Abstract
A sequence of 11 consecutive arginine residues (11R) is one of the best protein transduction domains for introducing proteins into cell membranes. Heme-oxygenase-1 (HO-1) is involved in heme catabolism and reduces the contractile effect of hemoglobin after subarachnoid hemorrhage (SAH). Therefore, we constructed 11R-fused HO-1 protein to achieve successful transduction of the protein into the cerebral arteries and examined the therapeutic effect of the 11R-HO-1 protein for cerebral vasospasm (CV) after SAH. We injected the 11R-HO-1 protein into the cisterna magna of male rats and, several hours after the injection, performed immunofluorescence staining and western blotting analysis of the rat basilar arteries (BAs) to determine transduction efficacy. We also assessed intraarterial HO-1 activity as cGMP (cyclic guanosine 3′, 5′-cyclic monophosphate) accumulation in SAH and determined whether protein transduction of 11R-HO-1 quantified the therapeutic effect in a rat double-hemorrhage model of SAH. The BAs expressed significantly more HO-1 in the group injected with 11R-HO-1 (3.56±0.54 (11R-HO-1) versus control (saline)), and transduction of 11R-HO-1 resulted in higher activity (>3.25-fold) in rat BAs with SAH. Moreover, the results of the rat double-hemorrhage model showed that the 11R-HO-1 protein significantly attenuated CV after SAH (317.59±23.48 μm (11R-HO-1) versus 270.08±14.66 μm (11R-fused enhanced green fluorescent protein), 252.05±13.95 μm (saline), P<0.01).
Keywords: cerebral vasospasm, heme-oxygenase-1 (HO-1), protein transduction domain (PTD), subarachnoid hemorrhage (SAH), 11 consecutive arginines (11R)
Introduction
Cerebral vasospasm (CV) with delayed ischemic neurologic deficit occurs in 30% to 70% of patients with aneurysmal subarachnoid hemorrhage (SAH) (Adams et al, 1987). In spite of promising therapeutic approaches such as endothelin receptor antagonists (Chitaley and Webb, 2002; Vatter et al, 2004), calcium antagonists, or sodium nitroprusside (Edvinsson et al, 1979; Thomas et al, 1999; Raabe et al, 2002), CV still remains a major cause of morbidity and mortality and an important cause of cerebral ischemia and stroke after SAH (Solenski et al, 1995; Miller and Diringer, 1995). Clearly, to improve clinical outcomes after SAH, the development of more effective therapies is required.
Gene therapy is a promising strategy for treating cerebrovascular diseases including CV after SAH. Several genes that encode vasoactive proteins have been transferred through cerebrospinal fluid into cerebral arteries in experimental animal models (Toyoda et al, 2003). Gene therapy using HO-1 (heme-oxygenase-1) is a candidate for treating CV, because HO-1 is involved in heme catabolism and reduces the contractile effect of hemoglobin after SAH (Suzuki et al, 1999). The recombinant HO-1 gene can be transferred using adenoviral vectors into the vascular wall, and the recombinant HO-1 protein can be functionally expressed in cerebral arteries after experimental SAH (Marton et al, 2000). However, HO-1 transgene expression is a multistep process, and the product is expressed mainly in the adventitia overlying cerebral vessels (Ono et al, 2000). Moreover, virus-mediated gene therapy has some critical limitations in general, such as inflammatory responses, viral cytotoxicity, and the random integration of viral vector DNA into host chromosomes (Verma and Somia, 1997).
Protein transduction (protein therapy) has recently been studied as a means to overcome these disadvantages. Only very small therapeutic proteins (typically <600 Da) can be efficiently delivered into cells in vivo (Scheld, 1989). However, conjugation with protein transduction domains (PTDs) (comprising 10 to 20 amino-acid peptides) allows the transduction of even very large proteins into any type of cell (Schwarze et al, 1999). A sequence of 11 arginines (11R) has been shown to be an effective transduction domain applicable not only to cultured cells but also to tissue slices and in vivo (Matsushita et al, 2001; Michiue et al, 2005; Inoue et al, 2006). We also found in a previous study that 11R-fused enhanced green fluorescent protein (11R-EGFP) penetrated all layers of the rat basilar artery (BA) (Ogawa et al, 2007). A recent regenerative medicine study has shown that somatic cells can be fully reprogrammed into pluripotent stem cells by direct delivery of 11R-fused reprogramming proteins (Zhou et al, 2009).
Therefore, using the 11R protein transduction method, we examined whether HO-1 fused to 11R (11R-HO-1) can be efficiently transduced into rat BAs and whether protein transduction of 11R-HO-1 ameliorates cerebral vasoconstriction after SAH in vivo.
Materials and methods
Plasmid Construction (pET-21a(+)-11R-HO-1)
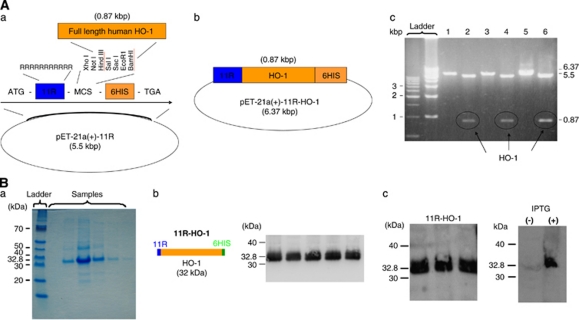
Full-length human HO-1 cDNA was subcloned into double-digested (Bam I and Hind III) pET-21a(+)-11R vector as detailed below (Figures 1A and 1B). Previously, we constructed a pET-21a(+)-p53-11R plasmid from pET-21a(+)-11R. This pET-21a(+)-11R was produced by modification of a pET-21a(+) vector (Novagen, Madison, WI, USA) (Takenobu et al, 2002). In brief, the vector was tagged with a 6-histidine leader, followed by an 11-arginine flanked by glycine and glutamic acid residues (for free bond rotation of the domain) in the COOH terminal. This pET-21a(+)-p53-11R (0.26 μg/μL) plasmid was cut with BamHI and Hind III to remove p53. The cut vector with free cohesive ends was purified using PCR purification kits (Qiagen, Valencia, CA, USA). Inserted HO-1 cDNA was prepared by PCR using the forward and reverse primers, GGATCCATGGAGCGTCCGCAACCCGACAGC and AAGCTTGCATGGCATAAAGCCCTACAGCAAC, respectively. PCR products were also purified. The HO-1 gene was ligated to the Topo TA vector (Invitrogen, Carlsbad, CA, USA), and the sequence was checked to determine whether some sites had mutated during PCR. The HO-1 DNA was cut from the Topo TA vector by overnight digestion with BamHI and Hind III. The HO DNA fragment was purified from agarose gels using Gel extraction kits (Qiagen). Ligated DNA (5 μL) was transformed into Topo 10 Escherichia coli bacteria (Invitrogen) and then spread on ampicillin-resistant plates. Colonies that developed overnight were purified using DNA purification kits (Qiagen). Whether human HO was inserted into the pET21a(+)-11R vector and whether some sequences had mutated during the process were checked by sequencing (using the ABI 3100 sequencer (Applied Biosystems, Foster City, CA, USA)) and could prove that there were no mutations in the alignments. In addition, pET-21a(+)-11R-HO-1 was incubated at 37°C for 3 hours with Bam I and Hind III and then electrophoretically resolved (n=10). In Figure 1A(c), the bands of pET-21a(+)-11R-HO-1 (6.37 kbp), pET-21a(+)-11R (5.5 kbp), and human HO-1 plasmid (0.87 kbp) can be seen.
