Abstract
The metabotropic glutamate receptor subtype 5 (mGluR5) has been implicated in the pathophysiology of mood and anxiety disorders. Recently, a positron emission tomography (PET) tracer exhibiting high selectivity and specificity for mGluR5, 3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-11C-methyl-oxime ([11C]ABP688), was developed. In this work, eight healthy adult male humans were imaged twice to assess within-subject [11C]ABP688 binding variability using PET. In seven of the eight subjects, significantly higher binding was observed during the second (retest) scan. This binding increase could not be definitively explained by differences in ligand injected mass or dose, or changes in metabolism between scans. In addition, this type of systematic binding increase was not observed in a [11C]ABP688 test–retest study performed by our group on anaesthetized baboons. It is therefore possible that the increased binding was because of physiological changes occurring between scans, such as changes in endogenous glutamate levels. If PET imaging with [11C]ABP688 could detect such differences, as preliminary evidence suggests, it could be used to help uncover the role of glutamate in the pathophysiology of brain disorders. However, regardless of its ability to detect endogenous glutamate differences, [11C]ABP688 binding variability could make accurate assessments of drug occupancy or group differences using this ligand difficult.
Keywords: brain imaging, glutamate, molecular imaging, neurotransmitters, positron emission tomography
Introduction
Glutamate is the primary neurotransmitter in the brain, found in as many as 90% of brain neurons, with 80% to 90% of synapses being glutamatergic (Siegel, 2006). Perhaps because of its universality, initial investigations of the neurobiology of mood disorders focused on monoamine dysfunction. Increasingly, however, diverse studies have implicated glutamatergic, in addition to monoaminergic, pathway dysfunction in the pathophysiology of mood disorders (Kendell et al, 2005). It is now believed that the availability of glutamate in the brain contributes to its impact in a spectrum of neurological and psychiatric disorders (Kendell et al, 2005). As an example of this, biochemical and electrophysiological studies have shown that glutamate receptors are influenced by traditional antidepressant treatments, which primarily target monoaminergic transmission (Pilc et al, 2008). Because of their important role in brain function, elucidating the mechanism of action of glutamate receptors would clarify glutamate's role in physiological and pathological conditions.
There are two main types of glutamate receptors—ionotropic, responsible for fast excitatory transmission, and metabotropic (mGlu), which exert a modulatory role on the central nervous system (Palucha and Pilc, 2007). One mGlu receptors in particular, the mGlu receptor subtype 5 (mGluR5), has been localized in moderate to high levels in brain regions associated with depression (Spooren et al, 2003). Antagonists of mGluR5 have potential as effective antidepressants (Palucha and Pilc, 2007). In addition, mGluR5 may be a therapeutic target for schizophrenia (Lindsley et al, 2006; Liu et al, 2008), psychostimulant addiction (Gass et al, 2009; Markou, 2009), Parkinson's disease (Aguirre et al, 2005; Turle-Lorenzo et al, 2005), attention-deficit and hyperactivity disorder (Elia et al, 2010), as well as fragile X syndrome, the most common inherited form of mental retardation and autism (Dolen and Bear, 2008).
Although the importance of mGluR5 has been established, its role in the pathophysiology of disease and its effectiveness as a therapeutic target is difficult to assess without the ability to measure mGluR5 expression or mGluR5 antagonist occupancy. To this end, 3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-11C-methyl-oxime ([11C]ABP688), a highly selective allosteric antagonist of mGluR5 in vitro with high selectivity for mGluR5 in vivo, has been developed for use in humans (Ametamey et al, 2006). The use of this compound as a positron emission tomography (PET) tracer provides the ability to visualize and study mGluR5 binding and distribution in vivo. In two human studies, this PET ligand was shown to have the highest uptake in mGluR5-rich regions and to have favorable kinetics (Ametamey et al, 2007; Treyer et al, 2007).
Although interindividual variance of [11C]ABP688 binding has been evaluated using PET, the only way to assess intraindividual binding variation is by performing repeated [11C]ABP688 scans on the same subject. One such study was recently performed by our group on anesthetized Papio anubis. In that study, [11C]ABP688 test–retest volume of distribution was found to be remarkably stable, with the average percent difference varying between 4.3% and 8.2% across all brain regions (DeLorenzo et al, 2011). To determine whether this binding consistency remains in awake humans, in this study, [11C]ABP688 binding in eight healthy adult male humans was assessed using a test–retest paradigm.
Materials and methods
Subjects
Eight healthy men between the ages of 19 and 34 years completed this study (mean age: 25.4±5.8 years). Three subjects (37.5%) reported their race as Hispanic, three (37.5%) as black, one (12.5%) as Asian, and one (12.5%) as white (non-Hispanic). Inclusion criteria were assessed by the following: history, Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID), review of systems, physical examination, routine blood tests, urine toxicology, and electrocardiography. The inclusion criteria consisted of: (1) men between the ages of 18 and 65 years and (2) capacity to provide written informed consent. Exclusion criteria included: (1) lifetime history of alcohol or substance abuse or dependence, (2) an Axis I or Axis II Cluster B disorder diagnosis, as assessed by the SCID, (3) presence and/or history of a clinically significant major neurological or psychiatric disorder, (4) presence and/or history of first-degree relative with history of major depression, schizophrenia, schizoaffective disorder, or suicide attempt, (5) presence and/or history of two or more first-degree relatives with a history of substance dependence, (6) laboratory tests with clinically significant abnormalities or positive urine toxicology screen, (7) evidence of human immunodeficiency virus, (8) history of head trauma with prolonged loss of consciousness (>10 minutes), any neurological condition including stroke or seizure (excluding a single childhood febrile seizure), or a history of migraine headaches, (9) history of adverse drug reactions or intolerance to more than three types of systemically administered medications, (10) use of any prescription or over-the-counter medication (including herbal remedies or diet aids) within 14 days of the imaging session (multivitamins are permitted), or (11) subject is a Lilly employee. The Institutional Review Boards of Columbia University Medical Center and the New York State Psychiatric Institute approved the protocol. Subjects gave written informed consent after receiving an explanation of the study.
