Abstract
Contiguous regions along the mammalian gastrointestinal tract, from the esophagus to the rectum, serve distinct digestive functions. Some organs, such as the esophagus and glandular stomach or the small bowel and colon, are separated by sharp boundaries. The duodenal, jejunal and ileal segments of the small intestine, by contrast, have imprecise borders. Because human esophageal and gastric cancers frequently arise in a background of tissue metaplasia and some intestinal disorders are confined to discrete regions, it is useful to appreciate the molecular and cellular basis of boundary formation and preservation. Here we review the anatomy and determinants of boundaries and transitions in the alimentary canal with respect to tissue morphology, gene expression, and, especially, transcriptional control of epithelial identity. We discuss the evidence for established and candidate molecular mechanisms of boundary formation, including the solitary and combinatorial actions of tissue-restricted transcription factors. Although the understanding remains sparse, genetic studies in mice do provide insights into dominant mechanisms and point the way for future investigation.
Keywords: digestive tract development, gastro-esophageal junction, gastro-duodenal boundary, transcriptional regulation of tissue identity, homeobox genes in gut development
The alimentary canal has variably sharp anatomic and functional boundaries between its contiguous segments: the esophagus, stomach, small intestine and colon (Figure 1). These boundaries often span a single row of cells and are established during developmental transitions of the embryonic gut tube. One guiding principle is that the mesenchyme beneath the luminal epithelium carries the bulk of positional information for regional identity and boundary formation. Here we summarize the morphologic and genetic markers that delimit gastrointestinal boundaries and the current understanding of how such boundaries are established and maintained.
Figure 1. Organization of regions in the gastrointestinal tract.
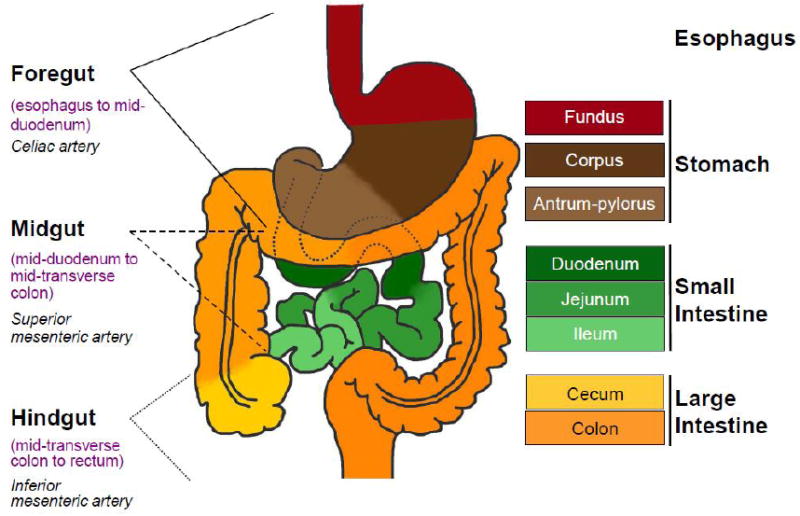
Left: The foregut, midgut and hindgut are classically defined according to the blood supply, as indicated. Right: Colors in the diagram refer to distinctive epithelia within the alimentary canal. In mice, the stratified squamous epithelium of the esophagus (crimson) extends into the dome-like forestomach, before forming a sharp, single-cell boundary with the glandular columnar epithelium of the gastric corpus (dark brown). As discussed in the text, the boundary between the gastric body and antral-pyloric epithelia (light brown) is less distinct, while that between the stomach and small intestine (green), marked by the pyloric sphincter, is sharp. Small intestine regions have different digestive functions and characteristic patterns of genes expression, without well-defined boundaries. The villous epithelium of small intestine transitions abruptly into a flat, non-villous epithelium at the ileo-cecal valve, which is followed by a specialized cecum (yellow) and the remainder of the colon (orange).
In classical embryology, gut tube regions are defined according to their blood supply. The celiac artery feeds the foregut, which ends at the junction of the liver and pancreatic primordia. The midgut, which extends until the cecum, and the hindgut receive blood from the superior and inferior mesenteric arteries, respectively (Figure 1, left). By contrast, here we consider boundaries to mean the epithelial transitions between organs of distinctive structure, function, cell morphology, and gene expression. For example, the junction between the esophagus and stomach, which are lined by distinctive squamous and columnar epithelia, respectively, and separated by a fibromuscular sphincter, constitutes a firm boundary. Junctions between the duodenal, jejunal and ileal segments of the small intestine, or between the gastric corpus and antrum, are anatomically less sharp (Figure 1, right).