Figure 1.
(A) Structure of the 11R-HO-1 gene. (a) and (b) Structure of the pET21a(+)-HO-1-11R plasmid. (c) Results of electrophoresis. pET 21a(+)-HO-1-11R cut with Bam HI (1, 3, 5) and with Bam HI and Hind III (2, 4, 6). The cDNA encoding the therapeutic protein (human HO-1) is subcloned into pET 21a(+)-11R vectors digested two restriction enzymes. Plasmid pET-21a(+)-HO-1-11R was incubated at 37°C for 3 hours with Bam I and Hind III and then analyzed electrophoretically. (c) Bands of pET-21a(+)-11R-HO-1 (6.37 kbp), pET-21a(+)-11R (5.5 kbp), and human HO-1 plasmid (0.87 kbp). This result confirms human HO-1 cDNA subcloning into pET 21a(+)-HO-1-11R. ATG, start codon; 11R, 11 consecutive arginine residues (MRRRRRRRRRRR); MCS, multiple cloning site; 6HIS, 6 × histidine; TGA, stop codon. (B) 11R-HO-1 protein expression. Recombinant proteins were expressed in Escherichia coli BL-21 and purified using His Tag-Ni affinity under denaturing conditions. Proteins were dialyzed against PBS and used in biologic assays. Expression of the 11R-HO-1 protein was determined at 32.8 kDa by Coomassie brilliant blue staining of SDS-PAGE gels (a). Schema of the 11R-HO-1 protein (b, left). Protein was fused to 11R and a histidine tag for efficient protein purification. Western blotting proceeded using an anti-histidine antibody against the His tag part of the protein (b, right) and an anti-HO-1 antibody (c, left). After induction with 0.4 mmol/L IPTG, 11R-HO-1 was specifically expressed (c, right). 11R, 11 consecutive arginine residues; HO-1, heme-oxygenase-1; IPTG, isopropyl-1-thio-β--galactopyranoside; PBS, phosphate-buffered saline.
Purification of Protein Expressed by pET-HO-1-11R
The 11R fusion protein was expressed and purified as described previously (Matsushita et al, 2001). In brief, the constructed plasmids were transformed into BL21-DE3 E. coli cells that were then cultured with 2 L of fresh Lysogeny broth (LB) (Amp+) at 200 r.p.m. and 37°C until OD 600 reached 0.8 to 1.0. Proteins that were expressed in these cells after induction with 0.4 mmol/L isopropyl-1-thio-β--galactopyranoside were purified from bacterial sonicates using Probond Nickel-Chelating resin (Invitrogen), dialyzed overnight in 4 L of phosphate-buffered saline (PBS), and stored at −80°C.
Confirmation of 11R-HO-1 Protein Expression
Staining with Coomassie Brilliant Blue
Incubated samples were resolved on 10% SDS-PAGE gels and then stained with Coomassie brilliant blue. The staining revealed a robust 11R-HO-1 protein expression (32.8 kDa) (n=8) (Figure 1B(a)).
Western Blotting
Anti-polyhistidine: The protein contains 6-histidine. Therefore, we used anti-polyhistidine to detect the protein expression (Figure 1A(b)). Proteins were resolved on 10% SDS-PAGE gels and then transferred to nitrocellulose membranes where they were probed with primary antibodies against polyclonal anti-polyhistidine (1:2,000, Sigma-Aldrich, St Louis, MO, USA) and peroxidase horseradish peroxidase-coupled goat anti-mouse secondary antibodies (1:2,000, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA). Bands were visualized (LAS-3000, Fujifilm, Tokyo, Japan) using a commercial Immobilon western chemiluminescent horseradish peroxidase substrate kit (Millipore Corporation, Billerica, MA, USA) (n=9). A robust band can be seen at 32.8 kDa, proving the successful expression of the protein (Figure 1B(b)).
Anti-HO-1: Western blotting also proceeded using anti HO-1 antibody. The first antibody was anti-HO-1 (HSP32) antibody (developed in rabbits; IgG fraction of antiserum, 1:1,000, Sigma-Aldrich) and the second was horseradish peroxidase-coupled goat anti-rabbit antibody (1:2,000, Jackson ImmunoResearch Laboratories Inc.) (n=9). The results also illustrate the successful expression (Figure 1B(c)).
Cytotoxicity Assays
The cytotoxicity of 11R-HO-1 was evaluated by (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, tetrazole (MTT) assays using cell proliferation kits before starting animal experiments in vivo (Roche Applied Science, Mannheim, Germany). The assay is based on cleavage of the yellow tetrazolium salt MTT to purple formazan crystals by metabolically active cells (Mosmann, 1983; Michiue et al, 2005; Tada et al, 1986; Denizot and Lang, 1986). Human fibroblasts, human coronary artery endothelial cells (Promocell, Heidelberg, Germany), or human coronary artery smooth muscle cells (Promocell) were incubated with 0.1 μmol/L 11R-HO-1, 11R-EGFP, 11R proteins, or 1% Triton X as a positive control (n=102 each) at 37°C for 24 hours followed by yellow MTT solution for 4 hours. The plates were then incubated at 37°C for 24 hours to solubilize formazan crystals, and then the formazan product in the resulting colored solution was quantified by using spectrophotometry to scan the solution at absorbance and reference wavelengths of 550 and 655 nm, respectively.