Radiochemistry
Radiosynthesis of [11C]ABP688 was achieved using the procedure outlined by Ametamey et al (2006), with slight modification. [11C]MeOTf was trapped into an acetone (400 mL) solution containing 0.5 mg of desmethyl-ABP688 and 10 μL NaOH (5 ) at room temperature for 5 minutes. The crude product was loaded into a semipreparative high performance liquid chromatography (HPLC) column (Phenomenex C18, Phenomenex, Torrance, CA, USA), eluted with 50:50 (acetonitrile/0.1 mol/L AMF, 10 mL/min), and the product fraction (major diastereomer) was collected between 9 and 10 minutes based on a γ-detector. The collected fraction was then diluted with deionized water (100 mL), passed through a C-18 SepPak (Waters, Milford, MA, USA), and washed with water (2 × 20 mL). After the water rinse, the product was eluted from the SepPak with 1 mL of ethanol. A small portion of the product was analyzed with analytical HPLC for chemical and radiochemical purities, specific activity, and other quality control indices. The remaining ethanol solution was diluted with 9 mL of normal saline, filtered through a 0.22-μm filter, and used for further studies. The total time required for the synthesis of [11C]ABP688 was 30 minutes from the end of bombardment.
Positron Emission Tomography
All subjects underwent two identical PET scans, test and retest, on the same day, separated by an ∼2-hour break. The one exception was subject no. 4 who, because of scheduling constraints, underwent PET imaging on two consecutive days. PET imaging was performed with an ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN, USA), with 63 slices covering an axial field of view of 15.5 cm and axial sampling of 2.425 mm, in 3D mode. A 10-minute transmission scan was acquired before injection. At the end of the transmission scan, [11C]ABP688 was administered intravenously as a bolus over 30 seconds. After injection of [11C]ABP688, emission data were collected for 60 minutes in listmode. The listmode data were then binned into 20 frames (10 at 1 minute duration and 10 at 5 minute duration). Images were reconstructed using attenuation correction from the transmission data, as previously described (DeLorenzo et al, 2009b).
Magnetic Resonance Imaging
Magnetic resonance images (MRIs) were acquired on a 3-T Signa Advantage system (GE Healthcare, Waukesha, WI, USA), as previously described (Ogden et al, 2007). The final voxel size was 1.02 × 1.02 × 1.00 mm, with an acquisition time of 11 minutes.
Input Function Measurement
Before PET imaging, catheters were inserted in the radial artery and forearm veins for arterial blood sampling and radioisotope injection, respectively. Arterial samples were collected automatically every 10 seconds for the first 2 minutes, followed by every 20 seconds until minute four, then manually at 5, 8, 10, 15, 20, 30, 40, 50, and 60 minutes, for a total of 27 samples. Radioactivity of 200 μL aliquots of centrifuged plasma samples was measured in a 1480 Wizard 3 M automatic γ counter (Wallac, Turku, Finland).
Free fraction measurements were performed using an ultrafiltration technique as previously described (Ogden et al, 2007). However, these measurements yielded inconsistent results, preventing accurate free fraction determination. A SepPak assay of five of the arterial blood samples (at 2, 5, 15, 30, and 60 minutes) was used to establish unmetabolized parent compound levels. To perform this assay, 200 μL of plasma was pipetted into 2.5 mL of deionized water in a polypropylene tube. The mixture was vortexed for 10 seconds and the diluted plasma was loaded onto a SepPak tC18 cartridge (6 cc/1 gm; 37 to 55 μm; Waters). The SepPak was eluted dropwise with 5 mL of water and the leftover parent and metabolite (in the combined eluted solution) activity were measured.
Unmetabolized parent fraction levels were fit with a Hill function (Wu et al, 2007). The input function was calculated as the product of the interpolated parent fraction and the total plasma counts. These combined data were then fit as the combination of a straight line and the sum of three exponentials, describing the function before and after the peak, respectively.
Image Analysis
All images were analyzed using MATLAB (MathWorks, Natick, MA, USA). The last 13 frames of an individual PET study were registered to the eighth frame using the FMRIB linear image registration tool (FLIRT), version 5.0 (FMRIB Image Analysis Group, Oxford, UK), to correct for subject motion during the scan. Automatic regions of interest were obtained using nonlinear registration techniques to warp 18 manually outlined MRIs to the target image. A total of 34 regions were previously manually traced by experienced technicians on the 18 MRIs, as previously described (Ogden et al, 2007). Before applying the 18 templates to the target images, each target subject's MRI was first preprocessed with SPM5 for segmentation into gray matter, white matter, and CSF (Wellcome Trust Centre for Neuroimaging, London, UK), and with an automated skull-stripping algorithm (atropos; Avants et al, 2010). Each of the 18 templates was registered to the target brain MRI using the Automatic Registration Toolbox (ART; Ardekani et al, 2005), which was a top performer in an evaluation of 14 nonlinear brain registration algorithms (Klein et al, 2009). The regional label for each target voxel was then determined by evaluating the percentage of the 18 normalized brains that were labeled as that region. The labels are therefore probabilistic and these probabilities are used in the calculation of the time–activity curves (TACs).
The mean PET image was coregistered to the subject's MRI to apply regions of interest to the mean PET image, and individual PET frames. PET-to-MRI transformations were computed using FLIRT with a mutual information cost function, six degrees of freedom, and trilinear interpolation. Eight different coregistration possibilities with varying source/target images and weighting masks were performed, as previously described (DeLorenzo et al, 2009a). The optimum transformation was chosen as the one that maximized a mutual information cost function and verified by visual inspection. Time–activity curves were generated by plotting the measured activity within a region over the time course of the PET acquisition.
Outcome Measure Calculation
The outcome measure closest to the receptor density is BPF (Bavail/KD), where Bavail is the density of available receptors and KD is the equilibrium dissociation constant (Innis et al, 2007). As the determination of BPF requires accurate measurement of the free fraction (fp) and [11C]ABP688 free fraction measurements were unreliable, BPF estimations were unreliable. Therefore, in this work, the measurement closest to receptor density, while maintaining reliability, was BPP fp Bavail/KD. BPP can be calculated as VT−VND, where VT is the volume of distribution (ratio of the concentration of the ligand in the region to that in the plasma at equilibrium) and VND is the volume of distribution of the reference region, representing the non-displaceable VT (Innis et al, 2007). The reference region used in this work was cerebellar gray matter, which was found to be the most suitable reference region for this ligand (DeLorenzo et al, 2011). Although BPP is the outcome measure of choice when the free fraction is not measured, the advantage of using VT as an outcome measure is that it is not influenced by estimations of VND.
In addition to VT and BPP, the outcome measure BPND ((VT−VND)/VND) was calculated (Innis et al, 2007). As BPND represents the concentration of ligand in the region of interest relative to the reference region, it is insensitive to arterial input function quantification and can therefore provide another important measure by which to evaluate ABP688 binding.
