Abstract
Intracerebral hemorrhage (ICH) has the highest mortality of all stroke subtypes, yet treatments are mainly limited to supportive management, and surgery remains controversial. Despite significant advances in our understanding of ICH pathophysiology, we still lack preclinical models that accurately replicate the underlying mechanisms of injury. Current experimental ICH models (including autologous blood and collagenase injection) simulate different aspects of ICH-mediated injury but lack some features of the clinical condition. Newly developed models, notably hypertension- and oral anticoagulant therapy-associated ICH models, offer added benefits but further study is needed to fully validate them. Here, we describe and discuss current approaches to experimental ICH, with suggestions for changes in how this condition is studied in the laboratory. Although advances in imaging over the past few decades have allowed greater insight into clinical ICH, there remains an important role for experimental models in furthering our understanding of the basic pathophysiologic processes underlying ICH, provided limitations of animal models are borne in mind. Owing to differences in existing models and the failed translation of benefits in experimental ICH to clinical practice, putative neuroprotectants should be trialed in multiple models using both histological and functional outcomes until a more accurate model of ICH is developed.
Keywords: animal models, experimental, inflammation, intracerebral hemorrhage, translational medicine
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a significant cause of morbidity and mortality, accounting for 10% to 20% of all strokes (Feigin et al, 2009) and affecting at least 25 per 100,000 people annually. It has the highest mortality of all stroke subtypes (Feigin et al, 2009) and <40% of ICH survivors regain independence (van Asch et al, 2010). Despite improvements in patient care and better control of hypertension (a major risk factor for ICH), it is remarkable that the incidence and case fatality of ICH have not changed in the last 30 years (van Asch et al, 2010). The rapid reversal of abnormal coagulation and control of significantly elevated blood pressure are recommended (Morgenstern et al, 2010; National Collaborating Centre for Chronic Conditions, 2008), but these interventions are only indicated in a small minority of patients. At present, there are no proven pharmacological therapies for ICH, and the role of surgery remains controversial (Mendelow et al, 2005). As such, ICH represents a major unmet health burden worldwide.
Major progress has been made in the understanding of ICH in the clinical setting, facilitated by modern imaging techniques. Alongside this, experimental ICH studies have revealed some of the cellular and molecular mechanisms underlying ICH and this could not have been achieved in the clinical setting alone. Successful translation of findings between the two settings is vital and there is much to be learned from the widely discussed failure to translate promising findings in experimental ischemic stroke to the clinical setting (Fisher et al, 2009). This failure has occurred for several reasons, including inadequately powered animal studies often lacking randomization or blinding, and poor modeling not reflecting the true clinical situation (Fisher et al, 2009); although there are of course major differences in the pathophysiology of cerebral ischemia and ICH, many of these shortcomings are as applicable to experimental ICH as they are to ischemic stroke models.
In a systematic review of 1,026 neuroprotective strategies for stroke, an inverse relationship between study quality (as determined by adherence to STAIR (Stroke Therapy Academic Industry Roundtable) criteria (Fisher et al, 2009)) and reported efficacy of treatment was observed (O'Collins et al, 2006). Further, a recent review of study design quality in experimental cerebrovascular research found flaws in the design, reporting, and statistics of many studies (Vesterinen et al, 2011); such deficiencies may contribute to the translational roadblock experienced in experimental stroke (Fisher et al, 2009). This roadblock has resulted in wasted resources on multiple negative trials and delayed benefits to patients.
Although relatively speaking, translational research in ICH is in its infancy, it is crucial that lessons are learnt from experiences in cerebral ischemia to prevent history repeating itself. If experimental ICH does not accurately reproduce the pathophysiology of clinical ICH, any conclusions reached from such studies may lack clinical relevance. With these factors in mind, this review aims to describe and discuss current approaches to experimental ICH, with suggestions for changes in how this condition is studied in the laboratory.
Clinical intracerebral hemorrhage: what are we trying to model?
A Heterogeneous Condition with Multiple Etiologies
To model ICH accurately in the laboratory, an understanding of what leads to ICH in humans is important. There are numerous causes, risk factors, and precipitating/contributing factors and each case is usually multifactorial in origin. The major underlying causes include the pathophysiological changes in deep perforating arteries associated with chronic hypertension (which may include lipohyalinosis and microaneurysms), amyloid angiopathy, and vascular malformations such as aneurysms, arteriovenous malformations, and cavernomas (Qureshi et al, 2009). Important precipitating/contributing factors may include acute hypertension, abnormal coagulation, and thrombolysis. Amyloid angiopathy increases in incidence with age (Miller et al, 1999), whereas in young patients with ICH, vascular malformations predominate (Ruiz-Sandoval et al, 1999). While hypertension is the biggest risk factor for ICH, other risk factors include alcohol consumption, diabetes, the menopause, and cigarette smoking (Feldmann et al, 2005).
Complications Are Common Following Intracerebral Hemorrhage in Humans
Hematoma expansion is common following ICH in humans (Figure 1), even in those with normal coagulation profiles, and correlates with outcome (Brott et al, 1997). A recent study found hematoma expansion to be present in ∼73%, and clinically significant in up to 35% of ICH patients within the first 3 hours postictus (Davis et al, 2006). Perihematomal edema increases early following clinical ICH (Butcher et al, 2004; Gebel et al, 2002a), and may be closely related to outcome (Gebel et al, 2002b), though a larger, more recent study did not find edema to be a predictor of outcome, independent of hematoma size (Arima et al, 2009). Intraventricular hemorrhage (IVH) occurs in almost half of ICH patients by 24 hours postictus (Steiner et al, 2006); the presence of IVH and hydrocephalus are both independent predictors of poor outcome following ICH in humans (Mayer et al, 2005; Mendelow et al, 2005). Extension of blood into the subarachnoid space can also occur (Qureshi et al, 2001b), especially with lobar bleeds (Miller et al, 1999).
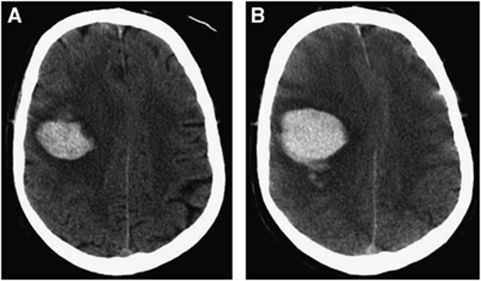
Figure 1.
Computed tomography (CT) brain scans from an acute intracerebral hemorrhage (ICH) patient at 1.5 hours (A) and 4.5 hours (B) after symptom onset demonstrating early hematoma expansion. Hematoma expansion is a common complication of ICH in the clinical setting and an important therapeutic target but is not well reproduced in experimental ICH.
A Perihematomal Penumbra
A contentious issue within ICH research is whether an ischemic penumbra (that is, a hypoperfused, functionally impaired, but potentially viable area of tissue) surrounds an intracerebral hematoma (Kirkman, 2011). This is an important question clinically, as the presence of perihematomal ischemia would favor a role for surgery and argue against blood pressure lowering to prevent hematoma expansion on the grounds that this may exacerbate perihematomal hypoperfusion (Broderick et al, 2007). Animal studies initially suggested the existence of an ischemic penumbra evidenced by decreased perihematomal blood flow (Bullock et al, 1988; Mendelow et al, 1984). Several experimental (Qureshi et al, 1999) and clinical studies of small and medium sized hemorrhages utilizing magnetic resonance imaging (Schellinger et al, 2003), computed tomography perfusion (Fainardi et al, 2005; Rosand et al, 2002), and positron emission tomography (Zazulia et al, 2001) subsequently found perihematomal hypoperfusion but no evidence of perihematomal ischemic tissue. It is now thought that these areas of perihematomal hypoperfusion arise as a consequence of decreased metabolic demand as opposed to ischemia, as evidenced by a normal or reduced oxygen extraction fraction in perihematomal tissue measured by positron emission tomography imaging in acute clinical ICH (Schellinger et al, 2003; Zazulia et al, 2001). In addition, small reductions in blood pressure have no significant effect on global or perihematomal cerebral blood flow (CBF) (Powers et al, 2001). The implication is thus that ischemia in the perihematomal tissue does not exist, at least in smaller hemorrhages.
A Note on Clinical Studies and Their Limitations
We have been able to study ICH in humans through postmortem studies, clinical assessments, and access to peripheral blood as well as cerebrospinal fluid (where accessible due to clinical indications). Further, sophisticated noninvasive means of studying the pathophysiology of clinical ICH have been developed over the last few decades—including advanced computed tomography, magnetic resonance imaging, and positron emission tomography. In situations where questions can be reliably answered using these powerful imaging tools and other clinical research methodologies, there should be little that testing the same questions in experimental ICH will add. However, there are some difficulties encountered with the study of ICH in the clinical setting. For example, ICH patients are often critically ill, requiring physiological support, and even if this is not the case, they may be drowsy and confused. Of note, in the acute setting the majority of patients with ICH are unsuitable for magnetic resonance imaging due to medical instability (Singer et al, 2004). Though sophisticated imaging studies can be successfully performed in such patients (Zazulia et al, 2009), this requires a very well set up and staffed service to conduct such research safely and to obtain useable and reliable data. Such difficulties therefore limit what can be achieved in the clinical setting.
Experimental intracerebral hemorrhage: the current situation
Experimental ICH offers the potential to do things that would clearly be impossible in humans—for example, histology in survivors of ICH (except where samples are taken during surgery), the initial testing of novel interventions, gene knockouts, homogeneous experimental groups, and a predictable onset of ICH allowing the hyperacute stage to be prospectively studied. As such, there remains a role for experimental ICH models to help in our understanding of the basic pathophysiological processes leading to the poor outcomes following clinical ICH.
