Abstract
About 15 million strokes occur each year worldwide. As the number one cause of morbidity and acquired disability, stroke is a major drain on public health-care funding, due to long hospital stays followed by ongoing support in the community or nursing-home care. Although during the last 10 years we have witnessed a remarkable progress in the understanding of the pathophysiology of ischemic stroke, reperfusion induced by recombinant tissue-type plasminogen activator (tPA—Actilyse) remains the only approved acute treatment by the health authorities. The objective of the present review is to provide an overview of our present knowledge about the impact of tPA on the neurovascular unit during acute ischemic stroke.
Keywords: acute stroke, clinical trials, excitotoxicity, interventional neuroradiology, neurovascular unit, thrombolysis
Introduction
Stroke concerns 15 million people every year worldwide and causes 5.7 million deaths, accounting for 9% of all deaths. The majority of ischemic strokes, the most common type of stroke, result from an acute thrombosis. A thrombus consists in blood cells trapped in a matrix of fibrin. Dissolution of this fibrin clot (fibrinolysis) is enzymatically driven by the trypsin-like serine protease, plasmin. The active form plasmin is generated from a precursor (zymogen), known as plasminogen. The conversion of plasminogen into plasmin requires proteolytic cleavage by naturally occurring plasminogen activators, which in mammals can be tissue-type plasminogen activator (tPA) or urokinase-type plasminogen activator (Rijken and Sakharov, 2001). Tissue-type plasminogen activator, urokinase-type plasminogen activator, plasmin (or a genetically modified plasmin named microplasmin), and Desmoteplase (DSPA) (a recombinant plasminogen activator derived from bat salivary glands) are available through recombinant technologies (for review on mechanisms of action of fibrinolytic agents, see Lijnen and Collen, 1995). In addition, other bacterial/microbial thrombolytics exist, including streptokinase and staphylokinase.
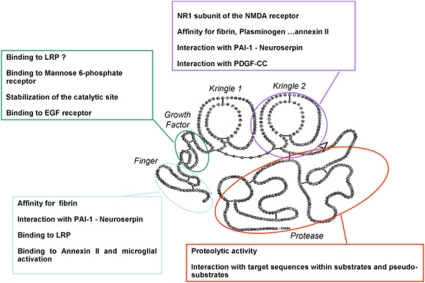
The tPA (Figure 1) is a 69-kDa glycoprotein, which consists in a single polypeptide chain of 527 or 530 amino acids. It is synthesized within cells as a polypeptide chain held by 34 disulfides bonds and released as a single chain enzyme (sctPA). Limited proteolysis by plasmin in the extracellular space cleaves the chain between arginine 275 and isoleucine 276, converting it into a two-chain (tctPA) form (chain A or heavy chain and chain B or light chain). Chain A contains four distinct domains: a finger (F) domain composed by residues 6 to 43, homologous to the first domain of the fibronectin. This domain has a high affinity for the fibrin; an epidermal growth factor (EGF) domain, composed by residues 44 to 92, homologous with the human and murine EGF. This domain is involved in the hepatic clearance with the finger domain; two kringle (K) domains homologous to the K domains of plasminogen consisting in residues 93 to 176 and 176 to 275. The K2 and F domains form an interaction site for fibrin. The B chain contains the catalytic protease (P) domain and is composed of the last 230 residues. The active site is constituted of Histidine 322, Asparagine 371, and Serine 478. Due to its high affinity for fibrin, tPA activates clot-bound plasminogen 100-fold more efficiently than circulating plasminogen, although it has a very short half-life in the blood (3 to 5 minutes) (Tanswell et al, 1989). These characteristics have led to successful clinical trials of thrombolytic therapy in acute stroke treatment (NINDS-tPA Study Group, 1995).
Figure 1.
Tissue-type plasminogen activator (tPA) is a ‘JANUS' protease. tPA is a five domain serine protease including a Finger domain, an epidermal growth factor (EGF)-like domain, two kringle domains, and a catalytic domain. Pleiotropic functions of tPA as related to the specificity of each of these domains. LRP, lipoprotein receptor-related protein; PAI-1, plasminogen activator inhibitor type 1; NMDA receptor, N-methyl--aspartate receptor; PDGF-CC, platelet-derived growth factor-CC.
However, growing body of evidence suggests that, in the context of acute ischemic stroke, tPA affects the integrity of the neurovascular unit, a functional entity involving microvessels, pericytes, astrocytes, neurons, axons, and other supporting cells such as microglia and oligodendrocytes. Indeed, both protective and deleterious effects of tPA on this ‘unit' have been reported. This paper will review the current knowledge of benefits and risks of tPA in patients with acute ischemic stroke, preclinical evidences that could explain the suboptimal efficacy/safety ratio of tPA, and promising approaches that could enhance the efficiency of thrombolysis.
Tissue-Type Plasminogen Activator-Mediated Thrombolysis in Ischemic Stroke: Bed Side
Two placebo-controlled randomized trials have shown substantial benefit of early treatment with intravenous recombinant tPA (rtPA) in patients with acute ischemic stroke (NINDS Study Group, 1995; Hacke et al, 2008), and several meta-analyses of randomized trials support the use of rtPA within 4.5 hours after ischemic stroke onset (Lees et al, 2010; Wardlaw et al, 2009; Lansberg et al, 2009). The National Institute of Neurological Disorders and Stroke (NINDS) trial was the first to test rtPA within 3 hours of stroke onset at the dosing regimen of 0.9 mg/kg (maximum 90 mg), with 10% given as an initial bolus and the remainder infused over 1 hour. In this study, the absolute benefit of tPA treatment ranged from 11% to 13% depending on the stroke outcome scale. For every 100 patients treated, as compared with placebo, ∼32 benefited in a clinically important manner (Saver et al, 2010). Following these results, rtPA has been approved in several countries and recommended in most guidelines for the management of patients with acute stroke (Adams et al, 2007). However, widespread adoption of rtPA therapy proceeded slowly. Among the different potential obstacles restricting the use of rtPA, the time window has been the most important. Many patients do not arrive at the hospital within 3 hours after stroke onset, and even then, the time required to thoroughly evaluate the patient and make the treatment decision may jeopardize the chances of initiating thrombolysis.
Expansion of the Time Window up to 4.5 Hours: European Cooperative Acute Stroke Study III
To explore a longer time window, a pooled analysis of six trials (2,775 patients) that had differing time windows (up to 6 hours) for enrollment (NINDS Parts 1 and 2, European Cooperative Acute Stroke Study (ECASS) I and II, and ATLANTIS Parts A and B) was performed (Hacke et al, 2004). This pooled analysis showed that the benefit of rtPA diminished as time elapsed and that a favorable outcome was still observed if treatment was given between 3 and 4.5 hours, with an odds ratio for favorable outcome of 1.4 with rtPA as compared with placebo.
In 2002, the European Medicines Agency was cautious and granted license for the use of rtPA in ischemic stroke within 3 hours of symptoms onset on condition of the completion of a prospective registry of (1) patients treated with rtPA within the 3-hour time window (Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) study, see below) and (2) a randomized, placebo-controlled trial of rtPA between 3 and 4.5 hours after stroke onset. Thus, the ECASS III multicenter, prospective, randomized trial was conducted in Europe and tested rtPA 3 to 4.5 hours after stroke onset in 821 patients (Hacke et al, 2008). The dosing regimen was the same as in NINDS trial. The proportion of patients who had a favorable outcome (modified Rankin Scale score<2) at 90 days was significantly greater with rtPA than with placebo (52.4% vs. 45.2% odds ratio=1.34, 95% confidence interval (95% CI)=1.02 to 1.76; risk ratio=1.16, 95% CI=1.01 to 1.34), corresponding to number needed to treat to benefit of 14 (absolute improvement of 7.2%). Additional outcomes and subgroup analyses published in a separate paper showed that rtPA was effective across a broad range of subgroups of patients, including those aged 65 years or more and those with a severe stroke at baseline (Bluhmki et al, 2009).
The ECASS III trial has represented an important step forward in the treatment of acute stroke, allowing an increase in the number of eligible patients. However, the ECASS III trial results do not mean that we have more time to initiate the treatment (Saver, 2006). From the moment a patient arrives at the door, the focus must remain on the door-to-needle time (Lyden, 2008). The updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials (3,670 patients) have demonstrated that the benefit of rtPA decreased substantially over time from stroke onset. In this analysis, no benefit was seen after around 4.5 hours (Lees et al, 2010). On the basis of ECASS III and SITS-MOST studies results, the European Stroke Organization and subsequently the American Heart Association and American Stroke Association changed their guidelines (Del Zoppo et al, 2009; Ringleb et al, 2008).