For regional analysis, outcome measures were calculated using an unconstrained two-tissue compartment (2TC) model. According to previous human studies (Treyer et al, 2007), 2TC models are superior to one-tissue compartment models for modeling [11C]ABP688 tracer kinetics. In addition, the test–retest experiments performed in non-human primates indicated that the unconstrained 2TC model optimally fit [11C]ABP688 TACs (DeLorenzo et al, 2011). Because of the computational expense of the 2TC method, the Logan graphical approach (Logan et al, 1990), which results in outcome measures highly correlated to those found by the 2TC (Treyer et al, 2007), was used for voxel analysis.
Statistical Analysis
To determine the significance of detected differences in test–retest outcome measures, a linear mixed effects model with region as a fixed effect (for all regions listed in Table 1) and subject and scan (nested within subject) as random effects was applied to the data. Subsequently, post hoc analysis was performed on the outcome measures from each region individually using a two-tailed, paired t-test. In both cases, significance level was defined as 0.05.
Table 1. Volume of distribution (VT, mL/cc), and two measures of binding potential (BPP, mL/cc, and BPND, unitless) for test and retest scans.
| Test | Retest | Percent difference (%) | P value | ICC | |
|---|---|---|---|---|---|
| Insula | |||||
| VT | 2.29±0.55 | 2.69±0.51 | 19.7±13.7 | 0.02 | 0.55 |
| BPP | 1.06±0.38 | 1.36±0.27 | 31.3±21.0 | 0.02 | 0.40 |
| BPND | 0.87±0.28 | 1.03±0.15 | 22.9±22.5 | 0.08 | 0.37 |
| Ventral striatum | |||||
| VT | 2.22±0.54 | 2.60±0.42 | 20.0±17.1 | 0.05 | 0.40 |
| BPP | 0.99±0.40 | 1.27±0.18 | 35.4±26.0 | 0.07 | 0.15 |
| BPND | 0.82±0.30 | 0.97±0.18 | 31.4±21.9 | 0.17 | 0.25 |
| Temporal lobe | |||||
| VT | 2.09±0.57 | 2.53±0.54 | 20.7±17.1 | 0.01 | 0.55 |
| BPP | 0.86±0.38 | 1.20±0.31 | 36.8±28.8 | 0.01 | 0.41 |
| BPND | 0.70±0.26 | 0.90±0.19 | 30.5±27.6 | 0.04 | 0.30 |
| Cingulate | |||||
| VT | 2.09±0.56 | 2.45±0.52 | 19.1±17.2 | 0.04 | 0.57 |
| BPP | 0.86±0.34 | 1.12±0.28 | 32.1±29.1 | 0.04 | 0.38 |
| BPND | 0.70±0.22 | 0.85±0.17 | 24.4±28.6 | 0.11 | 0.19 |
| Medial prefrontal cortex | |||||
| VT | 2.07±0.51 | 2.43±0.51 | 18.9±13.4 | 0.02 | 0.61 |
| BPP | 0.84±0.31 | 1.10±0.25 | 34.1±21.7 | 0.02 | 0.41 |
| BPND | 0.69±0.21 | 0.83±0.11 | 26.8±24.1 | 0.09 | 0.14 |
| Dorsal putamen | |||||
| VT | 2.06±0.47 | 2.36±0.44 | 17.0±14.1 | 0.05 | 0.57 |
| BPP | 0.83±0.28 | 1.02±0.20 | 29.0±20.3 | 0.06 | 0.36 |
| BPND | 0.68±0.20 | 0.78±0.14 | 23.2±18.9 | 0.20 | 0.36 |
| Entorhinal cortex | |||||
| VT | 2.03±0.52 | 2.39±0.51 | 17.3±16.6 | 0.02 | 0.60 |
| BPP | 0.80±0.36 | 1.06±0.32 | 32.0±26.0 | 0.01 | 0.60 |
| BPND | 0.66±0.26 | 0.81±0.23 | 25.8±24.1 | 0.04 | 0.63 |
| Posterior parahippocampal gyrus | |||||
| VT | 2.00±0.40 | 2.34±0.47 | 19.7±14.3 | 0.05 | 0.39 |
| BPP | 0.77±0.24 | 1.00±0.24 | 34.9±22.9 | 0.04 | 0.17 |
| BPND | 0.64±0.20 | 0.76±0.18 | 25.4±20.8 | 0.11 | 0.44 |
| Parietal lobe | |||||
| VT | 1.93±0.48 | 2.30±0.48 | 18.9±13.7 | 0.01 | 0.62 |
| BPP | 0.70±0.29 | 0.97±0.25 | 34.5±22.9 | <0.01 | 0.48 |
| BPND | 0.57±0.19 | 0.73±0.14 | 27.6±23.5 | 0.02 | 0.30 |
| Orbital prefrontal cortex | |||||
| VT | 1.91±0.52 | 2.29±0.49 | 19.7±14.3 | 0.01 | 0.64 |
| BPP | 0.68±0.33 | 0.96±0.27 | 38.9±35.1 | 0.01 | 0.45 |
| BPND | 0.55±0.22 | 0.73±0.16 | 36.6±34.8 | 0.06 | 0.18 |
| Dorsolateral prefrontal cortex | |||||
| VT | 1.92±0.50 | 2.28±0.51 | 18.6±13.6 | 0.01 | 0.65 |
| BPP | 0.70±0.29 | 0.94±0.27 | 34.0±25.6 | 0.01 | 0.53 |
| BPND | 0.56±0.19 | 0.71±0.14 | 25.6±28.5 | 0.04 | 0.34 |
| Amygdala | |||||
| VT | 1.95±0.43 | 2.21±0.45 | 14.8±9.1 | 0.02 | 0.73 |
| BPP | 0.72±0.31 | 0.88±0.25 | 27.9±20.5 | 0.03 | 0.73 |
| BPND | 0.60±0.25 | 0.67±0.18 | 24.8±25.9 | 0.30 | 0.70 |
| Dorsal caudate | |||||
| VT | 1.82±0.48 | 2.20±0.49 | 22.1±12.9 | 0.01 | 0.59 |
| BPP | 0.59±0.32 | 0.87±0.26 | 49.3±33.9 | <0.01 | 0.49 |
| BPND | 0.48±0.24 | 0.65±0.13 | 39.3±39.6 | 0.02 | 0.42 |
| Occipital lobe | |||||
| VT | 1.84±0.47 | 2.16±0.45 | 16.9±13.5 | 0.01 | 0.68 |
| BPP | 0.61±0.27 | 0.82±0.21 | 34.3±21.7 | <0.01 | 0.56 |
| BPND | 0.49±0.17 | 0.62±0.12 | 29.4±21.6 | 0.03 | 0.32 |
| Hippocampus | |||||
| VT | 1.81±0.52 | 2.14±0.48 | 18.4±16.9 | 0.01 | 0.68 |
| BPP | 0.58±0.33 | 0.81±0.27 | 44.0±39.4 | 0.01 | 0.59 |
| BPND | 0.46±0.24 | 0.61±0.18 | 39.8±39.5 | 0.07 | 0.43 |
| Thalamus | |||||
| VT | 1.42±0.37 | 1.66±0.34 | 17.7±12.5 | 0.01 | 0.69 |
| BPP | 0.19±0.17 | 0.33±0.11 | 82.6±81.4 | <0.01 | 0.48 |
| BPND | 0.15±0.13 | 0.25±0.08 | 73.4±84.9 | 0.02 | 0.38 |
| Cerebellar gray matter | |||||
| VT | 1.23±0.28 | 1.34±0.30 | 13.5±10.6 | 0.14 | 0.76 |
| BPP | 0.00±0.00 | 0.00±0.00 | — | — | — |
| BPND | 0.00±0.00 | 0.00±0.00 | — | — | — |
ICC, intraclass correlation coefficient.
