Abstract
We hypothesize that beneficial effects of estradiol on cognitive performance diminish with age and time following menopause due to a progressive decline in basal forebrain cholinergic function. This study tested whether galanthamine, a cholinesterase inhibitor used to treat memory impairment associated with Alzheimer’s disease, could enhance or restore estradiol effects on cognitive performance in aged rats that had been ovariectomized in middle-age. Rats were ovariectomized at 16–17 months of age. At 21–22 months of age rats began receiving daily injections of galanthamine (5 mg/day) or vehicle. After one week, half of each group also received 17ß-estradiol administered subcutaneously. Rats were then trained on a delayed matching to position (DMP) T-maze task, followed by an operant stimulus discrimination/reversal learning task. Treatment with galanthamine + estradiol significantly enhanced the rate of DMP acquisition and improved short-term delay-dependent spatial memory performance. Treatment with galanthamine or estradiol alone were without significant effect. Effects were task-specific in that galanthamine + estradiol treatment did not significantly improve performance on the stimulus discrimination/reversal learning task. In fact, estradiol was associated with a significant increase in incorrect responses on this task after reversal of the stimulus contingency. In addition, treatments did not significantly affect hippocampal choline acetyltransferase activity or acetylcholine release. This may be an effect of age, or possibly is related to compensatory changes associated with long-term cholinesterase inhibitor treatment. The data suggest that treating with a cholinesterase inhibitor can enhance the effects of estradiol on acquisition of a DMP task by old rats following a long period of hormone deprivation. This could be of particular benefit to older women who have not used hormone therapy for many years and are beginning to show signs of mild cognitive impairment. Potential mechanisms for these effects are discussed.
Keywords: spatial learning, hormone therapy, cholinesterase inhibitor, surgical menopause, Cognitive Aging, Acetylcholine, Alzheimer’s Disease, Nicotinic Receptors
INTRODUCTION
Animal studies show that ovariectomy and estrogen replacement significantly affect the structure and function of hippocampal and cortical circuits with corresponding effects on the performance of wide variety of cognitive tasks (Daniel and Bohacek, 2010; Fernandez et al., 2008; Sandstrom and Williams, 2001; Sandstrom and Williams, 2004; Spencer et al., 2008; Wallace et al., 2006; Woolley, 2007). Studies in humans likewise have demonstrated beneficial effects of estrogen therapy on specific cognitive tasks in younger surgically menopausal and perimenopausal women, particularly in the realm of verbal memory and executive functioning (Sherwin and Henry, 2008). A recent study also shows that women who experience an early menopause are at significantly greater risk for age-related cognitive decline and dementia, and this risk is mitigated by early estrogen therapy (Shuster et al., 2010). Animal studies, however, suggest that many of the beneficial effects of estradiol on cognitive performance diminish with age and time following ovariectomy when initiation of therapy is delayed (Daniel et al., 2006; Gibbs et al., 2009; Markowska and Savonenko, 2002; Talboom et al., 2008). This is consistent with human trials reporting limited benefit when estrogen therapy is initiated in older women. In fact, several large trials including the very large Women’s Health Initiative Memory Study (WHIMS), have reported either no beneficial effect or increased harm for women receiving hormone therapy (HT) in old age (Resnick et al., 2006; Shumaker et al., 2004; Shumaker et al., 2003). These findings have given rise to the critical period hypothesis, which proposes that estrogen therapy must be initiated within a window of time following menopause in order to produce beneficial effects on brain function and cognition (Daniel and Bohacek, 2010; Maki, 2006; Sherwin, 2009).
We hypothesize that this window of opportunity is defined, at least in part, by the function of cholinergic projections originating in the septum and nucleus basalis magnocellularis, and innervating the hippocampus and cortex (Gibbs, 2010). Acetylcholine release in the hippocampus and cortex increases wakefulness and attention, plays a role in stimulus-reward behavior, and has important effects on learning (Parikh and Sarter, 2008; Pepeu and Giovannini, 2010; Schliebs and Arendt, 2006). The function of cholinergic afferents declines with age (Baskerville et al., 2006; Fischer et al., 1992b) as well as with specific neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (Lanari et al., 2006; Linstow and Platt, 1999; Smith et al., 1999). This is demonstrated by decreases in the number and size of cholinergic neurons in the MS, DBB, and NBM (Altavista et al., 1990; Fischer et al., 1992a; Fischer et al., 1989; Mesulam et al., 1987; Stroessner-Johnson et al., 1992), decreases in high affinity choline uptake (Kristofiková et al., 1992; Sherman and Friedman, 1990), acetylcholine release (Araujo et al., 1990; Moore et al., 1996; Takei et al., 1989; Wu et al., 1988), and cholinergic synaptic transmission (Taylor and Griffith, 1993). These neurons also are adversely affected by loss of ovarian function as demonstrated by decreases in ChAT and TrkA expression beyond the effects of normal aging (reviewed in Gibbs, 2010). Studies also show that some of the increases in hippocampal plasticity and cognitive performance that are produced by estradiol in young rats are lost or attenuated by cholinergic deafferentation (Gibbs, 2007; Lam and Leranth, 2003; Rudick et al., 2003), or by pharmacological inhibition of cholinergic receptors (Daniel et al., 2005).
Recently we showed that in rats that had been ovariectomized as young adults, treating with donepezil (a cholinesterase inhibitor) at advanced age restored beneficial effects of estradiol on acquisition of a delayed matching-to-position (DMP) T-maze task (Gibbs et al., 2009). Likewise, the combination of donepezil + estradiol significantly enhanced acquisition as well as delay-dependent memory in young rats with partial loss of septal cholinergic neurons (Gibbs et al., 2011). These findings suggest that addition of a cholinesterase inhibitor can enhance or restore beneficial effects of estradiol that have been compromised by a decrease in basal forebrain cholinergic function. The purpose of the present study was to extend these findings by testing whether galanthamine, another cholinesterase inhibitor commonly used to treat Alzheimer’s disease, can restore effects of estradiol on cognitive performance in aged ovariectomized rats and to determine whether the behavioral effects are associated with effects on cholinergic function.
MATERIALS AND METHODS
Animals
Forty-eight female Fisher/Brown Norway rats were purchased at 15 months of age from Harlan Sprague-Dawley, Inc. out of the National Institute on Aging rodent colony. Rats were maintained on a 12 hour:12 hour light/dark schedule with lights on at 0700 and with free access to food and water. All procedures were consistent with PHS guidelines on the care and use of laboratory animals, and with the approval of the University’s Institutional Animal Care and Use Committee.
Treatments
At 16–17 months of age, rats were anesthetized with a combination of ketamine and xylazine and the ovaries were surgically removed. Rats received ketofen (1 mg, 2x/day s.c.) for three days following surgery to alleviate discomfort. At 21–22 months of age (5 months after ovariectomy), rats began receiving daily injections of galanthamine hydrobromide (Gal, 5mg/kg; Tocris, Inc.) or sterile saline delivered i.p. Injections continued until rats were euthanized. Galanthamine (4aS,6R,8aS)-4a,5,9,10,11,12- Hexahydro-3-methoxy-11-methyl- 6H-benzofuro[3a,3,2-ef] [2]benzazepin-6-ol) is a competitive, reversible cholinesterase inhibitor that is approved for the treatment of mild to moderate Alzheimer’s disease and vascular dementia. Galanthamine inhibits acetylcholinesterase in the brain with an IC50 of approximately 2.8–3.2 µM for the frontal cortex and the hippocampus (Thomson et al., 1991) and has a half-life of approximately 7 hrs. In addition to its anticholinesterase activity, galanthamine acts as an allosteric enhancer at multiple nicotinic receptor subtypes (Samochocki et al., 2003; Schilstrom et al., 2006). The dose of 5 mg/Kg was selected based on studies by Geerts et al. (2005) suggesting that doses in the range of 1.5–5.0 mg/Kg in rats produce optimal brain concentrations for the allosteric enhancing effect, and on a study by Woodruf-Pak et al. (2003) showing that a dose of 3 mg/Kg reversed the effects of the nicotinic receptor blocker mecamylamine on conditioned eye blink response in rabbits. Injections were administered at the end of the day (~5:00 PM) to avoid the effects of mild stress on learning (Fitz et al., 2006). One week after the beginning of treatment, half of each group (Gal or sterile saline) received a silastic capsule (6 mm length, 1.98mm I.D., 3.18 mm O.D) packed with 3mm of 100% powdered 17ß-estradiol (Sigma-Aldrich, Inc.), implanted s.c. The remaining rats received an empty capsule as a control.
