Abstract
Multipotent mesenchymal stromal cells (MSCs) increase tissue plasminogen activator (tPA) activity in astrocytes of the ischemic boundary zone, leading to increased neurite outgrowth in the brain. To probe the mechanisms that underlie MSC-mediated activation of tPA, we investigated the morphogenetic gene, sonic hedgehog (Shh) pathway. In vitro oxygen and glucose deprivation and coculture of astrocytes and MSCs were used to mimic an in vivo ischemic condition. Both real-time-PCR and western blot showed that MSC coculture significantly increased the Shh level and concomitantly increased tPA and decreased plasminogen activator inhibitor 1 (PAI-1) levels in astrocytes. Inhibiting the Shh signaling pathway with cyclopamine blocked the increase of tPA and the decrease of PAI-1 expression in astrocytes subjected to MSC coculture or recombinant mouse Shh (rm-Shh) treatment. Both MSCs and rm-Shh decreased the transforming growth factor-β1 level in astrocytes, and the Shh pathway inhibitor cyclopamine reversed these decreases. Both Shh-small-interfering RNA (siRNA) and Glil-siRNA downregulated Shh and Gli1 (a key mediator of the Shh transduction pathway) expression in cultured astrocytes and concomitantly decreased tPA expression and increased PAI-1 expression in these astrocytes after MSC or rm-Shh treatment. Our data indicate that MSCs increase astrocytic Shh, which subsequently increases tPA expression and decreases PAI-1 expression after ischemia.
Keywords: astrocytes, multipotent mesenchymal stromal cells (MSCs), plasminogen activator inhibitor 1 (PAI-1), sonic hedgehog (Shh), stroke, tissue plasminogen activator (tPA)
Introduction
Multipotent mesenchymal stromal cells (MSCs) promote functional recovery after stroke (Chen et al, 2001a; Hardy et al, 2008), in part through tissue plasminogen activator (tPA) activity (Shen et al, 2011). Mesenchymal stromal cells increase tPA activity by concomitantly increasing tPA expression and decreasing plasminogen activator inhibitor 1 (PAI-1) expression in astrocytes in the ischemic boundary zone (Xin et al, 2010). The increase in tPA activity thereby increases axonal remodeling in this area (Xin et al, 2010). Mesenchymal stromal cells in the ischemic boundary zone secrete soluble factors and directly interact with parenchymal cells (Xin et al, 2006); however, the signaling pathways by which MSCs regulate tPA and PAI-1 expressions have not been investigated.
Hedgehog proteins act as morphogens in many tissues during embryonic development and postnatal repair (Ming et al, 1998; Simpson et al, 2009; Varjosalo and Taipale, 2008). As the most broadly expressed mammalian hedgehog signaling molecule, Sonic hedgehog (Shh) has a major role in various organs including the nervous system during development (McMahon et al, 2003; Varjosalo and Taipale, 2008). In addition, in the adult, acute brain injury induces activation of the Shh signaling pathway in reactive astrocytes (Amankulor et al, 2009), and increased Shh expression is found in the subventricular zone (Jiao and Chen, 2008; Wang et al, 2009) and in the hippocampus (Sims et al, 2009) after focal cerebral ischemia, suggesting that the Shh signaling pathway contributes to the repair process in the brain tissue. Mesenchymal stromal cell treatment also increases activation of the Shh signaling pathway after stroke, which may benefit recovery of neurologic function (Zhang et al, 2009).
Interaction between the Shh signaling pathway and tPA activity has been reported previously (Garcia de Veas et al, 1998).
From a basic science perspective, the interaction of MSCs, as a model of cell therapy, with damaged brain parenchymal cells is important, because benefit appears to derive from the stimulation of parenchymal cells by exogenous cells, with MSCs acting almost as catalysts for parenchymal cell response. The astrocyte, as the most numerous parenchymal cell, likely has an important if not a primary role in mediating recovery of function, whether it is by neurite remodeling and/or by some other mechanisms. Therefore, in this study, we initially selected astrocytes as our in vitro target for oxygen and glucose deprivation (OGD) and coculture of astrocytes and MSCs to mimic the in vivo ischemic condition. We investigated the effects of MSC treatment of stroke on the Shh signaling pathway and the relationship between Shh and tPA activity in astrocytes. As transforming growth factor (TGF)-β1 regulates PAI-1 expression (Milei et al, 2004; Vivien and Ali, 2006), we therefore also investigated the crosstalk between the Shh and TGF-β1 signaling pathways. It is our belief that this study may facilitate the clinical application of MSCs for treatment of stroke and may shed new light on the neglected function of astrocytes in brain repair.