Recanalization Rates on Tissue-Type Plasminogen Activator
Data on recanalization rate after intravenous rtPA are limited to observational studies, as none of the major randomized trials has assessed the baseline occlusion status or recanalization after treatment. Yet, it is clearly established that early recanalization (assessed until 24 hours from symptoms onset) is the strongest predictor of good clinical outcome. Depending on timing when recanalization status was assessed and on technique used, the recanalization rates after intravenous rtPA varied widely, with an overall estimate of ∼46% (Rha and Saver, 2007). The speed of clot dissolution by thrombolysis appears to affect the evolution of the ischemic lesion and clinical outcome (Delgado-Mederos et al, 2007). Vessel recanalization rates also vary depending on thrombus location with lower rates in proximal vs. distal arteries, suggesting that the burden of thrombus is important (Rha and Saver, 2007; Del Zoppo et al, 1992; Saqqur et al, 2007). Patients with extracranial large vessel occlusion have also very low rates of recanalization (Saqqur et al, 2007; Bhatia et al, 2010). Data on recanalization according to stroke etiologic subtype are limited. It has been suggested that patients with cardioembolic ischemic stroke are more likely to have recanalization, which is moreover faster and more complete, than patients with noncardioembolic stroke (Molina et al, 2004). Indeed considering the rtPA pharmacology, the drug is more likely to be active on fibrin-rich clots than on platelet-rich clots (see below).
Innovative Strategies to Enhance Recombinant Tissue-Type Plasminogen Activator's Effects
Thus, although the overall recanalization rate with intravenous rtPA is about twice the spontaneous rate, it is still <50%. This low recanalization rate is in agreement with the fact that only about one third of patients benefit of rtPA and has encouraged novel techniques to increase this relatively modest effect of intravenous fibrinolytic therapy. Development of catheters suitable for navigation into brain vasculature has opened the possibility for endovascular therapies including intraarterial fibrinolysis. As intraarterial approach allows a direct access to the clot, the technique should theoretically permit the use of lower doses of fibrinolytic, be combined with mechanical thrombus disruption devices, and therefore offer the potential of restoring blood flow more efficiently than intravenous therapy. Although intraarterial fibrinolysis has been used for decades in acute stroke patients, this therapy is not approved by the US Food and Drug Administration or by the European Medicines Agency. No single randomized clinical trial has demonstrated a statistically significant beneficial effect (defined by a modified Rankin Scale ⩽1 or ⩽2) of intraarterial fibrinolysis compared with control groups and not all studies used rtPA (Del Zoppo et al, 1998; Furlan et al, 1999; Keris et al, 2001; Macleod et al, 2005; Ogawa et al, 2007). However, despite differences in fibrinolytic drug used and in associated interventions (intravenous thrombolysis or mechanical disruption), the meta-analysis of these trials shows that intraarterial fibrinolysis is associated with high recanalization rates and better outcomes (Lee et al, 2010). Symptomatic intracerebral hemorrhage (ICH) is significantly more frequent in intraarterial fibrinolysis compared with control groups (8.9% vs. 2.3%), but mortality is similar in both groups (20.5% vs. 24.0%). Partial or complete recanalization was obtained in 64.6% of patients treated with intraarterial fibrinolysis, but complete recanalization was obtained in only 19.0% in mean, showing that intraarterial fibrinolytic therapy alone may not be a definitive solution to rapidly and fully recanalize occluded cerebral arteries.
The main limitations of intraarterial thrombolysis are the need for availability of an interventionalist and the additional time required to begin thrombolysis. Thus, a combined intravenous and intraarterial approach was explored in pilot studies (Lewandowski et al, 1999; Ernst et al, 2000; Hill et al, 2002; Suarez et al, 2002). The Interventional Management of Stoke (IMS) program began in 2001 aiming at further investigate the feasibility and safety of a combined intravenous–intraarterial approach within 3 hours of stroke onset in patients with moderate-to-large ischemic stroke (NIHSS⩾10). IMS I and II studies (IMS Study Investigators, 2004; IMS II Trial Investigators, 2007) used a similar protocol with initial intravenous rtPA (0.6 mg/kg, 60 mg maximum over 30 minutes) followed by additional rtPA at the site of the thrombus (up to 22 mg) in case of visible arterial clot on early angiography for up to 7 hours from stroke onset. Unlike IMS I, IMS II used the EKOS microinfusion catheter consisting in end-hole infusion lumen with a 1.7-MHz radially emitting ultrasound transducer in place of classical microcatheter contrast injection leading to increased recanalization rates (Tomsick et al, 2008). These 2 nonrandomized studies enrolled 80 patients each. In comparison with historical control subjects from the NINDS trial, the results suggested that the combined approach to recanalization might be more effective than standard intravenous rtPA alone. The RECANALISE study has evaluated the combined approach using a before–after design (Mazighi et al, 2009). In comparison with 107 patients treated with intravenous rtPA only in the first period, the 53 patients treated with intravenous–intraarterial approach had higher recanalization rates (87% vs. 52% risk ratio=1.49, 95% CI=1.21 to 1.84) and were more likely to have an early neurologic improvement defined as National Institute of Health Stroke Scale score of 0 or 1 or improvement of 4 points at 24 hours (60% vs. 39% risk ratio=1.36, 95% CI=0.97 to 1.91). However, there was no significant difference in the 90-day favorable outcomes defined as a Rankin Scale of 0 to 2 (57% vs. 44%, P=0.35) between the two groups. In addition, the study showed that there was also a strong association between better clinical early and 3-month clinical outcomes and shorter time from symptom onset to recanalization. IMS I and II have also shown that favorable clinical outcome was related to complete recanalization (Khatri et al, 2005; Tomsick et al, 2008) and to time to angiographic reperfusion (Khatri et al, 2009). The ongoing International Management of Stroke III randomized trial (IMS-III) currently addresses whether combined intravenous–intraarterial approach is superior to intravenous rtPA treatment alone when initiated within 3 hours of stroke onset. Interestingly, intraarterial approach allows the use of mechanical thrombus disruption devices in combination to rtPA. However, these devices have not yet been evaluated in randomized studies (see Nogueira et al, 2009 for review).
Ultrasound-enhanced thrombolysis is a promising approach including improvement in drug delivery, increase in binding of rtPA to fibrin, and reversible alteration of the fibrin structure (Daffertshofer and Hennerici, 2003). So far, three different ultrasound technologies have been used: transcranial Doppler, transcranial color-coded duplex, and low-frequency ultrasound. A recent meta-analysis of small randomized trials and nonrandomized studies has shown that sonothrombolysis with high-frequency ultrasounds appears to be safe, leads to higher recanalization rates (odds ratio=2.99; 95% CI=1.70 to 5.25) and higher likelihood of independence when compared with intravenous thrombolysis alone (Tsivgoulis et al, 2010), as it was suggested by the first results of the CLOTBUST trial (Alexandrov et al, 2004). However, the single trial using low-frequency ultrasounds was stopped because ultrasounds were associated with a significant increase in risk of symptomatic ICH (including atypical subarachnoid hemorrhages; Daffertshofer et al, 2005). The potential benefit of high-frequency ultrasounds results needs to be confirmed in new and larger randomized-controlled studies.
Another way to enhance fibrinolysis and prevent the risk of reocclusion is the use of antiplatelet agents in combination with rtPA. The most widely used antiplatelet agent is aspirin, but there is currently no randomized trial that has assessed the effect of aspirin in combination with rtPA and it is even recommended that aspirin should not be started within the 24 hours after thrombolysis (Adams et al, 2007). However, ∼30% of stroke patients treated by intravenous rtPA receive antiplatelet agents at the time of their stroke. In the Safe Implementation of Treatments in Stroke (SITS) International Stroke Thrombolysis Register (SITS-ISTR), patients who received aspirin, clopidogrel, aspirin and clopidogrel, or aspirin and dipyridamole at the time of their stroke were no more likely to have a good prognosis than those who did not receive antiplatelet agents after adjustment for baseline characteristics (Diedler et al, 2010). The results were in agreement with those of previous studies (Cucchiara et al, 2009). The issue of whether intravenous rtPA in combination to aspirin is superior to intravenous rtPA alone is currently addressed in the randomized ARTIS trial (Zinkstok et al, 2010). Combining glycoprotein IIb/IIIa inhibitors with intravenous or intraarterial tPA has been shown to be feasible and apparently safe in small observational studies, but no large randomized studies are available (Deshmukh et al, 2005; Mangiafico et al, 2005; Qureshi et al, 2006). Considering that the use of abciximab within 5 hours of stroke onset is associated with an increased risk of symptomatic or fatal ICH (Adams et al, 2008), the approach combining abciximab and intravenous rtPA is likely to be illusory. The randomized CLEAR study assessed the safety of the eptifibatide in combination with intravenous rtPA within 3 hours of acute stroke (Pancioli et al, 2008). The combination was apparently safe and, although there was no trend toward efficacy, a new trial is currently ongoing (http://www.strokecenter.org/trials/trialDetail.aspx?tid=995).
Based on experimental data and early human experience, hypothermia appears a promising neuroprotective therapy for acute ischemic stroke. Hypothermia could improve the efficacy and potentially extends the time window for tPA-induced reperfusion. The recent ICTuS-L trial assessed the feasibility and safety of induced hypothermia after ischemic stroke and thrombolysis. Fifty-nine patients were randomized to receive either hypothermia (33°C) immediately after rtPA-induced thrombolysis for 24 hours, followed by 12 hours of controlled rewarming or normothermia (Hemmen et al, 2010). Target temperature was reached in 20/28 patients. At 3 months, 18% of patients treated with hypothermia had good outcome compared with 24% in the normothermia group. A higher rate of pneumonia was reported in the hypothermia group (50% vs. 10%, P=0.001). A large European randomized trial assessing the efficacy of hypothermia in acute ischemic stroke is ready to begin (Macleod et al, 2010).
