Summary
The maintenance of H3K9 and DNA methylation at imprinting control regions (ICRs) during early embryogenesis is key to the regulation of imprinted genes. Here, we reveal that ZFP57, its cofactor KAP1, and associated effectors bind selectively to the H3K9me3-bearing, DNA-methylated allele of ICRs in ES cells. KAP1 deletion induces a loss of heterochromatin marks at ICRs, whereas deleting ZFP57 or DNMTs leads to ICR DNA demethylation. Accordingly, we find that ZFP57 and KAP1 associated with DNMTs and hemimethylated DNA-binding NP95. Finally, we identify the methylated TGCCGC hexanucleotide as the motif that is recognized by ZFP57 in all ICRs and in several tens of additional loci, several of which are at least ZFP57-dependently methylated in ES cells. These results significantly advance our understanding of imprinting and suggest a general mechanism for the protection of specific loci against the wave of DNA demethylation that affects the mammalian genome during early embryogenesis.
Highlights
► ZFP57/KAP1 bind all methylated imprinted control regions in ES cells ► ZFP57/KAP1 bind other nonimprinted methylated sequences in ES cells ► ZFP57/KAP1 are necessary for DNA and histone methylation maintenance ► ZFP57 recognizes a methylated hexanucleotide with two C2H2 zinc fingers
Introduction
In higher mammals, most autosomal genes are expressed from alleles inherited from both the father and the mother. Imprinted genes are exceptions to this rule, being transcribed from only one parental allele along patterns that are determined during gamete formation. Imprinted genes play key developmental roles and are generally organized in clusters controlled by CpG-rich sequences known as imprinting control regions (ICRs) (Weaver et al., 2010). ICRs are targeted by parental allele- specific DNA methylation imprints that are established during late gametogenesis, maintained in the zygote and somatic cells, and erased in primordial germ cells (Reik, 2007). ICRs can exert their effect through various mechanisms. Several ICRs with maternally inherited methylation correspond to the promoters of long noncoding and antisense RNAs with in cis silencing activity toward the imprinted genes of the same cluster (Koerner et al., 2009). In contrast, ICRs with paternally inherited methylation do not contain promoters but at least two of them display enhancer-blocking (insulator) activity. Notably, the promoter or insulator activities of ICRs are manifest only on the allele that is not methylated.
In embryonic stem cells (ES cells), the chromatin associated with the ICRs carries allele-specific histone modifications (Kacem and Feil, 2009). In general, H3K9me3, H4K20me3, and H4/H2AR3me2 are associated with methylated ICR alleles, whereas H3K4me2/3 and H3/H4 acetylation are found on their nonmethylated counterparts. Furthermore, depletion of the H3K9 methyltransferase G9a and Polycomb group protein-mediated H3K27 methylation affect the expression of imprinted genes in a gene- and tissue-specific manner (Mager et al., 2003; Nagano et al., 2008; Sanz et al., 2008; Wagschal et al., 2008). In addition, removal of H3K4 methylation by the histone demethylase KDM1B is necessary for establishment of DNA methylation imprints at some loci in oocytes (Ciccone and Chen, 2009).
Gene knockout experiments in mice have recently implicated the Krüppel-associated box-containing zinc-finger protein (KRAB-ZFP) ZFP57 in the establishment and maintenance of several imprinted loci (Li et al., 2008), and loss-of-function mutations in the human Zfp57 gene are associated with hypomethylation at multiple imprinted regions in individuals affected by transient neonatal diabetes (Mackay et al., 2008). The genomes of higher vertebrates encode close to 400 KRAB-ZFPs. These proteins all harbor a so-called KRAB domain that is situated upstream of an array of 2–40 C2H2 zinc fingers, which can provide sequence-specific DNA-binding ability. KRAB recruits KRAB-associated protein 1(KAP1), also known as TRIM 28, Tif1b, or KRIP-1, which acts as a scaffold for various heterochromatin-inducing factors, such as heterochromatin protein 1 (HP1), the histone methyltransferase SETDB1 (also known as ESET), the nucleosome remodeling and histone deacetylation (NuRD) complex, and the nuclear receptor corepressor complex 1 (N-CoR1) (Schultz et al., 20001, 2002). Therefore, the binding of KRAB-ZFPs to specific chromosomal loci results in nearby transcriptional repression and establishes heterochromatin marks, such as H3K9me3. Correspondingly, KRAB-ZFPs can no longer mediate transcriptional repression when KAP1 is inactivated (Groner et al., 2010). KRAB-ZFPs exhibit tissue-specific patterns of expression, and ZFP57 can be detected in ES cells, ovaries, testes, and the nervous system (Alonso et al., 2004; Li et al., 2008). Mouse and human ZFP57 contain three and six C2H2 zinc fingers, respectively, two of which are conserved as amino acids predicted to govern nucleotide sequence-specific recognition.
Several pieces of evidence link KRAB-ZFPs and KAP1 to DNA methylation during the early embryonic period. First, KRAB-mediated tethering of KAP1 to the vicinity of promoters during the first few days of embryogenesis leads to their permanent silencing via CpG methylation (Wiznerowicz et al., 2007). Second, KAP1 controls endogenous retroviruses in ES cells and, together with ZFP809, is responsible for the methylation and silencing of the murine leukemia virus (MLV) in these targets (Rowe et al., 2010; Wolf and Goff, 2009). Finally, the replacement of wild-type (WT) KAP1 by a mutant defective for HP1 binding in embryonic carcinoma cells leads to loss of methylation and upregulation of the imprinted MEST gene (Riclet et al., 2009).
The present work further investigates the roles of ZFP57 and KAP1 in the regulation of imprints during the early embryonic period. Our results demonstrate that, in embryonic stem cells, ZFP57/KAP1 interact in a parental allele-specific fashion with the ICRs and that their presence is necessary for the recruitment of chromatin and DNA modifiers at the imprinted loci. Furthermore, we unveil the role of a methylated hexanucleotidic motif responsible for recruiting ZFP57/KAP1 to ICRs and to a number of additional loci, at least some of which are also methylated in a ZFP57-dependent fashion.
Results
KAP1 Deletion in Embryonic Stem Cells Leads to Chromatin Changes at Imprinting Control Regions
We examined the status of imprinted genes in previously described ES cell lines, allowing for tamoxifen (4-OHT)-induced KAP1 deletion. When the KRAB-ZFP cofactor is removed, these cells lose pluripotency markers, markedly upregulate endogenous retroviruses, undergo differentiation, and stop dividing (Rowe et al., 2010). The levels of H3K9 methylation and H3 acetylation were investigated in control and KAP1-deleted ES cells by chromatin imunoprecipitation (ChIP) combined with quantitative polymerase chain reaction (qPCR)-mediated DNA amplification, using primers specific for several ICRs and isolated imprinted genes (Figures 1A and 1B). In control ES cells, both H3K9me3 and H3K9ac were present at these loci, consistent with previous studies (Kacem and Feil, 2009). Seventy-two hours after KAP1 deletion, levels of H3K9ac were markedly increased, and H3K9me3 was almost completely lost at all tested ICRs. DNA methylation, as assessed by combined bisulfite restriction analysis (COBRA) (Figure S1A available online), was unaffected in Kap1-knockout ES cells, as expected in view of their rapid growth arrest (Figures S1C and S1D).