DMP Training
One week after receiving the silastic capsule, rats were food restricted to 85% body weight and then trained on a delayed matching to position (DMP) T-maze task as described previously (Gibbs et al., 2011; Gibbs and Johnson, 2007; Gibbs et al., 2009). Rats were trained to traverse the maze and enter the goal arms by using a series of 6 forced “choice” trials per day for at least 4 days, each of which was rewarded with 4 food pellets (45 mg, formula 5TUM, Test Diets, Inc.). Right and left arms were alternated randomly in order to avoid the introduction of a side bias. DMP training was then performed in trial pairs. Each rat received 8 trial pairs per day. The first trial of each pair consisted of a “forced” choice in which one goal arm was blocked, requiring the rat to enter the open arm to receive a food reward (2 pellets). The rat was then immediately returned to the start box. All arms of the maze were wiped with 50% ethanol, and both goal arms were opened for the second trial. A choice was defined as an animal placing both front legs and at least one rear leg into a goal arm. Returning to the same arm as the previous “forced” trial resulted in food reward (4 pellets). Entering the opposite arm resulted in no reward and confinement to the arm for 10 seconds. During each day of training, four arms on the right and four arms on the left were selected in random order for the “forced” trials. After each trial pair, the rat was returned to its cage for 5–10 min. Each rat continued to receive 8 trial pairs per day until it reached a criterion of 15/16 correct choices over two consecutive days, or until it received 30 days of training.
Post-criterion testing
All rats that reached criterion also received post-criterion testing. One day after reaching criterion, animals received a probe trial during which the T-maze was rotated 180 degrees (relative to extramaze cues) between the forced and open trials. This probe trial was done to determine whether rats were utilizing a place strategy (i.e., were utilizing extramaze cues) or a response strategy (i.e., were utilizing internal or kinetic cues) to perform the task. An animal using extramaze cues is expected to turn in the opposite direction (i.e., enter the opposite physical arm now located in the same position of the room as the goal arm during the “forced” trial), while an animal using internal or kinetic cues would be expected to turn in the same direction (i.e., enter the same physical arm as during the “forced” trial) even though the arm occupies a different position in the room. Selecting the arm located in the same position of the room as during the “forced” trial was assigned a score of 0, while selecting the opposite arm was assigned a score of 1. One day after the probe trial, animals received four days of additional DMP training to assess delay-dependent effects on performance. On these days animals received 8 trial pairs/day and the delay between the “forced” and “open” trial was increased each day (day 1=minimal delay as during training, day 2=30 seconds, day 3=60 seconds, day 4=90 seconds).
Analysis of DMP Performance
Days to criterion (DTC) were analyzed by ANOVA using Treatment as the between factor. The effects of rotating the maze 180° during the probe trial were analyzed by contingency table and Chi-square test. Performance during increased intertrial delays was analyzed by ANOVA with repeated measures on‘Delay’.
We have observed that after several days of DMP training, some rats adopt a persistent turn whereby they consistently enter either the right or left arm of the maze. This behavior increases significantly with age (Gibbs et al., 2009) and following septal cholinergic lesions (Gibbs et al., 2011; Gibbs and Johnson, 2007). This was quantified by counting the total number of days during training that an animal chose the same arm of the maze 15 out of 16 times over a two-day period. Any animal that met this criterion was defined as having adopted a persistent turn. Chi-Square analysis was used to compare the number of animals that adopted a persistent turn between groups, and ANOVA was used to compare the number of days that this pattern persisted among the treatment groups. To evaluate the contribution that the number of days engaged in a persistent turn made to effects on DTC, the duration of the persistent turn was subtracted from DTC for each animal and the results analyzed using ANOVA.
Operant Discrimination and Reversal Learning Task
After completing DMP training, all rats were trained on an operant discrimination/reversal learning task. This task was chosen because (a) it is a non-spatial task, (b) it uses the same food reward as the DMP task, (c) it requires the ability to discriminate between sensory stimuli and to associate stimuli with food reward, and (d) it tests cognitive flexibility, i.e., the ability to alter behavior in response to a reversal of stimulus-reward contingency. Training was performed in operant chambers (Med. Associates, Inc., Georgia, VT) connected to a computer running Med-PC software. Each operant chamber contained a dim red house light, a ventilation fan, a 6W stimulus panel light, a speaker calibrated to present a 1500 Hz tone, a pellet dispenser, and a recessed food cup located immediately below the panel light. Entry into the food cup was monitored by a photosensor. Rats were adapted to the chamber by receiving one 60 min session during which they received a total of 16 food pellets delivered at intervals ranging from 2 to 6 min. Testing began the following day. Each rat received one training session per day for a total of 17 days. During the session, rats received 30 presentations of a tone stimulus and 30 presentations of a light stimulus. Rats were randomly divided into 2 groups and trained to respond to only one of the stimuli (either tone or light). If the rat entered the food cup within 10 sec of the presentation of the reinforced stimulus, a food reward (one 45 mg pellet) was delivered. If an animal entered the food cup during the non-reinforced stimulus, the stimulus was discontinued, the house light turned off for 60 sec, and no food was delivered. Stimuli were presented in random order throughout the session and occurred at one of 30 randomly selected intertrial intervals ranging from 12 to 70 sec. After 7 days of testing, the stimulus contingency was reversed such that rats were now rewarded for responding to the initially non-reinforced stimulus. Rats were trained using the reversed contingency for a total of 10 days.
The number of correct and incorrect responses committed on each day before and after reversal was tabulated for each rat. These data were analyzed by ANOVA with repeated measures on‘Day’. Post-hoc comparisons were made using a Tukey test.
In vivo microdialysis
At the completion of all behavioral training, six rats from each group were randomly assigned to undergo in vivo microdialysis to assess effects on acetylcholine (ACh) release. These rats were anesthetized with a combination of ketamine and xylazine, and a cannulae (CMA 12 Elite Guides, CMA Microdialysis, Inc.) was inserted into the brain with the tip positioned at the surface of the right hippocampus (−3.4 mm bregma, 1.18 mm lateral, −3.4 mm ventral based on Paxinos & Watson (Paxinos and Watson, 1986). The cannula was fixed to the skull with dental cement.
Microdialysis was conducted on awake rats 3–5 days after cannula placement. Concentric, 3 mm microdialysis probes were used (CMA 12 Elite Probes, CMA Microdialysis, Inc.). Probes were perfused at a rate of 1 µl/min with artificial cerebrospinal fluid (ACSF; 144.3 mM NaCl, 4.0 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 1.7 mM Na2HPO4, 0.5 mM NaH2PO4) containing 0.2 µM neostigmine bromide. This concentration of neostigmine has been shown to facilitate the ability to detect increases in ACh release in rats during food-rewarded T-maze training (Chang et al., 2006). On each day of microdialysis, probes were first dialyzed for 30 minutes against a solution of ACSF containing 10 pmol ACh/25 µL. This sample was used to calculate probe efficiency. The probe was then inserted through the cannula into the hippocampus. The rat was placed into a large plastic container for the duration of the experiment. Dialysate was collected continuously. Samples were collected and frozen every 30 min. After waiting 90 minutes for basal ACh release to stabilize, two additional samples were collected over one hour and were averaged to represent basal ACh release. Rats were then given two 45 mg food pellets every 5 minutes for 15 minutes and dialysate was collected for 30 minutes. This was done to assess ACh release in response to the food reward. Next, probes were perfused with ACSF containing elevated levels of potassium (60 mM) in order to assess potassium-stimulated release. These samples were collected after 30 and 60 min.
Tissue Collection
All rats were anesthetized with an overdose of ketamine and xylazine and then decapitated. Blood was collected for the determination of serum estradiol levels. Brains were removed and hippocampi were dissected and frozen at −80°C for analysis of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) activities. All rats were included in the neurochemical assays.