In Vivo Study
All animal studies were approved by the local Committee for Animal Experimentation (Düsseldorf, Germany) and the Animal Research and Care Committee at the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences. Male Sprague–Dawley rats weighing 300 to 400 g were randomly assigned to the following experiments. The whole experimental design schematics are provided in Supplementary Figures 1 to 4 online.
Protein Transduction
We examined the transduction efficacy of the 11R-HO-1 protein, which consists of HO-1, 6-histidine tag, and 11 consecutive arginines (11R), into the cerebral arteries of rats after transcisternal injection. In brief, rats were intraperitoneally anesthetized with a mixture of ketamine (Pfizer Pharma GmbH, Berlin, Germany) and xylazine (Bayer Health Care, Leverkusen, Germany) (100 and 5 mg/kg body weight, respectively). A 27-G needle was inserted into the cisterna magna through the atlanto-occipital membrane. Cerebrospinal fluid (200 μL) was withdrawn and 200 μL of 11R-HO-1 (0.1 mg/mL) or saline was infused over 5 minutes. Animals were killed at 6, 8, 12, 24, and 48 hours after the protein injection and transcardially perfused with 100 mL (PBS) at physiologic blood pressure.
Western Blots of 11R-HO-1 Expressed In Vivo
The dissected brains, brain stems, and BAs of rats 6 hours after 11R-HO-1 injection were boiled and sonicated in 1% SDS. Sonicates were resolved by electrophoresis on 10% SDS-PAGE gels, and then separated proteins were transferred onto nitrocellulose membranes and probed with primary antibodies against polyclonal anti-histidine (1:500, Sigma-Aldrich) (7 rats) or anti-HO-1 (1:500, Sigma-Aldrich) (7 rats per group) and peroxidase-coupled secondary antibodies (1:5,000, Jackson ImmunoResearch Laboratories Inc.). Proteins of interest were visualized using the Immobilon western chemiluminescent horseradish peroxidase substrate kit (Millipore Corporation).
Immunohistochemistry of Rat Basilar Arteries In Vivo
We examined the fluorescence intensity of BAs at 2, 6, 8, 24, and 48 hours after the injection of 11R-HO-1 (7 rats per time point) to determine the transduction efficacy of 11R-HO-1. Sections of BAs and brain stems were washed in 0.1 mol/L PBS and immersed in 10% bovine serum albumin. The BA sections were incubated with anti-histidine antibodies (1:200), washed, and incubated with fluorescein isothiocyanate-conjugated secondary antibodies (1:100, Jackson ImmunoResearch Laboratories Inc.) for 1 hour. Fluorescent staining was visualized using a fluorescence microscope. We additionally used the anti-HO-1 antibody (1:200) to test whether HO-1 was really translocated into the rat BAs, visualized with a fluorescent Cy3-conjugated secondary antibody (1:100, Jackson ImmunoResearch Laboratories Inc.).
Effect of Protein Transduction on Basilar Artery Diameter In Vivo
The baseline diameter of the BAs in normal rats was examined 6 hours after a transcisternal injection of 11R-HO-1 (200 μL, 0.15 mg/mL), 11R-EGFP (200 μL, 0.15 mg/mL), or saline (7 rats per group).
Heme-Oxygenase-1 Activity
Heme-oxygenase-1, which comprises 288 amino acids with a molecular mass of 32,800 Da is an enzyme that is involved in heme catabolism as it cleaves heme to form biliverdin and carbon monoxide (CO) (Yoshida et al, 1988). The CO relaxes blood vessels by activating soluble guanylyl cyclase and increasing intracellular levels of cyclic guanosine-3′,5-monophosphate (cGMP). Accordingly, we examined the amount of cGMP in the cerebral arteries as an indicator of HO-1 activity with the previously confirmed method by using a rat single-hemorrhage model (Shimada et al, 2009). On day 0, 200 μL of cerebrospinal fluid was aspirated and then 250 μm of autologous arterial blood was injected into the cisterna magna. On day 2, 11R-EGFP or 11R-HO-1 (200 μL, 0.15 mg/mL) was injected into the cistern magna over 5 minutes. The BAs were then extracted from rats in 6 hours, washed three times with PBS, homogenized, and assayed with cGMP enzyme immunoassay kits (Assay Designs/Stressgen, Butler, PA, USA) (7 rats per group). We additionally examined the activity for an extended period of time (5 or 7 days). For the 5-day examination period, the 11R-HO-1 protein was injected on day 0 and autologous blood was injected on day 3. The activity in the rat BAs was then examined on day 5. For the 7-day period, the 11R-HO-1 protein was injected on day 0 and autologous blood on day 5. The activity in the rat BAs was then examined on day 7.
The Rat Double-Hemorrhage Model
We assessed whether 11R-HO-1 protein transduction prevents vasospasm in vivo in a rat double-hemorrhage model of SAH using established methods (Gules et al, 2002). On day 0, animals were intraperitoneally anesthetized with a mixture of ketamine (Pfizer Pharma GmbH) and xylazine (Bayer Health Care) (100 and 5 mg/kg body weight, respectively) and allowed to breathe spontaneously, after which 200 μL of cerebrospinal fluid was aspirated and then 250 μL of autologous blood was injected into the cisterna magna over a 5-minute period. Rats were anesthetized again 2 days later (day 2) and the same procedure was repeated. These rats were then randomly assigned into four groups and HO-1, 11R, 11R-EGFP, 11R-HO-1 (200 μL, 0.15 mg/mL), or saline (considered the untreated SAH control) was injected into the cisterna magna of rats over a period of 5 minutes at different time points. The first group, group 1, was injected 48 hours before killing. Proteins were injected into the cisterna magna of rats on day 5 and rats were killed with a pentobarbital (Sanofi-Aventis, Frankfurt, Germany) overdose on day 7. Group 2 was injected 24 hours before killing. Proteins were injected on day 6 and rats were killed on day 7. Group 3 was injected 8 hours before killing. Proteins were injected on day 7 and rats were killed 8 hours after the protein injection. Finally, group 4 was injected 6 hours before killing. Proteins were injected on day 7 and rats were killed 6 hours after the protein injection.