Materials and methods
All experimental procedures were carried out in accordance with the NIH (National Institutes of Health) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Henry Ford Hospital.
Cell Culture of Mesenchymal Stromal Cells and Astrocytes
The MSCs were provided by Theradigm Inc. (Baltimore, MD, USA). In brief, the bone marrow harvested from the hind legs of C57/Bl6 mice (aged 2 to 3 months) were prepared as described previously (Shichinohe et al, 2010). Mesenchymal stromal cells were cultured with α-modified Eagle's medium (Hyclone, Logan, UT, USA) containing 20% fetal bovine serum (Gibco Laboratory, Grand Island, NY, USA) and penicillin–streptomycin on 75-cm2 tissue culture flasks (Corning, St Louis, MO, USA). Mouse cortical astrocytes, C8-D1A (Astrocyte type I clone from C57/BL6 strains), were obtained from the American Type Culture Collection (CRL-2541, Arlington, VA, USA). Cells were cultured in high-glucose Dulbecco's modified Eagle's medium (Invitrogen, San Diego, CA, USA) with 10% fetal bovine serum, containing penicillin–streptomycin on 75-cm2 tissue culture flasks, and all cells were placed in a moist incubator and cultured at 37 °C with 5% CO2.
Oxygen and Glucose Deprivation and Treatments of Astrocytes
For OGD treatment, astrocytes (1 × 105) were seeded in each well of a 6-well plate containing the normal medium. After cells grew to 70% confluence, the medium was replaced with nonglucose culture media and cultured in an anaerobic chamber (model 1025, Forma Scientific, Marietta, OH, USA) for 2 hours. Astrocytes were then cultured under normal conditions with or without MSC coculture. For astrocytes cocultured with MSCs, an upper chamber of the transwell insert dish (Becton Dickinson Labware, Franklin Lakes, NJ, USA) was seeded with MSCs at a ratio 1:100 to astrocytes. For exogenous factor treatment of astrocytes, recombinant mouse Shh (rm-Shh), amino-terminal peptide (rm-Shh, 100 ng/mL, R&D Systems Inc. Minneapolis, MN, USA; catalog no. 461-SH) or recombinant human TGF-β1 (0.1 ng/mL, R&D Systems Inc. catalog no. 240-B) were directly added into the culture media at the demanded concentration. The inhibitors used in this study were cyclopamine, Veratrum californicum (for the Shh signaling pathway, 20 μmol/L, Calbiochem, Wilmington, MA, USA; catalog no. 239803), and recombinant human TGF-β1 latency-associated peptide (for the TGF-β1 signaling pathway, 200 ng/mL, R&D Systems Inc., catalog no. 246-LP). Mouse Shh-small-interfering RNA (siRNA) (siGENOME SMARTpool M-042008-01-0005, Mouse Shh, NM_009170, Dharmacon, Lafayette, CO, USA) and mouse Gli1-siRNA (siGENOME SMARTpool, Mouse Gli1, M-047917-01-0005, Dharmacon) were used to downregulate endogenous Shh and Gli1 expressions in astrocytes, respectively. All treatments were performed for 24 hours, and then the astrocytes in the plate and MSCs in the insert were detached and collected for extraction and used to measure the RNA and protein.
Quantitative Real-Time PCR
Quantitative real-time-PCR was performed with the isolated total RNA transcribed into cDNA using poly-dT oligonucleotides following the manufacturer's instructions. Quantitative PCR for total cDNAs was performed in the ABI Prism 7000 sequence detection system, using the standard protocols with the Quantitec SYBY Green PCR kit (Qiagen, Valencia, CA, USA). The following primers for quantitative real time-PCR were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA): mouse Shh, forward: CCTTTACCCTACAAGCAGTTTATTGC, reverse: GTAATTGGGGGTGAGTTCCTTAAATC; mouse tPA, forward: CTGAGGTCACAGT CCAAGCA, reverse: ACAGATGCTGTGAGGTGCAG; mouse PAI-1, forward: GTCTTTCCGACCAAGAGCAG, reverse: ATCACTTGGCCCATGAAGAG; and mouse GAPDH (glyceraldehyde 3-phosphate dehydrogenase), forward GTCTACTGGTGTCTTCACCACCAT, reverse: GTTGTCATATTTCTCGTGGTTCAC. To obtain a representative figure of mRNA expression, the traditional method using agarose gel for detection of PCR amplification at the final phase or end point of the PCR reaction was also performed in the study (Russell and Sambrook, 2001).