Estradiol assay
Serum levels of 17ß-estradiol (E2) were determined using a sensitive LC-MS/MS assay recently developed in our department. Samples were spiked with internal standard (2,4,16,16,17-d3–17ß-estradiol) and then extracted with n-butyl chloride. After centrifugation and evaporation, the residue was derivatized in 0.1 mL buffered dansyl chloride solution (pH 10.5). E2 was eluted from a Waters Acquity UPLC BEH C18, 1.7 um, 2.1 × 150 mm reversed-phase column, with an acetonitrile:water (0.1% formic acid) gradient. Detection and quantitation were achieved in the positive mode with a Thermo Fisher TSQ Quantum Ultra mass spectrometer interfaced via an electrospray ionization (ESI) probe with the Waters UPLC Acquity solvent delivery system. Transitions used for analysis were 506 → 171 for E2, and 511 → 171 for the deuterated internal standard. Area under the peak was quantified and used to determine absolute levels of E2/mL of sample by comparison with a series of standards. The limit of detectability for this assay is 2.5 pg/mL Intra-assay statistics show errors below 8.1% and relative standard deviations below 10.4%. Inter-assay statistics show errors below 5.0% with relative standard deviations below 7.4%.
ChAT Assay
ChAT activity in hippocampal tissues was analyzed as previously described (Gibbs and Johnson, 2007). Tissues were dissociated by sonication in a medium containing EDTA (10 mM) and Triton X-100 (0.5%) and diluted to a concentration of 10 mg tissue/mL An aliquot of each sample was used for the determination of total protein. Three 5 µl aliquots of each sample were incubated for 30 min at 37°C in a medium containing 3[H] acetyl-CoA (Perkin Elmer, Inc., 25,000–30,000 d.p.m./tube, final concentration 0.25mM acetyl-CoA), choline chloride (10.0mM), physostigmine sulfate (0.2mM), NaCl (300mM), sodium phosphate buffer (pH 7.4, 50mM), and EDTA (10 mM). The reaction was terminated with 4ml sodium phosphate buffer (10 mM) followed by the addition of 1.6ml of acetonitrile containing 5mg/ml tetrephenylboron. The amount of [3H] ACh produced was determined by adding 8ml of Insta-Fluor scintillation cocktail (Packard Instruments, Meriden, CT) and counting total cpm in the organic phase using an LKB beta-counter. Background was determined using identical tubes to which no sample was added. For each sample, triplicates were averaged and the difference between total cpm and background cpm was calculated. This was used to estimate the total amount of ACh produced per sample. ChAT activity was then calculated for each sample as pmol ACh manufactured/h/µg protein. This assay produced a linear response as a function of sample dilution (i.e. protein content; see Supplemental Figure 1A). Treatment effects were evaluated by ANOVA. Post-hoc comparisons were made using a Tukey test.
AChE Assay
AChE activity in the hippocampus was measured using a method adapted from Ellman et al. (Ellman et al., 1961). Three 5-µL aliquots of each sonicate (see above) were placed into separate wells of a 96-well plate containing 175 µL of reaction solution composed of sodium phosphate buffer (pH 7.4, 50 mM), tetraisopropyl pyrophosphoramide (IsoOMPA, 0.03 mM), and 5,5’-dithiobis(2-nitrobenzoic acid) (0.33 mM). Plates were pre-incubated for 30 min at 37°C to inactivate non-specific esterases. The reaction was initiated by adding 20 µL of acetylthiocholine iodide to a final concentration of 0.5 mM. Plates were immediately mixed and scanned at 410 nm using an Emax plate reader (Molecular Devices, Menlo Park, CA, USA). Plates were incubated in the dark for an additional 30 min at 37°C, and then scanned again at 410 nm. AChE activity for each sample was calculated as the average net (OD410 × reaction volume) / (extinction coefficient (13.6 mmol/µL) × sample concentration × sample volume × incubation time) and expressed as mmol/h/µg protein. Mean AChE activity for each animal was then calculated. This assay produces a linear response as a function of both incubation time and protein concentration for OD values less than 2.0 (see Supplemental Figures 1B & 1C). Treatment effects were evaluated by ANOVA. Post-hoc comparisons were made using a Tukey test.
HPLC analysis of ACh
Dialysates were analyzed using HPLC, enzymatic conversion, and electrochemical detection as previously described (Rhodes et al., 1996). An ESA HPLC system was used. Flow rate was 0.30 µL/min. Mobile phase consisted of 100 mM di-sodium hydrogen phosphate anhydrous (Fluka, Inc.), 1-Octanesulfonic acid (Sigma-Aldrich, Inc.), Reagent MB (ESA, Inc), and the pH was adjusted to 8.0 using phosphoric acid. Samples were passed through a Shiseido Capcell Pak C18 MGII column, 100A, 3micron, 2.0 × 35 mm, and then through a solid-phase reactor (ESA, Inc.) containing acetylcholinesterase and choline oxidase at 35 °C. The resulting H2O2 was detected with a model M5041 electrochemical cell attached to a Coulochem model 5300 detector (ESA, Inc.). Chromatograms were analyzed using EasyChrome software. ACh standards were prepared in ACSF. Twenty-five microliters of each sample was injected. All standards were run in duplicate. Quantity of ACh was determined by measuring area under the ACh peak. This assay produced a linear response as a function of ACh content, and was able to detect as little as 30 fmol of ACh per 25 µL sample (see Supplemental Figure 1D). Values were expressed as picomoles ACh per 25 µl sample. The two samples collected prior to presenting food were averaged as an estimate of basal ACh release. Subsequent samples were used to calculate percent change in ACh release relative to baseline for each rat. In this way, each rat served as its own control for assessing effects on release. Percent change that was calculated from the two samples collected over 60 minutes after switching to high potassium were averaged as a measure of potassium-stimulated release. This was done for each rat. Average basal, food-stimulated release and potassium-stimulated release were then calculated for each treatment group. Effects of treatment on basal and potassium-stimulated release were analyzed using ANOVA followed by a Tukey test.
RESULTS
Animal Attrition
Six rats died or had to be euthanized for health reasons prior to completion of the study. Five rats would not run the T-maze. One rat in the Gal-treated group failed to reach criterion on the task. All of these rats were excluded from the final analysis. Resulting group sizes are Controls (n=10), Gal (n=8), E2 (n=9), and Gal+E2 (n=9).
Serum levels of estradiol
Levels of estradiol in non-E2-treated rats were below the level of detection. Mean serum levels of estradiol in E2-treated rats were 39.1 ± 4.6 pg/mL and 28.4 ± 3.9 pg/mL for E2- and Gal+E2-treated rats. These values did not differ significantly from each other.
Effects on DMP acquisition
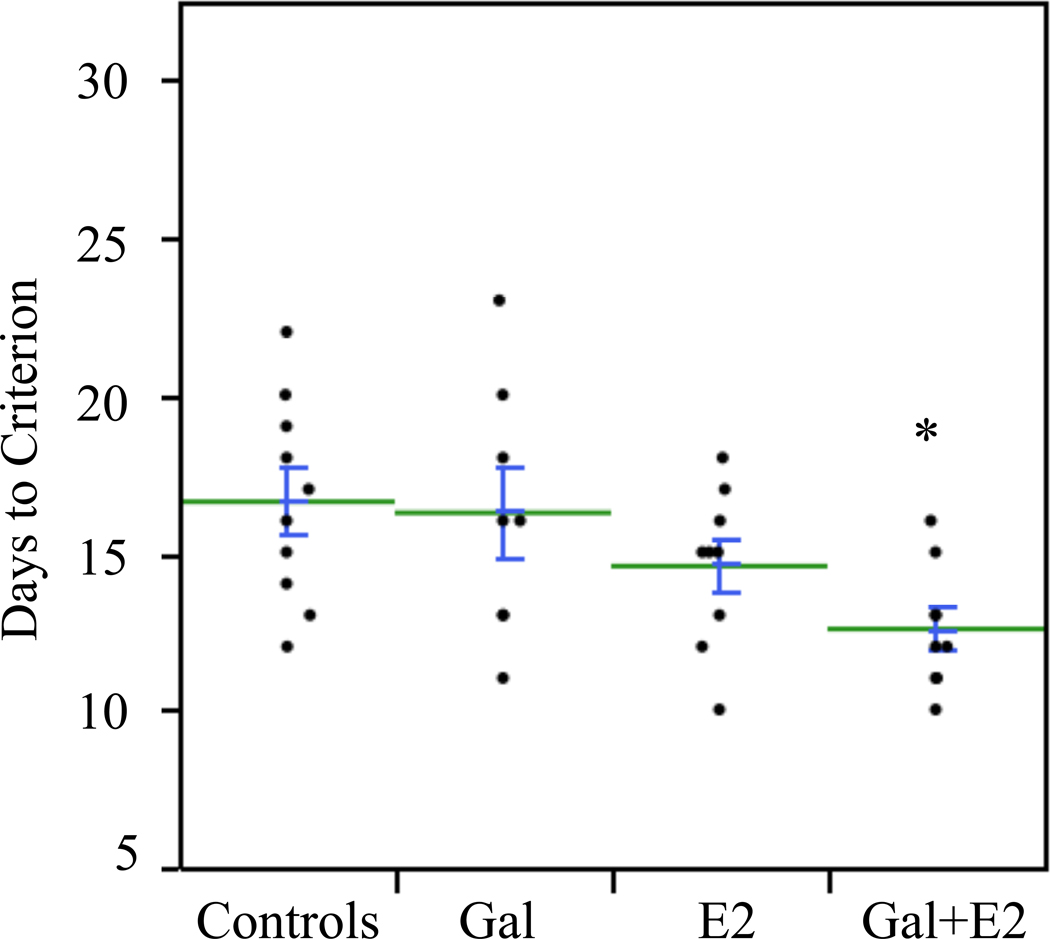
Treatments significantly affected the number of days required to reach criterion on the DMP task (F[3,32]=3.5, p<0.05). Results are illustrated in Figure 1. On average, controls took the longest number of days to reach criterion (16.6 ± 1.0). Post-hoc analysis revealed that rats treated with Gal+E2 took significantly fewer days to reach criterion (12.6 ± 0.6) than controls. Treatment with Gal or E2 alone produced intermediate results which did not differ significantly from controls. These results demonstrate that treating aged rats that were ovariectomized at middle-age with Gal+E2 significantly improved the rate of DMP acquisition whereas treating with Gal or E2 alone did not.