Pathophysiology of Intracerebral Hemorrhage
Much has been learnt about the pathophysiology of ICH from experimental studies in animals. The initial rupture of a blood vessel in ICH leads to hematoma formation, with mechanical trauma to the neurones and glia, and resulting neurotransmitter release, mitochondrial dysfunction, and membrane depolarization (Kim-Han et al, 2006; Qureshi et al, 2001b, 2009). Secondary (delayed) injury results from the coagulation cascade and hemoglobin breakdown products including thrombin, which precipitates early microglial activation (within 4 hours) (Xi et al, 2006). The activated microglia (Wang and Tsirka, 2005) release detrimental substances causing blood–brain barrier (BBB) breakdown, vasogenic edema, and apoptosis in neurones and glia (Aronowski and Hall, 2005; Gong et al, 2001; Qureshi et al, 2003a, 2003b). These findings have mainly come from experimental ICH studies, but analysis of human perihematomal tissue from surgically evacuated hematomas has confirmed the role of edema, necrosis, apoptosis, and inflammatory cells in ICH (Qureshi et al, 2003b).
Animal Models of Intracerebral Hemorrhage
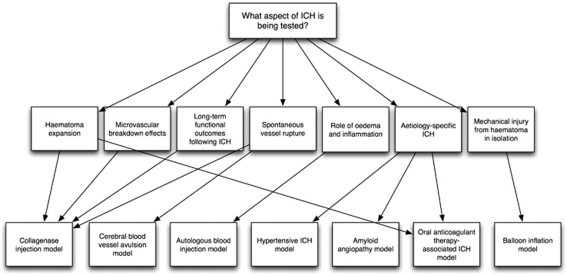
The commonest methods of inducing ICH in animals are the injection of either autologous blood or bacterial collagenase into the cerebrum (Table 1). Other ICH models include balloon inflation (Lopez Valdes et al, 2000; Mendelow, 1993) and cerebral blood vessel avulsion (Funnell et al, 1990), but neither is commonly used. Etiology-specific models of ICH related to oral anticoagulant therapy, hypertension, and amyloid angiopathy also exist. Each model mimics different aspects of clinical ICH, including the physical injury caused by an expanding mass within the brain parenchyma, the role of blood components, and hematoma expansion (Figure 2). In addition to various models of ICH, there is a selection of species that these models are used in; however, rodents are the commonest, and the focus of this review.
Table 1. Comparison of the two commonest ICH modeling techniques.
| Autologous blood injection | Collagenase injection |
|---|---|
| Main purpose | |
| Investigating mechanisms of hemorrhagic damage | Assessing long-term functional outcomes following ICH |
| Advantages | |
| Produces consistent hemorrhage volume | Stimulates human disease more accurately |
| A clinically relevant comparison | Simple procedure |
| Simple procedure | Can be used in multiple species |
| Can be used in multiple species | Dose-dependent hemorrhage size |
| May imitate bleeding–rebleeding phenomenon | |
| Mimics a spontaneous bleed | |
| Disadvantages | |
| Excessive inflammatory response? | Excessive inflammatory response? |
| Does not emulate small vessel rupture | Mechanism different to human condition |
| Not a spontaneous bleed | Neurotoxicity of collagenase |
| Not suitable for evaluating rebleeding | |
| Cannot evaluate microvascular breakdown effects | |
| Not suitable for studying long-term effects of ICH | |
ICH, intracerebral hemorrhage.
Figure 2.
Schematic illustrating the role of various models in researching different aspects of intracerebral hemorrhage (ICH)-mediated injury.
Autologous blood model
The autologous blood injection model was one of the earliest ICH models developed. Initially used in rats in the 1980s (Bullock et al, 1984; Nath et al, 1986), this model was later adapted for use in rabbits (Kaufman et al, 1985), dogs (Qureshi et al, 2001a), pigs (Wagner et al, 1996), and mice (Nakamura et al, 2004b). Blood is taken from a superficial vessel and stereotactically injected into the brain, usually into the striatum as injections into the cortex are often complicated by SAH (subarachnoid hemorrhage) and thus inconsistent hematoma volumes (Xue et al, 2000). However, in clinical practice, many lobar bleeds result in the subarachnoid extension of blood (Miller et al, 1999). This simple model is clinically relevant as it directly mirrors the rapid accumulation of intraparenchymal blood observed in the clinical setting, and allows for control of hematoma volume (homogeneity). However, in early studies, ventricular rupture and backflow of infused blood along the needle track led to IVH and/or SAH (Yang et al, 1994), again producing inconsistent hematoma volumes. In addition, extension into adjacent white matter occurred in 25% of animals (Xue and Del Bigio, 2003).
Double injection, where slow infusion of a small blood volume into the striatum allows blood clotting along the needle track, followed by the remaining blood to generate the hematoma, results in reproducible hematoma volumes (Deinsberger et al, 1996). In addition, the autologous blood injection model produces a consistent neurologic deficit, brain swelling, and cortical hypoperfusion (Belayev et al, 2003). Slow injection of 50 μL blood in rodents is equivalent to a 30-mL bleed in humans (Mendelow, 1993; Xi et al, 2001; Xue and Del Bigio, 2000). Faster injections of larger volumes may lead to raised intracranial pressure, systemic side effects, and direct tissue damage in a larger area than desired. These events often occur in clinical ICH and whether they are desirable in the experimental setting depends on the question being addressed. They may not be ideal when trying to create consistency in an experimental setting (Kingman et al, 1988) but may be important when testing a novel therapy.
Bacterial collagenase model
Injection of bacterial collagenase into the basal ganglia of rats to breakdown the basal lamina of blood vessels was first introduced in the early 1990s (Clark et al, 1998; Rosenberg et al, 1990). In the collagenase model, bleeding begins around 10 minutes after injection, but develops slowly into a full hematoma 4 to 24 hours later (Rosenberg et al, 1990). An advantage of this simple and reproducible model is that the hemorrhage occurs from in situ vessels (Wang and Tsirka, 2005), and it may imitate hematoma expansion (the bleeding–rebleeding phenomenon). Collagenase injection is simpler than blood injection and not complicated by the backflow of blood along the needle track (leading to IVH±SAH).
Balloon inflation model
Stereotactic insertion and inflation of a needle-mounted microballoon is a mechanical ICH model that has been used in rodents primarily to study mass effect of a hematoma and its removal on brain injury (Lopez Valdes et al, 2000; Mendelow, 1993; Sinar et al, 1987). The variables of balloon volume and duration of inflation are easily reproducible. The mechanical inflation model causes cell death involving apoptosis 6 to 24 hours following deflation (Nakashima et al, 1999). This model does not assess the role of blood and its breakdown products on ICH-related injury but is valuable for studying the effects of physical injury in isolation.
Avulsion of cerebral blood vessels
Surface cortical veins can be exposed through craniotomy and avulsed using a bent-tip needle, resulting in cortical hemorrhages (Funnell et al, 1990; Xue and Del Bigio, 2003).
Translational gaps
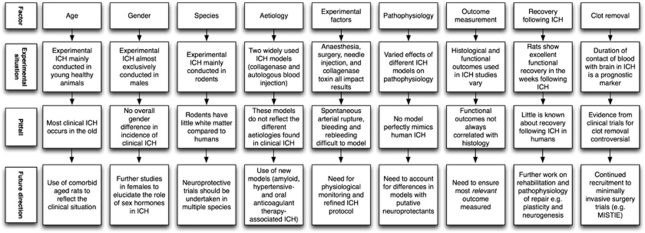
The heterogeneity of clinical ICH must be reflected in the experimental setting and potential treatments must be tested at a clinically relevant dose, route and time, and with clinically relevant outcomes. There are several areas where experimental ICH differs from human ICH, and/or where further translational work is required (Figure 3). Each area will now be discussed in turn.
Figure 3.
Schematic illustrating pitfalls in translational intracerebral hemorrhage (ICH) research and possible solutions.
Age and Comorbidity
Both the incidence and mortality of ICH increase with age in humans (van Asch et al, 2010), yet most experimental ICH is conducted in young healthy animals lacking comorbidities. Risk factors for ICH become more common with increasing age, namely amyloid angiopathy (O'Donnell et al, 2000) and chronic hypertension (Ariesen et al, 2003). When compared with young rats, ICH in aged rats causes more profound neurologic deficits, autophagy, brain swelling, microglial activation, and induction of heat shock proteins (Gong et al, 2004, 2011). Age is an important predictor of functional outcome (Daverat et al, 1991), and thus likely to be a significant determinant of brain injury, following ICH in humans. Importantly, aged rats exhibit a different quantitative and temporal inflammatory profile compared with their younger counterparts (Wasserman et al, 2008). It is therefore important that putative neuroprotectants are trialed in aged animals to maximize translatability.
Replicating the clinical milieu in which ICH occurs is likely to be important, as not doing so may have major and unpredictable effects on experimental outcomes. For example, experiments studying deep ICH are often performed in young healthy animals but should be performed in aged, spontaneously hypertensive animals as this subtype of ICH predominates in humans with these risk factors. This pitfall may have led to difficulties in translating promising preclinical findings to clinical trials. Preclinical data suggested recombinant factor VIIa reduces early hemorrhage growth during ICH (Kawai et al, 2006), corroborated by a phase IIb clinical trial (Mayer et al, 2005), which confirmed these findings and that of improved neurologic outcomes. However, while a subsequent phase III clinical trial of recombinant factor VIIa confirmed its ability to reduce hematoma growth, it failed to improve survival or functional outcome following ICH (Mayer et al, 2008). The phase III study identified an increase in serious vascular complications with active treatment, including myocardial infarction. An animal model where comorbidity was reproduced might have identified this, at least in terms of increased mortality, whereas testing in healthy young animals would have been much less likely to reveal this potential problem.