Western Blot Assay
The total protein was used for western blot assay following the standard western blotting protocol (Molecular Clone, Edition II). The concentrations of the primary antibodies used were: Shh (1:2,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; sc-1194), tPA (1:2,000, Santa Cruz Biotechnology Inc.; sc-15346), PAI-1 (1:2,000, Santa Cruz Biotechnology Inc.; sc-8979), and β-actin (1:5,000, Santa Cruz Biotechnology Inc.; sc-1616). Respective horseradish peroxidase-labeled secondary antibodies were applied, and enhanced chemiluminescence detection was used according to the manufacturer's instructions (Pierce, Rockford, IL, USA). The integrated density mean gray value of the bands was analyzed under ImageJ (National Institutes of Health, Bethesda, MD, USA) software, and the corresponding relative expression ratio was calculated as protein of interest/β-actin.
Enzyme-Linked Immunosorbent Assay Detection of the Transforming Growth Factor β1 Expression in Astrocytes
A mouse TGF-β1 assay kit (Bender MedSystems, San Diego, CA, USA; catalog no. BMS608/2) was used to measure the TGF-β1 level in lysates from astrocytes. After the manufacturer's assay procedures, lysates from the control and various treated astrocytes were added into the wells of enzyme-linked immunosorbent assay plates, as well as various diluted tPA standards. The primary and second antibodies were added sequentially. The reaction was quenched by the addition of 1 mol/L H2SO4, and absorbance values were read at 450 nm.
Statistical Analysis
Data are expressed as mean±s.e. The differences between mean values were evaluated with the two-tailed Student's t-test (for two groups) and the analysis of variance (for more than two groups). All calculations and statistical tests were performed by the computer programs Microsoft Excel 2000 (Microsoft, Redmond, WA, USA) or SPSS 11.5 (SPSS, Chicago, IL, USA). P<0.05 was considered significant for all analyses.
Results
Mesenchymal Stromal Cells Concomitantly Increase Shh and Tissue Plasminogen Activator Expression in Cultured Astrocytes
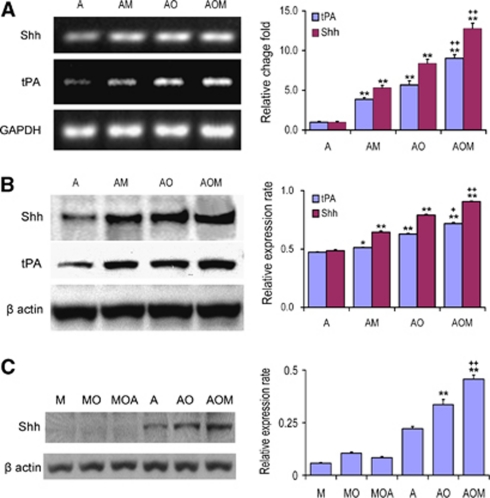
Using an in vitro OGD culture system to mimic the in vivo ischemic condition, the mRNA level (Figure 1A) and protein level (Figure 1B) of Shh in astrocytes subjected to OGD were significantly increased compared with normal cultured astrocytes; coculture of normal and OGD astrocytes with MSCs substantially increased the Shh expression both at mRNA and protein levels compared with normal cultured and OGD cultured astrocytes, respectively. In parallel with the Shh expression, similar changes in tPA expression were found in astrocytes. The tPA expression in astrocytes was increased after OGD, and MSC coculture increased the tPA level both in normal cultured astrocytes and in OGD-cultured astrocytes. These data suggest that tPA expression is regulated by Shh. As Shh is a secreted protein, parenchymal Shh may also derive from MSCs. To measure the Shh expression in MSCs, western blot was performed and showed that Shh expression in MSCs was very low under normal culture conditions (Figure 1C). The Shh expression in OGD MSCs and OGD MSCs cocultured with astrocytes (1:100) were significantly lower than those in OGD astrocytes and in OGD astrocytes cocultured with MSCs (100:1), respectively (Figure 1C). These data imply that Shh is generated in astrocytes.
Figure 1.