Figure 1.
Plot summarizing the effects of treatment on acquisition of the delayed matching-to-position task. Values indicate the number of days required to reach criterion. Each dot represents one rat. Lines indicate group mean ± s.e.m. Rats treated with Gal+E2 required significantly fewer days to reach criterion than controls (*p<0.05).
Effects on Persistent Turn
Next we tested whether treatments affected the predisposition of rats to adopt and maintain a persistent turn while performing the DMP task and whether this might account for the effects of Gal+E2 on DMP acquisition. Most rats adopted a persistent turn at some point during DMP acquisition. Within each group, the number of rats that adopted a persistent turn were: Controls (9/10), Gal (4/8), E2 (7/9) and Gal+E2 (5/9). These ratios did not differ significantly from each other (X2(3)=4.5, p>0.2). Nor was there a significant effect of treatment on the number of days that rats engaged in a persistent turn (F[3,32]=1.8, p=0.16). When analyzed for an independent effect of galanthamine, rats treated with Gal (Gal or Gal+E2) were significantly less likely to adopt a persistent turn than rats that received vehicle or E2 alone (X2(1)=4.1, p<0.05). There also was a trend for rats treated with Gal to spend fewer days engaged in a persistent turn (2.6 ± 0.7 vs. 5.0 ± 1.0; t(32)=1.9, p=0.06). In contrast, there was no evidence for an effect of E2 on the predisposition to adopt a persistent turn or on the number of days that rats engaged in a persistent turn. Subtracting the number of days that rats engaged in a persistent turn from DTC did not eliminate the significant effect of treatment on performance (F[3,32]=2.9, p=0.05), and rats treated with Gal+E2 still differed significantly from controls (p<0.05 by Tukey test). These data suggest that the effects of Gal+E2 on performance were not due to an effect on the predisposition to adopt or maintain a persistent turn.
Probe Trial
All rats that reached criterion received a probe trial in which the maze was rotated 180° between the forced and open choice. As mentioned above, this probe trial was done to determine whether, after reaching criterion, rats were utilizing a place strategy (i.e., were utilizing extramaze cues) or a response strategy (i.e., were utilizing internal or kinetic cues) to perform the task. An animal using extramaze cues is expected to turn in the opposite direction (i.e., enter the opposite physical arm now located in the same position of the room) as the goal arm during the “forced” trial, while an animal using internal or kinetic cues would be expected to turn in the same direction (i.e., enter the same physical arm) as during the “forced” trial even though the arm occupies a different position in the room. Likewise, any rat engaged in persistent turning behavior would be expected to turn in the same direct as during the ‘forced’ trial. After rotating the maze, 8/10 controls selected the arm located in the same location of the room as was entered during the forced choice, consistent with using a place strategy. In comparison, 5/8, 5/9, and 5/9 rats from the Gal-, E2, and Gal+E2-treated groups selected the same physical arm of the maze that was entered during the forced choice, even though the arm was located in a different position of the room. These ratios did not differ significantly from chance (p>0.05 in all cases), and did not differ significantly from each other (x2(3)= 6.0, p=0.11).
Delay-Dependent Performance
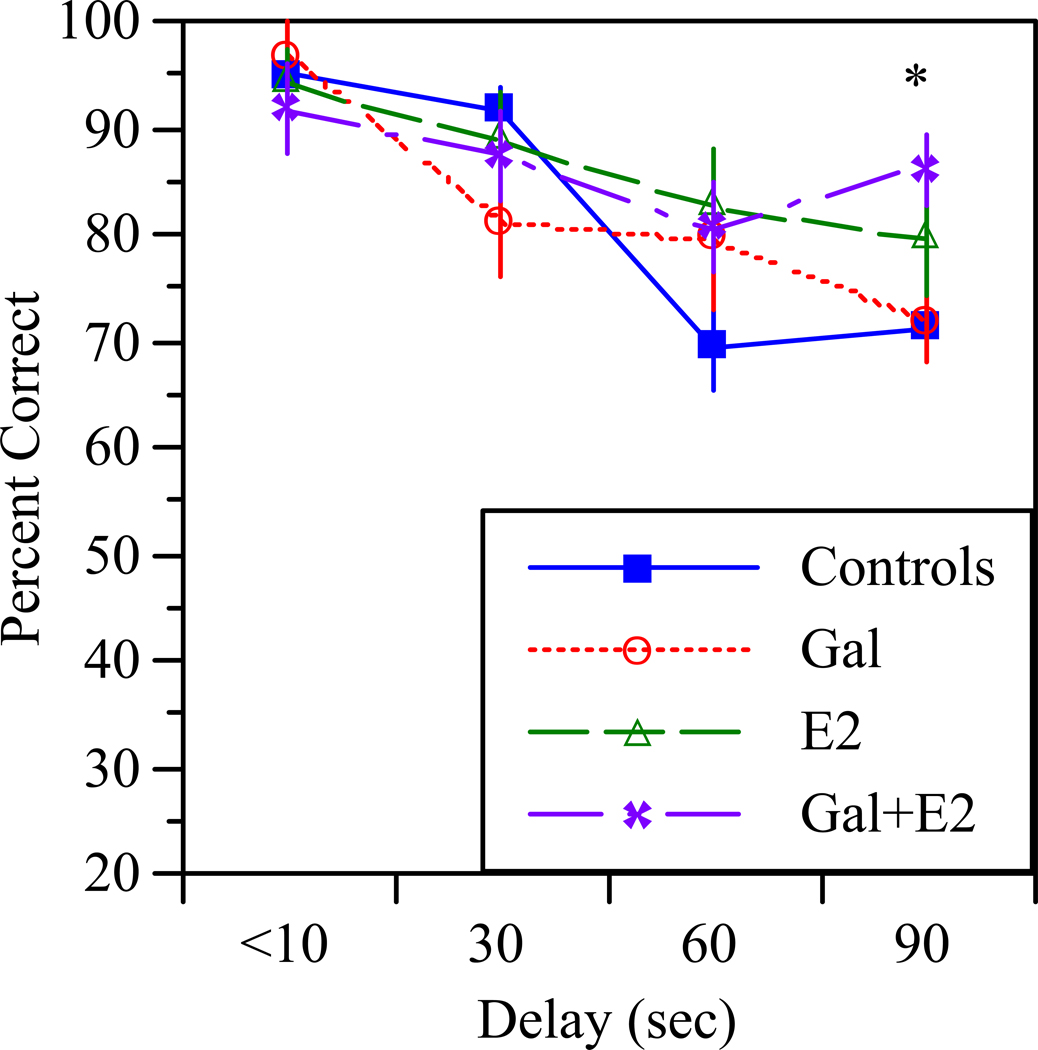
Following the probe trial, rats underwent testing with increasing delays between the forced and open trials (Figure 2). ANOVA with repeated measures on delay revealed a significant effect of delay (F[3,90]=20.7, p<0.0001) as well as a significant treatment × delay interaction (F[9,90]=2.1, p<0.05). Post-hoc analysis showed that rats treated with Gal+E2 performed significantly better than controls at the 90 second delay, indicating a beneficial effect of Gal+E2 on delay-dependent performance.