Gender
It is of note that almost all experimental ICH is conducted in male animals, yet the overall incidence in humans is no higher in males (van Asch et al, 2010). There are two main factors that have influenced this preference for using male animals. The first is the lack of an estrus cycle in males, which potentially reduces experimental variability between subjects. However, even in male rats, the influence of testosterone in experimental stroke appears to be age dependent; castration protects the young and testosterone supplementation protects the middle aged (Cheng et al, 2009). The second factor is the contentious issue of estrogen being a neuroprotectant; in humans, the menopause is an independent risk factor for the development of ICH (Feldmann et al, 2005), but the specific effects of the menopause on ICH-induced injury in females are unknown (Xi et al, 2006). It is postulated that estrogen may exert a neuroprotective effect in experimental stroke through decreased oxidative stress, apoptosis, inflammation, regulation of growth factors, and vascular modulation (Strom et al, 2011). However, when given at the wrong dose and by the wrong route, studies have found estrogen to be neurotoxic, resulting in increased inflammation, excitotoxicity, and oxidative stress (Strom et al, 2011). Most work on estrogen's neuroprotective role has been conducted using models of cerebral ischemia as opposed to ICH. However, a study of autologous blood injection in male and female mice found female mice to have significantly less brain edema at day 3 post-ICH compared with males (Nakamura et al, 2004b). Interestingly, following systemic administration of 17β-estradiol in male mice, the brain water content at day 3 was also significantly reduced, as was recovery time from the brain injury compared with normal males (Nakamura et al, 2004b). Similar results have been found in autologous blood injection in rats (Nakamura et al, 2006), suggesting that 17β-estradiol could be a potential therapeutic agent in ICH. It is of note that 17β-estradiol offered no further protection in female rats, suggesting that estrogen levels in female rats are sufficient for maximal neuroprotection. In female rats, the estrogen receptor is involved in limiting injury following ICH (Nakamura et al, 2006). Recent experimental work has identified tamoxifen, a selective estrogen receptor modulator, to have neuroprotective effects although this may be at the expense of a higher risk of developing hydrocephalus (Xie et al, 2011).
Relatively little attention has been paid to the role of other hormones on outcome following ICH. Recent evidence in male rats suggests that progesterone may reduce perihematomal brain edema and improve functional outcomes and testosterone may be deleterious following ICH (Chen et al, 2011). It is also of note that little attention has been paid to the effect of gender on the role of coagulation and blood breakdown products in ICH pathophysiology. However, both the coagulation cascade and iron handling differ between the sexes (Nanji et al, 2001). We do know, however, that estrogen protects against iron-induced cell death in vitro and brain edema in vivo (Gu et al, 2010). Further work is required to explore the underlying basis for gender differences in outcome following ICH-mediated injury, and to clarify the roles of estrogen and progesterone as putative neuroprotectants.
Species
Mice and rats are the mainstay of modeling in ICH (Clark et al, 1998; Sinar et al, 1987), although models in the pig (Mun-Bryce et al, 2001; Shi et al, 2010), dog (Coulter and Gooch, 1993; Wu and Zhong, 2010), cat (Kobari et al, 1988; Plotnikov et al, 1984), rabbit (Kaufman et al, 1985; Thai et al, 2006), baboon (Del Zoppo et al, 1986), and primate (Bullock et al, 1988) also exist. Advantages and disadvantages of the different species in modeling ICH are highlighted in Table 2. Generally speaking, larger animals such as dogs and monkeys are best for neurophysiologic and surgical studies, but are expensive to house. Rodents have the advantage of being the most commonly used species, with a vast array of reagents for immunohistochemistry and molecular biology, well-developed paradigms for testing functional outcomes, and the potential for transgenic and knockout animals for study (Wagner, 2007). The main disadvantage of rodents is their paucity of white matter relative to humans (Fisher et al, 2009). Thus, the optimal species of animal chosen for any given experiment depends on the question that requires an answer. However, as per the STAIR guidelines (Fisher et al, 2009), multiple species should be used in assessing putative neuroprotectants to ensure their validity.
Table 2. Relative advantages and disadvantages of different species in ICH modeling.
| Advantages | Disadvantages |
|---|---|
| Canine | |
| Useful for surgical and neurophysiological studies Historical use | Require large animal housing ‘Companion status' Expensive to use |
| Feline | |
| Useful for physiological and neurophysiological studies | ‘Companion status' Not historically used Availability of other more useful animal models |
| Lapine | |
| Easily housed Relatively inexpensive Largest ‘small animal' model | No transgenic systems Not historically used |
| Murine | |
| Cost-effective Widely used Easily housed Extensive reagents available for immunohistochemistry and molecular biology Well-developed neurobehavioral testing paradigms Transgenic systems (mice) | Paucity of white matter relative to humans Difficult to study physiological aspects Small brains |
| Porcine | |
| White-to-gray matter ratio similar to humans Useful for surgical, physiological, and neurophysiological studies | Expensive to use Require large animal housing |
| Primate | |
| Closely related to humans Useful for surgical and physiological studies | Complex to house Expensive to use Increasing regulatory oversight |
ICH, intracerebral hemorrhage.
Adapted from James et al (2008) and Wagner (2007).
Multiple Models for Multiple Etiologies
Outcomes following ICH vary depending on cause and location of ICH within the brain. Different causes tend to favor certain locations, such as lobar ICH in amyloid angiopathy and deep ICH in hypertensive bleeds. This heterogeneity in clinical ICH needs to be acknowledged in the design of experimental ICH studies, and the testing of potential treatments in several different models designed to closely mirror the ‘typical case' that is seen with each common cause of ICH seems warranted. Although the final common event in ICH is extravasation of blood into the brain parenchyma, the underlying cause can have a major impact on subsequent events. For example, there is pathological evidence that small vessels adjacent to the site of initial vessel rupture may also be damaged and bleed due to physical stresses (Takebayashi and Kaneko, 1983). If ICH is induced in animals without hypertension, will similar changes occur?
There has been a move toward the development of models that more correctly replicate the different underlying etiologies of ICH, including hypertension, oral anticoagulant therapy, and amyloid angiopathy. While the final common pathway of all these models is blood in the brain, the adoption of different models allows the corroboration of any findings. This may prevent the expense associated with a therapy deemed promising in one ICH model later disappointing at the clinical trial stage. Three etiology-specific ICH models are described below.
Hypertensive intracerebral hemorrhage
Limited progress in understanding the pathophysiology of spontaneous ICH is in part because current models do not accurately mimic spontaneous ICH in hypertensive humans (NINDS ICH Workshop Participants, 2005). While stroke-prone, spontaneous hypertensive rats are useful for studying stroke, they tend to develop ischemic stroke and, when hemorrhage occurs, this is usually secondary to hemorrhagic transformation of an infarct (Sadoshima et al, 1981). In humans, chronic hypertension is a major risk factor for ICH, and recent evidence suggests that development of an acute hypertensive episode superimposed on chronic hypertension may trigger ICH, possibly through activation of the renin–angiotensin and sympathetic nervous systems (Metoki et al, 2006; Vaughan and Delanty, 2000). A new model of ICH in hypertensive mice has thus been proposed (Wakisaka et al, 2010). Incorporating AngII (angiotensin II) and L-NAME (an inhibitor of nitric oxide synthase) in drinking water produces chronic hypertension, and giving injections of either AngII or noradrenaline creates further acute rises in systolic blood pressure. Despite noradrenaline resulting in larger blood pressure surges, AngII injections were more likely to cause spontaneous ICH, perhaps secondary to AngII-mediated oxidative stress, resulting in increased matrix metalloproteinase-9 and alteration of cerebral blood vessel matrices (Wakisaka et al, 2010). Oxidative stress is a recognized contributor to vascular injury in hypertension (Heistad, 2006). In this hypertensive ICH model, distribution of ICH location is similar to hypertensive humans—with the basal ganglia, thalamus, brain-stem, cerebellum, and cerebral cortex predominating (Xi et al, 2006). Inhibition of the renin–angiotensin system is therefore a potential target in preventing spontaneous ICH in hypertensive patients.
Oral anticoagulant therapy-associated intracerebral hemorrhage
The increasing incidence of atrial fibrillation, partly a reflection of the aging population, will lead to increased use of oral anticoagulants (typically warfarin, at present) in ischemic stroke prevention, and increased OAT-ICH. Although newer direct thrombin inhibitors may reduce the use of warfarin, their lack of an antidote (with the exception of dabigatran) makes further research on anticoagulant-associated hemorrhage even more important. Oral anticoagulant therapy-ICH carries a short-term mortality rate of over 50% that has failed to improve with time (Aguilar et al, 2007; Sjoblom et al, 2001). Of note, rebleeding is commoner in OAT-ICH compared with ICH in the setting of normal coagulation (Flibotte et al, 2004). Since coagulation cascade activation and thrombin production are important steps in cellular damage and disruption of the BBB following ICH, it seems logical that OAT-ICH will result in a different tissue response than spontaneous ICH as hematomas may contain noncoagulated blood (Levine et al, 2007; Xi et al, 2006). A new model of OAT-ICH incorporates water-soluble warfarin into mouse feeding bottles (Foerch et al, 2008). Once coagulation assays are within the human therapeutic range, ICH is induced using bacterial collagenase. Surprisingly, only one of the 66 mice developed SAH, despite impaired coagulation. Anticoagulation with warfarin increased the hematoma volume at 24 hours by 2.5-fold relative to nonanticoagulated mice (Foerch et al, 2008), and a temporal increase in hematoma growth was observed, suggesting that this model may be useful for studying the rebleeding phenomenon. Treatment with prothrombin complex concentrate after 45 minutes successfully reduces hematoma volume (Foerch et al, 2009). More work is required to address whether differences between the pathophysiology of OAT-ICH and spontaneous ICH exist in terms of neuronal death, edema formation, BBB damage, and inflammation. In addition, while more work is needed to fully validate this model, the creation of a more refined model of OAT-ICH that does not rely on collagenase injection is also required.