Figure 1.
Altered Chromatin Status at Imprinted Control Regions in Kap1-Deleted Embryonic Stem Cells
Histone 3 lysine 9 acetylation (A) and methylation (B) were assessed by ChIP/PCR in WT (sham, dark bars) and KAP1-deleted (4OH-tamoxiphen-treated, light bars) ES cells, using primers specific for incited genomic sequences. (C) ChIP/PCR analysis of control (ES, dark bars) and HA-tagged KAP1-expressing (ES + 6HAKAP1, light bars), using a HA-specific monoclonal antibody and primers for the indicated genomic sequences. Error bars indicate standard deviation; the difference between control and 4OHTam-treated cells was always significant (p < 0.01, n = 3), except for the GAPDH locus. KV, KvDMR1 ICR.
Having found that KAP1 binding correlates with H3K9me3 deposition at ICRs, we tested its association with these loci. We first engineered an ES cell line that expresses a modified KAP1 protein carrying six hemagglutinin (HA) tags at its N-terminus. This 6HA-KAP1 derivative was fully functional, as it could prevent the drop in pluripotency markers and the accumulation in G1 that were otherwise induced by deleting endogenous KAP1 (Figures S1C and S1D). Thus, we performed ChIP with a HA-specific monoclonal antibody in 6HA-KAP1-expressing ES cells, followed by qPCR analysis with ICR-specific primers. KAP1 was found to bind all tested ICRs (Figure 1C).
ZFP57 Mediates KAP1 Recruitment to DNA-Methylated Imprinting Control Regions
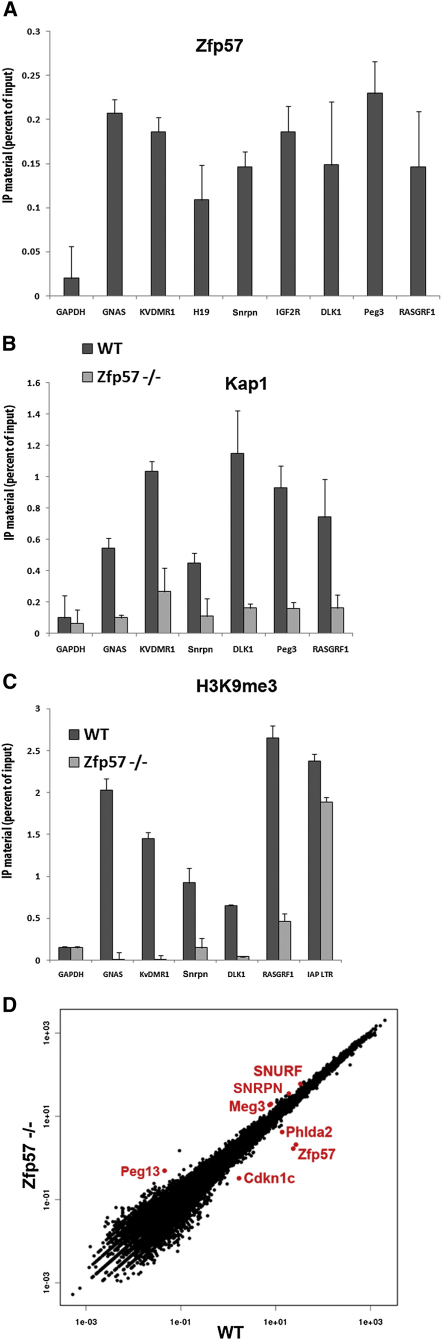
ZFP57 was previously detected at only a limited number of ICRs (Li et al., 2008). Using ChIP-PCR with a 6HA-tagged ZFP57 introduced in ES cells by lentiviral vector-mediated transduction, we found that ZFP57 associated with all tested ICRs (Figure 2A and data not shown). To confirm this result, we repeated the KAP1-specific ChIP studies in Zfp57−/− ES cells (Akagi et al., 2005). Although the master regulator was normally recruited to intracisternal A particles (IAPs), a subgroup of endogenous retroviruses (ERVs) in these cells, it was no longer associated with ICRs, which were also devoid of H3K9me3 (Figures 2B and 2C). Furthermore, a deep-sequencing analysis of polyadenylated RNA revealed that only few genes were dysregulated in Zfp57−/− cells and that the most significantly altered genes were the upregulated Meg3, Peg13, Snrnp, and Snurfs and the downregulated Cdkn1c and Phlda2, which are all parts of known imprinted clusters (Figure 2D and Figure S2).
Figure 2.
KAP1 ICR Recruitment in Embryonic Stem Cells Requires ZFP57
(A) ZFP57 binds to ICRs. HA-specific ChIP in ES cells expressing a HA-tagged ZFP57 protein, using primers specific for indicated genomic sequences. Error bars indicate standard deviation, and the difference between Gapdh locus and ICRs is always significant (p < 0.01, n = 3).
(B) No KAP1 ICR recruitment in the absence of ZFP57. Same analysis as in (A), in control and Zfp57 knockout ES cells expressing a HA-tagged form of KAP1. Error bars indicates standard deviation, error bars indicate standard deviation, and the difference between control and Zfp57−/− cells is always significant (p < 0.01, n = 3), except for the Gapdh locus.
(C) H3K9me3 is lost at ICRs in Zfp57-defective ES cells. Same analysis as in (B), using an antibody specific for this chromatin mark. Error bars indicates standard deviation, error bars indicate standard deviation, and the difference between control and Zfp57−/− cells is always significant (p < 0.01, n = 3), except for the Gapdh and IAP LTR loci.
(D) Transcriptional deregulation of several imprinted genes in Zfp57 knockout ES cells. Scatter plot showing the gene expression data extracted from RNA-seq analysis; highlighted in red are Zpf57 and imprinted genes that are most markedly affected. Error bars indicate standard deviation. KV, KvDMR1 ICR.
