Abstract
Chemokines are chemotactic cytokines that play an important role in cell migration and are thought to play an important role in a broad range of inflammatory diseases. The availability of chemokine receptor blockers makes them an important therapeutic target. In vitro, chemokines are shown to modulate neurotransmission. However, it is not very clear if chemokines play a role in behavior and cognition. Here we evaluated the role of CC chemokine receptor 5 (CCR5), in various behavioral tasks in mice using Wt (Ccr5+/+) and Ccr5-null (Ccr5−/−) mice. Ccr5−/− mice showed enhanced social recognition. Administration of CC chemokine ligand 3 (CCL3), one of the CCR5-ligands, impaired social recognition. Since the social recognition task is dependent on the sense of olfaction, we tested olfactory recognition for social and non-social scents in these mice. Ccr5−/− mice had enhanced olfactory recognition for both these scents indicating that enhanced performance in social recognition task could be due to enhanced olfactory recognition in these mice. Spatial memory and aversive memory were comparable in Wt and Ccr5−/− mice. Collectively, these results suggest that chemokines/chemokine receptors might play an important role in olfactory recognition tasks in mice and to our knowledge represents the first direct demonstration of an in vivo role of CCR5 in modulating social behavior in mice. These studies are important as CCR5 blockers are undergoing clinical trials and can potentially modulate behavior.
Keywords: chemokine, CC-chemokine receptor 5, CC-chemokine ligand 3, behavior, social recognition, N-Methyl D-Aspartate Receptor 1
Introduction
There is growing evidence for an important role of chemokine system in mediating homeostatic neurologic functions (Adler and Rogers, 2005, Miller et al., 2008). It has been hypothesized that by interacting with members of the neurotransmitter and neuropeptide systems, the chemokine system that is endogenous to the brain might play an important role in governing its function (Adler and Rogers, 2005, Miller et al., 2008). This postulate is based on two major findings. First, akin to neurotransmitters, the distribution of certain chemokines and their cognate receptors in the brain is non-random (Banisadr et al., 2005, Magdaleno et al., 2006). Second, chemokines have the ability to cross-desensitize other receptors in the nervous system such as the opioid receptors as well as transactivate transient receptor potential (TRP) channels and modulate pain in vivo (Szabo et al., 2002, Jung et al., 2008). Further supporting a role of the chemokine system in normal brain function, in vitro studies demonstrate that chemokines, including CC chemokine receptor 5 (CCR5) and its ligands, can influence neurotransmitter release, ion channel gating and long term potentiation (Meucci et al., 1998, Oh et al., 2002, Dong and Xiong, 2006) and the literature showing role of chemokines in modulating neuroendocrine function is growing (Rostène et al., 2011).
Emerging evidence also suggests that chemokine receptors and chemokines might modulate cognition and behavior in mice. A study by Parachikova et al. found that mice treated with an antagonist of the CXC chemokine receptor 4 (CXCR4) had impaired novel object and place recognition as well as impaired performance on T-maze (Parachikova and Cotman, 2007). Intra-nigral administration of CXC chemokine ligand 12 (CXCL12), a ligand for CXCR4 induced circling behavior. This behavior was blocked by CXCR4 antagonist AMD 3100 similar to the effect of dopamine release (Skrzydelski et al., 2007). Dopamine plays important role in modulating behavior. We have previously shown CC-chemokine CCL3, a CCR5 ligand, is constitutively expressed by the dopaminergic neurons in substantia nigra (Kalkonde et al., 2007). However, the role of CCR5 in modulating behavior is not very clear. Further investigation of such a role for CCR5 is important as CCR5 blockers are undergoing clinical trials in patients with HIV infection (Pett et al., 2009) and can potentially modulate behavior. Additionally, approximately 1% individuals of Caucasian descent have a 32 base-pair deletion (Δ32) in CCR5 which results in a truncated protein. This truncated protein is not expressed on the cell surface creating a null phenotype (Liu et al., 1996). Thus the carriers of this mutation in CCR5 may have a distinct neurobehavioral phenotype which is hitherto unknown.
Based on data demonstrating that CCR5 is expressed in the nervous system (Banisadr et al., 2005, Magdaleno et al., 2006) and the link between CCR5 ligands and neurotransmission, we tested the hypothesis that CCR5 and CCL3, a CCR5 agonist, will influence behavior and cognitive processes. To test this hypothesis, we determined whether mice genetically inactivated for CCR5 have alterations in behavioral and cognitive tasks such as social recognition, spatial memory and fear conditioning. As a corollary, we determined whether systemic administration CCL3 affects the social recognition task in wild-type mice.
Experimental Procedures
Reagents
Crystal violet was obtained from Sigma Chemicals Co. (St. Louis, Missouri, USA). Anti -N-methyl D-aspartate receptor 1(NMDAR-1) antibody was from Chemicon International (Temecula, CA, USA). Anti- cAMP response element-binding (CREB) and anti-phosphorylated-CREB (pCREB) antibodies were from Upstate (Lake Placid, NY, USA). Secondary antibodies were from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA). Recombinant murine chemokines were from PeproTech, Inc. (Rocky Hill, NJ, USA)
Animals
Experiments were with Ccr5+/+(Wt) and Ccr5−/− mice on the DBA1/J background that were generated as described previously (Quinones et al., 2004), and Ccr5+/+(Wt) mice on the C57BL6/J background (Jackson Laboratories, Bar Harbor, Maine, USA) ). There were no overt differences in the cortical or hippocampal morphology and neuronal density between the Wt and Ccr5−/− mice determined using Nissl, MAP-2 and synaptophysin staining (data not shown). Only male mice were used for experiments and they were housed in a pathogen free environment with free access to food and water in a climate controlled room with a 14/10 light/dark cycle. All experiments were performed in compliance with the rules of Institutional Animal Care and Use Committee at the University of Texas Health Science Center, San Antonio, TX.