Amyloid angiopathy-associated intracerebral hemorrhage
Cerebral amyloid angiopathy (CAA) is characterized pathologically by the deposition of extracellular congophilic β-amyloid in leptomeningeal and cerebral cortical vessels. Its incidence rises with increasing age and is associated with Alzheimer's disease (Miller et al, 1999). Clinically, it most commonly presents with lobar ICH, often extending into the subarachnoid space and ventricles (Miller et al, 1999). The first transgenic mouse model of CAA was reported in 2001 (Winkler et al, 2001). The model correctly mimics the age-related increase in CAA frequency and severity. In the first study utilizing this model, spontaneous hemorrhages were found in aged transgenic (APP23) mice with CAA but not the aged control mice, suggesting that CAA is the driving force for vessel rupture and hemorrhage (Winkler et al, 2001). There are, however, some drawbacks to this model. Although the model most commonly results in CAA and hence hemorrhage in the lobar region, in keeping with the clinical situation, thalamic CAA is found much more commonly in the model than in humans, and vice versa for cerebellar CAA. Overall, however, this model represents an important development in the study of CAA-associated ICH, a common cause of lobar ICH in the elderly.
Experimental Factors
There are several factors inherent in the process of the experimental setup of current ICH models that affect the outcomes observed following ICH in animals.
Mimicking spontaneous vessel rupture and hematoma expansion
In the clinical setting, ICH results from spontaneous blood vessel rupture (Qureshi et al, 2001b). Clearly, autologous blood injection does not mimic spontaneous rupture and the time taken to reach the desired hematoma volume is significantly shorter than the development of spontaneous ICH in humans (Rosenberg et al, 1990). Further, as autologous blood injection does not emulate small vessel rupture often seen in human ICH, it cannot be used to evaluate microvascular breakdown effects (James et al, 2008). The collagenase model and cerebral blood vessel avulsion model do give rise to ICH from in situ vessels, but both models have drawbacks. For example, the injection of collagenase has been postulated to result in an exaggerated inflammatory response, as discussed in section Minimizing excess inflammation. The cerebral blood vessel avulsion model induces ischemic infarction in addition to ICH, making it of doubtful clinical relevance and rendering comparisons with the other models of ICH difficult.
As described above, hematoma expansion is common following ICH in humans and adversely affects outcome. The autologous model is not characterized by hematoma expansion (Broderick et al, 1990), but the collagenase injection model is. Given that such expansion occurs and is clinically significant in up to 35% of ICH patients in the first 3 hours, it is important that this is taken into consideration when evaluating any data from experimental ICH.
Minimizing excess inflammation
The presence of excessive inflammation in the collagenase and autologous blood injection models compared with human ICH is widely disputed. It was initially thought that collagenase injection into the brain parenchyma might be directly toxic or result in an exaggerated immune and thus inflammatory response different from the mechanism producing human ICH (Del Bigio et al, 1996; Xue and Del Bigio, 2003). However, in vitro studies of inflammation and apoptosis simultaneously utilizing both autologous blood and collagenase models argue against this (Chu et al, 2004; Matsushita et al, 2000). In fact, recent evidence suggests that postictal inflammation is more exaggerated in the autologous blood injection model, especially in rats, as a result of hemoglobin crystallization, which is strongly chemotactic for neutrophils (Kleinig et al, 2009). In humans, hemoglobin only crystallizes if structurally abnormal, but hemoglobin crystallizes in most rat brains when ICH is induced using either the autologous blood or collagenase injection models, most markedly in the autologous blood injection model (Kleinig et al, 2009). Hemoglobin also crystallizes to a lesser degree in mice (Berman, 1967). Although crystallization could be minimized by heparinization, heparin antagonizes thrombin—a known mediator of secondary injury following ICH (Hua et al, 2007). It is thus pertinent that the potential for excess inflammatory response in the experimental setting is accounted for when looking at outcome data.
Replicating arterial bleeding
Most studies using the autologous blood injection ICH model have delivered intracerebral blood at a fixed flow rate (e.g., 10 μL/min). Early work involved intracerebral injection of blood at a fixed pressure of 100 mm Hg, corresponding roughly to the typical mean arterial pressure observed in the setting of human ICH (Bullock et al, 1984; Nath et al, 1986). One may assume that in ICH blood indeed extravasates at a rate determined by the cerebral perfusion pressure as opposed to a fixed flow. As such, flow is very likely to be greater initially and fall as increasing ICP reduces cerebral perfusion pressure. Work is therefore needed to compare whether fixed pressure or fixed flow more accurately replicates the human condition, and it would be useful to compare both methods in terms of histological and functional outcomes.
Minimizing physical damage caused by needle injection
In addition to these limitations, the extent of physical injury to the brain tissue induced through direct intraparenchymal injection with the metal needles currently used in experimental ICH models is significant (McCluskey et al, 2008). A study using a glass microneedle (tip diameter <50 μm) to inject both interleukin-1 and vehicle intracerebrally resulted in less mechanical injury, BBB breakdown and recruitment of neutrophils compared with a 26G (tip diameter ∼460 μm) metal Hamilton syringe (Hamilton, Carnforth, UK) (McCluskey et al, 2008). It therefore follows that use of a glass microneedle should be tested in experimental ICH models.
Effect of anesthesia and surgery
Anesthesia is essential for conducting experimental ICH, yet its use may alter the molecular environment and treatment response in an uncontrollable manner (Qureshi et al, 2009; Xi et al, 2006). For example, a study comparing bone marrow response to experimental stroke, sham surgery, and administration of isofluorane alone found systemic inflammatory changes and leukocyte responses in the bone marrow to be markedly affected even with isofluorane alone (Denes et al, 2011). The effect of isofluorane on various leukocyte populations occurs early within the first 20 to 30 minutes of anesthesia (Denes et al, 2011). While use of anesthesia is unavoidable in experimental ICH, this study highlights the need to account for the role of surgery and anesthesia when interpreting any results derived from such studies.
Anesthetic agents are generally considered to be neuroprotective as they reduce the metabolic rate and oxygen requirements of the central nervous system (Harvey and Paddleford, 1993). Inhalational anesthetics produce a generalized reversible CNS depression but the mechanism by which this is brought about is unknown. Anesthetics can also interact with putative neuroprotectants to improve apparent efficiency of the agent under investigation (Macleod et al, 2005). However, some induce apoptosis of neurones and are hence neurotoxic (Ikonomidou et al, 1999). Many variables influence the effect of an anesthetic on an individual. For example, the distribution and hence physiological impact of lipid-soluble anesthetics will vary according to fat content differences between species and also among different animals within species. Each anesthetic has its own side effect profile, which should be considered when undertaking experimental ICH (Table 3).
Table 3. Examples of some common anesthetic agents and their effects in experimental ICH.
| Anesthetic | Effect |
|---|---|
| Barbiturates (thiopental, thiobarbital, phenobarbital) | Phenobarbital can lower core body temperature by up to 4.5°C and brain temperature 0.4°C within an hour |
| Can cause excitatory response in some species e.g., cats | |
| Long half-life, resulting in prolonged anesthetic recovery period and affecting postoperative functional assessments | |
| Ketamine | Increases sympathetic tone (increasing heart rate and cardiac output) |
| Increases oxygen requirements, CBF, ICP, EEG activity | |
| Sevoflurane | Dose-dependent impairment of cerebrovascular autoregulation and decreases microglial activation and delays and decreases astrocyte activation |
| Propofol | Increases astrocyte and microglial cell activation |
| Isofluorane | Reduces the CNS metabolic rate and thus brain oxygen requirements, as well as suppressing burst activity on EEG |
| Halothane | Increases CBF by some 200%, thereby potentially increasing ICP |
| Causes myocardial depression and arrythmias | |
| Can affect cortical morphology and cause hepatotoxicity in rats | |
| Nitrous oxide (adjunctive anesthetic) | Increased CBF and oxygen requirements |
CBF, cerebral blood flow; CNS, central nervous system; EEG, electroencephalogram; ICH, intracerebral hemorrhage; ICP, intracranial pressure.
Temperature commonly decreases following anesthesia in small animals, principally due to their high surface area-to-body mass ratio making thermoregulation difficult. The use of unwarmed anesthetic gases exacerbates this problem (Haskins and Patz, 1980). Cooling can be profoundly neuroprotective (van der Worp et al, 2007), and the mechanism of action of a putative neuroprotectant itself may be related to a hypothermic process, as is thought for clomethiazole (Visser et al, 2005). In humans, however, hyperthermia is common following ICH and duration of fever is an independent prognostic indicator (Schwarz et al, 2000). Even mild hypothermia can reduce perihemorrhagic edema following ICH in humans (Kollmar et al, 2010). As such, any evaluation of a putative neuroprotectant should account for the effect of temperature changes on outcome.