The primitive gut tube arises from the definitive endoderm at the late gastrula stage, at embryonic day (E) 7.5 in mouse embryos and Hamburger-Hamilton (HH) stage 4 in chick embryos. Initial anterior-posterior (A-P) patterning and regional specification depend on repression of foregut, and induction of hindgut, fates mediated by a rostral to caudal gradient of Wnt, bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) signals directed from the mesoderm to the endoderm (reviewed in [1]). Hox-family homeodomain proteins and other regional transcription factors, including HHEX1, FOXA2 and SOX2 in the foregut and Caudal-family homeoproteins CDX1 and CDX2 in the hindgut, are among the regulators responsible for further regionalization (reviewed in [1]). We highlight studies late in development, during the establishment of recognized segmental boundaries.
The esophagus-stomach and internal gastric boundaries
The transit function of the esophageal squamous epithelium contrasts with the digestive functions of the glandular stomach mucosa; the two organs are separated by the most anterior and sharp boundary in the digestive tract. In mice the squamous epithelium extends into the dome of the stomach, pushing the boundary between stratified and columnar epithelia caudally, into the stomach (Figure 1). This boundary is important clinically because in humans it is the site of a precancerous condition known as Barrett’s metaplasia, wherein squamous tissue in the distal esophagus takes on the appearance and gene expression profile of intestine-type columnar cells (reviewed in [2]). Similar metaplasia often precedes cancer in the distal stomach but is rare elsewhere in the gut, suggesting that the esophagus-stomach boundary and diseased stomach epithelium retain an unusual degree of plasticity in fate. Despite this clinical importance, there is limited understanding of the determinants of this boundary. The transition from esophagus to stomach is abrupt: the first row of columnar cells, which express stomach-specific genes such as Atp4b and Pepsinogens, abuts the preceding (rostral) row of cells expressing squamous epithelial markers (Figure 2, right). The junction between the gastric corpus and antral epithelia is, by comparison, fuzzy. Gastrin-producing endocrine cells appear exclusively in the antrum and define the boundary; the antrum contains only scattered chief and parietal cells but is not devoid of these cell types. Epithelial stem cells in the antrum, but not those in the corpus, express the intestinal stem cell marker LGR5 [3].
Figure 2. Expression domains of transcription factors that participate in digestive tissue identity and boundary formation.
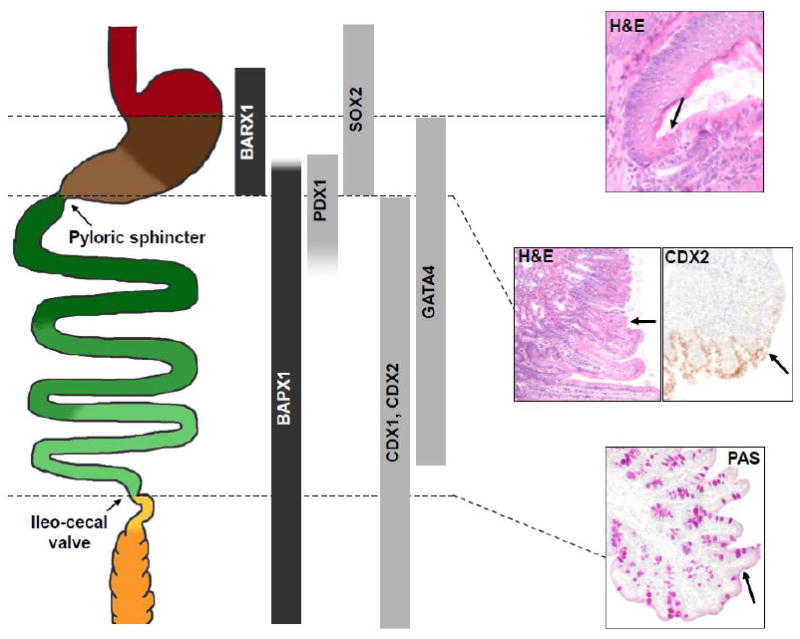
Left: Schematic diagram of the alimentary canal, stretched in the rostro-caudal axis, with discrete regions represented in the same colors as in Figure 1. Center: Expression domains of factors with known functions in epithelial specification or boundary formation. Factors expressed in the mesenchyme and epithelium are represented in black and gray, respectively. The many Hox-cluster genes expressed in the gut are not represented; see [39, 42, 48] for Hox gene expression. Right: Histologic demonstration of sharp anatomic boundaries at the mouse foregut squamo-columnar junction (top, H&E stain (adult)), gastroduodenal boundary (middle, H&E stain (adult), CDX2 immunohistochemistry (E16)), and ileo-cecal valve (bottom, Periodic acid Schiff stain (adult)). Arrows point to the respective junctions. At the foregut squamo-columnar junction, note the eosinophilic keratin lining the squamous epithelium, above the arrow, and its absence over glandular epithelium below the arrow.