Figure 2.
Effects of increasing the intertrial delay on performance. Note that increasing the intertrial delay reduced performance accuracy. Rats treated with Gal+E2 performed significantly better that controls at the 90 second delay (*p<0.05).
Effects on Operant Discrimination and Reversal Learning
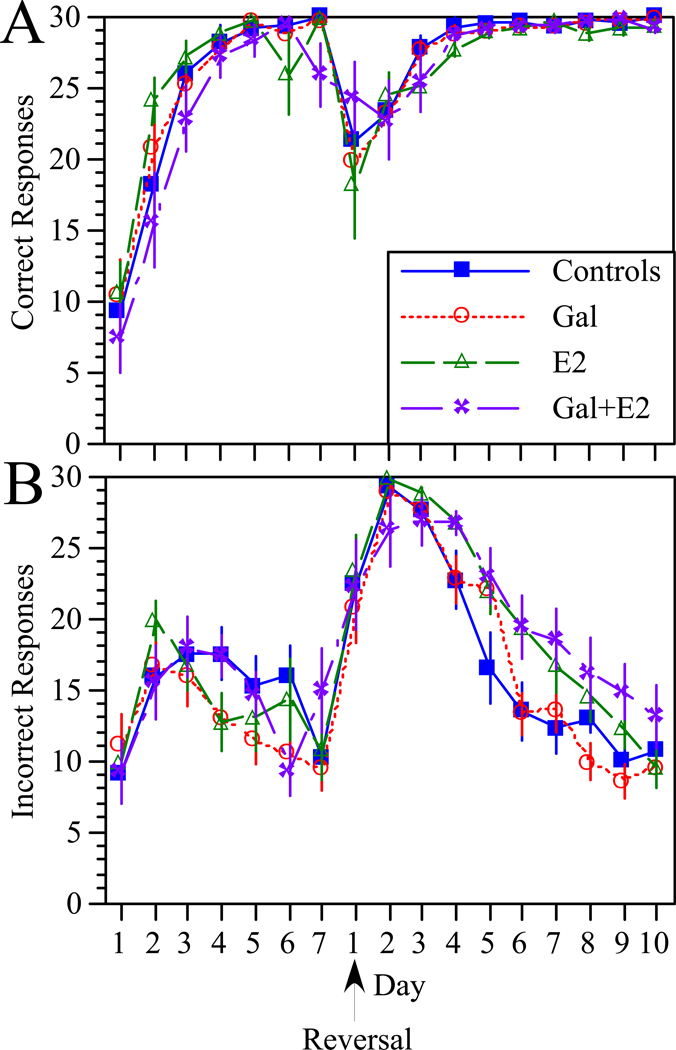
Numbers of correct and incorrect responses committed prior to and after reversal of the stimulus contingency are illustrated in Figure 3. All rats demonstrated the ability to learn to respond to the rewarded stimulus and not to the non-rewarded stimulus and to alter performance in response to the reversed contingency. Treatments produced no significant effects on the number of correct or incorrect responses committed prior to reversal, or on the number of correct responses committed after reversal. Likewise, no significant effect of treatment on the number of incorrect responses was detected following reversal; however, when data were collapsed according to hormone status, a significant effect of E2 was detected on the number of incorrect responses produced following reversal. ANOVA using ‘E2 treatment’ as the independent factor with repeated measures on ‘day’ revealed a significant effect of E2 (F[1,39]=6.3, p<0.02), a significant effect of day (F[9,351]=90.4, p<0.0001), and a significant E2 × day interaction (F[9,351]=2.6, p<0.01). Post-hoc analysis showed that rats that received E2 made significantly more incorrect responses on days 4 and 6–9 after reversal than rats that were not treated with E2 (supplemental Figure 2).
Figure 3.
Effects of treatment on the number of correct responses (A) and incorrect responses (B) on each day of the operant discrimination and reversal learning task. Symbols indicate group means ± s.e.m. The arrow indicates the day on which the stimulus-reward contingency was reversed. Note that rats in all groups quickly learned to respond to the reinforced stimulus and to suppress responses to the non-reinforced stimulus. No significant effects of treatment were detected prior to reversal. Following reversal, no effects of treatment on the number of correct or incorrect response were detected; however, when data were collapsed according to hormone status, E2 treatment was associated with a greater number of incorrect responses on days 4 and 6–9 following reversal compared with rats that did not receive E2 (see supplemental Figure 2 and text for details). A similar analysis of Gal revealed no effect.
Effects on Hippocampal ChAT and AChE activity and on ACh release
Mean levels of ChAT and AChE activity in the hippocampus of each treatment group are summarized in Table 1. No significant effects of treatment were observed.
Table 1.
Summary of Hippocampal Choline Acetyltransferase (ChAT) and Acetylcholinesterease (AChE) Activity by Treatment
| Tx | N | ChAT Activity pmol ACh produced/h/µg protein |
AChE Activity mmol ACh degraded/h/µg protein |
|---|---|---|---|
| Controls | 10 | 26.2 ± 1.5 | 1.4 ± 0.15 |
| Gal | 8 | 26.6 ± 1.3 | 1.3 ± 0.11 |
| E2 | 9 | 26.2 ± 2.0 | 1.2 ± 0.12 |
| Gal+E2 | 9 | 24.4 ± 2.1 | 1.3 ± 0.13 |
Effects of treatment on extracellular levels of ACh are summarized in Table 2. There was a strong trend for Gal treatment to be associated with increased basal extracellular levels of ACh (F[1,13]=4.1, p=0.06). In contrast, there was no evidence for an effect of E2 (F[1,13]=1.3, p=0.27), nor for an interaction between E2 × Gal (F[1,13]=1.2, p=0.29) on basal extracellular levels of ACh. In response to food, E2-treatment, but not Gal, was associated with a significant increase (~30%) in extracellular levels of ACh. ANOVA revealed a significant effect of E2 (F[1,11]=7.4, p<0.02), no significant effect of Gal (F[1,11]=0.04, p=0.85), and no E2 × Gal interaction (F[1,11]=0.09, p=0.77). In response to elevated potassium, levels of extracellular ACh increased approximately 2-fold in all treatment groups, with no differential effects of treatment.
Table 2.
Effects of Treatment on ACh Release in Hippocampus
| Tx | N | Basal Release (pmol/25 µL) |
Release Stimulated by Food (% Change from Basal) |
Release Stimulated by Elevated Potassium (% Change from Basal) |
|---|---|---|---|---|
| Controls | 6 | 0.29 ± 0.06 | −16.6 ± 10.0 | 149.4 ± 49.9 |
| Gal | 4 | 0.44 ± 0.07 | −0.5 ± 7.7 | 107.8 ± 36.4 |
| E2 | 4 | 0.26 ± 0.10 | 33.3 ± 17.5* | 124.6 ± 1.6 |
| Gal+E2 | 5 | 0.31 ± 0.04 | 28.5 ± 12.4* | 114.2 ± 28.1 |
DISCUSSION
DMP Acquisition
The primary finding of this study is that treating with a combination of Gal + E2 significantly enhanced the rate of DMP acquisition and improved short-term delay-dependent spatial memory performance in aged rats that were ovariectomized at middle-age. This was demonstrated by a significant decrease in the number of days required to reach criterion and by significantly better performance at the 90 second inter-trial delay, relative to vehicle-treated controls. The effects were task-specific in that no beneficial effect of Gal + E2 was detected on the operant discrimination/reversal learning task. In fact, E2 alone had a negative effect on reversal learning which was not attenuated by Gal. This is consistent with other recent studies showing that sustained E2 replacement produces significant deficits on other operant tasks including an operant delayed spatial alternation task, and a differential reinforcement of low rates of responding task (Wang et al., 2009; Wang et al., 2008). Notably, the effect of E2 on the operant delayed spatial alternation task is consistent across age (Wang et al., 2009). In addition to being task-specific, the effects on the DMP task likely are not due to affects on motivation since both the DMP task and the operant task were motivated by the same food reward. Nor are they likely due to effects on the ability to detect and discriminate between sensory cues.