Social recognition
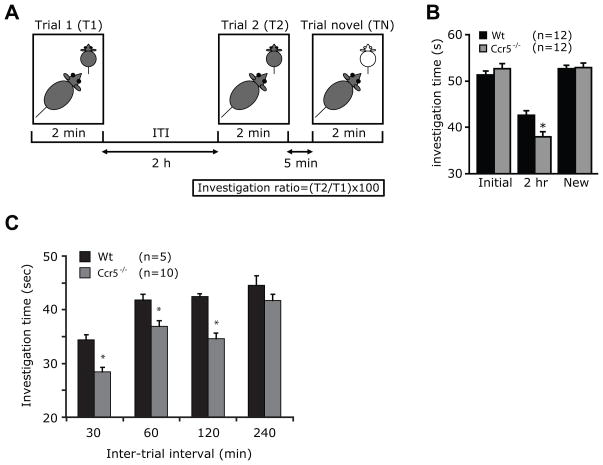
We tested social recognition in Wt and Ccr5−/− mice on the DBA1/J background (test mice) using previously described method (Nicot et al., 2004, Ohno et al., 2004). The overall experimental schema is shown in Fig. 1A. Briefly, each knock-out or wild-type littermate mouse to be tested was placed individually in a clean acrylic cage (similar to the cage used for housing and were 27 cm long × 16 cm wide × 12 cm high) in a quiet room and were allowed to acclimatize to the cage for 30 minutes. The target for the test mice was a male juvenile C57BL6/J mouse (18–21 days old). The target mouse was placed in a cage with the test mouse for an initial trial period of 2 minutes and the time spent investigating the mouse (t1) during the first trial (T1) was recorded using a digital timer by an observer who was blinded to the genotype of the mice. The juvenile mouse was then placed back into its cage. Following a 2 hour inter-trial interval (ITI), the same target juvenile mouse was placed back into the test mouse’s cage for a second trial (T2) for another 2 minutes and the time spent (t2) by the test mouse in investigating the target mouse was recorded. Thus, t1 and t2 indicate the length of time spent on social investigation behavior. This included direct contact with the juvenile target mouse such as when inspecting any part of the body surface (grooming, licking, and pawing), sniffing of the mouth, ears, tail, and ano-genital area, and closely following (within <1 cm) the juvenile mouse. We used juvenile male mice in these experiments as they provided a relatively neutral stimulus and tend to provoke minimal amounts of fighting and sexual behavior from the adult test mice. To test for specificity, after the second trial we determined the length of time the test mouse took to investigate a new juvenile mouse. This trial with a novel mouse was designated as a TN (Fig. 1A).
Figure 1.
Ccr5−/− mice have enhanced social recognition. (A) Experimental design to assess social recognition. Social recognition was tested by assessing the time spent (t1) by the test mouse (Wt or Ccr5−/−) investigating a juvenile male mouse (target mouse) during initial trial (T1) and a second trial (T2) after an inter-trial interval (ITI) of 2 hours (t2). Five minutes after T2, a novel target mouse was placed in the cage and investigation time was recorded again (tn, time for novel trial). (B) Social recognition is enhanced in Ccr5−/− mice as evident by reduced investigation time of target mouse. Effect of variation in inter-trial interval on (C) investigation time with variable ITI. Data are represented as mean ± SEM. *P < 0.05 for differences between Wt and Ccr5−/− mice (unpaired 2-sided t-test).
In some experiments, we changed the variables of this schema by varying the ITI between T1 and T2, using intervals of 30, 60, 120 and 240 minutes. Different batches of juvenile mice were used at each time point to limit habituation due to repeated exposure to the same test mouse.
To test the effect of the CCR5 ligand CCL3/MIP-1α on the retention of social recognition, after T1, mice were transiently anesthetized with isoflurane and 1μg of CCL3 in 50μl of PBS was administered intranasally as described (Lillard et al., 2003). As controls for these experiments, we also intranasally administered 50μl of CCL2/MCP-1 (ligand for chemokine receptor CCR2), heat inactivated CCL3 and phosphate buffered saline (PBS). Two hours after this intervention, social recognition was examined. We chose the intranasal route of administration as it allowed us to administer CCL3 by minimally disturbing the mice during the experiment. Also, this route of administration has been demonstrated to be one of the few non-invasive ways of delivering molecules to the brain (Liu et al., 2001, Born et al., 2002, De Rosa et al., 2005).
Olfactory recognition
For olfactory recognition, female urine (diluted 1:10 in water) or commercially available scents (vanilla and orange at 1:10 dilution in water) were used as odorants to test for social and non-social odor recognition tasks, respectively. 100 μl of these odorants were placed onto a circular piece of filter paper (Whatman grade 1) and the filter paper was inserted into an Eppendorf tube whose cap had been removed. Mice were housed individually, and the tube containing the odorant was placed at one corner of the home cage without disturbing the animal. The protocol for T1 and T2 was similar other than the fact that the odorants were used as targets. The time the test animals spent sniffing the tube was recorded. A decrease in investigation time during T2 was an indicator of olfactory recognition. To assess the specificity of odor recognition, the same animal was presented with urine from a different female or orange scent 5 minutes after the end of T2. For social odor recognition task the test mice were separated from rest of the colony for 24 hours to sensitize them to social odors.