MSCs concomitantly increase Shh and tPA expressions in cultured astrocytes. qRT-PCR and western blot show the (A) mRNA and (B) protein levels of tPA and Shh in cultured astrocytes. OGD treatment increased the Shh and tPA levels compared with normal cultured astrocytes. MSC coculture increased the Shh and tPA levels in both normal cultured and OGD cultured astrocytes. (C) The Shh expression in cultured MSCs was obviously lower than in astrocytes under different culture conditions. *P<0.05, **P<0.01, compared with group A; +P<0.05, ++P<0.01, compared with group AO. A: normal astrocytes; AM: normal astrocytes cocultured with MSCs; AO: OGD astrocytes; AOM: OGD astrocytes cocultured with MSCs; M: MSCs; MO: OGD MSCs; MOA: OGD MSCs cocultured with astrocytes. MSC, mesenchymal stromal cell; OGD, oxygen and glucose deprivation; qRT-PCR, quantitative real-rime PCR; Shh, sonic hedgehog; tPA, tissue plasminogen activator.
Mesenchymal Stromal Cells Increased the Shh Signaling Pathway and Regulates Tissue Plasminogen Activator and Plasminogen Activator Inhibitor 1 Expressions in Cultured Astrocytes
The smoothened protein (Smo) is a component of the hedgehog signaling pathway, and its activation triggers a series of intracellular events, leading to the activation of Gli-dependent transcription (Alexandre et al, 1996). The suppression of Smo is regulated by a multipass transmembrane protein, Patched (Ptch), and Shh binding to Ptch can alleviate the Ptch-induced suppression of Smo (Stone et al, 1996). To test whether the change in astrocytic tPA expression is mediated by Shh, we treated cultured astrocytes with cyclopamine, which inhibits the hedgehog signaling pathway by directly binding to Smo (Chen et al, 2002). Mesenchymal stromal cells and exogenous rm-Shh increased tPA expression in cultured astrocytes, and cyclopamine substantially blocked astrocyte tPA expression induced by MSCs and rm-Shh at mRNA (Figure 2A) and protein (Figure 2B) levels. We also investigated whether the Shh signaling pathway affects PAI-1 expression in vitro. Western blot data showed that PAI-1 was upregulated in OGD astrocytes (Figure 2C). Mesenchymal stromal cell coculture and rm-Shh treatment significantly decreased the PAI-1 expression in astrocytes under normal and OGD conditions, and this decrease was blocked by cyclopamine (Figure 2C).
Figure 2.
MSCs stimulated the Shh signaling pathway and thereby regulates tPA and PAI-1 expressions in cultured astrocytes. qRT-PCR and western blot show the (A) mRNA and (B) protein levels of tPA in cultured astrocytes. MSC treatment and addition of exogenous rm-Shh increased the tPA level in cultured astrocytes. The Shh signaling pathway inhibitor cyclopamine blocked the tPA increase in astrocytes with MSC coculture and rm-Shh treatment. (C) Western blot shows PAI-1 protein level in cultured astrocytes. MSC treatment and addition of exogenous rm-Shh decreased PAI-1 expression in cultured astrocytes. The Shh signaling pathway inhibitor cyclopamine increased PAI-1 expression in astrocytes with MSC coculture and rm-Shh treatment. *P<0.05, **P<0.01, compared with normal astrocytes; ^P<0.05, ^^P<0.01 compared with OGD astrocytes; #P<0.05, ##P<0.01, compared with MSC treatment; ++P<0.01, compared with rm-Shh treatment. A: astrocytes; AM: astrocytes cocultured with MSCs; AMC: astrocytes cocultured with MSCs and cyclopamine added; AS: astrocytes treated with rm-Shh; ASC: rm-Shh treated astrocytes with cyclopamine added. MSC, mesenchymal stromal cell; PAI-1, plasminogen activator inhibitor 1; qRT-PCR, quantitative real-rime PCR; rm-Shh, recombinant mouse Shh; Shh, sonic hedgehog; tPA, tissue plasminogen activator.