These findings are in agreement with two previous studies, one showing that donepezil + E2 enhances DMP acquisition in aged ovariectomized rats (Gibbs et al., 2009), and the other showing that donepezil + E2 enhances DMP acquisition and delay-dependent performance in young ovariectomized rats with partial loss of septal cholinergic neurons (Gibbs et al., 2011). In addition, the findings are consistent with data showing that cholinergic inputs to the hippocampus play an important role in mediating E2 effects on DMP acquisition (Gibbs, 2007) and other spatial learning tasks (Daniel and Dohanich, 2001; Daniel et al., 2005), and with the hypothesis that reduced basal forebrain cholinergic function contributes to a diminished beneficial effect of E2 on cognitive performance in aged ovariectomized rats. Most important, the findings demonstrate that combining E2 with cholinesterase inhibitor therapy is more effective at enhancing performance of aged ovariectomized rats on the DMP task than either treatment alone.
Effects on Learning Strategy and Perseveration
ACh release in the hippocampus and striatum have been shown to be differentially associated with the use of allocentric (relying on extramaze cues) vs. egocentric (relying on intramaze or kinetic cues) learning strategies (Marriott and Korol, 2003; McIntyre et al., 2003; Pych et al., 2005). In addition, cholinergic denervation of the hippocampus and cortex significantly increase perseverative behavior on certain types of tasks (Cutuli et al., 2009; Fitz et al., 2008; Gibbs and Johnson, 2007). For example, combined lesions of cholinergic neurons in the medial septum and nucleus basalis cause rats to be far less likely to rely on extramaze spatial cues to perform the DMP task (Gibbs and Johnson, 2007). Such lesions also significantly increase the predisposition of rats to adopt a persistent turn during DMP acquisition (Fitz et al., 2008; Gibbs and Johnson, 2007). Initially this increases performance to chance levels, but then retards further improvement. Similar effects are observed with age, such that middle-aged and aged rats show a progressive increase in persistent turn during DMP acquisition which corresponds with an increase in the number of days required to reach criterion (Gibbs et al., 2009). This is consistent with studies conducted over 30 years ago showing that aged rats are more likely to utilize a response strategy vs. a place strategy than young rats, and to engage in repetitive choice sequences when solving various spatial discrimination tasks (Barnes et al., 1980). The findings also are consistent with the documented decline in basal forebrain cholinergic function that occurs in rats with advanced age (Fischer et al., 1992b; Fischer et al., 1989; Kristofiková et al., 1992; Moore et al., 1996), and with the significant increase in perseverative behavior observed in patients with Alzheimer’s disease (Pekkala et al., 2008). These data suggest that changes in basal forebrain cholinergic function can have a substantial impact on learning strategy and perseveration in humans.
In the present study, results of the probe trial administered after reaching criterion suggest that treatments did not significantly affect the degree to which rats relied upon spatial cues to acquire the DMP task. Galanthamine did however produce a significant decrease in persistent turn, suggesting a reduction in perseverative behavior. The effect on persistent turn was not entirely responsible, however, for the beneficial effects of Gal + E2 on DMP acquisition. This is demonstrated by the fact that a significant effect of Gal + E2 on days to criterion was observed even after subtracting the number of days that rats engaged in a persistent turn. This differs from the effects of donepezil + E2 on DMP acquisition observed in our previous studies (Gibbs et al., 2011; Gibbs et al., 2009) and suggests that the effects of Gal + E2 on performance involve slightly different mechanisms.
Gal is a less potent cholinesterase inhibitor than donepezil (Thomson et al., 1991). Gal also acts as an allosteric potentiating ligand (APL) at nicotinic acetylcholine receptors (nAChRs) (Samochocki et al., 2003; Schilstrom et al., 2006). Many studies have shown that nicotine has anti-amnestic effects both in animals and in humans (Cincotta et al., 2008; Levin et al., 2006; Levin and Rezvani, 2002; Sarter et al., 2009), mediated by nACHRs. nAChRs are a family of ligand-gated ion channels composed of α and ß subunits which can combine in different conformations to produce receptors with distinct pharmacologies (Albuquerque et al., 2009; Jürgensen and Ferreira, 2009; Taly et al., 2009). The α4ß2 and α7 subtypes are the two most common nAChR subtypes in the mammalian brain (Lindstrom, 1997). The acute effect of activating nAChRs is the fast opening of a cationic channel permeable to Na+, K+ and sometimes Ca2+ ions. Thus, when activated, nAChRs induce neuronal membrane depolarization and directly or indirectly initiate calcium influx.
nAChRs receptors are located both postsynaptically and presynaptically, and presynaptic nAChRs have been shown to play an important role in modulating neurotransmitter release, including the release of glutamate and GABA (Albuquerque et al., 2000). Nicotinic APLs are weak non-competitive agonists that do not induce nicotinic responses on their own, but that potentiate nAChR activity induced by classical nAChR agonists (Maelicke et al., 2000). Recent studies show that Gal, acting as an APL on presynaptically located nAChRs that are weakly tonically activated, can induce long-lasting facilitation of glutamate and GABA transmission at CA1 neurons in rat hippocampal slices (Santos et al., 2002). These effects were not produced by other ChEIs that do not have nicotinic APL activity. This suggests that enhancing nAChR activity could be one mechanism by which Gal influences cognitive performance and perhaps facilitates positive effects E2.
Taylor & Maloney (Taylor and Maloney, 2010) recently showed that treating ovariectomized rats with nicotine + E2 was particularly potent at improving performance on a visuo-spatial orientation task. Sapronov et al. (Sapronov et al., 2006) reported that chronic treatment with a selective α4ß2-nicotinic receptor agonist in combination with E2 significantly improved performance in a rat model of Alzheimer’s-type dementia. This effect was blocked by mecamylamine, a nicotinic receptor antagonist. These data show that nicotinic receptor activity can interact with E2 to significantly enhance cognitive performance in normal rats and in a rat model of Alzheimer’s disease. Hence, the beneficial effects of Gal + E2 on DMP performance may be due to the nicotinic enhancing effect of Gal, rather than to the inhibition of cholinesterase. This may account for the ability to enhance performance independently of an effect on persistent turn.
Effects on Cholinergic Measures
One of our goals also was to assess the effects of treatment on measures of hippocampal cholinergic function. No significant effects of Gal or E2 on hippocampal ChAT or AChE activity were detected. This is in contrast to studies showing that short-term treatment with E2 alone increases ChAT activity in the hippocampus and frontal cortex of young adult rats (Gibbs, 1997; Luine, 1985; Singh et al., 1994). This effect appears to be dependent on both dose and duration of treatment (Gibbs, 1997) and may be disappear after long-term (8 weeks) treatment (Gibbs, 2000). This is despite the fact that effects on ACh release persist even after long-term treatment (Gabor et al., 2003). Recent studies also show that the effects of E2 on ChAT in the hippocampus are lost with age and time following ovariectomy (Bohacek et al., 2008; Gibbs et al., 2009). The reasons for these changes currently are unknown; however, they are consistent with the lack of effect on ChAT activity observed here. The ability of Gal to inhibit cholinesterase activity in the brain following short-term treatment is well documented. The failure in the present study to detect a reduction in hippocampal AChE activity in rats treated for over two months with Gal is somewhat surprising but not unprecedented. Other studies have demonstrated that up-regulation of AChE can occur in the brain in response to long-term cholinesterase inhibitor therapy (Darreh-Shori et al., 2006; Davidsson et al., 2001; Nordberg et al., 1999). This up-regulation of AChE may be one factor limiting the long-term effectiveness of cholinesterase inhibitor therapy in people with dementia. A compensatory up-regulation of AChE also may account for the lack of effect on hippocampal AChE activity in the current study.
Gal alone produced a modest increase in basal extracellular levels of ACh (P=0.06); however, no significant effects of treatment on potassium-stimulated ACh release were observed. Again, this is in contrast to studies showing that E2 increases potassium-stimulated ACh release in young ovariectomized rats (Gibbs et al., 2004; Gibbs et al., 1997; Marriott and Korol, 2003). This suggests that that, like the effect on ChAT, the effect of E2 on potassium-stimulated ACh release is lost with advanced age and long-term loss of ovarian function. Furthermore, these data show that the effects of E2 on ACh release are not restored by Gal.
E2 (but not Gal) did significantly increase ACh release in response to the food pellets that were used as a reward in both the DMP and operant discrimination tasks. This suggests that in E2-treated rats, food reward was associated with ACh release in the hippocampus. Other studies have demonstrated increased ACh release in the hippocampus, as well as in other cortical areas, during learning associated with food-reward (Chang et al., 2006; Iso et al., 1999; Orsetti et al., 1996; Pych et al., 2005). Parikh et al. (Parikh et al., 2007) recently showed that ACh release in the prefrontal cortex is associated with successful performance on a cue detection task. In that study, release specifically correlated with the timing of the expected delivery of a food reward, suggesting that ACh release plays a role in connecting the benefits of food reward with a particular behavioral context for subsequent use during a decision making event. In the current study, food reward increased ACh release only in rats treated with E2. It is possible that the effect of food reward on ACh release is diminished with age and ovariectomy and that this response is restored by E2. This in turn would contribute to beneficial effects on cognitive performance. A possible explanation for this could be effects on orexin-containing fibers originating from cells in the lateral hypothalamus.