Finally, another very promising approach is to improve the selection of patients who are most likely to benefit from thrombolysis, i.e., those who still have salvageable cerebral tissue (ischemic penumbra). EPITHET has shown that mismatch on magnetic resonance imaging treatment by tPA was associated with attenuation of infarct volumes and nonsignificant better functional recovery at 3 months (Davis et al, 2008). This tissue-based approach could also be applied in patients with unknown-onset or wake-up stroke (Mackey et al, 2011; Barreto et al, 2009). A few thrombolysis trials with penumbra assessment have been carried out, most showing encouraging results, and new trials are currently ongoing (see Donnan et al, 2009 for review).
Safety of Recombinant Tissue-Type Plasminogen Activator-Induced Thrombolysis
The most serious complication of rtPA treatment is the occurrence of a large ICH, most often in the core of the cerebral infarction. When this complication occurs, there is a fatality rate of ∼50%. There have been several definitions of symptomatic ICH after rtPA treatment in trials and safety-monitoring studies, all based on computerized tomography scan imaging. Most studies defined symptomatic ICH as any hemorrhage associated with neurologic deterioration, as indicated by an increase in the NIHSS score of 4 or more points (Hacke et al, 1995; Wahlgren et al, 2007). However, clinical deterioration in patients with ICH could also be due to an increase in cerebral infarction or cerebral edema, coinciding with hemorrhagic transformation. Thus, in ECASS III, the ICH had to have been identified as the predominant cause of the neurologic deterioration to be considered symptomatic (Hacke et al, 2008).
After the publication of the NINDS and other trials, there have been concerns over the applicability of rtPA in daily practice, especially regarding the risks of ICH. In NINDS trial, the risk of symptomatic ICH was 6.4% in the rtPA group compared with 0.6% in the placebo group (P<0.001). The largest safety-monitoring study is the SITS-MOST (Wahlgren et al, 2007; Ahmed et al, 2010). The study was requested in 2002 by the European Medicines Agency, along with ECASS III, in order to reassess the benefit/risk profile of rtPA for the thrombolytic treatment of acute ischemic stroke. The main aim of SITS-MOST was to investigate whether rtPA within 3 hours of ischemic stroke symptoms, in accordance with the European summary of product characteristics, was as safe as reported in randomized trials when incorporated into clinical practice. The study was part of an existing SITS-ISTR, an Internet-based academic interactive register, which also recruited sites from countries outside Europe and included patients who did not meet the inclusion or exclusion criteria of SITS-MOST, in particular those treated up to 4.5 hours after stroke onset. The first analysis of 6,483 patients treated within 3 hours of stroke onset (from 2002 to 2006) confirmed that the rate of symptomatic ICH and the clinical outcomes (proportion of patients with a good clinical outcome and death rates) were similar to those observed in clinical trials (Wahlgren et al, 2007). Moreover, the initial publication of the SITS-ISTR registry suggested that patients could be treated safely beyond the 3-hour time window (Wahlgren et al, 2008). After adjustment for baseline imbalances, the rate of symptomatic ICH was significantly higher in patients treated within 3 to 4.5 hours than in those treated within 3 hours according to the SITS-MOST and ECASS II definitions, but not according to the NINDS definition and absolute differences were small. However, patients treated within 3 to 4.5 hours had also a higher mortality and were less likely to be functionally independent at 3 months. In both time windows, the rate of death attributable to symptomatic ICH was 1%. In the pooled analysis of ATLANTIS, ECASS, EPITHET, and NINDS trials, the rate of symptomatic ICH did not increase with onset-to-treatment time whereas mortality did (Lees et al, 2010). The mechanisms for this excess mortality are debated, as there was no parallel time-related increase in severe parenchymal hemorrhage. Importantly, the SITS-ISTR analyses have also shown that there has been a rapid implementation of thrombolysis with rtPA beyond the 3-hour time window after the publication of the ECASS III trial and the SITS-ISTR study, but that the widening of the time window has not resulted in delayed treatment of patients.
Several clinical, biological, and imaging predictors of ICH have been identified (Derex and Nighoghossian, 2008). Although longer stroke onset-to-treatment time may theoretically be associated with a higher risk of ICH, most studies and the pooled analysis of randomized trials failed to show an association between timing of rtPA administration and symptomatic ICH. There is very few evidence on the benefit/risk profile of rtPA in patients over 80 years from randomized trials. Yet, ∼25% of stroke patients in developed countries are aged over 80 years, and this proportion will increase over the next decades. The NINDS included only 42 patients over 80 and other trials did not enroll those patients. Thus, thrombolytic therapy is not currently licensed for patients >80 years in the European Union. By contrast, most safety-monitoring studies have included patients without an age limit and most stroke centers use rtPA in elderly patients. In published safety-monitoring studies, old age is associated with poor clinical outcome and with an increased risk of symptomatic ICH (Wahlgren et al, 2008). Of note, a small uncontrolled retrospective study showed that patients aged over 90 years (n=22) have poor outcomes at 30 days and a high mortality rate after intravenous thrombolysis (Mateen et al, 2009). However, an analysis of the SITS-ISTR showed that age over 80 years was not associated with an increased risk of symptomatic ICH after adjustment for other ICH risk factors. Moreover, in an adjusted-controlled comparison with nonrandomized patients who did (SITS-ISTR) and did not (VISTA collaboration) receive rtPA, the net benefit of rtPA was maintained in very elderly people (Mishra et al, 2010). Additionally, a retrospective study comparing the outcome of patients aged over 80 years (n=33) with their younger counterparts (n=81) after intraarterial thrombolysis reported equivalent rate of symptomatic ICH (7% vs. 8%, respectively). Accordingly, data from the Canadian Alteplase For Stroke Effectiveness Study showed that aging was not associated with an increased risk of symptomatic ICHs (Sylaja et al, 2006). Finally, although patients over 80 years were not included in ECASS III, the benefit of rtPA did not decrease with age (Hacke et al, 2008). Taken together, these data suggest that well-selected elderly patients otherwise fulfilling the intravenous rtPA license criteria are appropriate candidates for thrombolysis (Ford et al, 2010). The International Stroke Trial-3 and the Thrombolysis in Elderly Stroke Patients trial are currently recruiting such patients (Sandercock et al, 2008; Toni and Lorenzano, 2009). Apart age, the main well-recognized risk factors for symptomatic ICH include clinical stroke severity (e.g., defined by a baseline NIHSS >20), elevated postthrombolysis blood pressure (Ahmed et al, 2009), history of diabetes mellitus or elevated pretherapeutic blood glucose (Derex and Nighoghossian, 2008; Ahmed et al, 2010), low platelet count (Tanne et al, 2002), the use of antiplatelet agents, and early ischemic changes occupying >33% of the middle cerebral artery territory (Larrue et al, 2001). In SITS-ISTR, the absolute excess of symptomatic ICH was small in patients who received antiplatelet agents (highest in those receiving aspirin and clopidogrel), but was not associated with a poor functional outcome or higher mortality rate at 3 months (Diedler et al, 2010). It is also important to underline that patients taking warfarin seem to have a higher risk of symptomatic ICH than those not taking warfarin despite an international normalized ratio <1.7 (as permitted in most guidelines) (Prabhakaran et al, 2010). Other radiological potential risk factors for symptomatic ICH are diffusion-weighted imaging lesion size, severity of decrease in apparent diffusion coefficient, presence of microbleeds, and leukoaraiosis (Derex and Nighoghossian, 2008; Touzé, 2009). Interestingly, early recanalization does not seem to increase the risk of symptomatic ICH (Saqqur et al, 2008).
A few studies have shown that the use of biomarkers, such as matrix metalloproteinases (MMPs, especially MMP-9), may help identify patients at high risk of symptomatic ICH (Derex and Nighoghossian, 2008). These proteinases, which are released at the acute stage of ischemia, increase the blood–brain barrier (BBB) permeability as they damage some components of the vessel and the basal lamina. High plasmatic levels of vascular adhesion protein 1 and cellular fibronectin have also been associated with symptomatic ICH after thrombolytic treatment (Hernandez-Guillamon et al, 2010; Castellanos et al, 2004, 2007). This approach seems promising, but more data are needed before biomarkers can be used to select the best candidates for thrombolysis.
In intravenous–intraarterial thrombolysis studies, the risk of symptomatic ICH was up to 10% according to ECASS-II definition, which is higher than that observed in patients treated with intravenous tPA alone (IMS Study Investigators, 2004; IMS II Trial Investigators, 2007; Mazighi et al, 2009). This higher rate of symptomatic ICH can be related to the severity of stroke and to the technique itself. Indeed, intraarterial procedure requires the use of heparin and microcatheter contrast injections increase the risk of ICH (Khatri et al, 2008). In IMS, internal carotid occlusion and atrial fibrillation were also independent risk factor for symptomatic ICH (Khatri et al, 2008).