Water Maze Test
The water maze test was performed as described by us previously (Pizarro et al., 2003). The water maze test apparatus consisted of a steel tank (150 cm in diameter and 60 cm in depth) painted white, filled with water (23°C) and made white with an opaque non-toxic water-soluble paint. Extra-maze cues surrounded the tank. The tank was divided into four quadrants and a 12 cm circular white platform was placed in one of the four quadrants. The platform was submerged 1 cm below the surface of the water. For each hidden platform training trial, the mouse was released randomly from one of four start locations (N, E, S, W) and was allowed 60 seconds to swim and search for the platform, which remained constant during all hidden platform training. If the mouse did not locate the platform within the 60 seconds, then the mouse was manually guided to the platform and placed there for 15 seconds. Mice were given four trials per day with a 10 minute inter-trial interval for five consecutive days. After the last training trial on the first, third and fifth day, each mouse received a probe trial. A probe trial consisted of removing the platform and allowing the mouse to swim freely for 60 seconds. The time spent in the correct quadrant as well as other quadrants were recorded by a video camera mounted from the ceiling and connected to a computerized tracking/image analyzer system (HVS-Image). Five days following training, each mouse received a retention trial. This retention trial was identical to the probe trial and the time spent in the correct quadrant (the quadrant with the hidden platform) was recorded. Increased time spent in the correct quadrant indicated retained spatial memory.
Trace Fear Conditioning
Trace fear conditioning was performed as previously described by us using The Video Monitoring of Fear Conditioning System (MED-VFC, MED Associates, VT) (Villarreal and Barea-Rodriguez, 2006). The apparatus consisted of a sound-attenuating cubicle, which held a conditioning chamber affixed with a stimulus light, ventilation fan and adjustable speaker for delivering acoustic stimuli. The video camera was mounted on the inside of the cubicle doors. The floor of the conditioning chamber consisted of stainless steel rods that were wired to a shock generator and scrambler for the delivery of shock. This conditioning apparatus was then placed within an isolated room. The testing chamber consisted of exactly the same equipment as that of the conditioning chamber except that it was not held within a sound-attenuating cubicle and was placed within an isolated room adjacent to the room used for conditioning. Both chamber setups were entirely automated and the data collected for the video camera was then recorded onto the PC hardware via the Video Freeze Software (MED-VFC, MED Associates, VT). In trace fear conditioning, each mouse was placed inside the conditioning chamber and was allowed 90 seconds to acclimate to the chamber. The light in the chamber was on for the duration of the conditioning and the doors of the isolation cubicle were closed. There was a coconut scent throughout the conditioning chamber. The coconut scent was unique to the chamber and helped to make the conditioning chamber different from the testing chamber. Each mouse received four trace trials of tone (80 decibels, 15 sec, 30 sec trace) and foot shock (1 sec, 0.4 mA) pairings with a 210 sec inter-trial interval. At 210 sec after the final shock, the mouse was then returned to its home cage. Each mouse was then tested for fear conditioning to the tone 24 hours after conditioning. For tone learning assessments, each mouse was returned to a novel test chamber and allowed 90 seconds to acclimate to the chamber. Small toys and bedding were placed into the chamber before testing to contribute to the novelty of the testing chamber. There were salient cues placed within the room along with a food scent within the chamber which was different from the coconut scent that was in the conditioning chamber. Five tone trials (80 decibels, 1 min) were then given without the foot shock, and the freezing response was then measured during this time.
Western blot analyses
Cortices and hippocampi were isolated from Wt and Ccr5−/− mice and homogenized in Radio Immuno Precipitation assay (RIPA) buffer. Thirty microgram of protein lysates were electrophesed in a 10% SDS gel. Proteins were transferred to a PVDF membrane and the membranes were probed using 1:2000 dilution of anti-NMDAR1 antibody followed by incubation with secondary antibody. To determine protein loading, membranes were probed with an antibody against GAPDH. Band intensities for NMDAR1 expression were analyzed using NIH ImageJ software and were adjusted for the protein loaded by dividing the intensity of the NMDAR1 band by the intensity for the GAPDH band.
Statistical analyses
Statistical analyses were performed using Stata 7 (StataCorp LP, College Station, TX, USA) statistical software. For group mean comparisons, data were analyzed using two-tailed unpaired Student’s t-tests when the data were normally distributed and Kruskall-Wallis test followed by Mann-Whitney U test when the data was not normally distributed. One way ANOVA was used to compare means of more than two groups for normally distributed data followed by Newman-Keuls post-hoc test for pair wise comparison. Repeated measures ANOVA was used to analyze behavioral data where indicated.
Results
Enhanced social recognition in Ccr5−/− mice
Mice have social recognition by which they can remember conspecifics (specific details of an individual). In this form of recognition, with repeated exposure to a novel social context such as a juvenile mouse (target mouse), the test mouse spends decreasing amounts of time investigating the same target mouse. The experimental design used for testing social recognition is as shown in Fig. 1A. The time spent by a male Wt or Ccr5−/− test mouse to investigate a male Wt juvenile target mouse was similar during the first social investigation trial (T1) (Fig. 1B). As expected, wild-type mice demonstrated a significant reduction in the amount of time spent investigating the same juvenile during the second trial (T2) compared with the initial exposure (T1) indicating social recognition (Fig. 1B, 2 hr). However, CCR5-null test mice spent even less time (38±1.02 sec) than Wt mice (42.5±1.03 sec) investigating the target juvenile mice during the second trial (t=4.2, P=0.0008) (Fig. 1B, 2hr). Specificity for these results was apparent by the finding that when exposed to a novel target mouse 5 minutes after the second trial, the social investigation times increased to the same levels as that recorded during the first trial, and there was no difference in the investigation times between animal groups (t=−0.2, P=0.8; Fig. 1B, compare new vs initial). These findings demonstrated that loss of CCR5 expression is associated with enhanced social recognition without affecting social interactions.