KAP1, ZFP57, SETDB1, and HP1 Colocalize with H3K9me3 at DNA-Methylated Imprinted Control Region Alleles
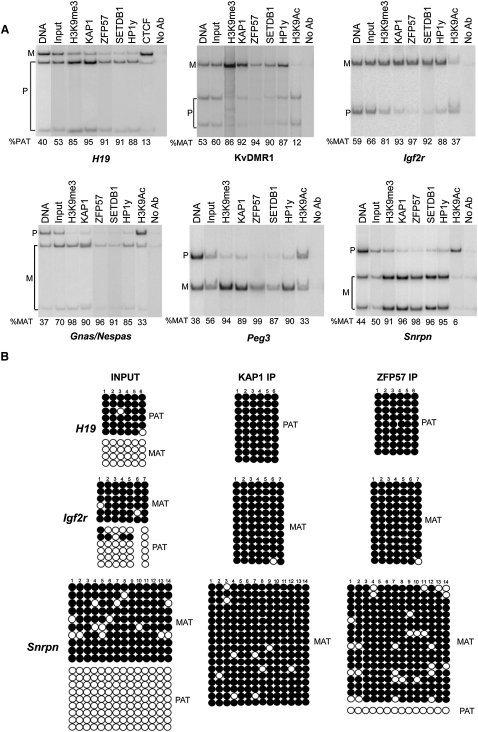
In order to investigate further the role of ZFP57, KAP1, and their known partners, such as the H3K9 methyltransferase SETDB1 and heterochromatin protein 1 (HP1), in the control of the parent of origin-dependent epigenetic status of the imprinted loci, we examined the potential allelic specificity of their recruitment to ICRs. The SF1-G ES cell line derives from a C57-Black6 × Mus spretus F1 hybrid, maintains genomic imprinting, and carries correct allele-specific DNA methylation and histone modifications at the ICRs (Delaval et al., 2007). In these cells, maternal and paternal ICR alleles can be distinguished, owing to sequence polymorphisms. Thus, we selected five maternally methylated ICRs (Snrpn, KvDMR1, Igf2r, Peg3, and Gnas/Nespas) and two paternally methylated ICRs (Igf2/H19 and Rasgrf1), and performed ChIP studies in SF1-G ES cells with antibodies that recognize the endogenous proteins to ask whether ICR recruitment of ZFP57, KAP1, and their associated proteins was parental allele-specific (Figures S3A–S3C). To determine the relative abundance of the maternal and paternal alleles, ChIP-PCR DNA products were digested with a restriction enzyme that cut only one allele or, for Rasgrf1, were run on capillary electrophoresis separating alleles of different lengths (Figures 3 and S3D). To ascertain the validity of our approach, we first analyzed CTCF, the multizinc-finger protein that is known to bind the H19 ICR on the maternal, nonmethylated allele (Stedman et al., 2008). The results confirmed a specific CTCF-H19 ICR interaction with a strong bias toward the maternal allele, revealing, in contrast, that KAP1, ZFP57, SETDB1, and HP1γ interacted preferentially with their methylated paternal counterparts (Figure 3A). When other ICRs were investigated, we found that these four factors were always preferentially immunoprecipitated with the paternal allele at the paternally methylated ICRs and with the maternal allele at the maternally methylated ICRs (Figure 3A). The presence of ZFP57/KAP1 and their associated factors also correlated with high levels of H3K9me3 and low levels of H3K9Ac that marked the opposite parental allele. To confirm and extend these results, we used bisulfite sequencing to determine directly the methylation status of the ZFP57/KAP1 recruiting allele of the H19, Igf2r, and Snrpn ICRs. Methylated and nonmethylated alleles of these elements were amplified with equal efficiencies from input DNA, but only methylated molecules derived from the paternal H19, and maternal Igf2r and Snrpn alleles were detected in the ZFP57- and KAP1-bound DNA (Figure 3B).
Figure 3.
KAP1, ZFP57, SETDB1, and HP1 Are Selectively Recruited to the Methylated Alleles of Imprinted Control Regions
(A) RFLP analysis of KAP1-, ZFP57-, SETDB1- and HP1-bound material. Following ChIP with indicated antibodies recognizing endogenous proteins and ICR-specific PCR amplification, products were digested with restriction enzymes that cut only one parental allele to measure the ratio of maternal (%MAT) or paternal (%PAT) allele relative to total. H3K9me3 (which is known to bind to the methylated ICRs allele) and H3K9Ac (which is known to bind to the unmethylated ICRs allele) were analyzed as control. No Ab, no specific primary antibody.
(B) Bisulfite sequencing of the KAP1- and ZFP57- bound H19, Igf2r and Snrpn ICRs. Methylated and unmethylated CpG dinuclotides are represented as open and filled circles, respectively. The input (pre-ChIP) DNA shows a 50/50 ratio of methylated and unmethylated ICR, although the KAP1- and ZFP57-immunoprecipitated (IP) material is totally or prevalently methylated. Each line corresponds to a single template DNA molecule cloned; each circle corresponds to a CpG dinucleotide. Filled circles designate the methylated cytosines; opened circles unmethylated cytosines. Differences are statistically significant (P-values < 0.05).
KAP1 Recruitment to Imprinting Control Regions is DNA Methylation-Dependent
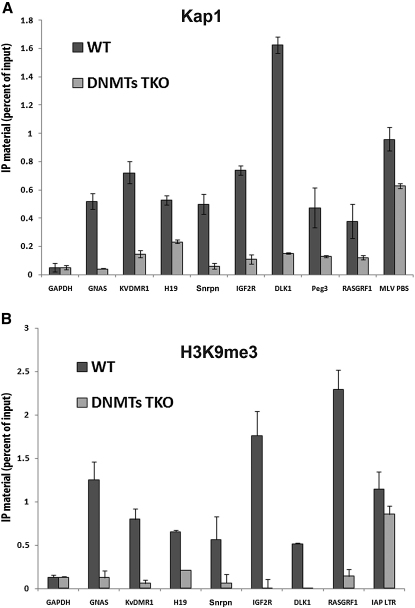
Because KAP1 exclusively binds the methylated allele of ICRs, we examined its status in cells deleted for the DNA methyltransferases DNMT1, DNMT3A, and DNMT3B. These cells have drastically reduced levels of DNA methylation over the entire genome, including at ICRs and ERV sequences (Tsumura et al., 2006). ChIP readily detected KAP1 and H3K9me3 at ICRs in parental but not in Dnmt triple-knockout ES cells, although both marks were present at ERVs in both settings (Figure 4). As KAP1 binding to DNA is usually indirect and mediated by KRAB-containing ZFPs (Sripathy et al., 2006), this result suggests that although some KRAB-ZFPs (e.g., ZFP57) require their DNA target to be methylated for binding, other members of this family (e.g., ERV-specific KRAB-ZFPs) recognize their cognate sequences as unmethylated DNA.
Figure 4.
KAP1 Recruitment and H3K9 Trimethylation at Imprinting Control Regions Require DNA Methylation
(A) HA-specific ChIP in control (WT) and Dnmt1/3a/3b-deleted (DNMTs TKO) ES cells expressing a HA-tagged form of KAP1 and transduced with an MLV-based retroviral vector, with primers for indicated genomic and virus-derived (MLV PBS) sequences. Error bars indicate standard deviation, and the difference between control and TKO cells is always significant (p < 0.01, n = 3), except for the GAPDH and MLV PBS.
(B) Same analysis with H3K9me3-specific antibody-mediated ChIP. Histone 3 trimethylation of ICRs, but not of IAP chromatin, is abolished in the absence of DNA methylation. Error bars indicate standard deviation, and the difference between control and TKO cells is always significant (p < 0.01, n = 3), except for the GAPDH and IAP LTR. KV, KvDMR1 ICR.