Tissue-Type Plasminogen Activator-Mediated Thrombolysis in Ischemic Stroke: Bench Side
Clinical trials and daily use of thrombolysis have highlighted that rtPA has several drawbacks in acute stroke setting. Most of the effects of rtPA can be replicated in ischemic stroke animal models. For instance, volumes of ischemic lesions are significantly smaller in tPA-deficient mice compared with nondeficient mice (Wang et al, 1998; Nagai et al, 1999). Similarly, inhibition of tPA in a murine stroke model results in a decrease in ischemic lesion volumes (Yepes et al, 2000). Also, we have shown that the use of rtPA in a thromboembolic stroke murine model was associated with deleterious effects when infused at a late stage after stroke onset (⩾4 hours) and that these deleterious effects could not be only explained by the increase in the risk of hemorrhage (Orset et al, 2007; Macrez et al, 2010; García-Yébenes et al, 2011). Consequently, tPA is a double-sided molecule, with beneficial effects due to its fibrinolytic activity and deleterious effects increasing with time after stroke onset that counteract the global benefits of reperfusion (Benchenane et al, 2004).
Deleterious Effects of Tissue-Type Plasminogen Activator in Experimental Stroke Include Both Actions in the Blood and in the Brain Parenchyma
In the circulation, main substrate for endogenous or exogenous tPA is plasminogen, which promotes thrombolysis (Figures 2 and 3). By contrast, in the brain tissue, tPA substrates extend above tPA/plasmin(ogen)-driven extracellular matrix degradation (Figure 3). Indeed, tPA has been reported to interact with the low density lipoprotein receptor-related protein (LRP), the N-methyl--aspartate (NMDA) receptor, and annexin-II both in glial cells and in neurons resulting in parenchymal cell death (Siao and Tsirka, 2002; Polavarapu et al, 2007, 2008; Nicole et al, 2001; Yepes et al, 2009).
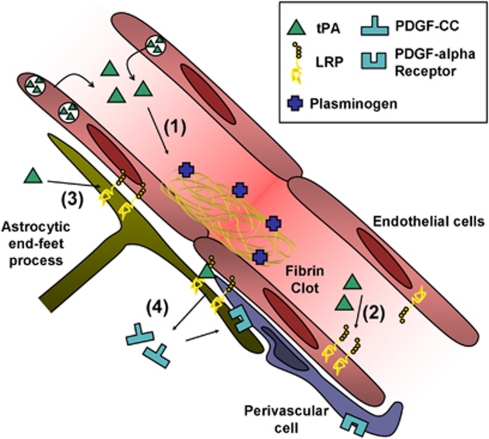
Figure 2.
Effect of exogenous and endogenous tPA at the blood–brain barrier (BBB). (1) Tissue-type plasminogen activator (tPA)-mediated plasminogen activation promotes clot lysis and subsequent blood flow restoration. (2) tPA interacts with endothelial LRPs, leading to NFκB activation and MMPs overexpression. (3) tPA can shed the ectodomain of astrocytic LRP receptors, inducing detachment of astrocytic end-feet processes. (4) tPA bound to LRP can activate latent PDGF-CC. Active PDGF-CC binds PDGF-Rα on perivascular cells leading to BBB opening. LRP, lipoprotein receptor-related protein; MMPs, metalloproteinases; PDGF-CC, platelet-derived growth factor-CC.
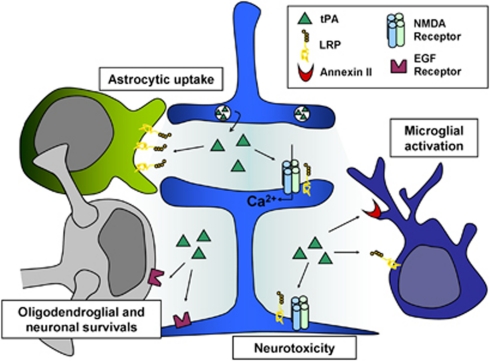
Figure 3.
Tissue-type plasminogen activator (tPA) in the brain parenchyma. In the brain parenchyma, tPA, released by neurons, was reported to potentiate NMDA receptors signaling to display either neurotrophic or neurotoxic effects, to reduce apoptotic neuronal and oligodendroglial deaths, to activate microglia after binding to annexin II and LRP and to be cleared from the extracellular space by astrocytes through an LRP-dependent mechanism. LRP, lipoprotein receptor-related protein; NMDA, N-methyl--aspartate receptor; EGF, epidermal growth factor.
Tissue-Type Plasminogen Activator at the Blood–Brain Barrier
A growing body of evidence indicates that tPA mediates increase in the permeability/leakage of the BBB (Lo et al, 2002). The pathways described are multiple and involve several components of the neurovascular unit (Figure 2). Indeed, in an embolic stroke animal model, treatment with rtPA induces the extravasation of Evans blue dye and overactivation of MMP-3 and MMP-9, two markers of BBB permeability. Studies in knockout mice argue that both MMP-9 and MMP-3 are responsible for tPA-induced parenchymal hemorrhages after stroke. Additionally, injection of rtPA into the cerebrospinal fluid induces a dose-dependent increase in permeability of the BBB, and this effect is inhibited by treatment with either the receptor-associated protein (an antagonist of the LRP) or by anti-LRP antibodies (Yepes et al, 2003). Since endothelial expression of LRP gradually increases during oxygen glucose deprivation (Suzuki et al, 2009), this could explain the observed time-dependent increase in hemorrhage rate after thrombolysis. In addition, it has been recently demonstrated in experimental models that expression of LRP also increased in perivascular astrocytes early after the onset of the ischemic insult onset and that its interaction with both endogenous and exogenous tPA-induced shedding of its ectodomain into the vascular basement membrane. Altogether, these events were associated with detachment of astrocytic end-feet from the basal lamina and subsequent increased BBB permeability (Polavarapu et al, 2007). In addition, it has been reported that rtPA could mediate the local activation of latent platelet-derived growth factor-CC (PDGF-CC) and subsequent activation of PDGF-α receptors on pericytes after cerebral ischemia (Macrez et al, 2011a, 2011b), an effect leading to hemorrhagic transformation (Su et al, 2008). Indeed, intraventricular injection of rtPA or active PDGF-CC, in the absence of ischemia, led to significant increase in BBB permeability. In contrast, coinjection of neutralizing antibodies to PDGF-CC with tPA blocked this increased permeability, indicating that PDGF-CC is a downstream substrate of tPA within the BBB. Since ischemia–reperfusion alone induces time-dependent changes in BBB integrity (Hamann et al, 1996), blood-derived tPA can reach the brain parenchyma and interact with all the components of the neurovascular unit.
Tissue-Type Plasminogen Activator Within the Brain Parenchyma
In the brain parenchyma, tPA had initially been shown to promote kainate-induced neurotoxicity (Tsirka et al, 1995) and then to potentiate NMDA receptor signaling, with several potential mechanisms: (1) an upregulation of NR2B-containing NMDA receptors (Pawlak et al, 2005); (2) a catalytic-independent activation of an ERK1/2-GSK3 signal transduction pathway (Medina et al, 2005); (3) the plasmin-independent proteolytic cleavage of the amino terminal domain of the NMDA receptor NR1 subunit (Nicole et al, 2001; Fernández-Monreal et al, 2004; Benchenane et al, 2007); (4) an overactivation of extrasynaptic NR2D-containing NMDA receptors (Baron et al, 2010). Although the fine mechanism remains debated, tPA should be now considered as a neuromodulator of glutamatergic signaling mediated by NMDA receptors (Benchenane et al, 2007; Samson and Medcalf, 2006) leading to an increased calcium influx, overactivation of MAP kinase ERK1/2 signaling and finally to an increased neurotoxicity. It is also interesting to note that NMDA-dependent nitric oxide production has been recently demonstrated to be dependent on tPA (Parathath et al, 2006), unveiling a possible role of tPA in the control of local cerebral perfusion. In contrast to its well-admitted exacerbating effect on neuronal excitotoxic necrosis (Nolin et al, 2008), tPA modulates apoptotic neuronal (Liot et al, 2006) and oligodendrocyte death independently of its proteolytic activity (Correa et al, 2011). Together with the previous observations that nonproteolytically active tPA can activate microglia after binding to annexin II (Siao and Tsirka, 2002), these antiapoptotic mechanisms of tPA strongly support the notion of a ‘cytokine-like' or even ‘neurotrophic-like' function of this molecule. However, these data are in contradiction with other reports (Pawlak et al, 2005; Liu et al, 2004), suggesting that tPA promoted NMDA-induced apoptosis. The protection of oligodendrocytes from apoptosis provided by exogenous tPA (Correa et al, 2011) raises the intriguing possibility that thrombolysis could improve stroke outcome even when arterial reperfusion fails. Since interaction of tPA with oligodendrocytes is mediated by its EGF domain, it is also interesting to note that other plasminogen activators lacking the EGF domain (such as reteplase) should be devoid of these protective effects.