Social recognition in mice, tested using the method described above is typically short lived (Ferguson et al., 2002). Increasing the interval between the first and second exposure to the same juvenile mouse would be expected to lead to a progressive increase in the amount of time spent investigating the target mouse suggesting decreased recognition. With long enough intervals, this would eventually lead to loss of recognition compared to the initial encounter. We varied the inter-trial interval (ITI) between the first and second trials from 30 to 240 minutes. Ccr5−/− mice spent significantly less time investigating target juvenile mice (Fig. 1C) except at an ITI of 240 minutes [(Genotype effect-F(1,33)=27, p<0.0001; ITI effect-F(3,54)=40, p<0.0001; post-hoc test- Wt vs Ccr5−/−, ITI of 30 min (P=0.008), 60 min (P=0.002) and 120 min (P<0.001), 240 minutes (P=0.2)]. These results suggest that Ccr5−/− mice have more robust social recognition after a single exposure to a target mouse. However, the amount of time this recognition is retained, appears to be similar in both wild-type and mutant mice with complete extinction by 4 hours.
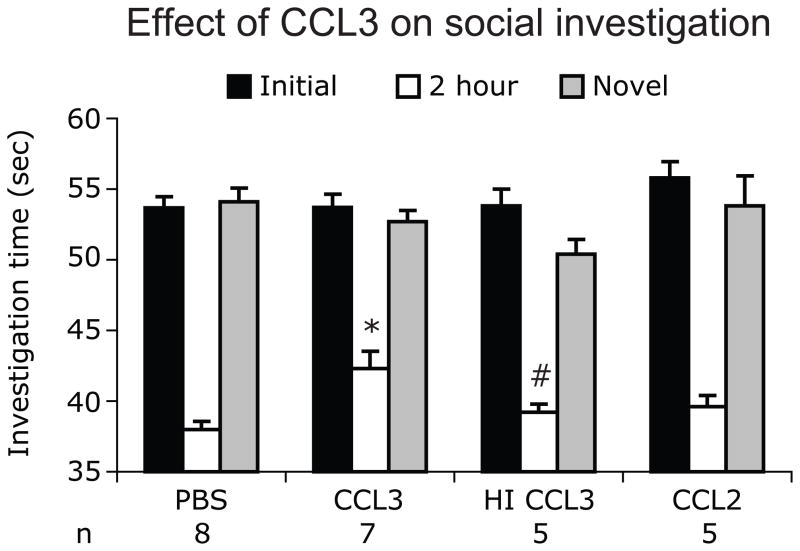
CCR5 ligand CCL3/MIP-1α negatively affects social recognition
The above data suggested that CCR5-mediated signaling inhibits social recognition and for this reason we hypothesized that CCR5 ligands will impair social recognition. We evaluated this hypothesis by administering CCL3 after the first trial and testing for social recognition after an ITI of 120 minutes. The investigation times for Wt animals treated with PBS or chemokines differed significantly [One way ANOVA, F (3,21)=7, P=0.002]. The investigation time for Wt animals treated with CCL3 (42.3±1s) was s higher than the time showed by Wt mice treated with PBS (38±0.6s, post-hoc test, P<0.01). Heat inactivation of CCL3 resulted in decrease in investigation time compared to that observed with CCL3 (39.2 ± 0.6s, P<0.05, post-hoc test) (Fig. 2). The investigation time for the Wt mice treated with heat inactivated CCL3 (39.2± 0.58s) and CCL2 (39.6 ± 0.81s) were comparable to the Wt mice treated with PBS (post-hoc test, P>0.05, Fig. 2). To determine the specificity of these findings, and to exclude that CCL3 administration induced lethargy, we placed the Wt animals with a new Wt juvenile test mouse (novel trial) 5 min after T2. We found that Wt target mice treated with PBS, CCL3, heat-inactivated CCL3 or CCL2 had spent similar amounts of time investigating the new juvenile target mouse [F(3,21)=1.5, P=0.25, Fig. 2]. Collectively, these results indicated that CCL3 modulates social recognition and supports the hypothesis that CCR5-mediated signaling inhibits social recognition without interfering with social interactions.
Figure 2.
