Abstract
A series of salicylate-based compounds were designed and synthesized based on the simple function group replacement from our previously reported catechol-containing inhibitors of methionine aminopeptidase (MetAP). Some of these salicylate derivatives showed similar potency and metalloform selectivity, and some showed considerable antibacterial activity. These findings are consistent with our previous conclusion that Fe(II) is the likely metal used by MetAP in bacterial cells and provide new lead structures that can be further developed as novel antibacterial agents.
Keywords: salicylic acid, metalloenzyme, inhibition, antibacterial, drug discovery
Proteins are synthesized in cells with a methionine at the N-terminus, and the methionine is removed cotranslationally in over 50% of the newly synthesized proteins.1 Methionine aminopeptidase (MetAP) is responsible for hydrolysis of the initiator methionine, and this critical protein maturation process is ubiquitously found in all prokaryotes and eukaryotes.1, 2 The essential role that MetAP plays in cell growth and survival is reflected in the lethal effect of MetAP gene deletion in Escherichia coli,3 Salmonella typhimurium,4 and Saccharomyces cerevisiae.5 Consequently, MetAP has become an appealing target for the development of broad-spectrum antibacterial and antifungal drugs with a novel mechanism of action.6
Hydrolytic cleavage of the peptide bond by MetAP is assisted by divalent metal ions that serve as cofactors for the catalytic reaction.7 When purified as an apoenzyme, MetAP can be reproducibly activated by a number of divalent metal ions, including Co(II), Mn(II), Ni(II), and Fe(II).8, 9 Based on X-ray structures, two metal ions are found at the bottom of a shallow catalytic site in a dinuclear arrangement.10 Most of the current MetAP inhibitors were discovered and characterized by using MetAP enzyme reconstituted with Co(II), because several MetAPs were initially identified as Co(II) enzymes,10 and this metal is among the best MetAP activators. Due to its relative chemical stability and consistent MetAP activation, Mn(II) has also been used extensively in the screening of MetAP inhibitors.11, 12 Even though several small molecules showed potent inhibition of the purified MetAP enzyme, almost all of them failed to show any significant antibacterial activity.13–15 We have shown that potent MetAP inhibitors of one metalloform may not inhibit the same MetAP enzyme in another metalloform.8, 11 Therefore, the failure may indicate that these MetAP inhibitors may not be able to effectively inhibit MetAP in a physiologically relevant metalloform in bacterial cells.
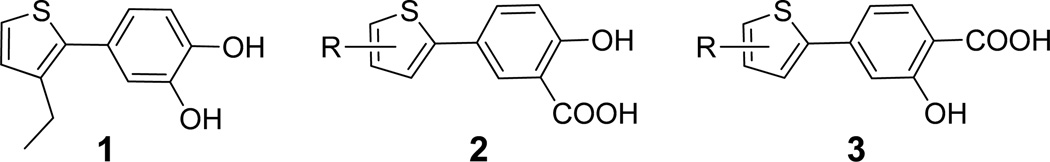
We have discovered potent inhibitors that selectively inhibit MetAP in either the Co(II), Mn(II) or Fe(II) form,8, 11, 16 and we demonstrated that only the Fe(II)-form selective MetAP inhibitors were able to halt bacterial growth.16 The compounds that are selective for the Fe(II)-substituted enzyme share a common catechol moiety (1, Fig. 1), and X-ray structure of E. coli MetAP in complex with one of the inhibitors showed that the catechol compounds coordinate with the catalytic metal ions through their hydroxyl groups.17 Salicylates are structurally similar to catechols, and salicylate derivatives, such as aspirin, are widely used as anti-inflammatory drugs. We envisioned that the salicylate scaffold may have better chemical stability and favorable pharmacokinetic properties and could potentially serve as a starting point for the development of novel MetAP inhibitors with antibacterial activity. In this study, we designed and synthesized a series of compounds based on the salicylate scaffold (2 and 3, Fig. 1), characterized them against the purified MetAP in different metalloforms, and evaluated their antibacterial activity against E. coli.
Figure 1.
Salicylate derivatives 2 and 3 were designed as MetAP inhibitors, based on the antibacterial MetAP inhibitor 1 with a catechol moiety.