Tissue-Type Plasminogen Activator and Cerebrovascular Inflammation
Interaction of tPA with glial cells raises questions about the influence of thrombolysis on poststroke inflammation. Indeed, cerebral ischemia/reperfusion rapidly leads to the release of pro-inflammatory cytokines (including TNF and IL-1β) (Zhang et al, 1999; Stoll et al, 1998) from injured brain tissues (especially from activated microglia). Subsequently, brain microvascular endothelial cells are activated and express adhesion molecules (ICAM-1, VCAM-1, E-Selectin) allowing transendothelial migration of circulating cells (Frijns and Kappelle, 2002). In the subacute stage, resident and infiltrating cells produce MMP (Gidday et al, 2005) and inflammatory cytokines. Altogether, these phenomena increase BBB permeability and further amplify inflammatory response, eventually leading to hemorrhagic transformation and neuronal death (for review of the role of MMPs in neuroinflammation, see Rosenberg, 2002). Interestingly, microglial activation was demonstrated to be significantly decreased in tPA-deficient mice (Rogove et al, 1999). Mice lacking LRP on microglia have reduced ischemic edema and reduced MMP-9 elevation after stroke (Zhang et al, 2009), suggesting that tPA interaction with microglial LRP contributes to the development of inflammation, BBB leakage, and cerebral edema. Notably, delayed tPA treatment in rats increases endothelial expression of ICAM-1 and neutrophil accumulation. Accordingly, strategies blocking leukocyte adhesion or activation increase therapeutic time window for thrombolysis in preclinical studies (Zhang et al, 1999; Bowes et al, 1995). It has been demonstrated that direct interaction of endothelial LRP receptors with tPA induces NFκB activation and subsequent overexpression of MMP-3, bypassing the need to cross BBB to induce inflammation (Suzuki et al, 2009). Moreover, crosstalk between inflammation and thrombosis could further induce deleterious effects (Evangelista et al, 1996). Indeed, delayed thrombolysis also increases tissue factor and Von Willebrand factor expression by endothelial cells, an effect associated with increased fibrin and platelets deposition in ischemic brain vessels (Zhang et al, 2005a, 2005b). By impairing microcirculation patency through platelets, fibrin and leukocytes accumulation, tPA-induced endothelial inflammation could counteract the beneficial effects of upstream arterial recanalization.
Tissue-Type Plasminogen Activator and Platelet-Derived Serpins
After arterial recanalization, platelets (among other blood components) accumulate on the injured vasculature and thus, could impair reperfusion. Since thrombolytic effects of tPA are mediated by plasmin, which degrades fibrin, tPA-induced thrombolysis is significantly more efficient on fibrin-rich clots than on platelet-rich clots (Levi et al, 1992; Zhu et al, 1999). But other factors may contribute to the limited efficiency of tPA on platelet-rich clots. Indeed, on activation, platelets release large amounts of plasminogen activator inhibitor type 1 (a serine protease inhibitor or serpin), which inhibits tPA proteolytic activity and blocks plasminogen activation. Accordingly, plasminogen activator inhibitor type 1 blockade promotes tPA-mediated thrombolysis in experimental models. Recently, it has also been shown that protease nexin 1, which accumulates in thrombus because of its presence in platelets, also impairs tPA-mediated thrombolysis by direct inhibition of fibrin bound plasmin (Boulaftali et al, 2011). Altogether, these mechanisms decrease thrombolysis efficiency on platelet-rich clots and could contribute to the low recanalization rate of ischemic stroke patient treated with tPA. Whether platelet-derived serpins could alleviate tPA-induced BBB injuries remains, however, to be investigated.
Tissue-Type Plasminogen Activator and Ageing
Whereas most of the preclinical studies are conducted in young and healthy animals, ischemic stroke mainly occur in elderly people with increased vascular risk factors and comorbidities (Jaramillo et al, 2006). Interestingly, it has been demonstrated that ageing was associated with a decrease in expression and activity of tPA in the brain parenchyma of mice, and that this was associated with a decrease ischemic lesion volumes and with protection of the barrier function of the neurovascular unit during cerebral ischemia (Roussel et al, 2009). Thus, although controlling the endogenous availability of tPA could be of therapeutic interest, its transcriptional regulation in nerve cells remains poorly investigated (Shin et al, 2004; Lee et al, 2008). Interestingly, it has been recently proposed that the transcription of the neuronal tPA may be influenced by epigenetic regulation (Obiang et al, 2011).
Can We Improve the Efficacy/Safety Ratio of Tissue-Type Plasminogen Activator?
The risk of hemorrhagic transformation carried by the use of tPA has initiated an era of investigation for alternative thrombolytic agents. With advances in DNA technology, chemistry and proteomics, several second and third generation of tPA derivatives, genetically engineered molecules targeting the blood clot and even ‘exotic' plasminogen activators have progressively reached clinical trials, including Streptokinase, DSPA, Tenecteplase (TNK-tPA), Microplasmin, Reteplase, Ancrod, and Pro-urokinase. However, none has demonstrated benefits so far. Conceptually, the ideal thrombolytic agent should be an effective fibrinolytic agent, devoid of harmful effects on brain cells and BBB. It has been recently demonstrated that the minimal structural requirements for tPA-mediated pro-excitotoxic activity is reteplase, i.e., K2 and protease domains (Lopez-Atalaya et al, 2008). Using an in vitro model of BBB, it has been shown that like tPA, DSPA can cross the intact BBB by an LRP-dependent transcytosis but that, unlike tPA, its passage remains LRP dependent when the BBB is leaky (López-Atalaya et al, 2007). Moreover, intravenous but not intrastriatal coadministration of DSPA antagonizes the pro-neurotoxic effect of intravenous tPA. This may be caused by a competition between DSPA and tPA for LRP binding at the BBB, thus effectively blocking tPA access to the brain parenchyma (López-Atalaya et al, 2007). Moreover, in contrast to tPA, DSPA, lacking a kringle 2 domain, has been shown to be devoid of pro-excitotoxic activity in vitro and in vivo (Liberatore et al, 2003; López-Atalaya et al, 2007). Accordingly, a tPA-derived molecule, with ‘inactive' K2 and with point mutations to prevent interaction with LRP would be in theory unable to cross the BBB and unable to exacerbate excitotoxic damages. In addition, the lack of Finger domain would also reduce microglial activation. Adding point mutations like those performed in Tenecteplase could also boost the overall benefit achievable with this ideal tPA-like molecule, but this remains to be established. Because the effect of tPA on BBB permeability seems to be a cell signaling event mediated by its interaction with LRP, the combined therapy of tPA and LRP antagonists (i.e., receptor-associated protein) could be useful not only to limit tPA crossing through the BBB, but also to block its interaction with astrocytic LRP, inhibiting in that way the activation of cell signaling pathways that may lead to increase in BBB permeability. By using a strategy of active immunization in mice, preventing the interaction of tPA with NMDA receptor, it has been shown that targeting the ability of tPA to potentiate NMDA receptor signaling led to reduced excitotoxic and ischemic brain injuries (Benchenane et al, 2007; Macrez et al, 2010, 2011a, 2011b). These data provide a proof of concept that targeting deleterious effects of tPA should improve the benefit of thrombolysis either by reducing its direct neurotoxicity or by increasing its therapeutic window. Interestingly, other combined strategies to improve stroke therapy have been reported in experimental models (Table 1). For example, a hexapeptide (EEIIMD) corresponding to amino acids 350 to 355 of plasminogen activator inhibitor type 1 known to bind to the docking site of tPA abolished the tPA-induced increase in infarct size and intracranial bleeding in both mechanical and embolic models of stroke in rats, and reduced brain edema and neuronal loss after traumatic brain injury in pigs (Armstead et al, 2006). Notably, the widely used HMG-CoA reductase inhibitors (statins) have been reported to enhance thrombolysis efficiency through multitargeted effects on cerebrovascular integrity (Zhang et al, 2005a, 2005b; Liu et al, 2006). Activated protein C, a serine protease with anticoagulant activities, has also been recently reported to be an interesting target, since it counteracts tPA toxic effects on neurons, endothelial cells, and BBB in vitro and in vivo (Cheng et al, 2006; Griffin et al, 2007).