Recombinant CCL3 impairs social recognition. Changes in the investigation times in Wt test mice treated immediately after the first trial (T1) with PBS, CCL3, heat-inactivated (HI) CCL3 and CCL2 are shown. Two hours after T1, test mice were presented with same target mouse as in T1 and social investigation times were recorded. Data are represented as mean investigation time (sec) ± SEM. (*P<0.05 for difference between PBS treated and CCL3 treated mice and #P<0.05 for difference between CCL3 and heat inactivated CCL3 treatment mice, using Newman Keul’s post-hoc test)
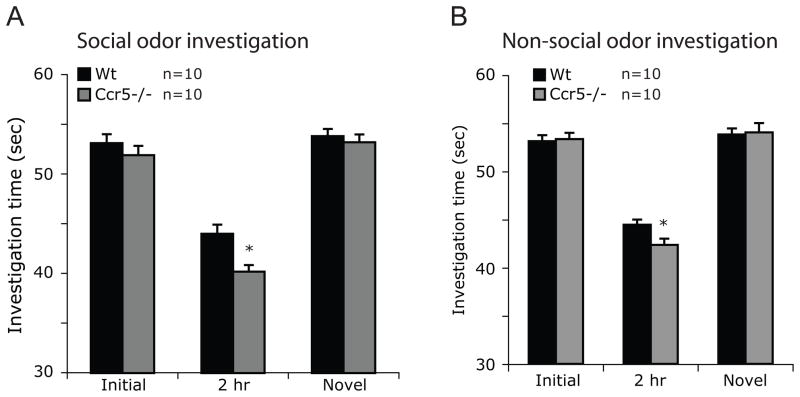
Ccr5−/− mice show enhanced olfactory recognition for both social and non-social odors
Since the task of social recognition is dependent, in part, on olfaction, we determined whether the enhanced social recognition observed in male Ccr5−/− mice was due to altered recognition of social scents in these mice. Mice lacking Ccr5 spent significantly less time investigating familiar social scent (female urine) compared to Wt mice (40.2±0.65s vs 44±0.91s, t=3.4, P=0.003; Fig. 3A, 2hr). To demonstrate the specificity of these findings, we conducted trials with female urine from a different mouse (novel social odor) and found that time spent investigating these odors by Wt and Ccr5−/− mice was similar (P>0.05, Fig. 3A). We then determined if the enhanced olfactory recognition is also seen for non-social scents indicating a global enhancement of olfactory recognition in Ccr5−/− mice. The olfactory recognition for non-social scents was enhanced in Ccr5−/− mice as these mice spent less time investigating familiar non-social scent (vanilla) compared to Wt mice (42.4±0.6s vs 44.5±0.5s, t=2.4, P=0.03; Fig. 3B, 2hr). Investigation time for a novel non-social scent (orange) was comparable in two groups (t= 0.6, P=0.6; Fig. 3B) indicating the specificity of non-social odor discrimination.
Figure 3.
Enhanced olfactory recognition of social (A) and non-social (B) odors in Ccr5−/− mice. Olfactory recognition was tested using the same protocol as shown in Figure 1 except that Eppendorf tubes containing odorants (female urine or vanilla/orange scent) on a blotting paper were used instead of a target mouse. A novel odorant was used to test the specificity of the results instead of a novel target mouse and investigation times were recorded. Data are represented as mean investigation time (sec) ± SEM. * P < 0.05 for differences between Wt and Ccr5−/− mice.
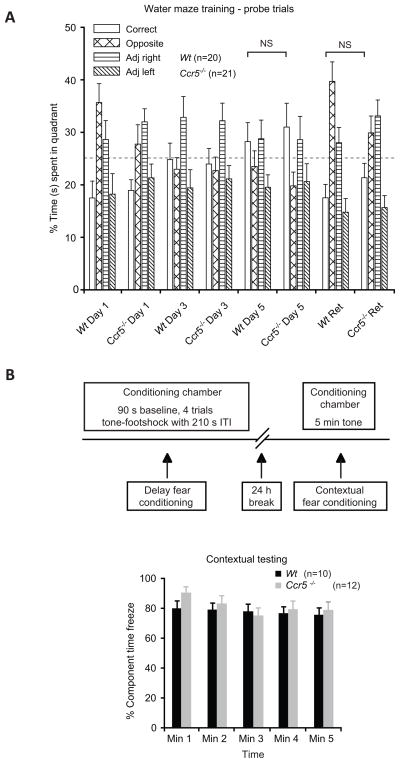
Spatial and fear conditioning memory are comparable in Wt and Ccr5−/− mice
Spatial memory was evaluated using visible and hidden platform tasks in a Morris water maze and no differences in this parameter were detected in Wt and Ccr5−/− mice (Fig. 4A, P>0.05). Fear conditioning memory in Wt and Ccr5−/− mice was assessed using cued and contextual fear learning elicited by pairing a neutral conditioned stimulus with an aversive unconditioned stimulus in a distinctive context (Fig.4B). We found that the freezing times were comparable in Wt and Ccr5−/− mice during context-elicited fear, indicating comparable fear conditioning memory in Wt and Ccr5−/− mice (Fig. 4B, P>0.05).
Figure 4.
Comparable spatial and fear conditioning memory in Wt and Ccr5−/− mice. (A) Spatial memory was tested using Morris water maze in Wt and Ccr5−/− mice. The percentage of time spent in the correct and the three other indicated quadrants were determined during the probe and the retention (Ret) trials for Wt and Ccr5−/− mice. Data is shown as mean percentage ±SEM. NS-statistically non-significant. (B) Fear conditioning memory in Wt and Ccr5−/− mice was tested using the approach outlined. Wt and Ccr5−/− mice showed similar levels of freezing during the 5 min tone testing 24 h after training. Data were recorded at 1 minute (Min) intervals and are expressed as mean percent freezing (±SEM).