ZFP57 is Necessary for the Maintenance of DNA Methylation at Imprinted Control Regions
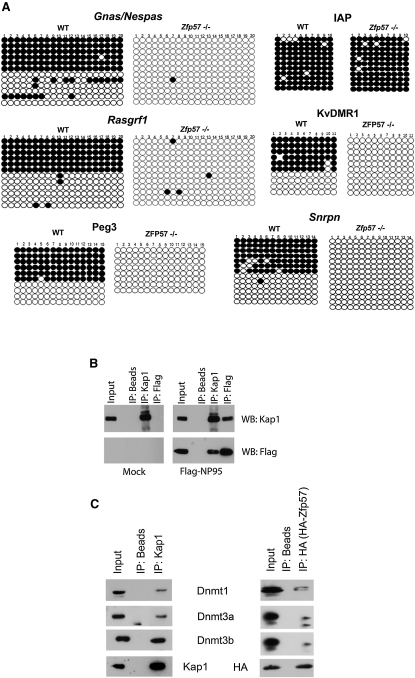
Upon examining Zfp57−/− embryos obtained by crossing a heterozygous male with a homozygous null female, Li et al. observed loss of DNA methylation at the DLK1 ICR (IG-DMR), indicating that methylation of a paternally methylated ICR cannot be maintained in the absence of both maternal and zygotic ZFP57 (Li et al., 2008). To investigate further the role of ZFP57 in the maintenance DNA methlylation at ICRs, we turned to the Zfp57−/− ES cell line, which was obtained by successively knocking out the two alleles with two different constructs (i.e., bearing two different antibiotic resistance genes) without passage through gametogenesis, thus achieving epigenetic reprogramming (Akagi et al., 2005). WT parental cells were used as controls because they allowed comparison with the methylation status of ICRs before ZFP57 removal. The Snrpn, KvDMR1, Rasgrf1, Peg3, and Gnas/Nespas ICRs exhibited the expected 50% DNA methylation rates in control ES cells, and IAPs were fully methylated in both these and their Zfp57−/− derivatives. In contrast, ICR DNA methylation was almost completely absent in the knockout cells (Figures 5A and S4). Notably, we could not test the effect of zfp57 deletion on the methylation of the Igf2/H19 and Igf2r ICRs because these sequences were already hypomethylated in the WT parental ES cell line (data not shown).
Figure 5.
Zfp57 Removal Leads to a Loss of DNA Methylation and ZFP57/KAP1 Interaction with NP95 and DNMTs
(A) Bisulfite sequencing analysis of DNA extracted from Zfp57 knockout and WT ES cells. ICR, but not IAP, DNA methylation is lost in Zfp57 knockout ES cells. Differences between Zfp57−/− and WT ES cells are statistically significant (p < 0.005).
(B) Total protein extracts (input) or immunoprecipitates recovered with either naked (IP: beads) or antibody-coated beads with indicated antibodies (IP:KAP1 and IP:Flag) from control (Mock, left) or Flag-NP95-expressing ES cells were analyzed by western blot with antibodies, indicated on the right.
(C) Same experiment in WT ES cells, using control, KAP1, and HA-specific beads for the IP and KAP1, HA, DNMT1, DNMT3A, or DNMT3B-specific antibodies for the western blot.
ZFP57/KAP1 Associate with NP95 and DNA Methyltransferases
A mass spectroscopic analysis of KAP1-associated proteins in ES cells revealed the presence of the multidomain protein NP95 (also known as UHRF1 or ICBP90; not illustrated). NP95 was previously demonstrated to help recruit DNMT1 to hemimethylated DNA, thereby playing an important role in the maintenance of DNA methylation in ES cells (Arita et al., 2008; Avvakumov et al., 2008; Bostick et al., 2007; Meilinger et al., 2009; Sharif et al., 2007). To confirm the KAP1-NP95 interaction, a FLAG-tagged NP95 variant was introduced in ES cells by lentivector-mediated transduction and immunoprecipitations were performed with KAP1- and FLAG-specific antibodies. Western blot analyses of the immunoprecipitates confirmed that KAP1 associates with NP95 in ES cells (Figure 5B). Furthermore, DNMT1, 3A, and 3B could also be coimmunoprecipitated with KAP1 and ZFP57 in ES cells (Figure 5C).
Identification of ZFP57-Dependently Methylated ZFP57/KAP1 Targets in Embryonic Stem Cells
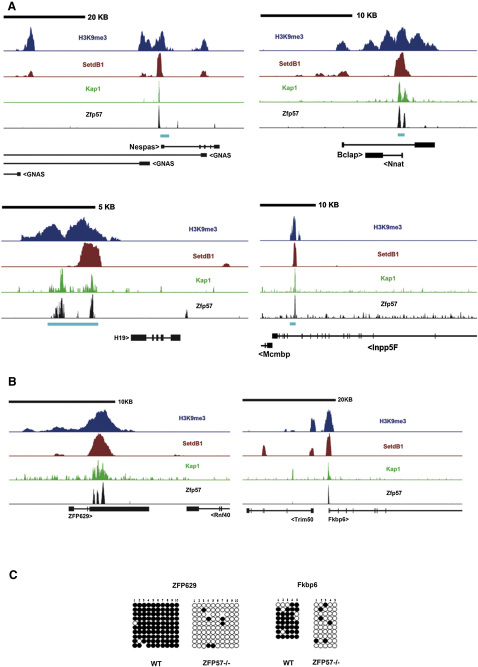
Having established the role of ZFP57/KAP1 in the maintenance of histone marks and DNA methylation at ICRs, we sought to explore the full range of DNA loci targeted by this phenomenon. Therefore, we performed ChIP-Seq analyses to identify all KAP1 and ZFP57 binding sites in ES cells. We superimposed these data with those previously obtained for SETDB1 (Bilodeau et al., 2009). Using a low-stringency peak-calling strategy, we detected approximately 11,000 of both KAP1- and ZFP57-specific peaks, among which 2,375 appeared common to the two markers. Of these, 216 also coincided with SETDB1 peaks, a number that was further reduced by direct examination and elimination of sites where ZFP57, KAP1, and SETDB1 peaks were not precisely colocalized. This high stringency criteria left 91 peaks, which included all known ICRs, thus supporting the validity of our approach (Figures 6A and S5 and data not shown). Two representatives of non-ICR peaks were examined in greater depth. The Zfp629 and Fkbp6 sites both recruited ZFP57, KAP1, and SETDB1, and both sites could also be immunoprecipitated with H3K9me3 antibodies (Figure 6B). Furthermore, DNA at these locations was heavily methylated in WT ES cells but was almost completely unmethylated in Zfp57 KO ES cells (Figure 6C). This suggests that ZFP57/KAP1 and associated proteins are involved in maintaining the chromatin status and DNA methylation not only at known ICRs but also at a number of additional selected loci.
Figure 6.
KAP1 Recognition of Imprinted Clusters Targets Imprinting Control Regions
(A) ChIP-seq using a polyclonal antibody against endogenous KAP1 and ChIP-seq using an antibody against HA tagged ZFP57. Four loci are illustrated, with KAP1 binding results in green and ZFP57 in black, superimposed on published SETDB1 and H3K9me3 data in red and blue, respectively (Bilodeau et al., 2009). The ICR position is indicated in light blue, between mRNAs and ZFP57.
(B) ChIP-seq data presented as (A) for nonimprinted loci predicted to bind ZFP57/KAP1.
(C) Bisulfite sequencing of CpG islands located at the binding sites presented in B. Differences between Zfp57−/− and WT ES cells are statistically significant (p < 0.001).