The mean and standard deviation of VT, BPP, and BPND values are shown for all test and retest scans (columns 2 and 3). For each test–retest pair, the percent difference was calculated as the absolute difference between VT (or BPP or BPND) values divided by the mean of the two VT (or BPP or BPND) values. The average of these percent differences over all subjects is indicated (column 4). For most regions, the increase in binding was significant, as indicated by the P value of the post hoc analysis (two-tailed, paired t-test, column 5). ICCs are indicated in the last column. Regions are listed in order from the highest to the lowest mean VT.
To compare within-subject to between-subject variability, the intraclass correlation coefficient (ICC) was calculated (Shrout and Fleiss, 1979). This measure varies between −1 (indicating low reliability) and 1 (indicating maximum reliability).
Results
Regional Uptake
The uptake of [11C]ABP688 appears greater in regions of the brain with known distributions of mGluR5, such as the insula, ventral striatum, temporal lobe, cingulate, and medial prefrontal cortex. Lower binding is observed in the thalamus and cerebellum.
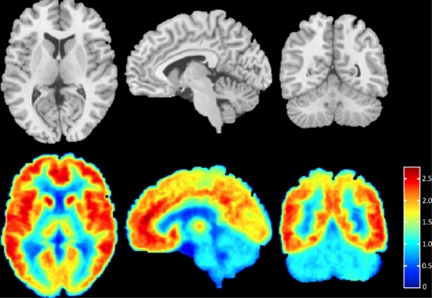
To visualize this binding pattern clearly, an aggregate image was created of all subjects (Figure 1). To create this aggregate image, VT was calculated at every PET image voxel for every subject and each voxel map was then aligned to the subject's MRI using the calculated coregistration transform (see Materials and methods—Image Analysis). The subject's MRI was then nonlinearly warped to a high-resolution template (Holmes et al, 1998) using ART, and the resulting nonlinear transform was used to bring each subject's VT voxel map into the space of the high-resolution template.
Figure 1.
Axial, sagittal, and coronal views of [11C]ABP688 binding. For each positron emission tomography image acquired (n=16), the volume of distribution (VT) was calculated at every voxel using the Logan graphical approach (Logan et al, 1990). The top row shows axial, sagittal, and coronal views of the magnetic resonance image template for anatomical reference. The bottom row shows the corresponding axial, sagittal, and coronal views of the mean [11C]ABP688 VT image (over all subjects), in the space of the high-resolution template (see text for details). The VT value associated with each color is indicated by the color bar.
Time–Activity Curve Fits
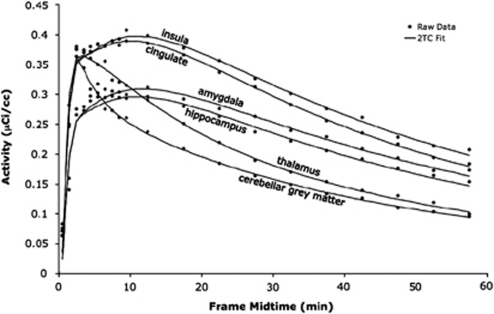
Examples of the unconstrained 2TC modeling method fits for high-, medium-, and low-binding regions are shown in Figure 2.
Figure 2.
Time–activity curve fits in one subject. The raw data (black circles, mean of the activity within the region) are shown for two regions with high binding (insula and cingulate), two with moderate binding (amygdala and hippocampus), and two with low binding (thalamus and cerebellar gray matter). These raw data were fit with an unconstrained, iterative two-tissue compartment model (2TC, black lines).
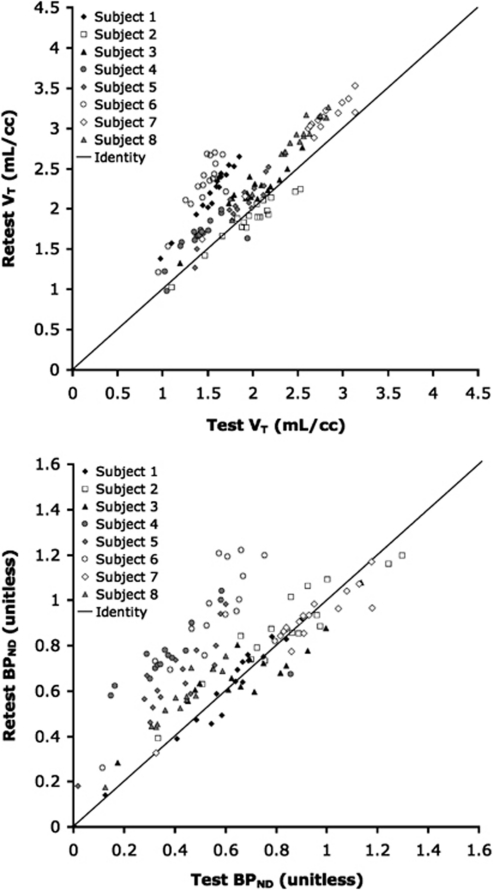
Test–Retest Results
Figure 3 shows the test–retest effect on the outcome measures VT and BPND. As evident from the graphs, an increase in binding (on retest) was observed across most regions and subjects. Applying the linear mixed effects model to the VT data (Figure 3, top) revealed a significant test–retest effect across all regions (P=0.01). When this linear mixed effects model was applied to the BPND data (Figure 3, bottom) for all regions except the reference region (where BPND cannot be calculated), a significant test–retest effect across all regions was also observed (P=0.05). For completeness, the linear mixed effects model was also applied to the BPP data, and results were similar (P=0.01).