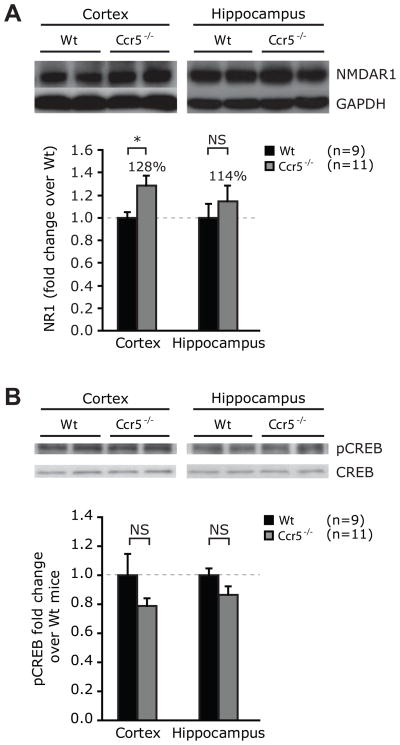
Increased expression of NMDA Receptor-1 in Ccr5−/− mice
Recent evidence suggests important role of NMDA receptor-1 in social recognition behavior. Inactivation of NMDAR-1 in mice results in impaired social recognition (Gao et al., 2009, Belforte et al., 2010) while administration of NMDA improves social recognition (Hlinák and Krejcí, 2002). We therefore evaluated expression of NMDAR-1 in the cortex and the hippocampi of Wt and Ccr5−/− mice. We chose cortex and hippocampi as the circuitry for social recognition is thought to involve these areas (Sánchez-Andrade et al., 2005). We found that the expression levels of NMDAR-1 were higher in the cortex of Ccr5−/− mice compared to controls (t=2.2, P=0.04; Fig. 5A). No significant differences in NMDAR-1 expression were detected in the hippocampus (t=0.8, P=0.4, Fig. 5A). We did not detect differences in levels of pCREB, a molecule involved in modulation of social recognition as well as memory tasks, in the hippocampus and the cortex of Ccr5−/− and Wt mice (Fig. 5B) (Kogan et al., 2000, Alberini, 2009).
Figure 5.
Expression levels of NMDAR1 and pCREB in the cerebral cortices and hippocampi of Ccr5−/− and Wt mice. (A) NMDAR1 and (B) pCREB expression in the cortices and the hippocampi of 6–8 week old male Wt and Ccr5−/− mice was determined by Western blotting. GAPDH expression was used to control for the amount of protein loaded in panels A and B, whereas CREB expression was used in panel B. Beneath each panel, the densitometric analysis of the radiograms is shown as fold difference in expression compared to Wt animals. Data are represented as mean ± SEM. * P < 0.05 for differences between Wt and Ccr5−/− mice. NS- statistically non-significant.
Discussion
There is a paucity of information on whether chemokines that are traditionally associated with inflammatory processes play a role in behavior and cognition. The results of this study demonstrated for the first time that CCR5 and its ligand CCL3 may modulate certain mice behaviors such as social recognition.
Social recognition was enhanced in Ccr5−/− mice using a paradigm of repeatedly presenting a juvenile mouse to the test mice. This test is classically used to test short-term recognition which is usually lost by 4 hours of ITI (Ferguson et al., 2002). This was evident in the Wt mice. The Ccr5−/− mice also showed gradual decline in the social recognition and had increasing investigation times with increasing ITIs. When this interval was increased to 240 minutes the investigation time continued to remain lower in Ccr5−/− mice but the difference in the investigation times between Wt and in Ccr5−/− mice was not statistically significant which is likely due to a ceiling effect in this short term social recognition paradigm. Collectively these results can be interpreted as a better social recognition in Ccr5−/− mice in this social recognition paradigm. In mice, olfactory input is pivotal for establishing social behaviors and regulating the preference for conspecifics (Liebenauer and Slotnick, 1996). Concomitantly, the enhanced social recognition in Ccr5−/− mice was associated with enhanced olfactory recognition as the recognition of novel social and non-social odors were enhanced in Ccr5−/− mice. Previous studies have shown that oxytocin, vasopressin and their receptors are important in social recognition and social interactions but not in nonsocial olfaction (Ferguson et al., 2000, Ferguson et al., 2001, Bielsky and Young, 2004, Bielsky et al., 2005). Since genetic inactivation of CCR5 altered both social and non-social odor recognition, the role played by CCR5 in modulating social behavior might be mediated through pathways other than that involving oxytocin or vasopressin.
Stimulation of NMDA receptors has been shown to improve social recognition (Hlinák and Krejcí, 2002, Sánchez-Andrade et al., 2005) while NMDA antagonist impair social recognition (Gao et al., 2009). Furthermore, expression levels of NMDAR-1 but not NMDAR-2, seem to influence social recognition in mice (White and Youngentob, 2004, Halene et al., 2009). A recent study showed that post-natal ablation of NMDAR-1 in mice resulted in impaired social memory and schizophrenia-like phenotype (Belforte et al., 2010). As CCR5 ligands can block NMDA-mediated effects in-vitro (Eugenin et al., 2003) we determined whether there might be a cross-talk between CCR5 expression levels and those of NMDAR-1 that, in turn, might provide a mechanistic basis for the effects of CCR5 on social recognition. Consistent with this possibility, we detected higher levels of NMDAR-1 expression in the cortex of Ccr5−/− mice compared to Wt mice. Our findings suggest that these effects of the chemokine system on behavior might thus be mediated by a cross-talk with other signaling systems such as NMDA receptors that are known to influence cognition and behavior. A recent study has shown that chronic administration of CCL3 resulted in increased expression of NMDAR-1 in cultured rat neurons (Kuijpers et al., 2010). Further studies will be needed to evaluate the CCR5, NMDAR-1 interactions in vivo.
One possible mechanism for the enhanced social recognition observed in Ccr5−/− mice is a compensatory developmental change caused by germ line inactivation of Ccr5. To exclude this possibility, we determined whether systemic administration of a CCR5 ligand would impair social recognition in Wt mice. We found that administration of CCL3 but not CCL2, a ligand for CCR2, influences social recognition. The latter finding suggests that not all the CC chemokines influence social recognition. Since CCL3 is a ligand for CCR1 and CCR5, we cannot exclude the possibility that CCR1 might also modulate social recognition and further studies would be needed to evaluate the role of CCR1 in social recognition. However, in conjunction with the results derived from CCR5-null mice, these findings suggest that CCR5 receptor-ligand axis might play a role in social recognition. It was noteworthy that the Ccr5−/− mice did not differ from Wt mice in other cognitive and behavioral tasks such as spatial memory and fear conditioning memory indicating that the CCR5/CCL3 axis might be specifically involved in modulation of social recognition.