To examine the long-term effectiveness of 11R-HO-1, we tested two more groups. Group 5 was injected 7 days before killing. Autologous blood (250 μL) and 11R-HO-1 protein (200 μL; 0.15 mg/mL) were injected into the cisterna magna of rats on day 0. The same volume of autologous blood was again injected on day 2. Rats were then killed on day 7. The second long-term treatment group, group 6, was injected 5 days before killing. Autologous blood was injected on day 0. A second injection of autologous blood and the 11R-HO-1 protein were injected on day 2. Rats were then killed on day 7. To examine the dose-response data for the 11R-HO-1 protein, we injected several concentrations (5 × 10−2, 1.5 × 10−2, 1.5 × 10−3, 1.5 × 10−4 mg/mL) of the 11R-HO-1 protein into the group of rats injected 6 hours before killing.
Therapeutic Effect of Proteins in the Rat Subarachnoid Hemorrhage Model
As we described above, all animals were killed on day 7 and were transcardially perfused with 100 mL of PBS at physiologic blood pressure. Frozen sections of BAs and brain stem were cut into 10-μm-thick sections using a cryostat. The group effect was assessed by measurement of the BAs. Cross-sections of the BAs were obtained for measurement at three locations: (1) 200 μm above the union of the vertebral arteries, (2) immediately below the anterior inferior cerebellar arteries, and (3) 200 μm below the BA bifurcation. The mean of the three points was taken as the diameter of the BA. At the same time, 11R-HO-1 transduction efficiency was assessed by immunofluorescence staining in the SAH model (seven rats per group). We used anti-HO-1 antibody (1:200, Sigma-Aldrich) as a primary antibody and a fluorescent Cy3-conjugated secondary antibody (1:100, Jackson ImmunoResearch Laboratories Inc.) for visualization.
Transduction of the Protein into the Brain for Longer Time Periods
We also examined protein transduction into the brain over a longer period of time. The brains were extracted from group 5 or 6 rats after transcardial perfusion with PBS. The same measurements were made in these rats as those described above for the BAs. To examine the pure transduction efficacy, anti-histidine antibody was used.
Statistical Analysis
Data are shown as means (±s.d.). Data were analyzed using one- or two-way ANOVA (analysis of variance), followed by planned comparisons of multiple conditions. When the P-value was <0.05, differences were considered statistically significant.
Results
Cell Viability Assay (MTT Assay)
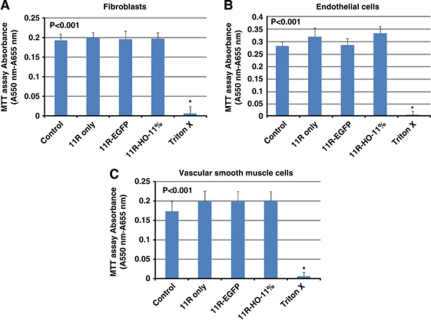
Before beginning the animal experiment in vivo, a cell viability assay (MTT assay) was performed in vitro. The results showed that the addition of 11R, 11R-EGFP, or 11R-HO-1 protein did not inhibit the cell growth of human fibroblasts, human coronary artery endothelial cells, or human coronary artery smooth muscle cells and revealed that the proteins had low cytotoxicity (n=102, each) (Figure 2). Cytotoxicity measures for each protein 11R, 11R-EGFP, 11R-HO-1, or positive control such as 1% Triton X versus control were assessed for human fibroblasts (104%±7.3%, 102%±10.4%, 102%±7.6%, 3%±9.9% versus 100%±8.3%) versus human endothelial cells (113%±12.6%, 101%±9.2%, 118%±10.3%, 1.2%±5.0% versus 100%±6.2%) and human smooth muscle cells (114%±15.3%, 115%±14.3%, 115%±13.8%, 3.7%±6.1% versus 100%±14.8%).
Figure 2.
Cytotoxicity of the proteins measured by MTT assay. Human fibroblasts (A), human endothelial cells (B), or human smooth muscle cells (C) were incubated with 11R, 11R-EGFP, 11R-HO-1, or 1% Triton X. Graph shows that 11R, 11R-EGFP, and 11R-HO-1 did not affect cell growth and have little toxicity in vitro. Control; intact cells. 11R-EGFP, 11R-fused enhanced green fluorescent protein; HO-1, heme-oxygenase-1.
Transduction of 11R-HO-1 into the Cerebral Arteries In Vivo
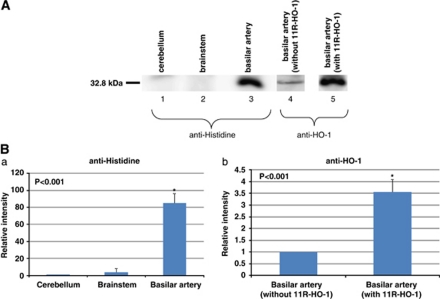
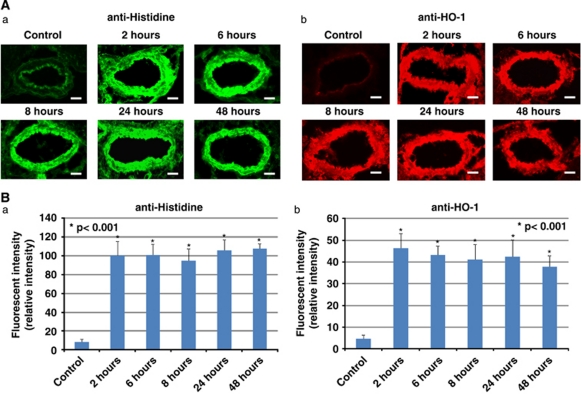
To examine the transduction efficacy of the 11R-HO-1 protein, western blotting analysis of rat BAs was performed (n=7, each). The anti-histidine antibody detected the 11R-HO-1 protein only in the rat BAs, indicating its selective entry into the cerebral arteries after transcisternal injection (BA (84.87±11.59), brain stem (3.71±4.76) versus cerebellum, relative ratio; P<0.001). The HO-1 labeling in the BAs significantly differed between the groups injected with saline (control) and 11R-HO-1, although BAs after saline injection also expressed some endogenous HO-1 protein (11R-HO-1 (3.56±0.54) versus control, relative ratio; P<0.001) (Figure 3). To examine the localization of the protein in the walls of the rat BAs, immunofluorescence staining with anti-histidine or anti-HO-1 antibody was also performed. The staining results showed that the 11R-HO-1 protein reached a high steady-state level in all layers of the BAs within 2 hours that persisted for >48 hours (n=7) (Figure 4).