Figure 3.
Comparison of volume of distribution (VT, top) and binding potential (BPND, bottom) between test and retest. VT and BPND were calculated using unconstrained, iterative two-tissue compartment method. In both graphs, the identity line is plotted for reference. In most cases, binding was higher in the retest scans than the test scans.
Table 1 shows the mean VT, BPP, and BPND values across all subjects, and the average percent difference between these values. It also lists both the P values associated with the regional post hoc analysis and ICC values within each region. The post hoc testing indicates that, for most regions, the test–retest difference is either significant or at trend level. The highest ICC value (0.76) was that of the reference region VT. Aside from that region, the range of ICCs was 0.39 to 0.73 (VT), 0.15 to 0.73 (BPP), and 0.14 to 0.70 (BPP). Within each region, VT ICC values were, in general, higher than BPP ICC values, and BPND ICC values were the lowest.
Although the unconstrained 2TC model fit the TACs well, in light of the observed unexpected increase in binding between test and retest, the data were modeled using four additional techniques: (1) a 2TC model constrained such that the ratio of kinetic constants, K1/k2, for each region is restricted to the ratio of K1 to k2 in the reference region (Parsey et al, 2000b), (2) a 2TC model fit using non-iterative methods in which the experimental data are matched to the most similar curve in a library of precalculated functions, rather than by performing a nonlinear least squares iterative fit, (3) Logan graphical analysis, in which outcome measures are calculated from the slope of the linear part of an integral plot (Logan et al, 1996), and (4) likelihood estimation in graphical analysis, which has been shown to reduce bias introduced by the Logan approach (Ogden, 2003). One-tissue compartment models were not used in the comparison because they did not fit the data well (data not shown). For all four models tested, the results were similar to those found using an unconstrained iterative 2TC method. That is, regardless of the chosen model, VT within each region of interest increased between test and retest by 11% to 21% on average (Table 2).
Table 2. Difference in volume of distribution (VT) between test and retest for four models.
|
Two-tissue compartment, constrained |
Two-tissue compartment, non-iterative |
Logan |
LEGA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test VT (mL/cc) | Retest VT (mL/cc) | PD (%) | Test VT (mL/cc) | Retest VT (mL/cc) | PD (%) | Test VT (mL/cc) | Retest VT (mL/cc) | PD (%) | Test VT (mL/cc) | Retest VT (mL/cc) | PD (%) | |
| Insula | 2.42±0.55 | 2.75±0.56 | 17±14 | 2.36±0.58 | 2.73±0.56 | 19±14 | 2.49±0.62 | 2.81±0.54 | 19±12 | 2.57±0.65 | 2.90±0.55 | 19±12 |
| Ventral striatum | 2.34±0.58 | 2.65±0.46 | 17±18 | 2.29±0.57 | 2.62±0.42 | 19±17 | 2.40±0.62 | 2.7±0.45 | 21±14 | 2.50±0.64 | 2.79±0.46 | 21±14 |
| Temporal lobe | 2.22±0.58 | 2.59±0.60 | 17±17 | 2.19±0.52 | 2.56±0.53 | 16±12 | 2.27±0.56 | 2.63±0.57 | 17±16 | 2.34±0.58 | 2.71±0.58 | 17±16 |
| Cingulate | 2.20±0.58 | 2.51±0.56 | 16±17 | 2.10±0.53 | 2.48±0.54 | 19±16 | 2.27±0.56 | 2.55±0.54 | 16±15 | 2.35±0.58 | 2.64±0.54 | 17±15 |
| Medial prefrontal cortex | 2.24±0.53 | 2.48±0.57 | 14±15 | 2.13±0.49 | 2.45±0.53 | 17±12 | 2.28±0.56 | 2.54±0.55 | 18±11 | 2.35±0.59 | 2.62±0.56 | 19±11 |
| Cerebellar gray Matter | 1.23±0.28 | 1.34±0.30 | 14±11 | 1.25±0.29 | 1.35±0.30 | 12±11 | 1.34±0.32 | 1.42±0.33 | 11±11 | 1.38±0.33 | 1.46±0.34 | 12±11 |
LEGA, likelihood estimation in graphical analysis; PD, percent difference; VT, volume of distribution.
The mean and standard deviation of VT values are shown for all test and retest scans. The five highest binding regions from Table 1, as well as the reference region, are listed.
Potential Causes of Observed Results
It is possible that the observed increases in binding could be because of differences in injected mass or dose of the ligand between test and retest scans. However, no significant correlation was found between injected mass and the VT of any region. For the non-reference regions listed in Table 1, the correlations between injected mass and regional VT varied between −0.27 and −0.10 (P values: 0.30 to 0.71). The correlation between injected mass and VT of the reference region was −0.48 (P=0.06). In addition, even if a correlation between injected mass and VT were found, injected masses were comparable across scans (Table 3). Mean injected mass was 4.33±1.73 μg and 4.47±1.09 μg for test and retest scans, respectively (P=0.84). Similarly, no significant differences were found in injected doses or specific activities between test and retest scans. Mean injected dose was 9.79±3.99 mCi and 12.71±3.95 mCi (P=0.14), and mean specific activity was 0.66±0.40 mCi/nmol and 0.76±0.41 mCi/nmol (P=0.54), for test and retest scans, respectively.
Table 3. [11C]ABP688-injected mass and clearance for test and retest scans.
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7 | Subject 8 | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| Test-injected mass (μg) | 5.31 | 5.30 | 4.98 | 4.94 | 0.67 | 5.57 | 5.18 | 2.72 | 4.33±1.73 |
| Retest-injected mass (μg) | 5.60 | 5.08 | 5.02 | 3.73 | 5.08 | 4.06 | 4.96 | 2.21 | 4.47±1.09 |
| Test clearance (L/h) | 57.1 | 69.5 | 74.4 | 78.8 | 79.2 | 74.7 | 75.9 | 87.8 | 74.7±8.8 |
| Retest clearance (L/h) | 77.6 | 58.4 | 94.4 | 69.8 | 93.0 | 92.5 | 75.4 | 88.2 | 81.2±13.0 |
The injected mass (μg) and clearance (L/h) are listed for both test and retest scans. There was no significant difference in mass injected (P=0.84) or clearance (P=0.20) between test and retest scans.