Recently, Lee et al. reported that Ccr5−/− mice demonstrate impaired performance on spatial memory and passive avoidance task (Lee et al., 2009). The authors attributed these findings to a compensatory increase in CCR2 expression in the astrocytes, increased astrocyte activation and increased Aβ1–42 accumulation in the cortex and hippocampi. However, this study did not test effect of CCR5 ligands such as CCL3 on spatial memory. Our findings differ from that of Lee et al. in that we did not find a difference in spatial memory and passive avoidance memory between Wt and Ccr5−/− mice. It was possible that some of the differences in our studies and those by Lee et al. could be due to different genetic backgrounds of mice. The studies by Lee et al. were conducted using Ccr5−/− mice on the B6 background while the studies reported here were performed using Ccr5−/− mice on DBA1/J background. However, a study by Passos et al. which used Wt and Ccr5−/− mice on the C57BL6 background did not find any difference in the spatial memory using the Morris water maze paradigm in these two groups (Passos et al., 2009). These findings are in agreement with our observations. Fear conditioning memory was not tested in the study by Passos et al. The reason for such differences in the finding remains unclear and could include subtle differences in genetic background and testing paradigms. Use of chemokine ligands in Wt mice in addition to the use of knockout mice, as was done in our study, can provide additional evidence to understand the role of chemokine receptors and their ligands in the nervous system.
We found enhanced olfactory recognition and increased expression of NMDAR1 in Ccr5−/− mice. It is possible that CCR5 also acts and modulates olfactory recognition at sites other than hippocampus such as olfactory bulb, piriform cortex and entorhinal cortex. Since the aim of this study was to explore potential mechanisms underlying enhanced olfactory recognition in the Ccr5−/− mice, we broadly chose cortex and hippocampus as targets. Thus if piriform cortex and entorhinal cortex in the Ccr5−/− mice had increased expression of NMDAR1, it is possible that we missed out on those differences. In the wake of our findings, we believe that further studies are needed to explore the role of CCR5 in olfactory recognition by a) evaluating expression of CCR5 in the olfactory bulb-piriform cortex-entorhinal cortex pathway in Wt mice, b) identifying the cells expressing CCR5 in this pathway and c) evaluating the levels of NMDAR1 in Wt and Ccr5−/− mice in the abovementioned pathway to look for enhanced expression of NMDAR1in Ccr5−/− mice.
From a broader context, it is important to recognize that inflammation restricted to brain as well as systemic inflammation is associated with changes in behavior in laboratory animals as well as in humans (Reichenberg et al., 2001, Wright et al., 2005, Teeling et al., 2007). Thus, the findings from the current study raise the possibility that in such settings, the chemokine system might concurrently influence inflammation and behavior. Also, impaired social behavior is seen in diseases like schizophrenia and Rett syndrome and social recognition is impaired in mouse models of Schizophrenia and Rett syndrome (Moretti et al., 2005, Belforte et al., 2010). Given the ability of CCR5/CCL3 axis in modulating social behavior, it would be interesting to test if CCR5/CCL3 receptor-ligand system plays a role in these diseases. Lastly, small molecule antagonists of CCR5 are currently used in the treatment of HIV infection. Thus, it will be important to determine whether such CCR5 blockers also affect behavior. Results of our study show that chemokine system could be a potential target to modulate behavior and additional studies would be needed to further understand their role.
Highlights.
We tested the role of CC chemokine receptor 5 (CCR5) in social behavior and memory
Ccr5−/− mice had better social and olfactory recognition than wild type (Wt) mice
Administration of CCL3, a ligand for CCR5, impaired social recognition in Wt mice
Spatial and fear conditioning memory were comparable in Wt and Ccr5−/− mice
NMDA-receptor 1 expression was higher in the hippocampi of Ccr5−/− than Wt mice
Acknowledgments
We thank William Kuziel for providing Ccr5−/− mice on C57BL6/J background, Timothy Freeman and Carlo Condello for help with experiments and Ivan Becerra, William Pate and Harjeet Singh for help with illustrations. This work was supported by the Veterans Administration Center on AIDS and HIV infection of the South Texas Veterans Health Care System, and a MERIT (R37046326) and other awards (AI043279 and MH069270) from the NIH to S.K.A. S.K.A. is a recipient of the Elizabeth Glaser Scientist Award and the Burroughs Wellcome Clinical Scientist Award in Translational Research. The funding sources had no role in the study design; data collection, analysis and interpretation; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Disclosure: The authors have reported no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler MW, Rogers TJ. Are chemokines the third major system in the brain? J Leukoc Biol. 2005;78:1204–1209. doi: 10.1189/jlb.0405222. [DOI] [PubMed] [Google Scholar]