Table 1. Relevant adjunctive therapy to tPA reported in the last 5 years.
| Study | Adjunctive therapy | Results |
|---|---|---|
| Macrez et al (2011a, 2011b) | Antibodies targeting the amino terminal domain of the NR1 subunit of the NMDA receptor (αATD-NR1) | αATD-NR1 prevents tPA-mediated excitotoxic neuronal death and increases the therapeutic window of thrombolysis |
| Zhu et al (2010) | Annexin A2 | Compared with high dose tPA alone, the combination of annexin A2 and low dose tPA extends the therapeutic window and attenuates hemorrhagic transformation |
| Liu et al (2010) | 17β-Estradiol (E2) | E2 blocks tPA-induced increases in MMP2/9. E2 extends the therapeutic window of tPA |
| Suzuki et al (2009) | Receptor-Associated Protein (RAP, an LRP antagonist) | RAP blocks tPA-mediated LRP-dependent MMP3 induction in endothelial cells and attenuates hemorrhagic transformation |
| Wang et al (2009) | Rosiglitazone (a PPARγ agonist) | It prevents MMP9 activation and attenuates inflammation, thus increasing the therapeutic window of tPA |
| Murata et al (2008) | Minocycline | Minocycline decreases plasma MMP9 levels, reduces infarction and ameliorates brain hemorrhage. It also increases the therapeutic window of tPA |
| Zhang et al (2008) | S-0139, an endothelin type A receptor antagonist | S-0139 suppresses ischemia- and tPA-triggered molecules related to thrombosis and increases the tPA therapeutic window |
| Wang et al (2008) | Melagatran (a direct thrombin inhibitor) | It enhances thrombus dissolution and prevents microthrombosis. Combination treatment is superior to each treatment performed alone |
| Su et al (2008) | Imatinib (PDGFR-α antagonist) | Imatinib reduces cerebrovascular permeability and hemorrhagic transformation after late thrombolysis |
| Okubo et al (2007) | FK-506 (tacrolimus) | FK-506 increases therapeutic window of tPA without increasing the risk of hemorrhagic transformation |
| Romanos et al (2007) | Uric acid (UA) | UA reduces tyrosine nitration, reduces brain neutrophil infiltration and further increases the benefits of thrombolysis |
| Cheng et al (2006) | Activated Protein C (APC) | APC inhibits tPA-induced MMP9 pathway and reduces tPA-mediated hemorrhage |
| Armstead et al (2006) | EEIIMD (hexapeptide) | EEIIMD abolishes tPA-induced increase in infarct size and intracranial bleeding |
| Chen et al (2006) | Melatonin | Melatonin attenuates BBB permeability and hemorrhagic transformation after thrombolysis |
NMDA, N-methyl--aspartate; tPA, tissue-type plasminogen activator; MMP, metalloproteinase; LRP, lipoprotein receptor-related protein; PPARγ, peroxisome proliferator-activated receptor gamma; PDGFR-α, platelet-derived growth factor receptor α BBB, blood–brain barrier.
Conclusion
Thrombolytic therapy using recombinant human tPA is a widely accepted therapy for acute stroke. However, despite an important advance in the management of stroke patients, more research is needed to increase the benefit and safety of tPA. First, a number of efforts remain to be done to ensure that patients are promptly identified and efficiently treated. Second, combined strategies to increase the rate of recanalization and prevent early reocclusion are warranted. Third, the selection of the best candidates for thrombolysis using imaging of the ischemic penumbra is very promising. Finally, there seems to be a potential for targeted strategies to prevent the induction of brain toxicity and hemorrhagic complications by tPA. Elaboration of such brain cocktails requires strong collaboration between researchers and clinicians.
The authors declared no conflict of interest. DV is involved in the development of pharmaceuticals that target reperfusion (Desmoteplase) and tPA toxicity (Macrez et al, 2011a, 2011b).
References
- Adams HPJ, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EFM. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- Adams HPJ, Leira EC, Torner JC, Barnathan E, Padgett L, Effron MB, Hacke W. Treating patients with ‘wake-up' stroke: the experience of the AbESTT-II trial. Stroke. 2008;39:3277–3282. doi: 10.1161/STROKEAHA.107.508853. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) Stroke. 2009;40:2442–2449. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Dávalos A, Eriksson N, Ford GA, Glahn J, Hennerici M, Mikulik R, Kaste M, Lees KR, Lindsberg PJ, Toni D. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR) Arch Neurol. 2010;67:1123–1130. doi: 10.1001/archneurol.2010.210. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moyé LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Nassar T, Akkawi S, Smith DH, Chen X, Cines DB, Higazi AA. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006;9:1150–1155. doi: 10.1038/nn1757. [DOI] [PubMed] [Google Scholar]
- Baron A, Montagne A, Cassé F, Launay S, Maubert E, Ali C, Vivien D. NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity. Cell Death Differ. 2010;17:860–871. doi: 10.1038/cdd.2009.172. [DOI] [PubMed] [Google Scholar]
- Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, Illoh K, Grotta JC, Savitz SI. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–832. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, López-Atalaya JP, Fernández-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Castel H, Boulouard M, Bluthé R, Fernandez-Monreal M, Roussel BD, Lopez-Atalaya JP, Butt-Gueulle S, Agin V, Maubert E, Dantzer R, Touzani O, Dauphin F, Vivien D, Ali C. Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J Cell Sci. 2007;120:578–585. doi: 10.1242/jcs.03354. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- Bluhmki E, Chamorro A, Dávalos A, Machnig T, Sauce C, Wahlgren N, Wardlaw J, Hacke W. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- Boulaftali Y, Ho-Tin-Noe B, Pena A, Loyau S, Venisse L, François D, Richard B, Arocas V, Collet J, Jandrot-Perrus M, Bouton M. Platelet protease nexin-1, a serpin that strongly influences fibrinolysis and thrombolysis. Circulation. 2011;123:1326–1334. doi: 10.1161/CIRCULATIONAHA.110.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–819. doi: 10.1212/wnl.45.4.815. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, Dávalos A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Sobrino T, Millán M, García M, Arenillas J, Nombela F, Brea D, Perez de la Ossa N, Serena J, Vivancos J, Castillo J, Dávalos A. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke. 2007;38:1855–1859. doi: 10.1161/STROKEAHA.106.481556. [DOI] [PubMed] [Google Scholar]
- Chen T, Lee M, Chen H, Kuo Y, Lin S, Wu T, Lee E. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J Pineal Res. 2006;40:242–250. doi: 10.1111/j.1600-079X.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernández JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, Hernangómez M, Montagne A, Liot G, Guaza C, Maubert E, Ali C, Vivien D, Docagne F. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiara B, Kasner SE, Tanne D, Levine SR, Demchuk A, Messe SR, Sansing L, Lees KR, Lyden P. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke. 2009;40:3067–3072. doi: 10.1161/STROKEAHA.109.554386. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Hennerici M. Ultrasound in the treatment of ischaemic stroke. Lancet Neurol. 2003;2:283–290. doi: 10.1016/s1474-4422(03)00380-6. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HPJ. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, Alberts MJ, Zivin JA, Wechsler L, Busse O. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- Delgado-Mederos R, Rovira A, Alvarez-Sabín J, Ribó M, Munuera J, Rubiera M, Santamarina E, Maisterra O, Delgado P, Montaner J, Molina CA. Speed of tPA-induced clot lysis predicts DWI lesion evolution in acute stroke. Stroke. 2007;38:955–960. doi: 10.1161/01.STR.0000257977.32525.6e. [DOI] [PubMed] [Google Scholar]
- Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. J Neurol Neurosurg Psychiatry. 2008;79:1093–1099. doi: 10.1136/jnnp.2007.133371. [DOI] [PubMed] [Google Scholar]
- Deshmukh VR, Fiorella DJ, Albuquerque FC, Frey J, Flaster M, Wallace RC, Spetzler RF, McDougall CG.2005Intra-arterial thrombolysis for acute ischemic stroke: preliminary experience with platelet glycoprotein IIb/IIIa inhibitors as adjunctive therapy Neurosurgery 5646–54.discussion 54–5 [DOI] [PubMed] [Google Scholar]
- Diedler J, Ahmed N, Sykora M, Uyttenboogaart M, Overgaard K, Luijckx G, Soinne L, Ford GA, Lees KR, Wahlgren N, Ringleb P. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke. 2010;41:288–294. doi: 10.1161/STROKEAHA.109.559724. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Baron J, Ma H, Davis SM. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 2009;8:261–269. doi: 10.1016/S1474-4422(09)70041-9. [DOI] [PubMed] [Google Scholar]
- Ernst R, Pancioli A, Tomsick T, Kissela B, Woo D, Kanter D, Jauch E, Carrozzella J, Spilker J, Broderick J. Combined intravenous and intra-arterial recombinant tissue plasminogen activator in acute ischemic stroke. Stroke. 2000;31:2552–2557. doi: 10.1161/01.str.31.11.2552. [DOI] [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Rotondo S, Martelli N, Polischuk R, McGregor JL, de Gaetano G, Cerletti C. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the beta 2 integrin CD11b/CD18. Blood. 1996;88:4183–4194. [PubMed] [Google Scholar]