Role of physiological monitoring
In addition to temperature, blood gas concentrations (Zausinger et al, 2002) and pH (Anderson and Meyer, 2002) also influence outcome following experimental stroke. Further, increases in blood pressure are associated with improved blood flow and oxygen metabolism (Shin et al, 2008). Anesthesia affects mean arterial pressure, arterial pO2 and pCO2, and venous flow, all of which in turn influence CBF. This highlights the importance of physiological monitoring during experimental ICH to minimize the undesirable effects of anesthesia. Monitoring of physiological parameters should be commenced in the preoperative period with baseline physiological measurements before anesthetic administration, and continue through to the postoperative period. Preoperative parameters to be measured include animal weight, temperature, heart rate, blood pressure, respiratory rate, and quality of respiratory effort. Intraoperative parameters may include heart rate, blood pressure, temperature, blood gas analysis, electrocardiogram, and electroencephalogram activity. Some recommend the measurement of both rectal and cranial temperatures (Busto et al, 1989), although there has been excellent correlation between the two with isofluorane anesthetic (R2=0.9996) (Zhu et al, 2009), suggesting that invasive brain temperature monitoring is unnecessary.
The measurement of CBF can be used to monitor cerebral ischemia, seizures, and the effect of drugs on CBF; although, as earlier described, true ischemia is not thought to occur in ICH unless the hematoma is of significant size (Xi et al, 2006). Through real-time monitoring not only can the presence of a cerebral injury be confirmed but also the period of which there is reduced or absent blood flow can be quantified. A drawback of CBF monitoring is that it requires placement of a probe necessitating a larger burr hole having to be drilled. In an ideal situation, we could monitor real-time changes in blood pressure, blood pH, anesthetic concentration, and partial pressures of O2 and CO2, to ensure that changes in values were brought about by ICH and not the experimental setup itself.
Pathophysiology
No current model perfectly mimics human ICH (James et al, 2008), and notable pathophysiological differences exist between the models themselves (NINDS ICH Workshop Participants, 2005). Interestingly, a study comparing cell death and inflammation following ICH in the autologous blood, collagenase, and cerebral vessel avulsion models demonstrated similar temporal profiles of cell death, inflammatory cell infiltration, and microglial reaction (Xue and Del Bigio, 2003). However, there are quantitative histological differences between the models. For example, the cerebral blood vessel avulsion model demonstrates more necrosis, less hemorrhage (Xue and Del Bigio, 2003), and less neutrophil infiltration (Xue et al, 2000) than both the autologous blood and collagenase injection models.
When comparing just the autologous blood and collagenase injection models, further differences arise; perihematomal neutrophil infiltration is more prominent in the collagenase model than the autologous blood injection model (Rosenberg et al, 1990; Xue and Del Bigio, 2000, 2003). The development of cerebral edema has a stronger temporal association with neurologic deterioration in the autologous blood injection model compared with the collagenase model, and is slower and more protracted in human compared with experimental ICH (Butcher et al, 2004; Gebel et al, 2002a; Hua et al, 2003; Qureshi et al, 2009; Rosenberg et al, 1990; Yang et al, 1994)—as is red cell resorption (Butcher et al, 2004; MacLellan et al, 2008; Xi et al, 2006). Further, when matched for hematoma size, the total loss of brain tissue can be twice as much in the collagenase model compared with the blood injection model (MacLellan et al, 2008). All of these differences suggest the need for the trialing of putative neuroprotectants with multiple models and outcome measures.
Outcome Measurement
An important aspect of designing experimental ICH studies is deciding which outcomes are relevant for assessment. The main outcomes assessed by ICH models include mortality, behavioral and functional outcomes, hematoma and perihematomal edema growth, neuroinflammation, changes in CBF and intracranial pressure, and extent of apoptosis. Hematoma volume, subsequent enlargement, and associated cerebral edema are strongly associated with outcomes in human ICH (Qureshi et al, 2009). Are these therefore the outcomes most relevant to the human condition? The STAIR Preclinical Recommendations indicate that both histological and behavioral outcomes should be assessed, with multiple end points and continuing assessment for at least 2 to 3 weeks postictus to demonstrate sustained benefit (Fisher et al, 2009). However, the methods used to assess functional recovery in animals are crude compared with those used in the clinical setting. In addition, most clinical and experimental studies looking at stroke recovery mechanisms are focused on ischemic stroke and hence use cortical injury assessments, whereas the striatum is the commonest injury site in ICH overall (MacLellan et al, 2011).
There is evidence to suggest poor correlation between functional and histological outcomes in different models of ICH. For example, when comparing the two commonest ICH models (autologous blood injection and collagenase injection) in a trial of therapeutic hypothermia, bleeding, tissue loss, and functional recovery differed between the two models (MacLellan et al, 2010). Further, in a trial of enriched rehabilitation, functional outcomes (assessed through a reaching task) in rats undergoing both autologous blood-induced ICH and collagenase-induced ICH were improved, but the former model resulted in no changes in lesion volume or contralateral dendritic morphology (MacLellan et al, 2010, 2011). Ongoing cell death observed in the collagenase model (MacLellan et al, 2008) is a potential target for neuroprotective agents and rehabilitation therapies (MacLellan et al, 2011). However, we know very little about the extent to which delayed cell death (in the order of days, weeks, or longer) occurs in human ICH—a sequential imaging study could help answer this (MacLellan et al, 2011). These data suggest that recovery methods vary between ICH models and highlight the need for testing of putative neuroprotectants in multiple models of ICH, utilizing both relevant functional and histological outcome measures, before progressing to clinical trials.
Recovery Following Intracerebral Hemorrhage
It is of note that spontaneous functional recovery is observed in clinical and experimental stroke (Cramer, 2008). Rodents demonstrate remarkable plasticity and functional recovery in the weeks following ICH (Hua et al, 2002; STAIR, 1999), but little is known as to whether this recovery results from neurogenesis, restoration of function to ipsilateral neurones, or the assimilation of new functions by ipsilateral or contralateral neurones (Hua et al, 2009). Even less is known about the mechanisms of recovery following ICH in humans, and most survivors retain motor or cognitive deficits; recovery following ICH can be protracted due to the slow resolution of an untreated hematoma, secondary medical complications, and perceptions of poor prognosis that leads to the withdrawal of support for patients (Zahuranec et al, 2007). Correlations between neurogenesis and functional improvements exist following experimental ICH, but it is not known whether the two are directly related. It is of note that young and aged rats exhibit identical temporal recovery profiles (Gong et al, 2004), suggesting that the worsened functional and pathophysiological outcomes observed in the latter group are due to aging rather than impairments in plasticity. While thrombin is a well-established mediator of secondary injury following ICH, there is now evidence that it has a role in brain recovery following ICH—affecting angiogenesis, neurogenesis, and plasticity (Hua et al, 2009). Further knowledge of the mechanisms underlying brain recovery following ICH will facilitate the development of neuroprotective therapies.
Rehabilitation following stroke is important for brain recovery and promoting plasticity. There were concerns regarding rehabilitation during the acute phase of ICH and the possibility of precipitating hematoma expansion, but in a rat model of ICH early treadmill training (24 hours following ICH) was found to be safe and promoted motor recovery (Park et al, 2010). However, further studies are needed to validate this finding for translation into clinical practice.
Clot Removal
Important prognostic indicators following ICH include hematoma size and duration of exposure of the brain to blood, the latter due to the release of toxic products from the hematoma including thrombin, and the lysis of extravasated erythrocytes releasing hemoglobin, heme, and iron. There has thus been a great research thrust (both clinical and experimental) toward early hematoma removal in an attempt to improve outcomes. In humans, a large randomized controlled trial comparing early surgery and conservative management for spontaneous supratentorial intracerebral hemorrhage (the STICH trial) found no overall benefit for surgery (Mendelow et al, 2005); post hoc subgroup analysis found benefit for those with superficial lobar ICH, and thus a further trial in this subgroup is currently underway (STICH II). Since open craniotomy has inherent risks in terms of recurrent bleeding and neural damage (Qureshi et al, 2009), minimally invasive techniques of clot evacuation have become increasingly popular.
There is evidence from porcine (Wagner et al, 1999) and rabbit (Wu et al, 2011a) ICH models of decreased tissue injury, BBB opening, and edema and hematoma volumes through minimally invasive clot aspiration and thrombolysis. A recent clinical randomized controlled trial showed benefits of stereotactic evacuation over conventional craniotomy in terms of functional outcomes and postoperative complications (Zhou et al, 2011), and a further randomized clinical trial (the Minimally Invasive Surgery plus Tissue Plasminogen Activator for Intracerebral Hemorrhage Evacuation, MISTIE trial) is currently ongoing. There is thus mounting evidence for a beneficial role of minimally invasive clot removal with thrombolysis and we await the results from the MISTIE trial.
Future directions
The optimal ICH model should be simple to create, permit the formation of reproducible volumes of hemorrhage, and induce hemorrhage with mechanisms reliably mirroring the clinical situation. In addition, this model should be able to produce ICH in different regions of the brain without altering the model drastically, and be financially viable as well as reproducible between different researchers and different laboratories. Further, it should be one in which the hemorrhagic event could be triggered on demand. The ideal model would allow consistency in the study of lobar localization, ventricular extension, vessel rupture, and the rebleeding phenomenon or continued hemorrhage. It would also help ascertain the impact of ICH on surrounding tissues, including local cerebral edema and hemispheric swelling (NINDS ICH Workshop Participants, 2005). The important issue of rebleeding or continued bleeding post-ICH has yet to be thoroughly studied in an animal model (NINDS ICH Workshop Participants, 2005).