Shh Regulates Plasminogen Activator Inhibitor 1 Expression in Astrocytes through the Transforming Growth Factor β1 Signaling Pathway
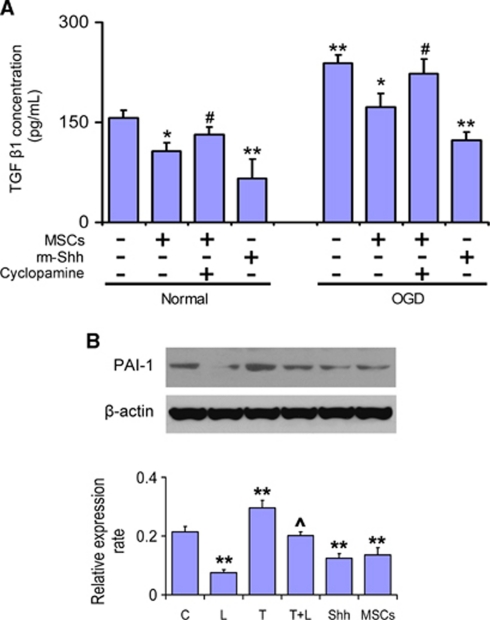
As TGF-β1 regulates PAI-1 expression (Milei et al, 2004; Vivien and Ali, 2006), we therefore also investigated the TGF-β1 signaling pathway in astrocytes after MSC treatment. Enzyme-linked immunosorbent assay data showed that the TGF-β1 level in astrocytes was significantly increased after OGD treatment. Recombinant mouse Shh significantly decreased the TGF-β1 level in normal and OGD astrocytes, and MSC treatment also decreased the TGF-β1 level in normal and OGD astrocytes. Cyclopamine abrogated the MSC-induced decrease in TGF-β1 levels (Figure 3A). Exogenous addition of rh-TGF-β1 significantly increased PAI-1 protein expression in astrocytes, and the TGF-β activity neutralizer TGF-β1 latency-associated peptide specifically downregulated the PAI-1 expression (Figure 3B). These data suggest that Shh affects PAI-1 expression in astrocytes through the TGF-β1 signaling pathway.
Figure 3.
Shh affected PAI-1 expression in astrocytes by regulating the TGF-β1 signaling pathway. ELISA detection shows that MSCs and rm-Shh significantly decreased TGF-β1 expression in cultured astrocytes. (A) The Shh signaling pathway inhibitor cyclopamine increased TGF-β1 level in astrocytes cocultured with MSCs. (B) Exogenous addition of rh-TGF-β1 significantly increased PAI-1 expression in astrocytes, and the TGF-β activity neutralizer TGF-β1 LAP specifically downregulated the PAI-1 expression. *P<0.05, **P<0.01, compared with normal cultured astrocytes; #P<0.05, compared with MSC treatment; ^P<0.05 compared with TGF-β1 treatment. C: control (normal cultured astrocytes); L: LAP treatment; T: TGF-β1 treatment; T+L: TGF-β1 and LAP treatment; Shh: rm-Shh treatment; MSCs: MSC treatment. MSC, mesenchymal stromal cell; PAI-1, plasminogen activator inhibitor 1; rm-Shh, recombinant mouse Shh; Shh, sonic hedgehog; TGF-β1, transforming growth factor-β1; ELISA, enzyme-linked immunosorbent assay.
RNA Interference Knock Down Indicates that the Shh Signaling Pathway Participates in the Regulation of Tissue Plasminogen Activator and Plasminogen Activator Inhibitor 1 Expression
To further confirm that the Shh signaling pathway regulates expressions of tPA and PAI-1, we used Shh-siRNA to downregulate endogenous Shh expression and Gli1-siRNA to downregulate the expression of Gli1, a key component of Shh signaling pathway transduction. Western blot showed that Shh-siRNA significantly downregulated Shh expression in cultured astrocytes and concomitantly and significantly decreased tPA expression and increased PAI-1 expression in those astrocytes (Figure 4A). Transfected Gli1-siRNA into astrocytes downregulated Gli1 expression (Figure 4B), and the downregulated expression of Gli1 inhibited the increase in tPA expression and the decrease in TGF-β and PAI-1 expression after MSC or rm-Shh treatment (Figure 4C). These data suggest that through Gli1, the Shh signaling pathway mediates the induction of tPA and inhibition of TGF-β in astrocytes after MSC treatment.
Figure 4.
