Ad.mda-7-induced ER stress and ceramide production might contribute to autophagy through a PERK-dependent pathway in prostate carcinoma cells.
Figure 1.
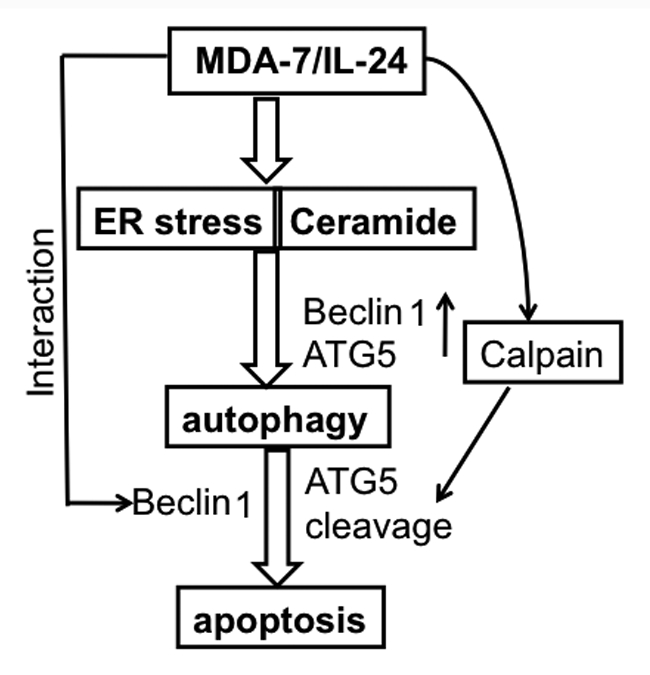
Model illustrating the possible molecular mechanism of ER stress- and ceramide-mediated autophagy promoted by MDA-7/IL-24 that switches to apoptosis in prostate cancer cells.
Cellular stress can promote autophagy and apoptosis in a number of ways including induction of autophagy/apoptosis sequentially, simultaneously, or in a mutually exclusive manner. Electron microscopy studies document autophagic features at 24 h followed by apoptotic features at 48 h post-Ad.mda-7 infection in DU-145 cells. To investigate the potential relationship between autophagy and apoptosis, we analyzed the growth inhibitory potential of Ad.mda-7 in DU-145 cells in the presence of the autophagy inhibitor 3-methyladenosine (3-MA). This experiment indicated that inhibition of autophagy can sensitize prostate tumor cells to the cytotoxic (apoptotic) actions of MDA-7/IL-24 and Ad.mda-7-induced autophagy might initially be a cytoprotective mechanism in prostate tumor cells. Additionally, the connection between Ad.mda-7-induced autophagy and apoptosis was investigated using Bcl-2 and Bcl-xL-overexpressing DU-145 cells (DU-Bcl-2 and DU-Bcl-xL). Ad.mda-7-infected DU-Bcl-2 and DU-Bcl-xL cells continue to exhibit higher autophagic phenotypes as compared with control DU-Neo clones, indicating that in DU-145 cells, Ad.mda-7-induced autophagy culminates in apoptosis, and when apoptosis is blocked by overexpression of Bcl-2 or Bcl-xL autophagy increases. To further confirm this possibility, we stained Ad.mda-7-infected DU-145 cells with MDC and PI and analyzed treated-cells by flow cytometry 48 h post-infection. We observed that autophagic cells also display apoptotic features as revealed by both MDC and PI staining in 25.8% of Ad.mda-7-infected cells as compared with 1.9% of Ad.vec-infected cells.
To investigate the role of the autophagy-related genes BECN1 and ATG5 in Ad.mda-7-induced apoptosis, we knocked down BECN1 and ATG5 expression with siRNA in DU-145 cells and examined Ad.mda-7 sensitivity. Beclin 1-deficient DU-145 cells exhibit increased sensitivity toward Ad.mda-7 when administered at 100 pfu/cell. In contrast, silencing of the ATG5 gene results in only a partial resistance to Ad.mda-7. These findings suggest that although at 24 h post-infection, Ad.mda-7 induces Beclin 1 and ATG5 to facilitate autophagy, at 48 h there might be inhibition of Beclin 1 and augmentation of ATG5 that then leads to apoptosis. During this predominantly apoptotic phase of Ad.mda-7-treatment the interaction between Beclin 1 and MDA-7/IL-24 might inhibit autophagy. To determine the role of ATG5 in the predominantly apoptotic phase at 48 h post-Ad.mda-7 infection, ATG5 mRNA and protein expressions were analyzed. At 48 h, Ad.mda-7 infection results in significant induction in ATG5 mRNA levels. Although the levels of the ATG5 protein do not significantly change upon Ad.mda-7 infection, a second band corresponding to a molecular weight 24-kDa protein is observed in Ad.mda-7-infected cells. Considering this finding, calpain activity was measured in Ad.mda-7-treated cells by calpain-Glo protease assay. Calpain activity is significantly higher in Ad.mda-7-infected cells when compared with control or Ad.vec-infected cells. Our study suggests a novel role for Beclin 1 and ATG5 in mediating a switch between protective autophagy and apoptosis in prostate cancer cells infected with Ad.mda-7. Based on our recent observations, employing strategies to block autophagy through promoting ER stress and ceramide production may represent a viable tactic for enhancing the antitumor activity of mda-7/IL-24 toward prostate and potentially other cancers.
Acknowledgements
The present study was supported in part by National Institutes of Health grant R01 CA097318 (P.B.F.), P01 CA104177 (P.B.F.) and R01 CA138540 (D.S.), the National Foundation for Cancer Research (P.B.F.), the Dana Foundation (D.S.) and the Korea Ministry of Education, Science and Technology through the National Research Foundation (2009-0063466) (S.G.L.). D.S. is a Harrison Endowed Scholar in Cancer Research in the VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Punctum to: Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70:3667–3676. doi: 10.1158/0008-5472.CAN-09-3647.