As injected masses, doses, and specific activities were similar between scans, subject metabolism changes were investigated. To assess this, each subject's clearance (injected dose divided by the area under the metabolite-corrected plasma curve; Hirvonen et al, 2008) was calculated. However, clearance rates did not significantly change between test and retest (Table 3). Average clearance rates were 74.7±8.8 L/h and 81.2±13.0 L/h for test and retest scans, respectively (P=0.20). Although the ICC of clearance was low (0.26), ICCs of area under the percent parent curve (0.77) and the time to peak of the plasma fit (0.74) indicated high reliability.
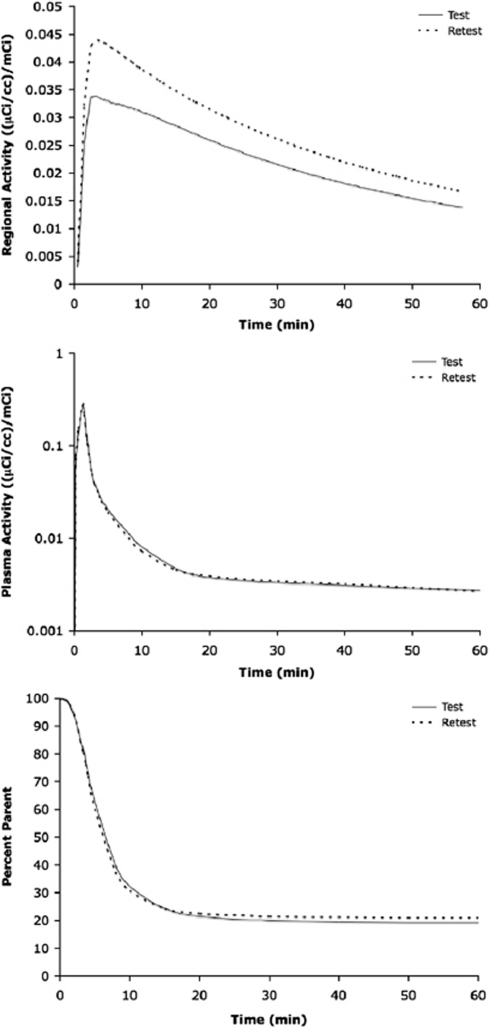
To illustrate how it is possible that measured binding can increase despite similar input functions, Figure 4 shows an example of a case in which the subject's metabolite and plasma activity curves were almost indistinguishable, yet the time–activity curves for test and retest scans still varied considerably. VT values estimated from these TACs varied by 19.9% (2.60 mL/cc versus 3.17 mL/cc for test and retest, respectively).
Figure 4.
Examination of time–activity curves divided by injected dose (top), plasma concentration divided by injected dose (middle), and percent parent (bottom) of [11C]ABP688 in the ventral striatum of one subject. Time–activity curves were fit with an unconstrained, iterative two-tissue compartment model. The plasma concentration was fit with a straight line and the sum of three exponentials before and after the peak, respectively. The metabolite data were fit with a Hill function. The injected doses for these scans were 15.52 and 14.59 mCi for test and retest, respectively. The clearance values calculated from metabolite-corrected plasma curve were 87.8 and 88.2 L/h for test and retest, respectively.
Discussion
Previous work has shown that the 2TC models fit [11C]ABP688 TACs better than the one-tissue compartment models (Treyer et al, 2007), and our test–retest [11C]ABP688 studies in baboons indicated that the optimal model for [11C]ABP688 is the unconstrained 2TC model (DeLorenzo et al, 2011). For these reasons, an unconstrained 2TC modeling method was the primary modeling technique used in this work. As Figure 2 indicates, this modeling method fit the data well. However, Table 2 suggests that outcome measure results were not strongly sensitive to model choice.
In previous in vitro, rodent, and primate studies, [11C]ABP688 exhibited high specificity and selectivity for mGluR5 (Ametamey et al, 2006; DeLorenzo et al, 2011; Hintermann et al, 2007; Wyss et al, 2007). In human studies, this PET ligand was shown to have the highest uptake in mGluR5-rich regions (Ametamey et al, 2007) and favorable kinetics (Treyer et al, 2007). However, VT estimates varied considerably between subjects, with coefficients of variation ranging between 16.7% and 32.1% across regions using an unconstrained 2TC model (Treyer et al, 2007). Without performing repeated PET scans on a single subject, as in a test–retest study, it is impossible to determine whether those binding differences were because of interindividual or within-subject variation in humans.
The current test–retest study indicates that the within-subject variation of [11C]ABP688 binding is high. However, the variation is not random. Instead, average [11C]ABP688 binding increases between scans. This robust test–retest finding is mostly uniform across regions (Table 1) and is significant, based on a linear mixed effects model, regardless of outcome measure used (VT, BPP, or BPND). In addition, VT and BPP of most individual regions show significant increases between test and retest in post hoc testing, and even BPND measures, which are not sensitive to tracer concentration in plasma or free fraction measurements, show mostly significant or trend-level increases in binding between scans.
This type of systematic increase in ligand binding between test and retest scans (Figure 3) has not been observed in other test–retest studies conducted in this group using a variety of ligands (and targets), such as [11C]DASB (serotonin transporter), [11C]CUMI (5-HT1A receptor), [11C]McN-5652 (serotonin transporter), [11C]PE2I (dopamine transporter), and [11C]WAY-100635 (5-HT1A receptor) (DeLorenzo et al, 2009b; Milak et al, 2008; Ogden et al, 2007; Parsey et al, 2000a, 2000b). Importantly, this increase was also not observed in the [11C]ABP688 baboon test–retest study performed by this group, with the same ligand preparation, scanning technique, and image analysis (DeLorenzo et al, 2011). Therefore, the cause of this systematic increase remains an open question. The variables most likely to cause binding differences—injected mass, injected dose, or clearance—did not significantly change between test and retest scans. Low-clearance ICC coupled with the nonsignificant difference between test and retest clearance rates suggests within-subject variation in clearance that, unlike [11C]ABP688 binding, is not in a single direction (i.e., clearance values do not all increase, Table 3). However, ICC values of the area under the metabolite curve and time to the plasma peak were high, indicating higher variance in these measures between subjects than within a subject. Even if the within-subject variation of plasma activity and metabolism is low, however, as Figure 4 indicates, it is possible to have nearly identical test and retest plasma activity and ligand metabolism, with drastically different TACs.
It should also be noted that [11C]ABP688 variability was not dependent on scan length (data not shown). This is not surprising given the stability of the data, as indicated by the TACs (Figure 2), and based on a simulation study performed by Treyer et al (2007), which showed stable VT values with as little as 20 minutes of scan time.