Since rodent models of ICH focus on gray matter injury, there is a need for more use of models that involve both gray and white matter brain injury—such as primate and swine models, which incorporate larger quantities of white matter.
The development and evaluation of neuroprotectants in stroke should be subject to rigorous evaluation along with recommendations from multiple sources (Corbett and Nurse, 1998; Dirnagl, 2006; Fisher et al, 2009; Gladstone et al, 2002; Stroke Therapy Academic Industry Roundtable, 1999). The recent development of the ARRIVE (Animal Research Reporting In Vivo Experiments) guidelines should improve the quality and reporting of animal studies (Kilkenny et al, 2010). Importantly, functional outcomes are a vital measure, as reductions in cell death, hematoma, or edema do not necessarily translate into improved recovery—the clinical end point of paramount importance (Corbett and Nurse, 1998; Stroke Therapy Academic Industry Roundtable, 1999). Also important is the consideration of negative as well as positive findings, while accounting for differences in ICH models used and effect sizes.
There is overwhelming evidence in young and old animals from several species of the ability of iron chelator desferrioxamine to reduce neurologic injury and improve functional recovery following ICH (Gu et al, 2009; Hua et al, 2006; Huang et al, 2002; Nakamura et al, 2004a; Okauchi et al, 2009; Song et al, 2007; Wan et al, 2006, 2009; Wu et al, 2011b). This remains the most promising putative neuroprotectant, having advanced to clinical trials (Selim, 2009) with a phase II currently underway. However, as already described, the translation of other compounds deemed promising from preclinical animal stroke into clinical practice has posed challenges. Collaboration between different laboratories performing preclinical studies, perhaps in a manner akin to a multicenter clinical trial, would ensure more robust and standardized methodologies allowing easy replication, as well as more data with more accurate findings. The ultimate aim of this would be to minimize potential sources of bias by randomization, concealment of allocation, blinding of surgery, and outcome assessment, and to ensure publication of all data (positive and negative). Although initially costly, this may indeed work out less expensive than running another clinical trial for a therapy that subsequently disappoints.
Conclusions
Intracerebral hemorrhage is a devastating condition that, despite significant advances in our understanding of the pathophysiology, still lacks any effective treatment and an animal model that accurately replicates the underlying mechanisms of injury. Available models simulate different aspects of ICH-mediated injury. Newly developed models, notably hypertension- and oral anticoagulant therapy-associated ICH models, offer added benefits but further study is needed to fully validate them. In addition, there are modifications that can be made to existing ICH models to improve their validity, as discussed in this article.
Despite advances in imaging over the past few decades, as long as the limitations of animal ICH models are borne in mind there is an important role for experimental ICH models in helping further our understanding of the basic pathophysiologic processes underlying ICH. The model chosen by researchers very much depends on the question requiring an answer. Owing to the differences in existing models and the failed translation of benefits in experimental ICH to clinical practice, until a more accurate model of ICH is developed putative neuroprotectants should be trialed in multiple models using histological and functional outcomes.
Literature search strategy and selection criteria
The references used in this review were obtained from searches of PubMed, MEDLINE, and Ovid between the time frame of January 1966 and May 2011. We used different combinations of the following search terms: ‘intracerebral hemorrhage', ‘animal model', ‘experimental', ‘inflammation', ‘translational', ‘hypertension', ‘warfarin', ‘anticoagulant', ‘amyloid angiopathy', ‘rat', ‘mice', ‘species', ‘outcome', ‘age', ‘anesthesia', ‘surgery', ‘autologous blood', and ‘collagenase'. In addition, articles were identified from the reference lists of articles, resulting from the literature search. Only papers written in English were reviewed. We selected papers for inclusion in the final reference list based on their methodological quality and importance to the topic at hand.
Acknowledgments
The authors thank Nancy Rothwell for her advice and comments on early drafts of this manuscript.
The authors declare no conflict of interest.
References
- Aguilar MI, Hart RG, Kase CS, Freeman WD, Hoeben BJ, GarcÖa RC, Ansell JE, Mayer SA, Norrving B, Rosand J, Steiner T, Wijdicks EFM, Yamaguchi T, Yasaka M. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Meyer FB.2002Protection of focal cerebral ischemia by alkalinization of systemic pH Neurosurgery 511256–1265.discussion 65–66 [DOI] [PubMed] [Google Scholar]
- Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Morgenstern LB, Chalmers J, Anderson CS, For the INTERACT Investigators Significance of perihematomal edema in acute intracerebral hemorrhage: The INTERACT trial. Neurology. 2009;73:1963–1968. doi: 10.1212/WNL.0b013e3181c55ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Belayev L, Saul I, Curbelo K, Busto R, Belayev A, Zhang Y, Riyamongkol P, Zhao W, Ginsberg MD. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke. 2003;34:2221–2227. doi: 10.1161/01.STR.0000088061.06656.1E. [DOI] [PubMed] [Google Scholar]
- Berman I. The ultrastructure of erythroblastic islands and reticular cells in mouse bone marrow. J Ultrasruct Res. 1967;17:291–313. doi: 10.1016/s0022-5320(67)80050-9. [DOI] [PubMed] [Google Scholar]
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage in Adults: 2007 Update: A Guideline From the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195–199. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- Bullock R, Brock-Utne J, van Dellen J, Blake G. Intracerebral hemorrhage in a primate model: effect on regional cerebral blood flow. Surg Neurol. 1988;29:101–107. doi: 10.1016/0090-3019(88)90065-1. [DOI] [PubMed] [Google Scholar]
- Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: description of technique, ICP changes and neuropathological findings. Neurol Res. 1984;6:184–188. doi: 10.1080/01616412.1984.11739687. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Ginsberg MD. The importance of brain temperature in cerebral ischemic injury. Stroke. 1989;20:1113–1114. doi: 10.1161/01.str.20.8.1113. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke. 2004;35:1879–1885. doi: 10.1161/01.STR.0000131807.54742.1a. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xi G, Mao Y, Keep RF, Hua Y. Effects of progesterone and testosterone on ICH-induced brain injury in rats. Acta Neurochir Suppl. 2011;111:289–293. doi: 10.1007/978-3-7091-0693-8_48. [DOI] [PubMed] [Google Scholar]
- Cheng J, Hu W, Toung TJ, Zhang Z, Parker SM, Roselli CE, Hurn PD. Age-dependent effects of testosterone in experimental stroke. J Cereb Blood Flow Metab. 2009;29:486–494. doi: 10.1038/jcbfm.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Jeong SW, Jung KH, Han SY, Lee ST, Kim M, Roh JK. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab. 2004;24:926–933. doi: 10.1097/01.WCB.0000130866.25040.7D. [DOI] [PubMed] [Google Scholar]
- Clark W, Gunion-Rinker L, Lessov N, Hazel K, Macdonald RL. Citicoline treatment for experimental intracerebral hemorrhage in mice editorial comment. Stroke. 1998;29:2136–2140. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- Corbett D, Nurse S. The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol. 1998;54:531–548. doi: 10.1016/s0301-0082(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Coulter DM, Gooch WM. Falling intracranial pressure: an important element in the genesis of intracranial hemorrhage in the beagle puppy. Biol Neonate. 1993;63:316–326. doi: 10.1159/000243948. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Daverat P, Castel JP, Dartigues JF, Orgogozo JM. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke. 1991;22:1–6. doi: 10.1161/01.str.22.1.1. [DOI] [PubMed] [Google Scholar]
- Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- Deinsberger W, Vogel J, Kuschinsky W, Auer LM, Boker DK. Experimental intracerebral hemorrhage: description of a double injection model in rats. Neurol Res. 1996;18:475–477. doi: 10.1080/01616412.1996.11740456. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Yan HJ, Buist R, Peeling J.1996Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates Stroke 272312–2319.discussion 9–20 [DOI] [PubMed] [Google Scholar]
- Del Zoppo G, Copeland B, Waltz T, Zyroff J, Plow E, Harker L. The beneficial effect of intracarotid urokinase on acute stroke in a baboon model. Stroke. 1986;17:638–643. doi: 10.1161/01.str.17.4.638. [DOI] [PubMed] [Google Scholar]
- Denes A, McColl BW, Leow-Dyke SF, Chapman KZ, Humphreys NE, Grencis RK, Allan SM, Rothwell NJ. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab. 2011;31:1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 2006;26:1465–1478. doi: 10.1038/sj.jcbfm.9600298. [DOI] [PubMed] [Google Scholar]