Figure 3.
Localization of the 11R-HO-1 protein 6 hours after intracisternal injection in rats. The localization was examined by western blotting with anti-histidine antibody and anti-HO-1 antibody. Representative immunoblots are shown in panel A. Basilar artery with 11R-HO-1 for anti-histidine antibody (lane 3), without (lane 4) or with 11R-HO-1 (lane 5) for anti-HO-1 antibody. Signals were analyzed using Scion image (B(a): relative to cerebellum and B(b): relative to control (basilar artery without HO-1)). HO-1, heme-oxygenase-1.
Figure 4.
Time-dependent changes in the level of transduced 11R-HO-1 in BAs in vivo. Several hours (2, 6, 8, 24, and 48 hours) after protein injection, rat BAs were immunostained and observed under a fluorescent microscope (A). Fluorescence intensity was also analyzed using Scion image (B). Exposure time was identical within each experiment. Bars, 50 μm. BA, basilar artery; HO-1, heme-oxygenase-1.
Effect of Injected Proteins on Basilar Artery Diameter
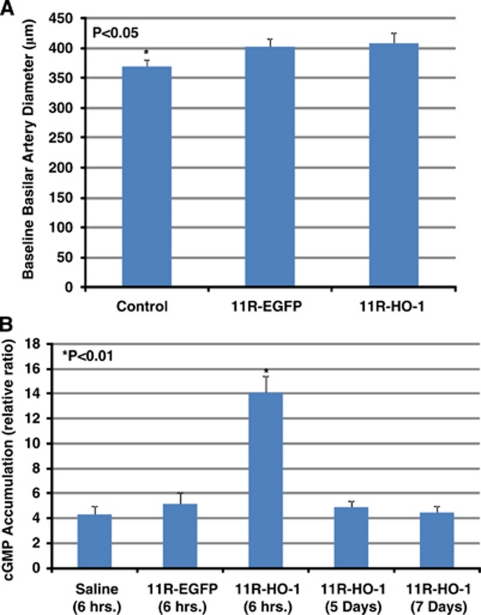
The diameter of the BA was examined 6 hours after intracisternal injection of 11R-HO-1 (n=7), 11R-EGFP (n=7), or saline (n=7). Physiological parameters were maintained within a normal range. The diameter of the BA was significantly larger in the 11R-HO-1 and 11R-EGFP groups than in the control group (408.91±17.74 μm and 402.67±13.81 μm versus 368.53±11.72 μm; P<0.01), although the diameter did not significantly differ between the 11R-HO-1 and 11R-EGFP groups (Figure 5A).
Figure 5.
Rat BA diameter 6 hours after injecting saline, 11R-EGFP, or 11R-HO-1. This graph shows significantly increased diameter after injection of either protein (11R-EGFP or 11R-HO-1) compared with saline, although 11R-HO-1 and 11R-EGFP groups do not differ significantly (A). Levels of cGMP in BAs in SAH were examined 6 hours after protein induction. BAs with 11R-HO-1 have higher cGMP levels than control and 11R-EGFP groups. However, the cGMP accumulations 5 or 7 days after protein transduction were decreased. All experiments were performed three times (B). BA, basilar artery; cGMP, cyclic guanosine 3′, 5′-cyclic monophosphate; 11R-EGFP, 11R-fused enhanced green fluorescent protein; HO-1, heme-oxygenase-1; SAH, subarachnoid hemorrhage.
Activity of 11R-HO-1 in Cerebral Arteries
To determine whether the 11R-HO-1 protein, which is transduced in cerebral arteries, has an active effect, we examined cGMP accumulation. The results of the cGMP enzyme immunoassay showed significantly increased accumulation in the group injected with 11R-HO-1 than with either saline (control) or 11R-EGFP (14.07±1.34 versus 4.29±0.65 or 5.15±0.92; 7 rats per group). However, the activity of 11R-HO-1 was decreased over longer time periods (5 days (4.87±0.50) and 7 days (4.48±0.53)) (Figure 5B).