- Fernández-Monreal M, López-Atalaya JP, Benchenane K, Cacquevel M, Dulin F, Le Caer J, Rossier J, Jarrige A, Mackenzie ET, Colloc'h N, Ali C, Vivien D. Arginine 260 of the amino-terminal domain of NR1 subunit is critical for tissue-type plasminogen activator-mediated enhancement of N-methyl-D-aspartate receptor signaling. J Biol Chem. 2004;279:50850–50856. doi: 10.1074/jbc.M407069200. [DOI] [PubMed] [Google Scholar]
- Ford GA, Ahmed N, Azevedo E, Grond M, Larrue V, Lindsberg PJ, Toni D, Wahlgren N. Intravenous alteplase for stroke in those older than 80 years old. Stroke. 2010;41:2568–2574. doi: 10.1161/STROKEAHA.110.581884. [DOI] [PubMed] [Google Scholar]
- Frijns CJM, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- García-Yébenes I, Sobrado M, Zarruk JG, Castellanos M, Pérez de la Ossa N, Dávalos A, Serena J, Lizasoain I, Moro MA. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin J, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Griffin JH, Fernández JA, Gale AJ, Mosnier LO. Activated protein C. J Thromb Haemost. 2007;5 (Suppl 1:73–80. doi: 10.1111/j.1538-7836.2007.02491.x. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Höxter G, Mahagne MH. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley ECJ, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, Wijman CA, Rapp KS, Grotta JC, Lyden PD. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Garcia-Bonilla L, Solé M, Sosti V, Parés M, Campos M, Ortega-Aznar A, Domínguez C, Rubiera M, Ribó M, Quintana M, Molina CA, Alvarez-Sabín J, Rosell A, Unzeta M, Montaner J. Plasma VAP-1/SSAO activity predicts intracranial hemorrhages and adverse neurological outcome after tissue plasminogen activator treatment in stroke. Stroke. 2010;41:1528–1535. doi: 10.1161/STROKEAHA.110.584623. [DOI] [PubMed] [Google Scholar]
- Hill MD, Barber PA, Demchuk AM, Newcommon NJ, Cole-Haskayne A, Ryckborst K, Sopher L, Button A, Hu W, Hudon ME, Morrish W, Frayne R, Sevick RJ, Buchan AM. Acute intravenous--intra-arterial revascularization therapy for severe ischemic stroke. Stroke. 2002;33:279–282. doi: 10.1161/hs0102.101900. [DOI] [PubMed] [Google Scholar]
- IMS Study Investigators Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35:904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- IMS II Trial Investigators The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- Jaramillo A, Góngora-Rivera F, Labreuche J, Hauw J, Amarenco P. Predictors for malignant middle cerebral artery infarctions: a postmortem analysis. Neurology. 2006;66:815–820. doi: 10.1212/01.wnl.0000203649.60211.0e. [DOI] [PubMed] [Google Scholar]
- Keris V, Rudnicka S, Vorona V, Enina G, Tilgale B, Fricbergs J. Combined intraarterial/intravenous thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2001;22:352–358. [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- Khatri P, Hill MD, Palesch YY, Spilker J, Jauch EC, Carrozzella JA, Demchuk AM, Martin R, Mauldin P, Dillon C, Ryckborst KJ, Janis S, Tomsick TA, Broderick JP. Methodology of the Interventional Management of Stroke III Trial. Int J Stroke. 2008;3:130–137. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40:2438–2441. doi: 10.1161/STROKEAHA.109.552547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim HJ, Lee WJ, Joo SH, Jeon S, Kim JW, Kim HS, Han S, Lee J, Park SH, Cheong JH, Kim W, Ko KH, Shin CY. Differential regulation of matrix metalloproteinase-9 and tissue plasminogen activator activity by the cyclic-AMP system in lipopolysaccharide-stimulated rat primary astrocytes. Neurochem Res. 2008;33:2324–2334. doi: 10.1007/s11064-008-9737-2. [DOI] [PubMed] [Google Scholar]
- Lee M, Hong K, Saver JL. Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke. 2010;41:932–937. doi: 10.1161/STROKEAHA.109.574335. [DOI] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- Levi M, Biemond BJ, van Zonneveld AJ, ten Cate JW, Pannekoek H. Inhibition of plasminogen activator inhibitor-1 activity results in promotion of endogenous thrombolysis and inhibition of thrombus extension in models of experimental thrombosis. Circulation. 1992;85:305–312. doi: 10.1161/01.cir.85.1.305. [DOI] [PubMed] [Google Scholar]
- Lewandowski CA, Frankel M, Tomsick TA, Broderick J, Frey J, Clark W, Starkman S, Grotta J, Spilker J, Khoury J, Brott T. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke. 1999;30:2598–2605. doi: 10.1161/01.str.30.12.2598. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Samson A, Bladin C, Schleuning W, Medcalf RL. Vampire bat salivary plasminogen activator (desmoteplase): a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke. 2003;34:537–543. doi: 10.1161/01.str.0000049764.49162.76. [DOI] [PubMed] [Google Scholar]
- Lijnen HR, Collen D. Fibrinolytic agents: mechanisms of activity and pharmacology. Thromb Haemost. 1995;74:387–390. [PubMed] [Google Scholar]
- Liot G, Roussel BD, Lebeurrier N, Benchenane K, López-Atalaya JP, Vivien D, Ali C. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J Neurochem. 2006;98:1458–1464. doi: 10.1111/j.1471-4159.2006.03982.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Cheng T, Guo H, Fernández JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu Q, He S, Simpkins JW, Yang S. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J Pharmacol Exp Ther. 2010;332:1006–1012. doi: 10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Roussel BD, Levrat D, Parcq J, Nicole O, Hommet Y, Benchenane K, Castel H, Leprince J, To Van D, Bureau R, Rault S, Vaudry H, Petersen K, Santos JSO, Ali C, Vivien D. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor-mediated neurotoxicity. J Cereb Blood Flow Metab. 2008;28:1212–1221. doi: 10.1038/jcbfm.2008.14. [DOI] [PubMed] [Google Scholar]
- Lyden P. Thrombolytic therapy for acute stroke--not a moment to lose. N Engl J Med. 2008;359:1393–1395. doi: 10.1056/NEJMe0806335. [DOI] [PubMed] [Google Scholar]
- López-Atalaya JP, Roussel BD, Ali C, Maubert E, Petersen K, Berezowski V, Cecchelli R, Orset C, Vivien D. Recombinant Desmodus rotundus salivary plasminogen activator crosses the blood-brain barrier through a low-density lipoprotein receptor-related protein-dependent mechanism without exerting neurotoxic effects. Stroke. 2007;38:1036–1043. doi: 10.1161/01.STR.0000258100.04923.84. [DOI] [PubMed] [Google Scholar]
- Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, Flaherty ML, Woo D, Khatri P, Adeoye O, Ferioli S, Khoury JC, Hornung R, Broderick JP. Population-based study of wake-up strokes. Neurology. 2011;76:1662–1667. doi: 10.1212/WNL.0b013e318219fb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, Davis SM, Mitchell PJ, Gerraty RP, Fitt G, Hankey GJ, Stewart-Wynne EG, Rosen D, McNeil JJ, Bladin CF, Chambers BR, Herkes GK, Young D, Donnan GA. Results of a multicentre, randomised controlled trial of intra-arterial urokinase in the treatment of acute posterior circulation ischaemic stroke. Cerebrovasc Dis. 2005;20:12–17. doi: 10.1159/000086121. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Petersson J, Norrving B, Hacke W, Dirnagl U, Wagner M, Schwab S. European Hypothermia Stroke Research Workshop. Int J Stroke. 2010;5:489–492. doi: 10.1111/j.1747-4949.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- Macrez R, Bezin L, Le Mauff B, Ali C, Vivien D. Functional occurrence of the interaction of tissue plasminogen activator with the NR1 Subunit of N-methyl-D-aspartate receptors during stroke. Stroke. 2010;41:2950–2955. doi: 10.1161/STROKEAHA.110.592360. [DOI] [PubMed] [Google Scholar]
- Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011a;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- Macrez R, Obiang P, Gauberti M, Roussel B, Baron A, Parcq J, Cassé F, Hommet Y, Orset C, Agin V, Bezin L, Berrocoso TG, Petersen KU, Montaner J, Maubert E, Vivien D, Ali C. Antibodies preventing the interaction of tissue-type plasminogen activator with N-methyl-D-aspartate receptors reduce stroke damages and extend the therapeutic window of thrombolysis. Stroke. 2011b;42:2315–2322. doi: 10.1161/STROKEAHA.110.606293. [DOI] [PubMed] [Google Scholar]
- Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D. Intravenous glycoprotein IIb/IIIa inhibitor (tirofiban) followed by intra-arterial urokinase and mechanical thrombolysis in stroke. AJNR Am J Neuroradiol. 2005;26:2595–2601. [PMC free article] [PubMed] [Google Scholar]
- Mateen FJ, Nasser M, Spencer BR, Freeman WD, Shuaib A, Demaerschalk BM, Wijdicks EFM. Outcomes of intravenous tissue plasminogen activator for acute ischemic stroke in patients aged 90 years or older. Mayo Clin Proc. 2009;84:334–338. doi: 10.1016/S0025-6196(11)60542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazighi M, Serfaty J, Labreuche J, Laissy J, Meseguer E, Lavallée PC, Cabrejo L, Slaoui T, Guidoux C, Lapergue B, Klein IF, Olivot J, Abboud H, Simon O, Niclot P, Nifle C, Touboul P, Raphaeli G, Gohin C, Claeys ES, Amarenco P. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–809. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- Medina MG, Ledesma MD, Domínguez JE, Medina M, Zafra D, Alameda F, Dotti CG, Navarro P. Tissue plasminogen activator mediates amyloid-induced neurotoxicity via Erk1/2 activation. EMBO J. 2005;24:1706–1716. doi: 10.1038/sj.emboj.7600650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NK, Ahmed N, Andersen G, Egido JA, Lindsberg PJ, Ringleb PA, Wahlgren NG, Lees KR. Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive. BMJ. 2010;341:c6046. doi: 10.1136/bmj.c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke. 2004;35:486–490. doi: 10.1161/01.STR.0000110219.67054.BF. [DOI] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- NINDS-tPA Study Group Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- Nolin WB, Emmetsberger J, Bukhari N, Zhang Y, Levine JM, Tsirka SE. tPA-mediated generation of plasmin is catalyzed by the proteoglycan NG2. Glia. 2008;56:177–189. doi: 10.1002/glia.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiang P, Maubert E, Bardou I, Nicole O, Launay S, Bezin L, Vivien D, Agin V. Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol Learn Mem. 2011;96:121–129. doi: 10.1016/j.nlm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, Miyamoto S, Sasaki M, Inoue T. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke. 2007;38:2633–2639. doi: 10.1161/STROKEAHA.107.488551. [DOI] [PubMed] [Google Scholar]