- Fainardi E, Borrelli M, Saletti A, Schivalocchi R, Russo M, Azzini C, Cavallo C, Ceruti S, Chieregato A, Tamarozzi R. Assessment of acute spontaneous intracerebral hematoma by CT perfusion imaging. J Neuroradiol. 2005;32:333–336. doi: 10.1016/s0150-9861(05)83164-5. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- Feldmann E, Broderick JP, Kernan WN, Viscoli CM, Brass LM, Brott T, Morgenstern LB, Wilterdink JL, Horwitz RI. Major risk factors for intracerebral hemorrhage in the young are modifiable. Stroke. 2005;36:1881–1885. doi: 10.1161/01.STR.0000177480.62341.6b. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, Lo EH. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39:3397–3404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerch C, Arai K, Van Cott EM, van Leyen K, Lo EH. Rapid reversal of anticoagulation reduces hemorrhage volume in a mouse model of warfarin-associated intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:1015–1021. doi: 10.1038/jcbfm.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell WR, Maysinger D, Cuello AC. Three-dimensional reconstruction and quantitative evaluation of devascularizing cortical lesions in the rat. J Neurosci Methods. 1990;35:147–156. doi: 10.1016/0165-0270(90)90104-n. [DOI] [PubMed] [Google Scholar]
- Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002a;33:2631–2635. doi: 10.1161/01.str.0000035284.12699.84. [DOI] [PubMed] [Google Scholar]
- Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002b;33:2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–883. doi: 10.1097/00006123-200104000-00037. [DOI] [PubMed] [Google Scholar]
- Gong Y, He Y, Gu Y, Keep RF, Xi G, Hua Y. Effects of aging on autophagy after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:113–117. doi: 10.1007/978-3-7091-0693-8_18. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Xi G, Liu W, Keep RF, Hua Y. Estrogen reduces iron-mediated brain edema and neuronal death. Acta Neurochir Suppl. 2010;106:159–162. doi: 10.1007/978-3-211-98811-4_29. [DOI] [PubMed] [Google Scholar]
- Harvey RC, Paddleford RR.1993Anesthesia for the central nervous systems and ophthalmic surgery Textbook of Small Animal Surgery(Slatter DS, ed). 2nd ed. vol. II.Philadelphia: W.B. Saunders; 2271–2278. [Google Scholar]
- Haskins SC, Patz JD. Effect of inspired-air warming and humidification in the prevention of hypothermia during general anesthesia in cats. Am J Vet Res. 1980;41:1669–1673. [PubMed] [Google Scholar]
- Heistad DD. Oxidative stress and vascular disease: 2005 duff lecture. Arterioscler Thromb Vasc Biol. 2006;26:689–695. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Gu Y, Xi G. Thrombin and brain recovery after intracerebral hemorrhage. Stroke. 2009;40:S88–S89. doi: 10.1161/STROKEAHA.108.533281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. A thrombin inhibitor reduces brain edema, glioma mass and neurological deficits in a rat glioma model. Acta Neurochir Suppl. 2003;86:503–506. doi: 10.1007/978-3-7091-0651-8_103. [DOI] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocritical Care. 2008;9:139–152. doi: 10.1007/s12028-007-9030-2. [DOI] [PubMed] [Google Scholar]
- Karwacki Z, Kowianski P, Dziewiatkowski J, Domaradzka-Pytel B, Ludkiewicz B, Wojcik S, Narkiewicz O, Morys J. The influence of sevoflurane on the reactivity of astrocytes in the course of the experimental intracerebral haemorrhage in rat. J Physiol Pharmacol. 2005;56:455–469. [PubMed] [Google Scholar]
- Karwacki Z, Kowianski P, Dziewiatkowski J, Domaradzka-Pytel B, Ludkiewicz B, Wojcik S, Narkiewicz O, Morys J. Quantitative analysis of influence of sevoflurane on the reactivity of microglial cells in the course of the experimental model of intracerebral haemorrhage. Eur J Anaesthesiol. 2006a;23:874–881. doi: 10.1017/S0265021506000603. [DOI] [PubMed] [Google Scholar]
- Karwacki Z, Kowianski P, Dziewiatowski J, Domaradzka-Pytel B, Ludkiewicz B, Wojcik S, Narkiewicz O, Morys J. The effect of propofol on astro- and microglial reactivity in the course of experimental intracerebral haemorrhage in rats. Folia Neuropathol. 2006b;44:50–58. [PubMed] [Google Scholar]
- Kaufman HH, Pruessner JL, Bernstein DP, Borit A, Ostrow PT, Cahall DL. A rabbit model of intracerebral hematoma. Acta Neuropathol. 1985;65:318–321. doi: 10.1007/BF00687015. [DOI] [PubMed] [Google Scholar]
- Kawai N, Nakamura T, Nagao S. Early hemostatic therapy using recombinant factor VIIa in a collagenase-induced intracerebral hemorrhage model in rats. Acta Neurochir Suppl. 2006;96:212–217. doi: 10.1007/3-211-30714-1_46. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Han JS, Kopp SJ, Dugan LL, Diringer MN. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke. 2006;37:2457–2462. doi: 10.1161/01.STR.0000240674.99945.4e. [DOI] [PubMed] [Google Scholar]
- Kingman TA, Mendelow AD, Graham DI, Teasdale GM. Experimental intracerebral mass: description of model, intracranial pressure changes and neuropathology. J Neuropathol Exp Neurol. 1988;47:128–137. doi: 10.1097/00005072-198803000-00005. [DOI] [PubMed] [Google Scholar]
- Kirkman MA. Debate—does a reversible penumbra exist in intracerebral haemorrhage. Br J Neurosurg. 2011;25:523–525. doi: 10.3109/02688697.2011.578773. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav. 2005;84:563–570. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kleinig TJ, Helps SC, Ghabriel MN, Manavis J, Leigh C, Blumbergs PC, Vink R. Hemoglobin crystals: a pro-inflammatory potential confounder of rat experimental intracerebral hemorrhage. Brain Res. 2009;1287:164–172. doi: 10.1016/j.brainres.2009.06.077. [DOI] [PubMed] [Google Scholar]
- Kobari M, Gotoh F, Tomita M, Tanahashi N, Shinohara T, Terayama Y, Mihara B. Bilateral hemispheric reduction of cerebral blood volume and blood flow immediately after experimental cerebral hemorrhage in cats. Stroke. 1988;19:991–996. doi: 10.1161/01.str.19.8.991. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Staykov D, Dorfler A, Schellinger PD, Schwab S, Bardutzky J. Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke. 2010;41:1684–1689. doi: 10.1161/STROKEAHA.110.587758. [DOI] [PubMed] [Google Scholar]
- Levine JM, Snider R, Finkelstein D, Gurol ME, Chanderraj R, Smith EE, Greenberg SM, Rosand J. Early edema in warfarin-related intracerebral hemorrhage. Neurocrit Care. 2007;7:58–63. doi: 10.1007/s12028-007-0039-3. [DOI] [PubMed] [Google Scholar]
- Lopez Valdes E, Hernandez Lain A, Calandre L, Grau M, Cabello A, Gomez-Escalonilla C. Time window for clinical effectiveness of mass evacuation in a rat balloon model mimicking an intraparenchymatous hematoma. J Neurol Sci. 2000;174:40–46. doi: 10.1016/s0022-510x(99)00288-9. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Plummer N, Silasi G, Auriat AM, Colbourne F. Rehabilitation promotes recovery after whole blood-induced intracerebral hemorrhage in rats. Neurorehabil Neural Repair. 2011;25:477–483. doi: 10.1177/1545968310395602. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41:S95–S98. doi: 10.1161/STROKEAHA.110.594457. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, Lo EH. Evidence for apoptosis after intracerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- McCluskey L, Campbell S, Anthony D, Allan SM. Inflammatory responses in the rat brain in response to different methods of intra-cerebral administration. J Neuroimmunol. 2008;194:27–33. doi: 10.1016/j.jneuroim.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Mendelow AD.1993Mechanisms of ischemic brain damage with intracerebral hemorrhage Stroke 24I115–I117.discussion I8–I9 [PubMed] [Google Scholar]
- Mendelow AD, Bullock R, Teasdale GM, Graham DI, McCulloch J. Intracranial haemorrhage induced at arterial pressure in the rat. Part 2: short term changes in local cerebral blood flow measured by autoradiography. Neurol Res. 1984;6:189–193. doi: 10.1080/01616412.1984.11739688. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149–154. doi: 10.1161/01.HYP.0000198541.12640.0f. [DOI] [PubMed] [Google Scholar]
- Miller JH, Wardlaw JM, Lammie GA. Intracerebral haemorrhage and cerebral amyloid angiopathy: CT features with pathological correlation. Clin Radiol. 1999;54:422–429. doi: 10.1016/s0009-9260(99)90825-5. [DOI] [PubMed] [Google Scholar]
- Morgenstern LB, Hemphill JC, III, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun-Bryce S, Wilkerson AC, Papuashvili N, Okada YC. Recurring episodes of spreading depression are spontaneously elicited by an intracerebral hemorrhage in the swine. Brain Res. 2001;888:248–255. doi: 10.1016/s0006-8993(00)03068-7. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004a;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004b;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Keep RF, Wang M, Nagao S, Hoff JT, Hua Y. Effects of endogenous and exogenous estrogen on intracerebral hemorrhage-induced brain damage in rats. Acta Neurochir Suppl. 2006;96:218–221. doi: 10.1007/3-211-30714-1_47. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yamashita K, Uesugi S, Ito H. Temporal and spatial profile of apoptotic cell death in transient intracerebral mass lesion of the rat. J Neurotrauma. 1999;16:143–151. doi: 10.1089/neu.1999.16.143. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- Nath FP, Jenkins A, Mendelow AD, Graham DI, Teasdale GM. Early hemodynamic changes in experimental intracerebral hemorrhage. J Neurosurg. 1986;65:697–903. doi: 10.3171/jns.1986.65.5.0697. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Chronic Conditions . Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA)) London: Royal College of Physicians; 2008. [PubMed] [Google Scholar]
- NINDS ICH Workshop Participants Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005;36:e23–e41. doi: 10.1161/01.STR.0000155685.77775.4c. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- O'Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, Ikeda D, Greenberg SM. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–1863. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Bang MS, Kwon BS, Park YK, Kim DW, Shon SM, Jeong SW, Lee DK, Kim DE. Early treadmill training promotes motor function after hemorrhagic stroke in rats. Neurosci Lett. 2010;471:104–108. doi: 10.1016/j.neulet.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Plotnikov MB, Korobova ZV, Chernysheva GA. [Effect of gutimine on the electroencephalogram and cerebral blood supply of cats with intracerebral hemorrhage] Farmakol Toksikol. 1984;47:36–39. [PubMed] [Google Scholar]