As the binding increases between scans could not be linked to any of the measured variables (or choice of modeling technique, Table 2), these increases are most likely attributable to a variable that was not measured, such as changes in free fraction or physiological variability. In this work, reliable estimates of BPF could not be attained because the free fraction was too low to be accurately measured. If the free fraction did vary between test and retest scans, this change could be responsible for the detected binding differences. Although this is possible, there is no intuitive reason for free fraction to significantly change in a consistent direction (i.e., to always increase between scans), especially when this effect was not observed in the baboon studies. In addition, it is likely that a change in free fraction would have resulted in a significant difference in reference region VT (which was not observed), and free fraction changes cannot explain the increases observed in BPND.
Although there is no reason to believe that binding changes were because of free fraction variation, this cannot be definitively ruled out without estimates of ABP688 free fraction. In this (and our previous baboon) work, the free fraction was determined using an ultrafiltration technique, owing to its short analysis time and lack of dilution effects or volume shifts (Kwon, 2001). However, the accuracy of this technique can suffer because of nonspecific binding of the drug to the plastic tube or ultrafiltration membrane (Kwon, 2001). This problem is exacerbated with highly protein-bound drugs such as ABP688, leading to inconsistent and unreliable results. To avoid this problem in future studies, plasma free fraction can be evaluated using equilibrium dialysis with volume shift correction (Lohman, 1986).
Another possibility that is often explored in connection with ligand binding differences are changes in P-glycoprotein (P-gp). The P-gp can be responsible for ligand efflux through the blood–brain barrier (Doze et al, 2000; Ishiwata et al, 2007). As such, modulators of P-gp, such as cyclosporine A, can increase the uptake of ligand in the brain (Doze et al, 2000; Ishiwata et al, 2007). If ABP688 were a substrate for P-gp, the increased binding observed in the retest scans could be because of diminished efflux of ABP688 from the brain. However, there is no evidence indicating that ABP688 is a P-gp substrate. In addition, the tracer doses in which ABP688 is administered are unlikely to cause such systemic effects to the blood–brain barrier, lasting several hours. Therefore, this possibility is also unlikely.
In addition to the above hypotheses, it is also possible that ABP688 binding increases were caused by physiological differences, such as changes in endogenous glutamate occurring in the brain between scans. A recently published pilot study performed in baboons suggests that [11C]ABP688 is sensitive to endogenous glutamate variation (Miyake et al, 2011). In this study, N-acetylcysteine was used to induce extrasynaptic glutamate release in baboons. N-acetylcysteine administration resulted in a decrease in [11C]ABP688 BPND between baseline and post-N-acetylcysteine PET scans. The posited mechanism of action (which remains to be tested) was a shift in affinity of the allosteric binding site for the tracer because of the N-acetylcysteine-induced glutamate increase.
Although further testing is required to definitely assess this possibility, if this tracer were sensitive to endogenous glutamate release, as suggested by the baboon pilot study, it may provide insight into the systematic increases observed in this study. It is possible that the stress of the first PET scan, including arterial and venous catheter placement, caused an increase in the levels of glutamate in the brain (Barkus et al, 2010; Cortese et al, 2010). This increased glutamate may have resulted in lower [11C]ABP688 binding, potentially because of an affinity shift of the receptor for the ligand. During the second scan, subjects were less likely to be anxious, because they were aware of the process and because the arterial and venous lines did not need to be replaced. (Note: The one exception to this is subject 4, who received PET scans on two different days. However, even this subject, having already experienced the process, may have been more relaxed during the second scan.) This may explain the higher binding observed in the retest scan. If this hypothesis is validated, the current study may be the first to report detection of glutamate level variation in humans using [11C]ABP688.
Similar to the use of dopamine antagonist PET tracers to monitor dopamine transmission in disorders such as schizophrenia and substance abuse (Guo et al, 2010), if changes in levels of endogenous glutamate affect [11C]ABP688 binding, this ligand could be used to monitor endogenous glutamate level variations in neurological and psychiatric disorders, as well as in response to treatment. This type of assessment may also help tailor treatments to individuals (by monitoring treatment-induced glutamate changes). Although there are numerous potential applications for a tracer that can detect endogenous glutamate variation, without the ability to compensate for this potential intrasubject variability, some conventional PET tracer applications become difficult. For example, typical applications of this tracer would include occupancy studies for mGluR5 agonists and antagonists, as well as group comparisons of mGluR5 distribution in healthy controls versus patients with psychiatric disorders. For those types of applications, good test–retest reliability and low variability are required; otherwise ligand-binding variability can obscure occupancy effects or group differences. However, as shown in Table 1, the ICCs of [11C]ABP688 outcome measures are low to moderate, meaning that within-subject variation is comparable to the between-subject variation. The significant intrasubject variation observed in the current study, as well as the variability between measurements observed in this and previous studies, regardless of its origins, may signal difficulties in using [11C]ABP688 for these types of applications in humans.
However, it is interesting to note that although [11C]ABP688 binding generally increases between test and retest scans, the binding variability (as indicated by standard deviation) decreases in almost all cases, across all outcome measures (Table 1). Consistent with the endogenous glutamate hypothesis, larger binding variation in the test (compared with retest) scans may be because of differences in the stress response between individuals. Therefore, lower variability in the retest scans may imply that, as the stress subsides, measures of binding become more consistent.
Conclusion
This test–retest study confirms that [11C]ABP688 binds to mGluR5-rich brain regions and that modeling [11C]ABP688 kinetics with an unconstrained 2TC model is appropriate in humans. The test–retest paradigm also allowed assessment of within-subject binding variability in humans, revealing an increase in binding between test and retest scans that was not because of the tracer injected mass, dose, or clearance. This binding variation could make quantification of drug occupancy using [11C]ABP688 difficult.
As this type of increase was not observed in [11C]ABP688 baboon test–retest studies (nor in the numerous human studies performed by our group using several other ligands), it may be because of glutamate level variation in response to the stress of the PET scans. This hypothesis requires further investigation. If this is the case, however, [11C]ABP688 may be able to monitor glutamate level variations in neurological and psychiatric disorders, as well as in response to treatment.
The authors declare no conflict of interest.