Shh signaling pathway-mediated induction of tPA expression and inhibition of TGF-β/PAI-1 expression through Gli1. Transfected with (A) Shh-siRNA downregulated Shh expression in cultured astrocytes and concomitantly tPA expression was decreased and PAI-1 increased in these astrocytes. (B) Gli1 expression was downregulated after transfected Gli1-siRNA into astrocytes. (C) The downregulated expression of Gli1 inhibited tPA increase and inhibited TGF-β and PAI-1 decrease after MSCs or rm-Shh treatment. *P<0.05, **P<0.01, compared with vector-transfected astrocytes; +P<0.05, compared with rm-Shh or MSCs treatment, respectively. MSC, mesenchymal stromal cell; PAI-1, plasminogen activator inhibitor 1; rm-Shh, recombinant mouse Shh; Shh, sonic hedgehog; TGF-β1, transforming growth factor-β1; tPA, tissue plasminogen activator.
Discussion
The therapeutic benefits of MSCs on stroke recovery have been shown previously (Chen et al, 2001b; Hardy et al, 2008). Mesenchymal stromal cells promote axonal remodeling in the ischemic boundary zone and enhance functional recovery after stroke through increase of tPA activity (Xin et al, 2010). Here, using the in vitro ischemic model, we found that MSCs stimulate the Shh signaling pathway in astrocytes, which leads to an increase in tPA and a concomitant decrease in PAI-1 expression.
The Shh has multiple actions during central nervous system development, including proliferation of neural precursors and control of axon growth (Marti and Bovolenta, 2002). During embryonic development, the notochord and later cells express Shh, which has a well-described role in determining cell fate in the ventral neural tube (Marti and Bovolenta, 2002). The ventral to dorsal gradient of Shh guides commissural axons approaching the midline floor plate (Gritli-Linde et al, 2001). Postnatal studies showed that activation of the Shh in the injured brain leads to cell proliferation, including angiogenesis and neural progenitor proliferation (Sims et al, 2009; Soleti and Martinez, 2009). The microenvironment after neural injury can promote regenerative sprouting, and the Shh protein is prominently localized within regenerating axons (Xu et al, 2008). Blockage of Shh signaling in retinal ganglion cells induced abnormal growth and navigation of contralateral projecting axons by influencing growth cone formation (Sanchez-Camacho and Bovolenta, 2008). Our in vitro studies show that increasing Shh subsequently increases tPA expression and concomitantly decreases PAI-1 expression in astrocytes. We blocked the Shh pathway by cyclopamine, which decreased tPA expression and increased PAI-1 expression in astrocytes subjected to MSC coculture or rm-Shh treatment. Using Shh-siRNA, we verified that Shh expression was downregulated in cultured astrocytes and concomitantly tPA expression was decreased and PAI-1 increased in these astrocytes. Tissue plasminogen activator activity promotes neurite outgrowth (Zhang et al, 2005). Gli1-siRNA knockdown data show that downregulation of Gli1 inhibits the increase of tPA and the decrease of TGF-β and PAI-1 after MSCs or rm-Shh treatment; thus, the Shh pathway may contribute to MSC-mediated improvement of neurologic function recovery after stroke. Our data show that exogenous Shh was more effective at reducing PAI-1 (and TGF-β) protein levels in astrocytes than was MSC coculture. The addition of Shh and MSC coculture was similarly effective at increasing tPA levels in astrocytes. This apparent discrepancy may be attributed to likely differential gene regulation of PAI-1 and tPA.
The Shh, Wnt, and TGF-β signaling pathways are all important for neural circuit assembly, including neuronal polarization, axon and dendrite development, and synaptogenesis during early development in animals (Sanchez-Camacho and Bovolenta, 2009). After ischemia-induced brain damage, TGF-β1 is strongly upregulated in the central nervous system (Docagne et al, 2003). Transforming growth factor-β1 induces PAI-1 expression in cultured cells and in vivo (Milei et al, 2004; Vivien and Ali, 2006). Transforming growth factor-β1-induced PAI-1 upregulation is mediated by reactive oxygen species and contributes to accumulation of extracellular matrix through suppression of plasmin activity (Ha and Lee, 2005). Mesenchymal stromal cells in the ischemic boundary zone reduce TGF-β1, subsequently downregulate PAI-1 in reactive astrocytes in this area, and thereby increase tPA activity, which may facilitate neurite outgrowth by proteolysis of the extracellular matrix (Tsirka, 2002). There is crosstalk between the Shh and TGF-β signaling pathways (Guo and Wang, 2009). During development and oncogenesis, these two pathways directly regulate key components of each other (Yoo et al, 2008). Our in vitro studies show that Shh decreased TGF-β1 expression and subsequently decreased PAI-1 level in astrocytes. Shh also directly increased the expression of tPA in astrocytes in vitro, suggesting that Shh and TGF-β signaling pathways regulate tPA activity in astrocytes in the ischemic brain, and contribute to the repair process after stroke. Mesenchymal stromal cells decreased the TGF-β1 level in astrocytes, and the Shh pathway inhibitor cyclopamine reversed this decrease, indicating that Shh affects TGF-β1 expression. Our data suggest that MSCs stimulate parenchymal cells to secrete Shh, which promotes cellular activation of tPA by (1) directly increasing tPA expression in astrocytes and (2) indirectly, by decreasing TGF-β1/PAI-1 in astrocytes. We provide the molecular basis for the intercellular communication between exogenous MSCs and parenchymal astrocytes and propose that Shh mediates the enhancement of tPA activity.