- Powers WJ, Zazulia AR, Videen TO, Adams RE, Yundt KD, Aiyagari V, Grubb RL, Jr, Diringer MN. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57:18–24. doi: 10.1212/wnl.57.1.18. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, Guterman LR, Hopkins LN. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003a;31:1482–1489. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ling GS, Khan J, Guterman LR, Hopkins LN. Absence of early proinflammatory cytokine expression in experimental intracerebral haemorrhage. Neurosurgery. 2001a;49:416–420. doi: 10.1097/00006123-200108000-00027. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MFK, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN, Bullock MR, Hodge CJ, Jr, Hlatky R, Valadka AB, Rutka JT. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003b;52:1041–1048. [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001b;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266–272. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- Rosand J, Eskey C, Chang Y, Gonzalez RG, Greenberg SM, Koroshetz WJ. Dynamic single-section CT demonstrates reduced cerebral blood flow in acute intracerebral hemorrhage. Cerebrovasc Dis. 2002;14:214–220. doi: 10.1159/000065681. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sandoval JL, Cantu C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke. 1999;30:537–541. doi: 10.1161/01.str.30.3.537. [DOI] [PubMed] [Google Scholar]
- Sadoshima S, Busija D, Brody M, Heistad D. Sympathetic nerves protect against stroke in stroke-prone hypertensive rats. A preliminary report. Hypertension. 1981;3:I124–I127. doi: 10.1161/01.hyp.3.3_pt_2.i124. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Fiebach JB, Hoffmann K, Becker K, Orakcioglu B, Kollmar R, Juttler E, Schramm P, Schwab S, Sartor K, Hacke W. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra. Stroke. 2003;34:1674–1679. doi: 10.1161/01.STR.0000076010.10696.55. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354–361. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- Selim M. Deferoxamine mesylate: a new hope for intracerebral hemorrhage: from bench to clinical trials. Stroke. 2009;40:S90–S91. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- Shi Y, Li Z, Zhang S, Xie M, Meng X, Xu J, Liu N, Tang Z. Establishing a model of supratentorial hemorrhage in the piglet. Tohoku J Exp Med. 2010;220:33–40. doi: 10.1620/tjem.220.33. [DOI] [PubMed] [Google Scholar]
- Shin HK, Nishimura M, Jones PB, Ay H, Boas DA, Moskowitz MA, Ayata C. Mild induced hypertension improves blood flow and oxygen metabolism in transient focal cerebral ischemia. Stroke. 2008;39:1548–1555. doi: 10.1161/STROKEAHA.107.499483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinar EJ, Mendelow AD, Graham DI, Teasdale GM. Experimental intracerebral hemorrhage: effects of a temporary mass lesion. J Neurosurg. 1987;66:568–576. doi: 10.3171/jns.1987.66.4.0568. [DOI] [PubMed] [Google Scholar]
- Singer OC, Sitzer M, du Mesnil de Rochemont R, Neumann-Haefelin T. Practical limitations of acute stroke MRI due to patient-related problems. Neurology. 2004;62:1848–1849. doi: 10.1212/01.wnl.0000125320.53244.fa. [DOI] [PubMed] [Google Scholar]
- Sjoblom L, Hardemark H-G, Lindgren A, Norrving B, Fahlen M, Samuelsson M, Stigendal L, Stockelberg D, Taghavi A, Wallrup L, Wallvik J. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567–2574. doi: 10.1161/hs1101.098523. [DOI] [PubMed] [Google Scholar]
- Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- STAIR (Stroke Theraphy Academic Industry Roundtable) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, Skolnick BE, Davis SM.2006Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII Neurosurgery 59767–773.discussion 73–74 [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Mechanisms of estrogens' dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int J Mol Sci. 2011;12:1533–1562. doi: 10.3390/ijms12031533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi S, Kaneko M. Electron microscopic studies of ruptured arteries in hypertensive intracerebral hemorrhage. Stroke. 1983;14:28–36. doi: 10.1161/01.str.14.1.28. [DOI] [PubMed] [Google Scholar]
- Thai QA, Pradilla G, Legnani FG, Kretzer RM, Hsu W, Tamargo RJ. Lysis of intracerebral hematoma with stereotactically implanted tissue plasminogen activator polymers in a rabbit model. J Neurosurg. 2006;105:424–429. doi: 10.3171/jns.2006.105.3.424. [DOI] [PubMed] [Google Scholar]
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356:411–417. doi: 10.1016/S0140-6736(00)02539-3. [DOI] [PubMed] [Google Scholar]
- Vesterinen HM, Egan K, Deister A, Schlattmann P, Macleod MR, Dirnagl U. Systematic survey of the design, statistical analysis, and reporting of studies published in the 2008 volume of the Journal of Cerebral Blood Flow and Metabolism. J Cereb Blood Flow Metab. 2011;31:1064–1072. doi: 10.1038/jcbfm.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SA, Pozarek S, Martinsson S, Forsberg T, Ross SB, Gabrielsson J. Rapid and long-lasting tolerance to clomethiazole-induced hypothermia in the rat. Eur J Pharmacol. 2005;512:139–151. doi: 10.1016/j.ejphar.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Wagner KR. Modeling intracerebral hemorrhage: glutamate, nuclear factor-kappa B signaling and cytokines. Stroke. 2007;38:753–758. doi: 10.1161/01.STR.0000255033.02904.db. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, Broderick JP, Brott TG. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27:490–497. doi: 10.1161/01.str.27.3.490. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, Brott TG. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–498. doi: 10.3171/jns.1999.90.3.0491. [DOI] [PubMed] [Google Scholar]
- Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Hua Y, Keep RF, Hoff JT, Xi G. Deferoxamine reduces CSF free iron levels following intracerebral hemorrhage. Acta Neurochir Suppl. 2006;96:199–202. doi: 10.1007/3-211-30714-1_43. [DOI] [PubMed] [Google Scholar]
- Wan S, Zhan R, Zheng S, Hua Y, Xi G. Activation of c-Jun-N-terminal kinase in a rat model of intracerebral hemorrhage: the role of iron. Neurosci Res. 2009;63:100–105. doi: 10.1016/j.neures.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005;36:613–618. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- Wasserman JK, Yang H, Schlichter LC. Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci. 2008;28:1316–1328. doi: 10.1111/j.1460-9568.2008.06442.x. [DOI] [PubMed] [Google Scholar]
- Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, Tolnay M, Staufenbiel M, Jucker M. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci. 2001;21:1619–1627. doi: 10.1523/JNEUROSCI.21-05-01619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Li C, Wang L, Mao Y, Hong Z. Minimally invasive procedures for evacuation of intracerebral hemorrhage reduces perihematomal glutamate content, blood-brain barrier permeability and brain edema in rabbits. Neurocrit Care. 2011a;14:118–126. doi: 10.1007/s12028-010-9473-8. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhong W. Effect of minimally invasive surgery for cerebral hematoma evacuation in different stages on motor evoked potential and thrombin in dog model of intracranial hemorrhage. Neurol Res. 2010;32:127–133. doi: 10.1179/016164109X12478302362617. [DOI] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011b;31:1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32:2932–2938. doi: 10.1161/hs1201.099820. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xie Q, Guan J, Wu G, Xi G, Keep RF, Hua Y. Tamoxifen treatment for intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:271–275. doi: 10.1007/978-3-7091-0693-8_45. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Comparison of brain cell death and inflammatory reaction in three models of intracerebral hemorrhage in adult rats. J Stroke Cerebrovasc Dis. 2003;12:152–159. doi: 10.1016/S1052-3057(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR, Muizelaar JP. Intracortical hemorrhage injury in rats: relationship between blood fractions and brain cell death editorial comment: relationship between blood fractions and brain cell death. Stroke. 2000;31:1721–1727. doi: 10.1161/01.str.31.7.1721. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, Garcia NM, Morgenstern LB. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–1657. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- Zausinger S, Baethmann A, Schmid-Elsaesser R. Anesthetic methods in rats determine outcome after experimental focal cerebral ischemia: mechanical ventilation is required to obtain controlled experimental conditions. Brain Res Brain Res Protoc. 2002;9:112–121. doi: 10.1016/s1385-299x(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Diringer MN, Videen TO, Adams RE, Yundt K, Aiyagari V, Grubb RL, Jr, Powers WJ. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–810. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Videen TO, Powers WJ. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral hemorrhage. Stroke. 2009;40:1638–1643. doi: 10.1161/STROKEAHA.108.536037. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang Y, Liu L, Huang Y, Tang Y, Su J, Hua W, Han X, Xue J, Dong Q. Minimally invasive stereotactic puncture and thrombolysis therapy improves long-term outcome after acute intracerebral hemorrhage. J Neurol. 2011;258:661–669. doi: 10.1007/s00415-011-5902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Ackerman JJ, Yablonskiy DA. Body and brain temperature coupling: the critical role of cerebral blood flow. J Comp Physiol B. 2009;179:701–710. doi: 10.1007/s00360-009-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]