Footnotes
Sources of Support: Eli Lilly and Company
References
- Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S, Andbjer B, Leo G, Medhurst AD, Agnati LF, Fuxe K. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005;1033:216–220. doi: 10.1016/j.brainres.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S, Auberson YP, Gasparini F, Schubiger PA. Radiosynthesis and preclinical evaluation of 11C-ABP688 asa probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med. 2006;47:698–705. [PubMed] [Google Scholar]
- Ametamey SM, Treyer V, Streffer J, Wyss MT, Schmidt M, Blagoev M, Hintermann S, Auberson Y, Gasparini F, Fischer UC, Buck A. Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med. 2007;48:247–252. [PubMed] [Google Scholar]
- Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Avants B, Klein A, Tusttison N, Woo J, Gee J.2010Evaluation of open-access, automated brain extraction method on multi-site multi-disorder data 16th Annual Meeting for the Organization of Human Brain MappingBarcelona, Spain
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Mitchell TR, Galloway MP, Prevost KE, Fang J, Moore GJ, Uhde TW. Region-specific alteration in brain glutamate: possible relationship to risk-taking behavior. Physiol Behav. 2010;99:445–450. doi: 10.1016/j.physbeh.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, Parsey RV.2009aA new method for assessing PET-MRI coregistration SPIE Medical Imaging Bellingham, WA: SPIE; 2009. 72592W-72598 [Google Scholar]
- DeLorenzo C, Kumar JS, Zanderigo F, Mann JJ, Parsey RV. Modeling considerations for in vivo quantification of the dopamine transporter using [(11)C]PE2I and positron emission tomography. J Cereb Blood Flow Metab. 2009b;29:1332–1345. doi: 10.1038/jcbfm.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo C, Milak MS, Brennan KG, Kumar JS, Mann JJ, Parsey RV. In vivo positron emission tomography imaging with [(11)C]ABP688: binding variability and specificity for the metabotropic glutamate receptor subtype 5 in baboons. Eur J Nucl Med Mol Imaging. 2011;38:1083–1094. doi: 10.1007/s00259-010-1723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doze P, Van Waarde A, Elsinga PH, Hendrikse NH, Vaalburg W. Enhanced cerebral uptake of receptor ligands by modulation of P-glycoprotein function in the blood-brain barrier. Synapse. 2000;36:66–74. doi: 10.1002/(SICI)1098-2396(200004)36:1<66::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, D'Arcy M, Deberardinis R, Frackelton E, Kim C, Lantieri F, Muganga BM, Wang L, Takeda T, Rappaport EF, Grant SF, Berrettini W, Devoto M, Shaikh TH, Hakonarson H, White PS. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2010;15:637–646. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, Slifstein M, Abi-Dargham A, Laruelle M, Javitch JA, Rayport S. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann S, Vranesic I, Allgeier H, Brulisauer A, Hoyer D, Lemaire M, Moenius T, Urwyler S, Whitebread S, Gasparini F, Auberson YP. ABP688, a novel selective and high affinity ligand for the labeling of mGlu5 receptors: identification, in vitro pharmacology, pharmacokinetic and biodistribution studies. Bioorg Med Chem. 2007;15:903–914. doi: 10.1016/j.bmc.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Johansson J, Teras M, Oikonen V, Lumme V, Virsu P, Roivainen A, Nagren K, Halldin C, Farde L, Hietala J. Measurement of striatal and extrastriatal dopamine transporter binding with high-resolution PET and [11C]PE2I: quantitative modeling and test-retest reproducibility. J Cereb Blood Flow Metab. 2008;28:1059–1069. doi: 10.1038/sj.jcbfm.9600607. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ishiwata K, Kawamura K, Yanai K, Hendrikse NH. In vivo evaluation of P-glycoprotein modulation of 8 PET radioligands used clinically. J Nucl Med. 2007;48:81–87. [PubMed] [Google Scholar]
- Kendell SF, Krystal JH, Sanacora G. GABA and glutamate systems astherapeutic targets in depression and mood disorders. Expert Opin Ther Targets. 2005;9:153–168. doi: 10.1517/14728222.9.1.153. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. Handbook of essential pharmacokinetics, pharmacodynamics, and drug metabolism for industrial scientists. New York: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr, Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piper idin-1-yl}-methano. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lohman JJHM. Plasma-protein binding of drugs—implications for therapeutic drug-monitoring. Pharm Weekblad-Sci Ed. 1986;8:302–304. [Google Scholar]
- Markou A. Accruing preclinical evidence aboutmetabotropic glutamate 5 receptor antagonists as treatments for drug dependence highlights the irreplaceable contributions of animal studies to the discovery of new medications for human disorders. Neuropsychopharmacology. 2009;34:817–819. doi: 10.1038/npp.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Severance AJ, Ogden RT, Prabhakaran J, Kumar JS, Majo VJ, Mann JJ, Parsey RV. Modeling considerations for 11C-CUMI-101, an agonist radiotracer for imaging serotonin 1A receptor in vivo with PET. J Nucl Med. 2008;49:587–596. doi: 10.2967/jnumed.107.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N, Skinbjerg M, Easwaramoorthy B, Kumar D, Girgis RR, Xu X, Slifstein M, Abi-Dargham A. Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [(11)C] ABP688 and N-acetylcysteine challenge. Biol Psychiatry. 2011;69:822–824. doi: 10.1016/j.biopsych.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Ogden RT. Estimation of kinetic parameters in graphical analysis of PET imaging data. Stat Med. 2003;22:3557–3568. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab. 2007;27:205–217. doi: 10.1038/sj.jcbfm.9600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A, Pilc A. Metabotropic glutamate receptor ligands aspossible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med. 2000a;41:1465–1477. [PubMed] [Google Scholar]
- Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, Guo NN, Van Heertum R, Mann JJ, Laruelle M. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. 2000b;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Siegel GJ.2006Basic Neurochemistry: Molecular, Cellular, and Medical Aspects7th ed.Amsterdam; Boston, MA: Elsevier [Google Scholar]
- Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U, Schmidt M, Gasparini F, Hock C, Buck A. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med. 2007;48:1207–1215. doi: 10.2967/jnumed.107.039578. [DOI] [PubMed] [Google Scholar]
- Turle-Lorenzo N, Breysse N, Baunez C, Amalric M. Functional interaction between mGlu 5 and NMDA receptors in a rat model of Parkinson′s disease. Psychopharmacology (Berl) 2005;179:117–127. doi: 10.1007/s00213-005-2202-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Ogden RT, Mann JJ, Parsey RV. Optimal metabolite curve fitting for kinetic modeling of 11C-WAY-100635. J Nucl Med. 2007;48:926–931. doi: 10.2967/jnumed.106.038075. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Ametamey SM, Treyer V, Bettio A, Blagoev M, Kessler LJ, Burger C, Weber B, Schmidt M, Gasparini F, Buck A. Quantitative evaluation of 11C-ABP688 asPET ligand for the measurement of the metabotropic glutamate receptor subtype 5 using autoradiographic studies and a beta-scintillator. Neuroimage. 2007;35:1086–1092. doi: 10.1016/j.neuroimage.2007.01.005. [DOI] [PubMed] [Google Scholar]