Therapeutic Effect of the 11R-HO-1 Protein in the Rat Subarachnoid Hemorrhage Model
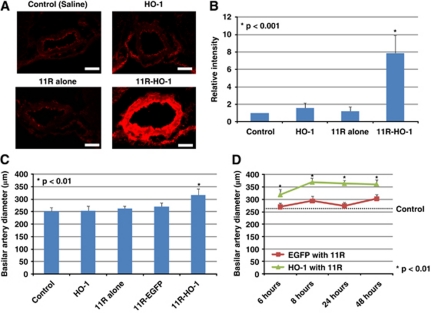
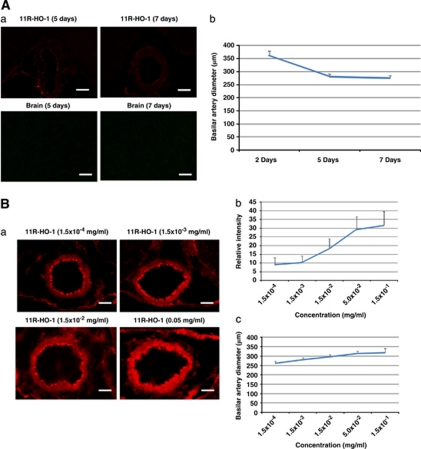
To investigate the transduction efficacy of the protein in the SAH model, immunofluorescence staining of BAs and brain stems in the rat double-hemorrhage model was performed. Physiological parameters were maintained within a normal range. The expression of HO-1 in BAs was also enhanced by 11R-HO-1 compared with saline (control), 11R, or HO-1 injection without 11R in the rat SAH model (11R-HO-1 versus controls, 11R, HO-1; 7.87±2.04 versus 1.00, 1.20±0.51, 1.60±0.54, P<0.001; 7 rats per group) (Figures 6A and 6B). The overexpression of active HO-1 in the rat cerebral arteries significantly diminished CV after SAH (Figure 6C). The diameter of the BA on day 7 was significantly larger 6 hours after injecting 11R-HO-1 than HO-1, 11R, 11R-EGFP, or saline (11R-HO-1 versus HO-1, 11R, 11R-EGFP, saline; 317.59±23.48 μm versus 254.07±18.81 μm, 262.45±9.05 μm, 270.08±14.66 μm, 252.05±13.95 μm, P<0.01; 7 rats per group). The BA diameters on day 7 were also increased at other time points (8, 24, and 48 hours after injecting 11R-HO-1 compared with 11R-EGFP (Figure 6D) (11R-HO-1 versus 11R-EGFP); 6 hours (317.59±23.48 μm versus 270.08±14.66 μm), 8 hours (368.86±12.90 μm versus 293.74±18.22 μm), 24 hours (363.28±13.68 μm versus 237.74±15.08 μm), 48 hours (360.72±18.40 μm versus 302.29±16.81 μm); 7 rats per group). However, at the long time periods, the expression of 11R-HO-1 was diminished and effectiveness reduced (11R-HO-1; 5 days (280.98±9.98 μm), 7 days (275.86±9.42 μm)). Protein transduction was also not found in the brains (Figure 7A). The expression and the effect of 11R-HO-1 were increased according to the concentration of the protein (Figure 7B) (1.5 × 10−4 mg/mL (8.96±4.10 μm, 261.62±11.43 μm), 1.5 × 10−3 mg/mL (10.31±3.78 μm, 280.88±11.45 μm), 1.5 × 10−2 mg/mL (18.06±5.93 μm, 296.04±10.8 μm), 5.0 × 10−2 mg/mL (29.23±7.33 μm, 313.61±10.77 μm)).
Figure 6.
Expression of the HO-1 protein in rat double-hemorrhage SAH model 6 hours after protein injection (A). Fluorescent images were obtained by fluorescence microscopy and relative intensity was analyzed using Scion image (relative to control) (B). Length of exposure was identical in each experiment. The diameter of BA after intracisternal protein injections 7 days after SAH in the double-hemorrhage model. Saline, HO-1 without 11R, 11R, 11R-EGFP, or 11R-HO-1 was injected into the cisterna magna of SAH rats at 6 hours (panel B, C) and at 6, 8, 24, and 48 hours (D) before measuring BA diameter. Bars, 50 μm. BA, basilar artery; 11R-EGFP, 11R-fused enhanced green fluorescent protein; HO-1, heme-oxygenase-1; SAH, subarachnoid hemorrhage.
Figure 7.
The expression and therapeutic effect of 11R-HO-1 protein over an extended time period. The expression was diminished and effectiveness decreased 5 or 7 days after the 11R-HO-1 protein injection. The protein was also not transduced in the brains (A). The dose–response relationships of 11R-HO-1 protein in the rat BAs. The expression and effectiveness were increased according to the concentration of the protein (B). BA, basilar artery.
Discussion
This study showed that (1) the 11R-fused HO-1 protein was transduced directly and immediately into all layers of the rat BAs; (2) the 11R-fused HO-1 protein was transduced selectively into the rat BAs by transcisternal injection; (3) both 11R-fused proteins (11R-EGFP, 11R-HO-1) increased rat BA diameters in normal rats; (4) the 11R-HO-1 protein injection increased cGMP accumulation (HO-1 activity) in the rat BAs of the SAH model; (5) 11R-HO-1 protein transduction resulted in attenuation of BA vasospasm in the rat double-hemorrhage model on day 7; and (6) the expression and the therapeutic effect of the 11R-HO-1 protein increased with increasing concentrations.
The protein transduction method has some advantages over viral vector-mediated gene therapy in terms of safety and transduction efficacy, as described in the ‘Introduction' section. With regard to viral-mediated HO-1 gene transfer, e.g., to show a positive effect on CV, CO gas produced by HO-1 must undergo a process of diffusion from the adventitia to the smooth muscle layer where it mediates vascular relaxation, because the gene can only reach the adventitia of cerebral vessels (Ono et al, 2002). Thus, the 11R protein transduction method might be more effective, because proteins can be directly transduced into the smooth muscle layer. Moreover, we also examined the cytotoxicity of 11R-fused EGFP or HO-1 protein in vitro and showed the absence of cytotoxic effects on normal cells (Figure 2), whereas adenovirus-mediated gene therapy is known to inhibit cell growth significantly (Michiue et al, 2005).
We used 11R-PTD in the present experiment, because 11R is a proven PTD. This 11R-PTD was developed from the transcription activator (TAT) protein of human immunodeficiency virus, which contains six arginine and two lysine residues (Matsushita et al, 2001). On the basis of this amino-acid sequence of TAT-PTD and other PTDs, it has been hypothesized that arginine is the most important factor for membrane penetration, and several lengths of peptides comprising only arginine were constructed (Matsushita et al, 2001; Matsui et al, 2003). Among these, the transduction ability of 11R was the most effective and even more powerful than that of the original TAT in cultured cells (Matsushita et al, 2001), suggesting that 11R-PTD is one of the most promising tools for protein transduction.
The mechanism of plasma membrane transduction by PTD is still controversial. A recent study of TAT has suggested that transduction is mediated by endocytosis through heparan sulfate proteoglycan receptors (Liu et al, 2000). In contrast, the oligopeptide penetratin derived from the homeodomain of Antennapedia has been shown to translocate across pure lipid bilayers (Thoren et al, 2000). Matsushita et al (2001) recently showed that the 11R domain requires differentiation by nerve growth factor for plasma membrane transduction in PC12 cells. These results support the idea that the uptake mechanism involves receptor- or transporter-dependent pathways. Therefore, they concluded that the expression level of receptors for 11R on the cell membrane is a critical factor for cell type specificity of protein transduction.