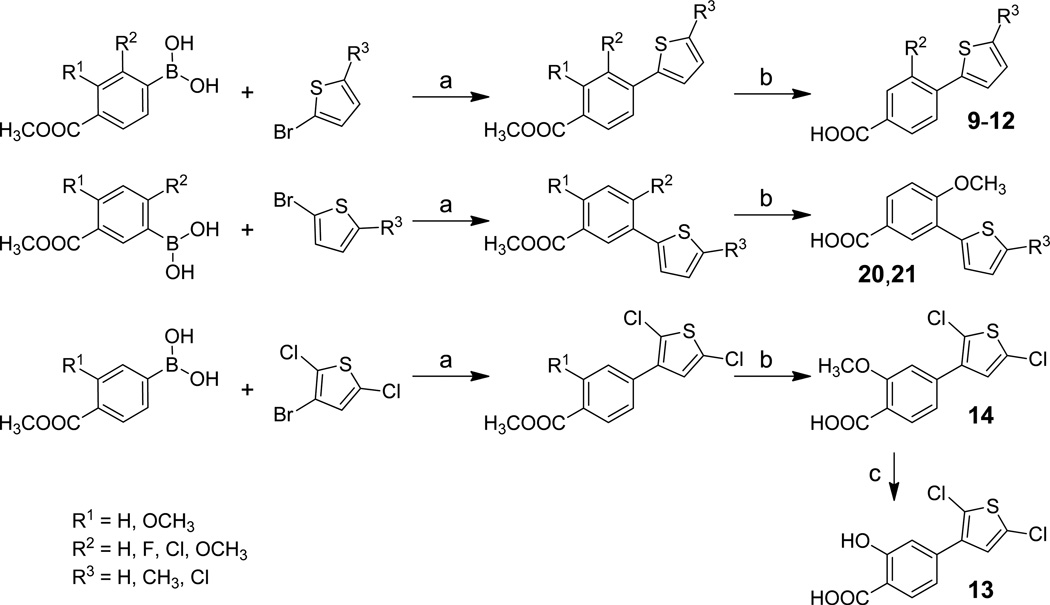
Syntheses of compounds 4–21 were outlined in Schemes 1 and 2. Suzuki coupling17 of appropriate phenylboronic acids with corresponding brominated thiophenes yielded phenyl thiophene intermediates. Hydrolysis of methyl esters yielded carboxylic acids 7–12, 14 and 18–21. Removal of methyl group from methoxyl compounds 7, 8, 14, 18 and 19 produced salicylate derivatives 4–6, 13, 15 and 16. Compounds 6 and 17 were similarly prepared by removing the methyl group in their precursors.
Scheme 1.
Reagent and condition: (a) Pd(PPh3)4, 2 N Na2CO3, DMF, 90 °C, yield 40%–80%; (b) LiOH, MeOH/H2O, yield 90%–100%.; (c) BCl3, CH2Cl2, −78 °C, yield 40%–70%.
Scheme 2.
Reagent and condition: (a) LiOH, MeOH/H2O, yield 90%–100%.; (b) BCl3, CH2Cl2, −78 °C, yield 40%–70%.
Assays for enzyme inhibition and bacterial cell growth inhibition were conducted as previously described.18 Inhibitory potencies of these salicylate derivatives on MetAP enzyme in the presence of either Co(II), Mn(II) or Fe(II) are presented in Table 1. For comparison, their inhibitory potencies on E. coli AS19 growth, as concentration at 50% inhibition (IC50) and minimum inhibitory concentration (MIC), are also presented.
Table 1.