A Hexanucleotidic Motif Is Responsible for the Methylation-Dependent Recruitment of ZFP57
Although our experiments thus far demonstrated that ZFP57 only binds the methylated allele of ICRs, whether it recognizes a specific DNA sequence remained to be determined. To do so, we subjected the 350 bp-long central fragments of the 91 identified ZFP57/KAP1/SETDB1 peaks to sequence alignment. This revealed that the TGCCGC hexanucleotide was present in 81 of these 91 identified ZFP57/KAP1/SETDB1 peaks, on average at two copies per locus (Figure 7A and Table S1). To confirm the role of this sequence in recruiting the ZFP57/KAP1 complex, we produced in E. coli a fragment of murine ZFP57 comprising the two highly conserved, adjacent C2H2 zinc fingers (Figure S6), fused to MBP or to GST, and tested the ability of this protein to bind in vitro a TGCCGC-containing double-stranded oligonucleotide as previously described (Renda et al., 2007). This ZFP57 derivative could bind the methylated form of this DNA more efficiently than its nonmethylated counterpart. Furthermore, although methylated hexanucleotide-containing sequences could compete for this binding, their effect was abolished by mutations in the motif (Figures 7B and S6D). Supporting a role for the TGCCGC hexanucleotide in imprinting control, it was found in all murine ICRs and also in at least some human ICRs with, for instance, two and three TGCCGC sequences, respectively, in the KvDMR and H19 ICRs from both species (Figure 7C).
Figure 7.
A Hexanucleotidic Motif Recognized by ZFP57 in a Methylation-Dependent Fashion
(A) Logo presentation of consensus binding site deduced from the analysis of sites binding ZFP57, KAP1, and SETDB1.
(B) Electrophoretic shift assay, using a recombinant MBP fusion protein encompassing the zinc fingers fragment Glu87-Ala195 from mouse ZFP57 to capture the illustrated double-stranded oligonucleotide including the consensus ZFP57 binding site (bold), in either native (unmet) or methylated (met) state. The met and unmet probes were assayed with or without a 50-fold excess of unlabeled WT met, WT unmet, mutant (mut1 and mut2), or nonspecific (NS) oligonucleotides. In addition to the methylation-dependent higher-mobility complex, a lower-mobility complex, possibly due to the binding of more than one ZFP57 protein to DNA, is also weakly detected with the unmet probe.
(C) Several copies of the TGCCGC consensus are found at all known mouse and human ICRs. The KvDMR and H19 ICRs are illustrated as examples.
Discussion
The parent-of-origin-specific expression of imprinted genes, which is required for normal embryonic development, remarkably correlates with asymmetric chromatin and DNA methylation signatures at ICRs. The present work demonstrates that, in ES cells, the tethering of ZFP57/KAP1 to the methylated allele of ICRs via the sequence-specific recognition of a hexanucleotidic motif is key to the maintenance of asymmetric histone modifications, heterochromatinization, and DNA methylation at these elements. Therefore, the ZFP57-dependent recruitment of KAP1 at ICRs is pivotal in the maintenance of epigenetic asymmetry in this cellular context, corroborating a recent demonstration that ZFP57 is required for the postfertilization maintenance of maternal and paternal methylation imprints at multiple imprinted domains (Li et al., 2008). Here, a first piece of evidence was obtained by observing that, in conditional Kap1-knockout ES cells, all ICRs largely lost the H3K9me3 chromatin mark and exhibited a rise in histone acetylation consistent with the known association of KAP1 with histone methyltransferases and histone deacetylases (Schultz et al., 2001, 2002). We then found that ZFP57, KAP1, and their associated chromatin modifiers, including SETDB1 and HP1, were recruited specifically to the DNA-methylated allele of ICRs. ZFP57 and KAP1 were bound to all ICRs. KAP1 no longer recruited ICRs in Dnmt triple-knockout cells and, in ZFP57-deleted ES cells, KAP1 no longer bound with ICRs, which were now unmethylated. Correlating this phenomenon, we found that the ZFP57/KAP1 complex associates with DNA methyltransferases and the hemimethylated DNA binding protein NP95. Finally, we identified the methylated TGCCGC motif as a motif recruiting ZFP57 at ICRs and a number of other loci, at least some of which are also ZFP57-dependently methylated in ES cells. On these bases, we propose a model whereby recognition of this motif results in the ZFP57-mediated tethering of a complex comprising KAP1, histone modifiers, and DNA methyltransferases that preserves the chromatin and DNA methylation status of ICRs and other selected loci during the epigenetic instability period that characterizes the first few days of embryogenesis.
Imprinted genes were deregulated in kap1−/− ES cells, but these cells exhibit massive transcriptional changes, probably as a consequence of the functional inactivation of the more than 200 different KRAB-ZFPs expressed in this setting (Rowe et al., 2010) (and data not shown). Thus, interpreting the deregulation of imprinted genes in this grossly perturbed context is delicate. In contrast, knocking out zfp57 had subtle consequences, with no gross phenotypic alteration (Akagi et al., 2005) and only a few deregulated transcripts, most of which mapped to known imprinted loci. In addition, the expression changes are those expected by the loss of ICR methylation. Indeed, in zfp57−/− ES cells, the genes (Snrpn, Snurf, Peg13, and Meg3) repressed by DNA methylation are overexpressed, although, conversely, those activated by DNA methylation (Cdkn1c and Phlda2) are downregulated. Even though the generally low basal level of activity of imprinted gene promoters in ES cells may dampen somewhat the consequences of removing ZFP57, these results are consistent with a loss of epigenetic allelic asymmetry of ICRs in the absence of the ICR-binding protein. Intriguingly, ZFP57 is expressed in the central nervous system, and transcriptional analyses of the hippocampus of mice deleted for kap1 in the adult forebrain revealed deregulation of imprinted genes, such as Mkrn3 (Jakobsson et al., 2008). Whether this was an indirect effect, or whether ZFP57 and KAP1 are involved in controlling imprinting, not only in early embryonic and germ cells but also in adult somatic cells warrants investigation.
Although ZFP57 associates with the methylated ICRs, it is reciprocally essential for the maintenance of their methylation in ES cells. ES cells are notorious for their epigenetic instability, notably exhibiting many so called bivalent promoters, which harbor both active and inactive chromatin marks. The concomitant expression in ES cells of TET proteins, which most likely partake in active DNA demethylation (Ficz et al., 2011; Ito et al., 2010), and de novo DNA methyltransferases implies that the methylation status of the genome is subjected to dynamic alterations in this setting. Our results demonstrate that a complex encompassing ZFP57 and KAP1, at least at specific sequences, is implicated in this process. This model is corroborated by the observed loss of DNA methylation at all tested ICRs in zfp57−/− ES cells. Importantly, as these cells were obtained by the elimination of ZFP57 from normally methylated ES cells, our finding of unmethylated ICRs can be attributed to a defect in the maintenance of this modification. Involvement of KAP1 in this phenomenon, albeit not directly demonstrated by our work, is supported by the observed loss of Mest promoter methylation in EC cells expressing an HP1-binding mutant of this protein (Riclet et al., 2009) and also by the Zfp809-dependent methylation of murine leukemia virus in embryonic cells (Wolf and Goff, 2009). Therefore, it may seem surprising that we did not observe a significant decrease of ICR DNA methylation following KAP1 deletion in ES cells. However, our RNA-seq analyses indicate that loss of KAP1 results in a marked downregulation of TET1 (data not shown), a major DNA demethylating enzyme (Ito et al., 2010). Furthermore, KAP1 removal rapidly leads to cell-cycle arrest (Figures S1C and S2D), likely depriving cells from the opportunity to lose methyl marks through repeated rounds of DNA replication. Nevertheless, our demonstration that KAP1 forms a complex with DNMTs and NP95, a protein that is required for the maintenance of DNA methylation in ES cells, notably at ICRs (Arita et al., 2008; Avvakumov et al., 2008; Bostick et al., 2007; Meilinger et al., 2009; Sharif et al., 2007), supports the involvement of KAP1 in this phenomenon.