The 11R protein transduction method seems particularly well suited for the characteristic phenomenon of CV after SAH. The 11R-PTD has already been studied mainly in the area of cancer therapy research, yielding positive results (Michiue et al, 2005; Inoue et al, 2006; Takenobu et al, 2002). Unlike the chronic pathology of cancers, however, the phenomenon of CV after SAH is acute and transient (Mayberg et al, 1994). In this study, we showed that the therapeutic protein 11R-HO-1 could be imported immediately into cerebral arteries (Figure 4). Therefore, this immediate penetration into cerebral arteries renders 11R-PTD potentially suitable for salvage therapy in manifest CV.
We have shown that injection of both 11R-EGFP and 11R-HO-1 increased BA diameter in normal rats in the present experiment. This may be associated with the vasodilatory effect of polyarginine (11R) itself (Figure 5A). Poly--arginine produces nitric oxide (NO) by activation of -arginine metabolism (Kinoshita and Katusic, 1997). This NO activates guanylate cyclase to increase smooth muscle levels of cGMP, causing smooth muscle relaxation (Ignarro, 1990).
However, in the SAH model, 11R-EGFP injection did not significantly increase the cGMP accumulation in BAs. The most probable explanation for this discrepancy in cGMP increase is because the effect of 11R could not sufficiently compensate for the reduction of NO, which is caused by scavenging of NO by hemoglobin in SAH.
Injection of 11R-HO-1 significantly increased cGMP accumulation in SAH (Figure 5B), revealing the therapeutic effect in the rat SAH model. Some possible mechanisms for the more powerful effectiveness of the 11R-HO-1 protein are (1) the CO produced by HO-1 increases cGMP levels directly as described in the ‘Materials and methods' section; (2) the CO could bind to hemoglobin, preventing hemoglobin from scavenging NO; and (3) the HO-1 metabolizes and reduces hemoglobin, which has itself contractile effects on cerebral arteries.
In this way, we confirmed that 11R-fused HO-1 exerts enzymatic HO-1 activity in cerebral arteries, showing that the protein retained the promising activity in cerebral arteries in this study. Previous studies with the TAT protein transduction system have also shown that after delivery of β-galactosidase or anti-apoptotic Bcl-xL protein into several organs, cultured cells remained physiologically active (Nagahara et al, 1998; Asoh et al, 2002). Other research on 11R-PTD proved that 11R-fused p53 and cAMP-dependent protein kinase A inhibitory peptide preserve their biologic activity in the cells (Matsushita et al, 2001; Michiue et al, 2005; Inoue et al, 2006).
Previous studies have shown that HO-1 assumes a crucial function and acts against CV in SAH. Levels of HO-1 mRNA in the BAs correlate with the degree of vasospasm and HO-1 is prominently involved in this pathology. To identify this, antisense HO-1 oligodeoxynucleotide was injected into the cisterna magna of rats where it significantly aggravated delayed CV after SAH (Suzuki et al, 1999). In addition, HO-1 overexpression inhibits arterial contractions induced by hemoglobin and reduced vasospasm after experimental SAH (Ono et al, 2002). More recently, it has been shown that intravenous administration of nicaraven, which is a synergistic enhancer of HO-1 induction, ameliorated CV in the rat SAH model (Shimada et al, 2009). In contrast, other studies have indicated that the level of HO-1 only reflects the intensity of oxidative stress (Clark et al, 2002), and HO-1 may even aggravate brain injury after intracerebral hemorrhage (Wang and Dore, 2007). In this study, 11R-HO-1 significantly dilated BA diameter compared with 11R-EGFP in the SAH model. This finding is attributable to the vasodilating action of HO-1 protein in cerebral arteries and supports the concept that HO-1 still has an important role and is beneficial for the treatment of CV in SAH.
The limitations and future perspectives of this study are: (1) because the duration of the expression and the therapeutic effect of the 11R-fused protein is relatively short, repeated or continuous administration of the protein may be necessary; (2) with intrathecal drug delivery, it is difficult to administer to a precise target location in the brain with pinpoint accuracy, meaning a high concentration of 11R-fused therapeutic protein may have to be given in the clinical setting; and (3) there were no large differences in neurologic findings between the experimental groups in this study. In the future, however, this should be investigated further and more clinically relevant data should be obtained for larger animals.
In conclusion, this is the first demonstration of the transduction and functional expression of a PTD-fused vasoactive protein in the cerebral arteries of an animal model of SAH. We delivered 11R-HO-1 into the BAs through transcisternal application and found high HO activity, which resulted in the prevention of CV. These results suggest that 11R-HO-1 protein transduction into the cerebral arteries will provide a promising therapeutic approach for the treatment of cerebrovascular diseases including CV after SAH.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported in part by the Alexander von Humboldt Foundation, Germany.