- Okubo S, Igarashi H, Kanamatsu T, Hasegawa D, Orima H, Katayama Y. FK-506 extended the therapeutic time window for thrombolysis without increasing the risk of hemorrhagic transformation in an embolic rat stroke model. Brain Res. 2007;1143:221–227. doi: 10.1016/j.brainres.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, del Zoppo G, Kleindorfer D, Woo D, Khatri P, Castaldo J, Frey J, Gebel JJ, Kasner S, Kidwell C, Kwiatkowski T, Libman R, Mackenzie R, Scott P, Starkman S, Thurman RJ. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39:3268–3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Melchor JP, Matys T, Skrzypiec AE, Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc Natl Acad Sci USA. 2005;102:443–448. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu R, An J, Zhang C, Yepes M. Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol. 2008;172:1355–1362. doi: 10.2353/ajpath.2008.070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Rivolta J, Vieira JR, Rincon F, Stillman J, Marshall RS, Chong JY. Symptomatic intracerebral hemorrhage among eligible warfarin-treated patients receiving intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2010;67:559–563. doi: 10.1001/archneurol.2010.25. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Harris-Lane P, Kirmani JF, Janjua N, Divani AA, Mohammad YM, Suarez JI, Montgomery MO.2006Intra-arterial reteplase and intravenous abciximab in patients with acute ischemic stroke: an open-label, dose-ranging, phase I study Neurosurgery 59789–796.discussion 796–7 [DOI] [PubMed] [Google Scholar]
- Rha J, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- Rijken DC, Sakharov DV. Basic principles in thrombolysis: regulatory role of plasminogen. Thromb Res. 2001;103 (Suppl 1:S41–S49. doi: 10.1016/s0049-3848(01)00296-1. [DOI] [PubMed] [Google Scholar]
- Ringleb P, Schellinger PD, Hacke W. European Stroke Organisation 2008 guidelines for managing acute cerebral infarction or transient ischemic attack. {Part 1] Nervenarzt. 2008;79:936–957. doi: 10.1007/s00115-008-2531-1. [DOI] [PubMed] [Google Scholar]
- Rogove AD, Siao C, Keyt B, Strickland S, Tsirka SE. Activation of microglia reveals a non-proteolytic cytokine function for tissue plasminogen activator in the central nervous system. J Cell Sci. 1999;112 (Part 22:4007–4016. doi: 10.1242/jcs.112.22.4007. [DOI] [PubMed] [Google Scholar]
- Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007;27:14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Macrez R, Jullienne A, Agin V, Maubert E, Dauphinot L, Potier M, Plawinski L, Castel H, Hommet Y, Munuera J, Montaner J, Yepes M, Ali C, Vivien D. Age and albumin D site-binding protein control tissue plasminogen activator levels: neurotoxic impact. Brain. 2009;132:2219–2230. doi: 10.1093/brain/awp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Sandercock P, Lindley R, Wardlaw J, Dennis M, Lewis S, Venables G, Kobayashi A, Czlonkowska A, Berge E, Slot KB, Murray V, Peeters A, Hankey G, Matz K, Brainin M, Ricci S, Celani MG, Righetti E, Cantisani T, Gubitz G, Phillips S, Arauz A, Prasad K, Correia M, Lyrer P. Third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke. Trials. 2008;9:37. doi: 10.1186/1745-6215-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, Akhtar N, Orouk FO, Salam A, Shuaib A, Alexandrov AV. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- Saqqur M, Tsivgoulis G, Molina CA, Demchuk AM, Siddiqui M, Alvarez-Sabín J, Uchino K, Calleja S, Alexandrov AV. Symptomatic intracerebral hemorrhage and recanalization after IV rt-PA: a multicenter study. Neurology. 2008;71:1304–1312. doi: 10.1212/01.wnl.0000313936.15842.0d. [DOI] [PubMed] [Google Scholar]
- Saver JL. Time is brain--quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- Saver JL, Gornbein J, Starkman S. Graphic reanalysis of the two NINDS-tPA trials confirms substantial treatment benefit. Stroke. 2010;41:2381–2390. doi: 10.1161/STROKEAHA.110.583807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CY, Kundel M, Wells DG. Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J Neurosci. 2004;24:9425–9433. doi: 10.1523/JNEUROSCI.2457-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siao C, Tsirka SE. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J Neurosci. 2002;22:3352–3358. doi: 10.1523/JNEUROSCI.22-09-03352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez JI, Zaidat OO, Sunshine JL, Tarr R, Selman WR, Landis DMD.2002Endovascular administration after intravenous infusion of thrombolytic agents for the treatment of patients with acute ischemic strokes Neurosurgery 50251–259.discussion 259–60 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- Sylaja PN, Cote R, Buchan AM, Hill MD. Thrombolysis in patients older than 80 years with acute ischaemic stroke: Canadian Alteplase for Stroke Effectiveness Study. J Neurol Neurosurg Psychiatry. 2006;77:826–829. doi: 10.1136/jnnp.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, Levine SR. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002;105:1679–1685. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- Tanswell P, Seifried E, Su PC, Feuerer W, Rijken DC. Pharmacokinetics and systemic effects of tissue-type plasminogen activator in normal subjects. Clin Pharmacol Ther. 1989;46:155–162. doi: 10.1038/clpt.1989.120. [DOI] [PubMed] [Google Scholar]
- Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, Khoury J. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29:582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni D, Lorenzano S. Intravenous thrombolysis with rt-PA in acute stroke patients aged >or=80 years. Int J Stroke. 2009;4:21–22. doi: 10.1111/j.1747-4949.2009.00242.x. [DOI] [PubMed] [Google Scholar]
- Touzé E. Thrombolytic therapy in ischemic strokes. Rev Neurol (Paris) 2009;165 (Spec No 2:F140–F152. [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, Eggers J, Ribo M, Perren F, Saqqur M, Rubiera M, Sergentanis TN, Vadikolias K, Larrue V, Molina CA, Alexandrov AV. Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke. 2010;41:280–287. doi: 10.1161/STROKEAHA.109.563304. [DOI] [PubMed] [Google Scholar]
- Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millán M, Muir K, Roine RO, Toni D, Lees KR. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Wang CX, Ding X, Shuaib A. Treatment with melagatran alone or in combination with thrombolytic therapy reduced ischemic brain injury. Exp Neurol. 2008;213:171–175. doi: 10.1016/j.expneurol.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A. Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. J Cereb Blood Flow Metab. 2009;29:1683–1694. doi: 10.1038/jcbfm.2009.87. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2009;4:CD000213. doi: 10.1002/14651858.CD000213.pub2. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M, Zivin JA. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke. 1999;30:624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, Schneider A, Schwaninger M. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005a;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, Hozeska A, Zhang C, Li L, Morris D, Zhang RL, Lu M, Chopp M. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005b;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang C, Zhang L, Roberts C, Lu M, Kapke A, Cui Y, Ninomiya M, Nagafuji T, Albala B, Zhang ZG, Chopp M. Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke. Stroke. 2008;39:2830–2836. doi: 10.1161/STROKEAHA.108.515684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, An J, Haile WB, Echeverry R, Strickland DK, Yepes M. Microglial low-density lipoprotein receptor-related protein 1 mediates the effect of tissue-type plasminogen activator on matrix metalloproteinase-9 activity in the ischemic brain. J Cereb Blood Flow Metab. 2009;29:1946–1954. doi: 10.1038/jcbfm.2009.174. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999;99:3050–3055. doi: 10.1161/01.cir.99.23.3050. [DOI] [PubMed] [Google Scholar]
- Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, Zhao S, Hajjar KA, Lo EH, Wang X. Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkstok SM, Vermeulen M, Stam J, de Haan RJ, Roos YB. A randomised controlled trial of antiplatelet therapy in combination with Rt-PA thrombolysis in ischemic stroke: rationale and design of the ARTIS-Trial. Trials. 2010;11:51. doi: 10.1186/1745-6215-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]