Potential Role of Orexins
Orexins are recently discovered neuropeptides that are produced in the hypothalamus and play an important role in regulating energy balance, reproduction, sleep-wake cycle, and vigilance (de Lecea, 2010; Nishino, 2011; Sakurai et al., 2010; Szekely et al., 2010). Orexins also are important for reward processing (Aston-Jones et al., 2010). Orexin-containing fibers project to many areas of the brain; however, studies suggest that the basal forebrain is a key site through which orexins activate cortical areas and promote behavioral arousal (Arrigoni et al., 2010). Studies show that orexin-positive fibers synapse onto both cell bodies and distal dendrites of cholinergic neurons located in the septum and substantia innominata (Espana et al., 2005; Fadel and Frederick-Duus, 2008; Peyron et al., 1998; Wu et al., 2004). Local infusion of orexins excites basal forebrain cholinergic neurons, and induces cortical release of ACh and promotes wakefulness (Eggermann et al., 2001; Espana et al., 2001; Fadel et al., 2005; Thakkar et al., 2001). Importantly, in rats conditioned to anticipate food, lesions of orexin neurons reduces cortical ACh release in anticipation of food (Frederick-Duus et al., 2007), suggesting a role for orexins in connecting food reward with a particular behavioral context. Studies also show that hypothalamic levels of orexins vary across the estrous cycle, are highest on proestrus, and are reduced in aged non-cycling rats (Porkka-Heiskanen et al., 2004). This suggests that orexin levels are regulated in part by E2. It is possible that E2 influences orexin signaling on the cholinergic neurons to enhance ACh release in association with food reward. This could result in a stronger association between the food reward and the behavioral context, resulting in a faster rate of learning on the DMP task. This mechanism may be impaired with age and compensated in part by the direct nAChR enhancing effect of Gal. This could account for the beneficial effect of E2 + Gal on DMP acquisition in aged rats.
Summary
Our data show that administering Gal + E2 to aged rats that were ovariectomized at middle-age significantly enhanced the rate of DMP acquisition and improved short-term delay-dependent spatial memory performance. Treatment with Gal or E2 alone were without significant effect. This is the third of a series of studies showing that combining E2 treatment with a cholinesterase inhibitor used to treat Alzheimer’s disease can significantly enhance cognitive performance in aged rats and in young rats with partial septal cholinergic lesions. These data support our hypothesis that beneficial effects of E2 on cognitive performance diminish with age and time following loss of ovarian function due to deficits in basal forebrain cholinergic function, and suggest that beneficial effects can be enhanced or restored by combining E2 with an appropriate cholinergic medication. Similar effects in people may be particularly beneficial for older women who have not used hormone therapy for many years and are beginning to show signs of mild cognitive impairment. Galanthamine may offer added benefits compared to other cholinesterase inhibitors such as donepezil or rivastigmine due to its ability to act as an allosteric enhancer at nAChRs. Galanthamine also may have added benefit due to its short half-life and the ability to maintain diurnal variations in cholinergic activity (Davis and Sadik, 2006). Clinical studies are needed to evaluate these effects. In addition, we propose that effects of E2 on cholinergic function and cognition may be due in part to effects on orexin signaling in the basal forebrain. Orexins have been shown to regulate cholinergic projections to the hippocampus and cortex in association with arousal and reward and may play an important role in mediating E2 effects on cholinergic function and cognitive performance. This hypothesis has not yet been tested and needs to be evaluated.
Highlights.
>Hypothesize that beneficial effects of estradiol on cognition are reduced in older women due to decline in basal forebrain cholinergic function. >Galanthamine + estradiol enhanced acquisition of a spatial task as well as delay-dependent memory performance in aged ovariectomized rats. >Suggests that combining cholinesterase inhibitor with estrogen therapy can benefit cognition.
Supplementary Material
Graphs showing characteristics of our biochemical assays. (A) ChAT assay results showing that the amount of acetylcholine (ACh) produced per minute increases linearly with protein content. (B & C) AChE assay results showing that signal (ΔOD) increases linearly as a function of protein content (B) and incubation time (C). (D) HPLC analysis of ACh. Graph is a typical standard curve showing a linear relationship between quantity of ACh and area under the peak. Each graph is derived from duplicate samples and shows best-fit regression line.
Effects of estradiol on the number of incorrect responses on each day of the operant discrimination and reversal learning task. Symbols indicate group means ± s.e.m. These are the same data shown in Figure 3B, but combined and analyzed according to hormone treatment. Note that E2 treatment was associated with a greater number of incorrect responses on days 4 and 6–9 following reversal of the stimulus-reward contingency. *p<;0.05
ACKNOWLEDGEMENTS
Supported by NIH grants R01AG021471 (RBG), S10RR023461 (RBG). HPLC equipment was provided by David Johnson of Duquesne University with support from NIH grant R01AG016261. We also wish to acknowledge the Small Molecules Biomolecular Core facility of the University of Pittsburgh School of Pharmacy for developing the estradiol assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose
LITERATURE CITED
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Mike A, Eisenberg HM, Maelicke A, Alkondon M. Neuronal nicotinic receptors in synaptic functions in humans and rats: physiological and clinical relevance. Behavioural brain research. 2000;113:131–141. doi: 10.1016/s0166-4328(00)00208-4. [DOI] [PubMed] [Google Scholar]
- Altavista MC, Rossi P, Bentivoglio AR, Crociani P, Albanese A. Aging is associated with a diffuse impairment of forebrain cholinergic neurons. Brain Research. 1990;508:51–59. doi: 10.1016/0006-8993(90)91116-x. [DOI] [PubMed] [Google Scholar]
- Araujo DM, Lapchak PA, Meaney MJ, Collier B, Quirion R. Effects of aging on nicotinic and muscarinic autoreceptor function in the rat brain: relationship to presynaptic cholinergic markers and binding sites. Journal of Neuroscience. 1990;10:3069–3078. doi: 10.1523/JNEUROSCI.10-09-03069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol (Oxf) 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Canadian journal of psychology. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- Baskerville KA, Kent C, Nicolle MM, Gallagher M, McKinney M. Aging causes partial loss of basal forebrain but no loss of pontine reticular cholinergic neurons. Neuroreport. 2006;17:1819–1823. doi: 10.1097/WNR.0b013e32800fef5a. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Savage LM, Gold PE. Microdialysis measures of functional increases in ACh release in the hippocampus with and without inclusion of acetylcholinesterase inhibitors in the perfusate. J Neurochem. 2006;97:697–706. doi: 10.1111/j.1471-4159.2006.03765.x. [DOI] [PubMed] [Google Scholar]
- Cincotta SL, Yorek MS, Moschak TM, Lewis SR, Rodefer JS. Selective nicotinic acetylcholine receptor agonists: potential therapies for neuropsychiatric disorders with cognitive dysfunction. Curr Opin Investig Drugs. 2008;9:47–56. [PubMed] [Google Scholar]
- Cutuli D, Foti F, Mandolesi L, De Bartolo P, Gelfo F, Federico F, Petrosini L. Cognitive Performances of Cholinergically Depleted Rats Following Chronic Donepezil Administration. J Alzheimers Dis. 2009;17:161–176. doi: 10.3233/JAD-2009-1040. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Darreh-Shori T, Meurling L, Pettersson T, Hugosson K, Hellstrom-Lindahl E, Andreasen N, Minthon L, Nordberg A. Changes in the activity and protein levels of CSF acetylcholinesterases in relation to cognitive function of patients with mild Alzheimer’s disease following chronic donepezil treatment. J Neural Transm. 2006;113:1791–1801. doi: 10.1007/s00702-006-0526-2. [DOI] [PubMed] [Google Scholar]
- Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, Hesse C. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer’s disease. Neurosci Lett. 2001;300:157–160. doi: 10.1016/s0304-3940(01)01586-5. [DOI] [PubMed] [Google Scholar]
- Davis B, Sadik K. Circadian cholinergic rhythms: implications for cholinesterase inhibitor therapy. Dement Geriatr Cogn Disord. 2006;21:120–129. doi: 10.1159/000090630. [DOI] [PubMed] [Google Scholar]