The focus of our work is on investigating mechanisms mediating neurorestoration after stroke by upregulating endogenous tPA in parenchymal cells, and not on exogenously administering tPA intravascularly as a thrombolytic agent to provide ‘neuroprotection'. For decades, the primary stratagem for stroke treatment has been focused on neuroprotection to reduce cerebral infarction. Unfortunately, except for thrombolysis using tPA, all neuroprotective agents developed in the laboratory have failed in clinical trials. At present, fewer than 5% of stroke patients in the United States receive tPA therapy. Therefore, there is a compelling need to develop neurorestorative therapies to enhance neurologic recovery, by primarily treating the brain during the recovery phases after stroke. The tPA has pleiotropic actions in the brain besides its well-established fibrinolytic action. It induces neural injury in the setting of acute stroke (Benchenane et al, 2004), but is also involved in synaptic plasticity (Samson and Medcalf, 2006), dendritic remodeling (Mataga et al, 2004), and axonal outgrowth (Minor et al, 2009). Thus, we hypothesized that administration of tPA during the recovery phase may provide beneficial effects on stroke recovery by promoting axonal remodeling. Our in vitro experiments were designed to dissect the interactions of MSCs with astrocytes. Our data provide a proof of principle that a cell-based therapy such as MSCs can induce astrocytic tPA, which promotes neurite outgrowth. In vitro studies simply allow us to identify MSC and astrocytes-specific secretions arising from normoxia and OGD that are not available from in vivo studies. Tissue plasminogen activator expression by MSCs is very low (Copland et al, 2009). The very small numbers of exogenously administered MSCs present in the brain stimulate tPA activity in parenchymal cells, most likely the numerous astrocytes. In this study, as a proof of principle, we provide in vitro data on a potential mechanism underlying MSC treatment-enhanced tPA activation in astrocytes. In vivo studies are warranted to investigate the effects of pharmacological inhibition of SHH on tPA and PAI-1 after MSC therapy.
Conclusions
In vitro data show that the increase of Shh by MSCs concomitantly increases tPA and decreases PAI-1 expression in astrocytes, thereby, increasing tPA activity.
Acknowledgments
The authors thank Theradigm Inc. for providing primary cultures of MSCs, and Cindi Roberts and Qing-e Lu for technical assistance.
The authors declare no conflict of interest.
Footnotes
This study was supported by RO1-AG037506.