Inhibition of E. coli MetAP enzyme activity and E. coli AS19 cell growth by salicylate-based derivatives
 | |||||||
|---|---|---|---|---|---|---|---|
| Comp | R1 | R2 | R3 | Inhibition of enzymatic activity, IC50, µM |
Inhibition of bacterial growth, IC50, µMb |
||
| Co(II) | Mn(II) | Fe(II) | E. coli AS19 | ||||
| 4 | OH | H | H | 65.2 | 80.2 | 39.1 | 112 (100) |
| 5 | OH | H | CH3 | 81.0 | 90.7 | 109.1 | 73.1 (47) |
| 6 | OH | H | Cl | 87.7 | 62.7 | 73.0 | 34.1 (28) |
| 7 | OCH3 | H | H | 254 | 89.8 | 500 | >1000 (>234) |
| 8 | OCH3 | H | CH3 | 256 | 160 | 270 | 550 (272) |
| 9 | H | H | H | 249 | 159 | >500 | >1000 (>204) |
| 10 | H | F | H | 251 | 143 | 267 | >1000 (>222) |
| 11 | H | F | CH3 | 329 | 336 | 253 | 871 (306) |
| 12 | H | F | Cl | 166 | 118 | 203 | 641 (297) |
| 13 | OH | 118 | 97.5 | 29.9 | 51.4 (33) | ||
| 14 | OCH3 | 290 | 97.3 | >500 | 829 (521) | ||
| 15 | OH | Cl | H | 88.1 | 33.7 | 3.1 | 74.6 (38) |
| 16 | OH | Cl | CH3 | 77.4 | 37.8 | 6.8 | 161 (87) |
| 17 | OH | Cl | Cl | 74.1 | 36.6 | 4.9 | 48.5 (30) |
| 18 | OCH3 | Cl | H | 203 | 19.1 | 208 | >1000 (>268) |
| 19 | OCH3 | Cl | CH3 | 70.9 | 11.4 | 106 | >1000 (>282) |
| 20 | H | OCH3 | H | >500 | 17.4 | 192 | >1000 (>234) |
| 21 | H | OCH3 | CH3 | 464 | 33.8 | 167 | >1000 (>248) |
MIC values in mg/L are in parentheses.
The relative inhibitory potencies on the three metalloforms of E. coli MetAP revealed the structural elements that are important in inhibition and metalloform selectivity. Compounds 15–17 showed good inhibitory potency and good selectivity for the Fe(II)-form of MetAP. Importantly, these potent and selective inhibitors 15–17 also showed good antibacterial activity on the E. coli strain, consistent with the enzyme inhibition. Although compounds 4–6 and 13 were not selective among the metalloforms, they did show considerable antibacterial activity. This may be due to the fact that they do maintain some potency on the Fe(II) form. Because the Fe(II) form is the likely cellular metalloform for MetAP in E. coli,16 these results are consistent with the concept that effectively inhibition of the cellular MetAP is important and inhibitors of the Fe(II) form of MetAP are more likely to show antibacterial activity. Wang et al. reported that human type 2 MetAP uses Mn(II) as its native cofactor,19 and potentially, the Fe(II) form selective MetAP inhibitors are desired lead compounds to optimize their antibacterial activity, while minimizing their human toxicity. It is interesting to note that all of the compounds with good enzyme inhibition and antibacterial activity (4–6, 13 and 15–17) have a hydroxyl group, along with a carboxyl group at the adjacent position, indicating the importance of the salicylate moiety in interaction with MetAP, probably through metal chelation. Compounds missing this hydroxyl group (9–12, 20 and 21) or compounds with this hydroxyl group replaced by a methoxyl group (7, 8, 14, 18 and 19) all showed significantly lower enzyme inhibition and low or no antibacterial activity. While this hydroxyl group is indispensable, the hydroxyl group alone is not sufficient for inhibition, because we showed previously that mono-hydroxyl analogs of the catechol series displayed no enzyme inhibition.18 Therefore, both the hydroxyl group and the carboxyl group in the salicylate derivatives are required for effective inhibition of MetAP. Compounds 18–21 showed noticeable potency and selectivity on the Mn(II)-form of MetAP. Lacking the hydroxyl group, they all have a carboxyl group, and their selectivity towards the Mn(II) form is consistent with the results from furan carboxylates we reported earlier.11 In that case, the carboxyl group directly coordinates with the catalytic metal ions, and the coordination contributes to their Mn(II)-form selectivity. However, their lack of antibacterial activity is possibly due to their poor potency on the Fe(II)-form of MetAP.