Our ChIP analyses revealed the binding of ZFP57, KAP1, and SETDB1 to all tested murine ICRs, always on the methylated allele. In addition, we could detect the three proteins at several tens of additional sites, at least some of which we found to be DNA-methylated in a ZFP57-dependent manner. The great majority of ZFP57-/KAP1-/SETDB1-binding sites contained at least two copies of the TGCCGC hexanucleotide. Confirming the role of this motif in recruiting the multimolecular complex, a recombinant ZFP57 derivative was able to bind in vitro a double-stranded oligonucleotide containing the methylated TGCCGC sequence, but only very weakly its nonmethylated counterpart. Specific recognition of the TGCCmetGC motif was demonstrated by competition with fragments containing methylated WT, unmethylated or point-mutated versions of this sequence. Thus, ZFP57 adds to the list of C2H2 zinc-finger proteins that preferentially bind to methylated DNA in a sequence-specific manner (Sasai et al., 2010). The TGCCGC consensus is found in all murine ICRs and also in at least some of their human homologs. Thus, our data explain not only how these loci are recognized by the ZFP57/KAP1 complex in both species, but also the parent-of-origin specificity of this recognition. Interestingly, the two zinc fingers incorporated in our TGCCmetGC-capturing recombinant protein are highly conserved between mouse and human orthologs of ZFP57, including at residues predicted to dictate nucleotide specificity (Figure S6B). This suggests that each one of these adjacent zinc fingers is responsible for recognizing one of the two triplets that comprise the hexanucleotide motif. It is also noteworthy that, at the H19/Igf2 ICR locus, the ZFP57-binding sequence partly overlaps with the motif recognized by CTCF (Figure S6C). Because this modification blocks CTCF binding, the methylation-dependence of ZFP57 recruitment (Hark et al., 2000) explains how the two factors each interact with a distinct allele of the H19/Igf2 ICR.
In addition to ICRs, we found at least two copies of the TGCCGC motif in more than 50 ZFP57-, KAP1-, and SETDB1-binding genomic sites, some of which were verified as DNA methylated in a ZFP57-dependent fashion. This result significantly broadens the range of sites subjected to ZFP57-dependent DNA methylation during early embryogenesis. It will be interesting to explore the functional significance of this phenomenon.
How precisely the ZFP57/KAP1 complex prevents demethylation of ICRs and other targeted loci remains undefined. Heterochromatin is often correlated with DNA methylation. For example, SUV39-H1 and -H2 contribute to the maintenance of DNA methylation at pericentric heterochromatin (Lehnertz et al., 2003). At ICRs, repressive H3K9 methylation was previously linked to the presence of DNA methylation (Kacem and Feil, 2009). Our data indicate a role for SETDB1 in this function, consistent with previous findings (Yuan et al., 2009). Similarly, KAP1-recruited SETDB1 is involved in proviral silencing in ES cells (Matsui et al., 2010), an event that culminates in the DNA methylation of these elements. KAP1 recruitment by ZFP809 also induces MLV repression, followed by DNA methylation, in ES cells (Wolf and Goff, 2009), and KAP1 is more broadly essential for the early embryonic control of ERVs, notably IAPs, which during this period are either protected from demethylation or immediately remethylated after losing methylated CpG marks (Lane et al., 2003; Rowe et al., 2010). We previously reported that if the KRAB domain is tethered to the vicinity of a promoter during the first 3–5 days of mouse embryogenesis, it leads to its irreversible silencing by CpG methylation (Wiznerowicz et al., 2007). Whether all these events proceed, or not, along the same general mechanism and what molecular interactions govern the specific contribution of given KRAB-ZFPs, KAP1, chromatin modifiers, and enzymes involved in regulating DNA methylation in each of these situations remain to be determined. Considering that a very high proportion of all KRAB-ZFPs are expressed in ES cells (Rowe et al., 2010), further deciphering their roles and that of KAP1 in the DNA methylation of specific genomic loci should yield important information about this crucial developmental period.
Experimental Procedures
Cell Culture and RNA-Seq
ES cells were maintained in DMEM supplemented with 2-mercaptoethanol, nonessential amino acids, sodium pyruvate, 10% fetal calf serum, and leukemia inhibitory factor (LIF) in gelatinized culture flasks. 4-hydroxy-tamoxifen (4-OHT, Sigma) used at a final concentration of 1μm. Zfp57−/− and WT parental cell lines were obtained from Dr. Yokota (Akagi et al., 2005). Dnmt TKO cells were obtained by Dr. Okano (Tsumura et al., 2006). SF1-G was obtained by crossing C57BL/6 female mice with M. spretus males as previously described (Dean et al., 1998). RNA-seq was performed using standard procedures. RNA was extracted with RNeasy Plus Mini Kit (QIAGEN), hydrolyzed, reverse-transcribed, and then deep-sequenced using Solexa (Illumina). The relative expression was normalized based on GAPDH expression.
Chromatin Immunoprecipitation
ES cells at 80% confluence were cross-linked with 1% formaldehyde for 10 min at room temperature. Cross-linking was quenched with 125 mM glycine, and whole-cell extracts were prepared for use in the chromatin immunoprecipitations. The fragmented chromatin from 107 cells was used for each reaction. DNA from the immunoprecipitates or from the input (1% of the input for quantification) was analyzed by real-time PCR using Power SBYRGreen PCR Master Mix (Applied Biosystem) performed on an Applied Biosystems 7900HT Real-Time PCR machine. Each reaction was performed in triplicate, and all presented results are representative of experiments performed at least twice. Primer sequences and antibodies are available in the Supplemental Information.
ChIP-Seq Analyses
Reads were aligned to the Mus musculus genome (assembly NCBI37/mm9) using bowtie (Langmead et al., 2009). Reads with more than four matches were excluded. Normalization of the WT data was performed using MACS (Zhang et al., 2008) and the knockout data for background normalization. Peaks were called using the ChIP-Seq Analysis Server (http://ccg.vital-it.ch/chipseq). The motif-finding program, MDmodule from the MotifRegressor software (Conlon et al., 2003), was used to identify possible consensus DNA motifs.