- Alberini C. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Belforte J, Zsiros V, Sklar E, Jiang Z, Yu G, Li Y, Quinlan E, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky I, Hu S, Ren X, Terwilliger E, Young L. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Xiong H. Human immunodeficiency virus type 1 gp120 inhibits long-term potentiation via chemokine receptor CXCR4 in rat hippocampal slices. J Neurosci Res. 2006;83:489–496. doi: 10.1002/jnr.20745. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Ferguson J, Aldag J, Insel T, Young L. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J, Young L, Hearn E, Matzuk M, Insel T, Winslow J. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Gao X, Elmer G, Adams-Huet B, Tamminga C. Social memory in mice: Disruption with an NMDA antagonist and attenuation with antipsychotic drugs. Pharmacol Biochem Behav. 2009;92:236–242. doi: 10.1016/j.pbb.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene T, Ehrlichman R, Liang Y, Christian E, Jonak G, Gur T, Blendy J, Dow H, Brodkin E, Schneider F, Gur R, Siegel S. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlinák Z, Krejcí I. N-methyl-D-aspartate improved social recognition potency in rats. Neurosci Lett. 2002;330:227–230. doi: 10.1016/s0304-3940(02)00802-9. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth P, White F, Miller R. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkonde Y, Morgan W, Sigala J, Maffi S, Condello C, Kuziel W, Ahuja S, Ahuja S. Chemokines in the MPTP model of Parkinson’s disease: absence of CCL2 and its receptor CCR2 does not protect against striatal neurodegeneration. Brain Res. 2007;1128:1–11. doi: 10.1016/j.brainres.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Kogan J, Frankland P, Silva A. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kuijpers M, van Gassen KL, de Graan PN, Gruol D. Chronic exposure to the chemokine CCL3 enhances neuronal network activity in rat hippocampal cultures. J Neuroimmunol. 2010;229:73–80. doi: 10.1016/j.jneuroim.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kwak D, Oh K, Nam S, Lee B, Yun Y, Kim Y, Han S, Hong J. CCR5 deficiency induces astrocyte activation, Abeta deposit and impaired memory function. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Liebenauer L, Slotnick B. Social organization and aggression in a group of olfactory bulbectomized male mice. Physiol Behav. 1996;60:403–409. doi: 10.1016/s0031-9384(96)80011-4. [DOI] [PubMed] [Google Scholar]
- Lillard JW, Jr, Singh UP, Boyaka PN, Singh S, Taub DD, McGhee JR. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood. 2003;101:807–814. doi: 10.1182/blood-2002-07-2305. [DOI] [PubMed] [Google Scholar]
- Liu R, Paxton W, Choe S, Ceradini D, Martin S, Horuk R, MacDonald M, Stuhlmann H, Koup R, Landau N. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH., 2nd Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Magdaleno S, Jensen P, Brumwell CL, Seal A, Lehman K, Asbury A, Cheung T, Cornelius T, Batten DM, Eden C, Norland SM, Rice DS, Dosooye N, Shakya S, Mehta P, Curran T. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol. 2006;4:e86. doi: 10.1371/journal.pbio.0040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Rostene W, Apartis E, Banisadr G, Biber K, Milligan E, White F, Zhang J. Chemokine action in the nervous system. J Neurosci. 2008;28:11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht J, Teague R, Paylor R, Zoghbi H. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SB, Endoh T, Simen AA, Ren D, Miller RJ. Regulation of calcium currents by chemokines and their receptors. J Neuroimmunol. 2002;123:66–75. doi: 10.1016/s0165-5728(01)00485-4. [DOI] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF. BACE1 Deficiency Rescues Memory Deficits and Cholinergic Dysfunction in a Mouse Model of Alzheimer’s Disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28:143–153. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos GF, Figueiredo CP, Prediger RD, Pandolfo P, Duarte FS, Medeiros R, Calixto JB. Role of the macrophage inflammatory protein-1alpha/CC chemokine receptor 5 signaling pathway in the neuroinflammatory response and cognitive deficits induced by beta-amyloid peptide. Am J Pathol. 2009;175:1586–1597. doi: 10.2353/ajpath.2009.081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett S, McCarthy M, Cooper D, MacRae K, Tendolkar A, Norris R, Strizki J, Williams K, Emery S. A phase I study to explore the activity and safety of SCH532706, a small molecule chemokine receptor-5 antagonist in HIV type-1-infected patients. Antivir Ther. 2009;14:111–115. [PubMed] [Google Scholar]
- Pizarro J, Haro L, Barea-Rodriguez E. Learning associated increase in heat shock cognate 70 mRNA and protein expression. Neurobiol Learn Mem. 2003;79:142–151. doi: 10.1016/s1074-7427(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A, Chenaux G, Reddick RL, Kuziel WA, Ahuja SS. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J Clin Invest. 2004;113:856–866. doi: 10.1172/JCI20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rostène W, Guyon A, Kular L, Godefroy D, Barbieri F, Bajetto A, Banisadr G, Callewaere C, Conductier G, Rovère C, Mélik-Parsadaniantz S, Florio T. Chemokines and chemokine receptors: new actors in neuroendocrine regulations. Front Neuroendocrinol. 2011;32:10–24. doi: 10.1016/j.yfrne.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Daugé V, Rovère C, Apartis E, Kitabgi P, Nahon JL, Rostène W, Parsadaniantz SM. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem. 2007;102:1175–1183. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99:10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Andrade G, James B, Kendrick K. Neural encoding of olfactory recognition memory. J Reprod Dev. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- Teeling J, Felton L, Deacon R, Cunningham C, Rawlins J, Perry V. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21:836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Villarreal J, Barea-Rodriguez E. ERK phosphorylation is required for retention of trace fear memory. Neurobiol Learn Mem. 2006;85:44–57. doi: 10.1016/j.nlm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- White T, Youngentob S. The effect of NMDA-NR2B receptor subunit over-expression on olfactory memory task performance in the mouse. Brain Res. 2004;1021:1–7. doi: 10.1016/j.brainres.2004.05.114. [DOI] [PubMed] [Google Scholar]
- Wright C, Strike P, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]