References
- Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29:10299–10308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Lopez-Atalaya JP, Fernandez-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001b;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland IB, Lord-Dufour S, Cuerquis J, Coutu DL, Annabi B, Wang E, Galipeau J. Improved autograft survival of mesenchymal stromal cells by plasminogen activator inhibitor 1 inhibition. Stem Cells. 2009;27:467–477. doi: 10.1634/stemcells.2008-0520. [DOI] [PubMed] [Google Scholar]
- Docagne F, Ali C, Lesne S, Nicole O, MacKenzie ET, Buisson A, Vivien D. [Does transforming growth factor-beta (TGF-beta) act as a neuroprotective agent in cerebral ischemia?] J Soc Biol. 2003;197:145–150. [PubMed] [Google Scholar]
- Garcia de Veas R, Schweigerer L, Medina MA. Modulation of the proteolytic balance plasminogen activator/plasminogen activator inhibitor by enhanced N-myc oncogene expression or application of genistein. Eur J Cancer. 1998;34:1736–1740. doi: 10.1016/s0959-8049(98)00285-8. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H, Lee HB. Reactive oxygen species amplify glucose signalling in renal cells cultured under high glucose and in diabetic kidney. Nephrology (Carlton) 2005;10 (Suppl::S7–10. doi: 10.1111/j.1440-1797.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- Hardy SA, Maltman DJ, Przyborski SA. Mesenchymal stem cells as mediators of neural differentiation. Curr Stem Cell Res Ther. 2008;3:43–52. doi: 10.2174/157488808783489471. [DOI] [PubMed] [Google Scholar]
- Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- Milei J, Cao G, Grana DR, Toblli JE. Plasminogen activator inhibitor-1 and transforming growth factor-beta 1 in carotid glomus and autonomic ganglia from spontaneously hypertensive rats. J Hypertens. 2004;22:1351–1359. doi: 10.1097/01.hjh.0000125434.28861.9b. [DOI] [PubMed] [Google Scholar]
- Ming JE, Roessler E, Muenke M. Human developmental disorders and the Sonic hedgehog pathway. Mol Med Today. 1998;4:343–349. doi: 10.1016/s1357-4310(98)01299-4. [DOI] [PubMed] [Google Scholar]
- Minor K, Phillips J, Seeds NW. Tissue plasminogen activator promotes axonal outgrowth on CNS myelin after conditioned injury. J Neurochem. 2009;109:706–715. doi: 10.1111/j.1471-4159.2009.05977.x. [DOI] [PubMed] [Google Scholar]
- Russell DW, Sambrook J.2001Molecular Cloning: A Laboratory Manual3rd edn., Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA [Google Scholar]
- Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Sanchez-Camacho C, Bovolenta P. Autonomous and non-autonomous Shh signalling mediate the in vivo growth and guidance of mouse retinal ganglion cell axons. Development. 2008;135:3531–3541. doi: 10.1242/dev.023663. [DOI] [PubMed] [Google Scholar]
- Sanchez-Camacho C, Bovolenta P. Emerging mechanisms in morphogen-mediated axon guidance. Bioessays. 2009;31:1013–1025. doi: 10.1002/bies.200900063. [DOI] [PubMed] [Google Scholar]
- Shen LH, Xin H, Li Y, Zhang RL, Cui Y, Zhang L, Lu M, Zhang ZG, Chopp M. Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodeling after stroke in mice. Stroke. 2011;42:459–464. doi: 10.1161/STROKEAHA.110.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Maruichi K, Osanai T, Sugiyama T, Chiba Y, Yamaguchi A, Iwasaki Y. Bone marrow stromal cells and bone marrow-derived mononuclear cells: which are suitable as cell source of transplantation for mice infarct brain. Neuropathology. 2010;30:113–122. doi: 10.1111/j.1440-1789.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- Simpson F, Kerr MC, Wicking C. Trafficking, development and hedgehog. Mech Dev. 2009;126:279–288. doi: 10.1016/j.mod.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, Moskowitz MA. Sonic Hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke. 2009;40:3618–3626. doi: 10.1161/STROKEAHA.109.561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleti R, Martinez MC. Microparticles harbouring Sonic Hedgehog: role in angiogenesis regulation. Cell Adh Migr. 2009;3:293–295. doi: 10.4161/cam.3.3.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, de Sauvage F, Rosenthal A. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Tsirka SE. Tissue plasminogen activator as a modulator of neuronal survival and function. Biochem Soc Trans. 2002;30:222–225. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Vivien D, Ali C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006;17:121–128. doi: 10.1016/j.cytogfr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Mao X, Xie L, Greenberg DA, Jin K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab. 2009;29:1644–1654. doi: 10.1038/jcbfm.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Chen X, Chopp M. Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485–1493. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J, Pourabdollah-Nejad DS, Zhang C, Zhang L, Jiang H, Zhang ZG, Chopp M. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One. 2010;5:e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu QG, Midha R, Martinez JA, Guo GF, Zochodne DW. Facilitated sprouting in a peripheral nerve injury. Neuroscience. 2008;152:877–887. doi: 10.1016/j.neuroscience.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Zhang ZG, Lu M, Borneman J, Buller B, Savant-Bhonsale S, Elias SB, Chopp M. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009;29:1166–1174. doi: 10.1038/jcbfm.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kanaho Y, Frohman MA, Tsirka SE. Phospholipase D1-promoted release of tissue plasminogen activator facilitates neurite outgrowth. J Neurosci. 2005;25:1797–1805. doi: 10.1523/JNEUROSCI.4850-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]