- de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf) 2010;198:203–208. doi: 10.1111/j.1748-1716.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres VA, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- Fadel J, Frederick-Duus D. Orexin/hypocretin modulation of the basal forebrain cholinergic system: insights from in vivo microdialysis studies. Pharmacol Biochem Behav. 2008;90:156–162. doi: 10.1016/j.pbb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in patial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Fischer W, Gage FH, Björklund A. Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur. J. Neurosci. 1989;1:34–45. doi: 10.1111/j.1460-9568.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Aversive stimulus attenuates impairment of acquisition in a delayed match to position T-maze task caused by a selective lesion of septo-hippocampal cholinergic projections. Brain Res Bull. 2006;69:660–665. doi: 10.1016/j.brainresbull.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Selective lesion of septal cholinergic neurons in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain Res Bull. 2008;77:356–360. doi: 10.1016/j.brainresbull.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Research. 2003;962:244–247. doi: 10.1016/s0006-8993(02)04053-2. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005;1033:186–193. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Research. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm Behav. 2011 doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997;749:143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem. 2007;88:19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso H, Ueki A, Shinjo H, Miwa C, Morita Y. Reinforcement enhances hippocampal acetylcholine release in rats: an in vivo microdialysis study. Behav Brain Res. 1999;101:207–213. doi: 10.1016/s0166-4328(98)00154-5. [DOI] [PubMed] [Google Scholar]
- Jürgensen S, Ferreira ST. Nicotinic Receptors, Amyloid-beta, and Synaptic Failure in Alzheimer’s Disease. J Mol Neurosci. 2009 doi: 10.1007/s12031-009-9237-0. [DOI] [PubMed] [Google Scholar]
- Kristofiková Z, Klaschka J, Tejkalová H, Benesová O. High-affinity choline uptake and muscarinic receptors in rat brain during aging. Arch. Gerontol Geriatr. 1992;15:87–97. doi: 10.1016/0167-4943(92)90043-4. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev. 2006;127:158–165. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology(Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr Drug Targets CNS Neurol Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Molecular neurobiology. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- Linstow Ev, Platt B. Biochemical dysfunction and memory loss: the case of Alzheimer’s dementia. Cell. Mol. Life Sci. 1999;55:601–616. doi: 10.1007/s000180050318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basalforebrain nuclei and projection areas of female rats. Exp. Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albuquerque EX. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Behavioural brain research. 2000;113:199–206. doi: 10.1016/s0166-4328(00)00214-x. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–322. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Rogers J. Age-related shrinkage of cortically projecting cholinergic neurons: a selective effect. Ann of Nerurol. 1987;22:31–36. doi: 10.1002/ana.410220109. [DOI] [PubMed] [Google Scholar]
- Moore H, Stuckman S, Sarter M, Bruno JP. Potassium, but not atropine-stimulated cortical acetylcholi Ne efflux, is reduced in aged rats. Neurobiology of Aging. 1996;17:565–571. doi: 10.1016/0197-4580(96)00075-9. [DOI] [PubMed] [Google Scholar]
- Nishino S. Hypothalamus, hypocretins/orexin, and vigilance control. Handb Clin Neurol. 2011;99:765–782. doi: 10.1016/B978-0-444-52007-4.00006-0. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Hellstrom-Lindahl E, Almkvist O, Meurling L. Activity of acetylcholinesterase in CSF increases in Alzhei- mer’s patients after treatment with tacrine. Alzheimers Rep. 1999;2:347–352. [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 1996;724:89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Atlas. London: Academic Press; 1986. [Google Scholar]
- Pekkala S, Albert ML, Spiro A, 3rd, Erkinjuntti T. Perseveration in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:109–14. doi: 10.1159/000112476. [DOI] [PubMed] [Google Scholar]
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and memory. Chem Biol Interact. 2010;187:403–408. doi: 10.1016/j.cbi.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur J Endocrinol. 2004;150:737–742. doi: 10.1530/eje.0.1500737. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Gold PE. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol Learn Mem. 2005;84:93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Li PK, Flood JF, Johnson DA. Enhancement of hippocampal acetylcholine release by the neurosteroid dehydroepiandrosterone sulfate: an in vivo microdialysis study. Brain Res. 1996;733:284–286. doi: 10.1016/0006-8993(96)00751-2. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, Lubbert H, Albuquerque EX, Maelicke A. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Santos MD, Alkondon M, Pereira EF, Aracava Y, Eisenberg HM, Maelicke A, Albuquerque EX. The nicotinic allosteric potentiating ligand galantamine facilitates synaptic transmission in the mammalian central nervous system. Molecular pharmacology. 2002;61:1222–1234. doi: 10.1124/mol.61.5.1222. [DOI] [PubMed] [Google Scholar]
- Sapronov NS, Fedotova YO, Kuznetsova NN. Antiamnestic effect of alpha7-nicotinic receptor agonist RJR-2403 in middle-aged ovariectomized rats with Alzheimer type dementia. Bull Exp Biol Med. 2006;142:700–702. doi: 10.1007/s10517-006-0455-y. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–67. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilstrom B, Ivanov VB, Wiker C, Svensson TH. Galantamine Enhances Dopaminergic Neurotransmission In Vivo Via Allosteric Potentiation of Nicotinic Acetylcholine Receptors. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301087. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Sherman KA, Friedman E. Pre- and post-synaptic cholinergic dysfunction in aged rodent brain regions: new findings and an interpretative review. Int. J. Dev. Neurosci. 1990;8:689–708. doi: 10.1016/0736-5748(90)90063-8. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Research. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroessner-Johnson HM, Rapp PR, Amaral DG. Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged Rhesus monkey. J. Neurosci. 1992;12:1936–1944. doi: 10.1523/JNEUROSCI.12-05-01936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely M, Petervari E, Balasko M. Thermoregulation, energy balance, regulatory peptides: recent developments. Front Biosci (Schol Ed) 2010;2:1009–1046. doi: 10.2741/s116. [DOI] [PubMed] [Google Scholar]
- Takei N, Nihonmatsu I, Kawamura H. Age-related decline of acetylcholinesterase release evoked by depolarizing stimulation. Neurosci Lett. 1989;101:182–186. doi: 10.1016/0304-3940(89)90527-2. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Maloney S. Gender differences and the role of estrogen in cognitive enhancements with nicotine in rats. Pharmacol Biochem Behav. 2010;95:139–145. doi: 10.1016/j.pbb.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Taylor L, Griffith WH. Age-related decline in cholinergic synaptic transmission in hippocampus. Neurobiol of Aging. 1993;14:509–515. doi: 10.1016/0197-4580(93)90110-w. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Thomson T, Kewitz H, Bickel V, Straschill M, Holl G. Preclinical and clinical studies with galantamine. In: Becker R, Giacobini E, editors. Cholinergic Basis of Alzheimer’s Disease. Boston: Birhauser; 1991. pp. 328–336. [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm Behav. 2009;56:382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, 3rd, Wenk GL. Mecamylamine interactions with galantamine and donepezil: effects on learning, acetylcholinesterase, and nicotinic acetylcholine receptors. Neuroscience. 2003;117:439–447. doi: 10.1016/s0306-4522(02)00872-2. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Wu CF, Bertorelli R, Sacconi M, Pepeu G, Consolo S. Decrease of brain acetylcholine release in aging freely-moving rats detected by microdialysis. Neurobiol Aging. 1988;9:357–361. doi: 10.1016/s0197-4580(88)80081-2. [DOI] [PubMed] [Google Scholar]
- Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphs showing characteristics of our biochemical assays. (A) ChAT assay results showing that the amount of acetylcholine (ACh) produced per minute increases linearly with protein content. (B & C) AChE assay results showing that signal (ΔOD) increases linearly as a function of protein content (B) and incubation time (C). (D) HPLC analysis of ACh. Graph is a typical standard curve showing a linear relationship between quantity of ACh and area under the peak. Each graph is derived from duplicate samples and shows best-fit regression line.
Effects of estradiol on the number of incorrect responses on each day of the operant discrimination and reversal learning task. Symbols indicate group means ± s.e.m. These are the same data shown in Figure 3B, but combined and analyzed according to hormone treatment. Note that E2 treatment was associated with a greater number of incorrect responses on days 4 and 6–9 following reversal of the stimulus-reward contingency. *p<;0.05