In summary, structure-function analysis of the newly synthesized salicylate derivatives as inhibitors of E. coli MetAP clearly indicates that replacement of one of the two hydroxyl groups to a carboxyl group on the catechol scaffold (change from 1 to 2 or 3, Fig. 1) can be accommodated by MetAP, and the potency and selectivity on the Fe(II)-form can be maintained. Both the hydroxyl group and the carboxyl group in the salicylate derivatives are involved in enzyme inhibition and required for antibacterial activity. The demonstrated activity of these salicylate derivatives on MetAP enzyme and on E. coli cells, coupled with their likely better chemical stability and favorable pharmacokinetic properties, provides a new starting point for the design and discovery of MetAP inhibitors as new antibacterial agents with a novel mechanism of action.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giglione C, Boularot A, Meinnel T. Cell Mol Life Sci. 2004;61:1455. doi: 10.1007/s00018-004-3466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradshaw RA, Brickey WW, Walker KW. Trends Biochem Sci. 1998;23:263. doi: 10.1016/s0968-0004(98)01227-4. [DOI] [PubMed] [Google Scholar]
- 3.Chang SY, McGary EC, Chang S. J Bacteriol. 1989;171:4071. doi: 10.1128/jb.171.7.4071-4072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CG, Kukral AM, Miller JL, Movva NR. J Bacteriol. 1989;171:5215. doi: 10.1128/jb.171.9.5215-5217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Chang YH. Proc Natl Acad Sci U S A. 1995;92:12357. doi: 10.1073/pnas.92.26.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan MD, Sampson PB, Honek JF. Curr Med Chem. 2002;9:385. doi: 10.2174/0929867023371102. [DOI] [PubMed] [Google Scholar]
- 7.Lowther WT, Matthews BW. Chem Rev. 2002;102:4581. doi: 10.1021/cr0101757. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Chen LL, Cui YM, Luo QL, Li J, Nan FJ, Ye QZ. Biochem Biophys Res Commun. 2003;307:172. doi: 10.1016/s0006-291x(03)01144-6. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza VM, Holz RC. Biochemistry. 1999;38:11079. doi: 10.1021/bi990872h. [DOI] [PubMed] [Google Scholar]
- 10.Lowther WT, Matthews BW. Biochim Biophys Acta. 2000;1477:157. doi: 10.1016/s0167-4838(99)00271-x. [DOI] [PubMed] [Google Scholar]
- 11.Ye QZ, Xie SX, Huang M, Huang WJ, Lu JP, Ma ZQ. J Am Chem Soc. 2004;126:13940. doi: 10.1021/ja045864p. [DOI] [PubMed] [Google Scholar]
- 12.Huang QQ, Huang M, Nan FJ, Ye QZ. Bioorg Med Chem Lett. 2005;15:5386. doi: 10.1016/j.bmcl.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Luo QL, Li JY, Liu ZY, Chen LL, Li J, Qian Z, Shen Q, Li Y, Lushington GH, Ye QZ, Nan FJ. J Med Chem. 2003;46:2631. doi: 10.1021/jm0300532. [DOI] [PubMed] [Google Scholar]
- 14.Schiffmann R, Heine A, Klebe G, Klein CD. Angew Chem Int Ed Engl. 2005;44:3620. doi: 10.1002/anie.200500592. [DOI] [PubMed] [Google Scholar]
- 15.Douangamath A, Dale GE, D'Arcy A, Almstetter M, Eckl R, Frutos-Hoener A, Henkel B, Illgen K, Nerdinger S, Schulz H, Mac Sweeney A, Thormann M, Treml A, Pierau S, Wadman S, Oefner C. J Med Chem. 2004;47:1325. doi: 10.1021/jm034188j. [DOI] [PubMed] [Google Scholar]
- 16.Chai SC, Wang WL, Ye QZ. J Biol Chem. 2008;283:26879. doi: 10.1074/jbc.M804345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]
- 18.Wang WL, Chai SC, Huang M, He HZ, Hurley TD, Ye QZ. J Med Chem. 2008;51:6110. doi: 10.1021/jm8005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Sheppard GS, Lou P, Kawai M, Park C, Egan DA, Schneider A, Bouska J, Lesniewski R, Henkin J. Biochemistry. 2003;42:5035. doi: 10.1021/bi020670c. [DOI] [PubMed] [Google Scholar]