Supplementary Material
References
- Adams HP, Jr, Kassell NF, Torner JC, Haley EC., Jr Predicting cerebral ischemia after aneurysmal subarachnoid hemorrhage: influences of clinical condition, CT results, and antifibrinolytic therapy. A report of the Cooperative Aneurysm Study. Neurology. 1987;37:1586–1591. doi: 10.1212/wnl.37.10.1586. [DOI] [PubMed] [Google Scholar]
- Asoh S, Ohsawa I, Mori T, Katsura K, Hiraide T, Katayama Y, Kimura M, Ozaki D, Yamagata K, Ohta S. Protection against ischemic brain injury by protein therapeutics. Proc Natl Acad Sci USA. 2002;99:17107–17112. doi: 10.1073/pnas.262460299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaley K, Webb RC. Microtubule depolymerization facilitates contraction of rat aorta via activation of Rho-kinase. Vascul Pharmacol. 2002;38:157–161. doi: 10.1016/s1537-1891(02)00163-5. [DOI] [PubMed] [Google Scholar]
- Clark JF, Reilly M, Sharp FR. Oxidation of bilirubin produces compounds that cause prolonged vasospasm of rat cerebral vessels: a contributor to subarachnoid hemorrhage-induced vasospasm. J Cereb Blood Flow Metab. 2002;22:472–478. doi: 10.1097/00004647-200204000-00011. [DOI] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Brandt L, Andersson KE, Bengtsson B. Effect of a calcium antagonist on experimental constriction of human brain vessels. Surg Neurol. 1979;11:327–330. [PubMed] [Google Scholar]
- Gules I, Satoh M, Clower BR, Nanda A, Zhang JH. Comparison of three rat models of cerebral vasospasm. Am J Physiol Heart Circ Physiol. 2002;283:H2551–H2559. doi: 10.1152/ajpheart.00616.2002. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Inoue M, Tomizawa K, Matsushita M, Lu YF, Yokoyama T, Yanai H, Takashima A, Kumon H, Matsui H. p53 protein transduction therapy: successful targeting and inhibition of the growth of the bladder cancer cells. Eur Urol. 2006;49:161–168. doi: 10.1016/j.eururo.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Katusic ZS. Nitric oxide and effects of cationic polypeptides in canine cerebral arteries. J Cereb Blood Flow Metab. 1997;17:470–480. doi: 10.1097/00004647-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Marton LS, Wang X, Kowalczuk A, Zhang ZD, Windmeyer E, Macdonald RL. Effects of hemoglobin on heme oxygenase gene expression and viability of cultured smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;279:H2405–H2413. doi: 10.1152/ajpheart.2000.279.5.H2405. [DOI] [PubMed] [Google Scholar]
- Matsui H, Tomizawa K, Lu YF, Matsushita M. Protein therapy: in vivo protein transduction by polyarginine (11R) PTD and subcellular targeting delivery. Curr Protein Pept Sci. 2003;4:151–157. doi: 10.2174/1389203033487270. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Tomizawa K, Moriwaki A, Li ST, Terada H, Matsui H. A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation. J Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, Sternau LL, Torner J, Adams HP, Jr, Feinberg W, Thies W. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25:2315–2328. doi: 10.1161/01.str.25.11.2315. [DOI] [PubMed] [Google Scholar]
- Michiue H, Tomizawa K, Wei FY, Matsushita M, Lu YF, Ichikawa T, Tamiya T, Date I, Matsui H. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction. J Biol Chem. 2005;280:8285–8289. doi: 10.1074/jbc.M412430200. [DOI] [PubMed] [Google Scholar]
- Miller J, Diringer M. Management of aneurysmal subarachnoid hemorrhage. Neurol Clin. 1995;13:451–478. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ono S, Ichikawa T, Arimitsu S, Onoda K, Tokunaga K, Sugiu K, Tomizawa K, Matsui H, Date I. Novel protein transduction method by using 11R: an effective new drug delivery system for the treatment of cerebrovascular diseases. Stroke. 2007;38:1354–1361. doi: 10.1161/01.STR.0000259887.70358.e0. [DOI] [PubMed] [Google Scholar]
- Ono S, Komuro T, Macdonald RL. Heme oxygenase-1 gene therapy for prevention of vasospasm in rats. J Neurosurg. 2002;96:1094–1102. doi: 10.3171/jns.2002.96.6.1094. [DOI] [PubMed] [Google Scholar]
- Ono S, Zhang ZD, Marton LS, Yamini B, Windmeyer E, Johns L, Kowalczuk A, Lin G, Macdonald RL. Heme oxygenase-1 and ferritin are increased in cerebral arteries after subarachnoid hemorrhage in monkeys. J Cereb Blood Flow Metab. 2000;20:1066–1076. doi: 10.1097/00004647-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Raabe A, Zimmermann M, Setzer M, Vatter H, Berkefeld J, Seifert V.2002Effect of intraventricular sodium nitroprusside on cerebral hemodynamics and oxygenation in poor-grade aneurysm patients with severe, medically refractory vasospasm Neurosurgery 501006–1013.discussion 1013-1004 [DOI] [PubMed] [Google Scholar]
- Scheld WM. Drug delivery to the central nervous system: general principles and relevance to therapy for infections of the central nervous system. Rev Infect Dis. 1989;11 (Suppl 7:S1669–S1690. doi: 10.1093/clinids/11.supplement_7.s1669. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Tsunoda H, Zang L, Hirano M, Oka T, Tanaka T. Synergistic induction of heme oxygenase-1 by nicaraven after subarachnoid hemorrhage to prevent delayed cerebral vasospasm. Eur J Pharmacol. 2009;620:16–20. doi: 10.1016/j.ejphar.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Solenski NJ, Haley EC, Jr, Kassell NF, Kongable G, Germanson T, Truskowski L, Torner JC. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995;23:1007–1017. doi: 10.1097/00003246-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kanamaru K, Tsunoda H, Inada H, Kuroki M, Sun H, Waga S, Tanaka T. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest. 1999;104:59–66. doi: 10.1172/JCI5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986;93:157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Takenobu T, Tomizawa K, Matsushita M, Li ST, Moriwaki A, Lu YF, Matsui H. Development of p53 protein transduction therapy using membrane-permeable peptides and the application to oral cancer cells. Mol Cancer Ther. 2002;1:1043–1049. [PubMed] [Google Scholar]
- Thomas JE, Rosenwasser RH, Armonda RA, Harrop J, Mitchell W, Galaria I. Safety of intrathecal sodium nitroprusside for the treatment and prevention of refractory cerebral vasospasm and ischemia in humans. Stroke. 1999;30:1409–1416. doi: 10.1161/01.str.30.7.1409. [DOI] [PubMed] [Google Scholar]
- Thoren PE, Persson D, Karlsson M, Norden B. The antennapedia peptide penetratin translocates across lipid bilayers—the first direct observation. FEBS Lett. 2000;482:265–268. doi: 10.1016/s0014-5793(00)02072-x. [DOI] [PubMed] [Google Scholar]
- Toyoda K, Chu Y, Heistad DD. Gene therapy for cerebral vascular disease: update 2003. Br J Pharmacol. 2003;139:1–9. doi: 10.1038/sj.bjp.0705217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatter H, Zimmermann M, Seifert V, Schilling L. Experimental approaches to evaluate endothelin-A receptor antagonists. Methods Find Exp Clin Pharmacol. 2004;26:277–286. [PubMed] [Google Scholar]
- Verma IM, Somia N. Gene therapy promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Biro P, Cohen T, Muller RM, Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988;171:457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.