Allele-Specific PCR Analyses
The binding of KAP1, SETDB1, HP1y, ZFP57, H3K9me3, and CTCF proteins to the maternal and paternal alleles of ICR was determined by typing for polymorphisms present between the two parental genomes of the SF1-G ES cells, which has a (C57BL/6 × Mus spretus) F1 genotype. ICRs were amplified and radiolabeled (α32PdCTP), then digested with a restriction enzyme that cuts only the paternal or maternal allele. The digested fragments were resolved on a nondenaturing polyacrylamide gel and quantified using PhoshorImager and ImageQuant software by Molecular Dynamics.
DNA Methylation Analyses
After chromatin immunoprecipitation using KAP1 and ZFP57 antibodies, DNA methylation of Snrpn ICR, was analyzed by bisulfite sequencing. All the immunoprecipitated DNA was treated with sodium bisulfate, using the EpiTect bisulfite kit (QIAGEN), and PCR-amplified. The PCR product was then cloned in Topo pCRII vector (Invitrogen) and the clones were sequenced. The maternal and paternal clones in the SF1-G ES cell line were discriminated using a single nucleotide polymorphism. Methylation in WT and Zfp57−/− ES cells was also investigated using bisulfite sequencing.
Protein Immunoprecipitation and Western Blot
Half a million cells were pelleted and resuspended on ice in lysis buffer (400 mM NaCl, 10 mM HEPES, pH 7.9, 0.1% NP-40, protease inhibitor, and 10mM N-Ethylmaleimide (Sigma)). Lysate was centrifugated 10 min at 10,000 g and supernatant was incubated with rabbit polyclonal anti-Kap1 antibody (Sripathy et al., 2006), followed by A-protein magnetic beads (Invitrogen), or directly immunoprecipitated with anti-HA Matrix (Roche) or α-FLAG M2 resin, (Sigma). Beads were washed three times with lysis buffer and elution was performed by boiling beads in Laemmli buffer. Western blot was performed with rabbit polyclonal anti-KAP1 antibody, anti-Dnmt1 (Abcam, ab87654), anti-Flag M2 (Sigma), and anti-HA antibody (Roche).
Electrophoretic-Mobility Shift Assay
A plasmid encoding Glu87-Ala 195 residues of mouse ZFP57 fused to the maltose binding protein (MBP) or to glutathion S-transferase (GST) was expressed in E. coli Bl21 and purified according to the manufacturer's protocol (NEB). Purified proteins (12 pmol) were incubated on ice for 10 min with 2.5 pmol of the specific 32P-labeled duplex oligonucleotide or with the same, but methylated, duplex. Incubation was performed in the presence of 25 mM HEPES pH 7.9, 50 mM KCl, 6.25 mM MgCl2, 1% Nonidet P-40, and 5% glycerol. After incubation, the mixture was loaded on a 5% polyacrylamide gel. Competition was performed in the presence of 50× excess unlabelled oligonucleotides. The NS oligonucleotide, 5′-AGGTTTGACAGTGTCACTTT-3′, was used as a nonspecific competitor.
Acknowledgments
We thank R. Feil and D. Schultz for the gift of reagents, J. Rougemont for his help with the genomic analyses, M. Okano for Dnmt TKO cells, T. Yokota for zfp57−/− cells, and members of the Trono lab for helpful discussions. A.R. and G.V. thank the IGB-SCFLab for assistance in ES cell culturing. This work was supported by grants from the Swiss National Science Foundation, the European Union (FP7 “PERSIST”), the European Research Council, and the Strauss Foundation to D.T., and by grants from MIUR PRIN 2007, Telethon-Italia N. GGP07086, and the Associazione Italiana Ricerca sul Cancro to A.R. and by Progetto Bandiera MIUR-CNR EpiGen-Epigenomica to A.R. and G.G.
Published: November 3, 2011
Footnotes
Supplemental Information includes six figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.molcel.2011.08.032.
Contributor Information
Andrea Riccio, Email: andrea.riccio@uunina2.it.
Didier Trono, Email: didier.trono@epfl.ch.
Accession Numbers
The Geo database accession number for the KAP1, ZFP57 Chip-seq and RNA-seq reported in this paper is GSE31183.
Supplemental Information
References
- Akagi T., Usuda M., Matsuda T., Ko M.S., Niwa H., Asano M., Koide H., Yokota T. Identification of Zfp-57 as a downstream molecule of STAT3 and Oct-3/4 in embryonic stem cells. Biochem. Biophys. Res. Commun. 2005;331:23–30. doi: 10.1016/j.bbrc.2005.03.118. [DOI] [PubMed] [Google Scholar]
- Alonso M.B., Zoidl G., Taveggia C., Bosse F., Zoidl C., Rahman M., Parmantier E., Dean C.H., Harris B.S., Wrabetz L. Identification and characterization of ZFP-57, a novel zinc finger transcription factor in the mammalian peripheral nervous system. J. Biol. Chem. 2004;279:25653–25664. doi: 10.1074/jbc.M400415200. [DOI] [PubMed] [Google Scholar]
- Arita K., Ariyoshi M., Tochio H., Nakamura Y., Shirakawa M. Recognition of hemi-methylated DNA by the SRA protein UHRF1 by a base-flipping mechanism. Nature. 2008;455:818–821. doi: 10.1038/nature07249. [DOI] [PubMed] [Google Scholar]
- Avvakumov G.V., Walker J.R., Xue S., Li Y., Duan S., Bronner C., Arrowsmith C.H., Dhe-Paganon S. Structural basis for recognition of hemi-methylated DNA by the SRA domain of human UHRF1. Nature. 2008;455:822–825. doi: 10.1038/nature07273. [DOI] [PubMed] [Google Scholar]
- Bilodeau S., Kagey M.H., Frampton G.M., Rahl P.B., Young R.A. SetDB1 contributes to repression of genes encoding developmental regulators and maintenance of ES cell state. Genes Dev. 2009;23:2484–2489. doi: 10.1101/gad.1837309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick M., Kim J.K., Estève P.O., Clark A., Pradhan S., Jacobsen S.E. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- Ciccone D.N., Chen T. Histone lysine methylation in genomic imprinting. Epigenetics. 2009;4:216–220. doi: 10.4161/epi.8974. [DOI] [PubMed] [Google Scholar]
- Conlon E.M., Liu X.S., Lieb J.D., Liu J.S. Integrating regulatory motif discovery and genome-wide expression analysis. Proc. Natl. Acad. Sci. USA. 2003;100:3339–3344. doi: 10.1073/pnas.0630591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaval K., Govin J., Cerqueira F., Rousseaux S., Khochbin S., Feil R. Differential histone modifications mark mouse imprinting control regions during spermatogenesis. EMBO J. 2007;26:720–729. doi: 10.1038/sj.emboj.7601513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficz G., Branco M.R., Seisenberger S., Santos F., Krueger F., Hore T.A., Marques C.J., Andrews S., Reik W. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- Groner A.C., Meylan S., Ciuffi A., Zangger N., Ambrosini G., Dénervaud N., Bucher P., Trono D. KRAB-zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet. 2010;6:e1000869. doi: 10.1371/journal.pgen.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hark A.T., Schoenherr C.J., Katz D.J., Ingram R.S., Levorse J.M., Tilghman S.M. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- Ito S., D'Alessio A.C., Taranova O.V., Hong K., Sowers L.C., Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson J., Cordero M.I., Bisaz R., Groner A.C., Busskamp V., Bensadoun J.C., Cammas F., Losson R., Mansuy I.M., Sandi C., Trono D. KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron. 2008;60:818–831. doi: 10.1016/j.neuron.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Kacem S., Feil R. Chromatin mechanisms in genomic imprinting. Mamm. Genome. 2009;20:544–556. doi: 10.1007/s00335-009-9223-4. [DOI] [PubMed] [Google Scholar]
- Koerner M.V., Pauler F.M., Huang R., Barlow D.P. The function of non-coding RNAs in genomic imprinting. Development. 2009;136:1771–1783. doi: 10.1242/dev.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N., Dean W., Erhardt S., Hajkova P., Surani A., Walter J., Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz B., Ueda Y., Derijck A.A., Braunschweig U., Perez-Burgos L., Kubicek S., Chen T., Li E., Jenuwein T., Peters A.H. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 2003;13:1192–1200. doi: 10.1016/s0960-9822(03)00432-9. [DOI] [PubMed] [Google Scholar]
- Li X., Ito M., Zhou F., Youngson N., Zuo X., Leder P., Ferguson-Smith A.C. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev. Cell. 2008;15:547–557. doi: 10.1016/j.devcel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D.J., Callaway J.L., Marks S.M., White H.E., Acerini C.L., Boonen S.E., Dayanikli P., Firth H.V., Goodship J.A., Haemers A.P. Hypomethylation of multiple imprinted loci in individuals with transient neonatal diabetes is associated with mutations in ZFP57. Nat. Genet. 2008;40:949–951. doi: 10.1038/ng.187. [DOI] [PubMed] [Google Scholar]
- Mager J., Montgomery N.D., de Villena F.P., Magnuson T. Genome imprinting regulated by the mouse Polycomb group protein Eed. Nat. Genet. 2003;33:502–507. doi: 10.1038/ng1125. [DOI] [PubMed] [Google Scholar]
- Matsui T., Leung D., Miyashita H., Maksakova I.A., Miyachi H., Kimura H., Tachibana M., Lorincz M.C., Shinkai Y. Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature. 2010;464:927–931. doi: 10.1038/nature08858. [DOI] [PubMed] [Google Scholar]
- Meilinger D., Fellinger K., Bultmann S., Rothbauer U., Bonapace I.M., Klinkert W.E., Spada F., Leonhardt H. Np95 interacts with de novo DNA methyltransferases, Dnmt3a and Dnmt3b, and mediates epigenetic silencing of the viral CMV promoter in embryonic stem cells. EMBO Rep. 2009;10:1259–1264. doi: 10.1038/embor.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T., Mitchell J.A., Sanz L.A., Pauler F.M., Ferguson-Smith A.C., Feil R., Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Renda M., Baglivo I., Burgess-Beusse B., Esposito S., Fattorusso R., Felsenfeld G., Pedone P.V. Critical DNA binding interactions of the insulator protein CTCF: a small number of zinc fingers mediate strong binding, and a single finger-DNA interaction controls binding at imprinted loci. J. Biol. Chem. 2007;282:33336–33345. doi: 10.1074/jbc.M706213200. [DOI] [PubMed] [Google Scholar]
- Riclet R., Chendeb M., Vonesch J.L., Koczan D., Thiesen H.J., Losson R., Cammas F. Disruption of the interaction between transcriptional intermediary factor 1beta and heterochromatin protein 1 leads to a switch from DNA hyper- to hypomethylation and H3K9 to H3K27 trimethylation on the MEST promoter correlating with gene reactivation. Mol. Biol. Cell. 2009;20:296–305. doi: 10.1091/mbc.E08-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H.M., Jakobsson J., Mesnard D., Rougemont J., Reynard S., Aktas T., Maillard P.V., Layard-Liesching H., Verp S., Marquis J. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature. 2010;463:237–240. doi: 10.1038/nature08674. [DOI] [PubMed] [Google Scholar]
- Sanz L.A., Chamberlain S., Sabourin J.C., Henckel A., Magnuson T., Hugnot J.P., Feil R., Arnaud P. A mono-allelic bivalent chromatin domain controls tissue-specific imprinting at Grb10. EMBO J. 2008;27:2523–2532. doi: 10.1038/emboj.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai N., Nakao M., Defossez P.A. Sequence-specific recognition of methylated DNA by human zinc-finger proteins. Nucleic Acids Res. 2010;38:5015–5022. doi: 10.1093/nar/gkq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D.C., Friedman J.R., Rauscher F.J., 3rd Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev. 2001;15:428–443. doi: 10.1101/gad.869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D.C., Ayyanathan K., Negorev D., Maul G.G., Rauscher F.J., 3rd SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002;16:919–932. doi: 10.1101/gad.973302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif J., Muto M., Takebayashi S., Suetake I., Iwamatsu A., Endo T.A., Shinga J., Mizutani-Koseki Y., Toyoda T., Okamura K. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- Sripathy S.P., Stevens J., Schultz D.C. The KAP1 corepressor functions to coordinate the assembly of de novo HP1-demarcated microenvironments of heterochromatin required for KRAB zinc finger protein-mediated transcriptional repression. Mol. Cell. Biol. 2006;26:8623–8638. doi: 10.1128/MCB.00487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman W., Kang H., Lin S., Kissil J.L., Bartolomei M.S., Lieberman P.M. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 2008;27:654–666. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura A., Hayakawa T., Kumaki Y., Takebayashi S., Sakaue M., Matsuoka C., Shimotohno K., Ishikawa F., Li E., Ueda H.R. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- Wagschal A., Sutherland H.G., Woodfine K., Henckel A., Chebli K., Schulz R., Oakey R.J., Bickmore W.A., Feil R. G9a histone methyltransferase contributes to imprinting in the mouse placenta. Mol. Cell. Biol. 2008;28:1104–1113. doi: 10.1128/MCB.01111-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J.R., Sarkisian G., Krapp C., Mager J., Mann M.R., Bartolomei M.S. Domain-specific response of imprinted genes to reduced DNMT1. Mol. Cell Biol. 2010;30:3916–3928. doi: 10.1128/MCB.01278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiznerowicz M., Jakobsson J., Szulc J., Liao S., Quazzola A., Beermann F., Aebischer P., Trono D. The Kruppel-associated box repressor domain can trigger de novo promoter methylation during mouse early embryogenesis. J. Biol. Chem. 2007;282:34535–34541. doi: 10.1074/jbc.M705898200. [DOI] [PubMed] [Google Scholar]
- Wolf D., Goff S.P. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature. 2009;458:1201–1204. doi: 10.1038/nature07844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Han J., Guo G., Orlov Y.L., Huss M., Loh Y.H., Yaw L.P., Robson P., Lim B., Ng H.H. Eset partners with Oct4 to restrict extraembryonic trophoblast lineage potential in embryonic stem cells. Genes Dev. 2009;23:2507–2520. doi: 10.1101/gad.1831909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.