Several laboratories have reported transcription factor profiles in the developing mouse esophagus and stomach [4, 5] but evidence that specific factors create a boundary is sparse. This is an important area for future investigation, to advance understanding of both development and cancer. Mouse fetal Sox2 expression initially encompasses both the fore- and glandular stomach but not the intestine; expression subsequently disappears from the corpus and antrum but increases in the stratified epithelium of the esophagus and forestomach, forming a sharp expression boundary [6]. The distal esophagus and its junction with the stomach are abnormal in human infants affected by the anophthalmia-esophageal-genital syndrome (OMIM 600992), which results from SOX2 heterozygosity [7], and in most neonatal mice carrying a Sox2 allele that expresses ~20% of normal levels [6]. Because respiratory columnar cells replace the stratified esophageal epithelium in the latter experimental model, it is difficult to know whether SOX2 helps determine the boundary per se but notably, reduced SOX2 levels activate glandular stomach epithelial genes in the esophagus [6]. Sox2 expression in the anterior foregut endoderm responds to FGF signaling from the underlying mesenchyme [6] and stratification of the squamous epithelium requires BMP signaling [8].
The stomach-duodenum boundary
An equally sharp boundary separates the stomach from the intestine, with the last row of glandular gastric cells that express stomach markers such as SOX2 [9] juxtaposed to the first row of villous cells that express intestinal markers such as CDX2 and VIL1 [10, 11] (Figure 2, right). Li et al. compared changes in global gene expression in the developing mouse stomach, pylorus, and duodenum between E14.5 and E16.5, when the pseudostratified fetal epithelium converts to a villiform columnar epithelium [11]. Gene expression differences were substantially larger between the stomach and intestine at E16.5 than at E14.5, driven largely by changes in intestinal transcription. Thus, although the alimentary canal is grossly patterned by E12, the gene expression program responsible for its mature function, which the authors call “intestinalization,” is executed days later. Notably, the intestine appears to be specified before the stomach, opposite to the trend for cytodifferentiation along the length of the intestine, which occurs in an anterior-to-posterior wave.
Inasmuch as cell specification represents the implementation of cell-specific gene expression programs, lineage-restricted transcription factors are thought to play a vital role in delineating cell types and establishing tissue boundaries. Gene profiling studies have also identified regionally restricted transcription factors that may, alone or in combination, contribute toward these processes [4, 11, 12]. One notable feature of the expression domains is that they often appear nested, with partial overlaps that suggest the possibility of networked interactions (Figure 2, center). The examples of BARX1, BAPX1 and PDX1, discussed below, begin to hint at the nature of such interactions.
The pyloric sphincter, a smooth-muscle structure, is an important feature of the stomach-duodenum boundary. In chick embryos, BMP4 signaling enhances expression of the homeobox gene Nkx2.5 in mesodermal cells fated to form this structure [13]. Research on the epithelial boundary between these organs illustrates several mechanisms that may also conspire to establish boundaries elsewhere. First, the combinatorial action of regulatory transcription factors likely mediates organ-specific gene expression. Second, distinctive cell signaling pathways operate in adjoining regions to delimit the gastro-intestinal boundary. Third, directed segregation might represent a means for intermingled cells that express stomach- or intestine-specific genes early in development to sort on either side of a sharp boundary.
Action of transcription factors
Late in mammalian development, the Caudal-family homeobox genes Cdx2 and Cdx1 are exclusively expressed in the intestine, and genetic studies in mice reveal their vital role in epithelial specification. Ectopic expression of either factor in the stomach epithelium induces intestinal heterotopia in transgenic mice [14, 15]. Conversely, CDX2 loss in the early endoderm converts the posterior intestinal lining into squamous epithelium of the esophageal type [16] and its loss later in development [17], or in combination with CDX1 in adult mice [18], derepresses stomach epithelial genes in the intestine. Notably, intestinal differentiation in the absence of CDX2 is overtly normal in the duodenum; esophageal or gastric features are most evident in the ileum, the site of highest CDX2 expression in wild-type animals [19]. These data implicate CDX2 in repressing anterior epithelial identities but the anatomic discontinuity argues against a boundary function per se. On the other hand, Cdx2 haploinsufficiency induces in the proximal colon a unique type of polyp, where the center lacks CDX2 expression altogether and shows stomach morphology [20], while surrounding Cdx2+/- cells show a graded small bowel morphology that mimics the normal morphologic gradient of contiguous intestinal segments [21]. We take such results to suggest that CDX proteins may contribute toward gastro-duodenal boundary formation but are dispensable for this activity. Nevertheless, this proposition does shift attention to the signaling and transcriptional mechanisms that restrict Cdx2 expression to the intestine, an important problem that has eluded resolution despite significant effort [22]. Control of spatially restricted expression is better understood for Pancreatic and duodenal homeobox 1 (Pdx1), a Parahox gene that is closely related to Cdx2 and Cdx1.
Unlike genes that are expressed only in the stomach or intestine, Pdx1 expression spans several organ and tissue boundaries across a short distance: levels are highest in the pancreas, but also substantial in the epithelium of the distal (antral-pyloric) stomach, with a sharp rostral margin, and the proximal duodenum, where the caudal margin is graded [23] (Figure 2, center). The gastro-duodenal border in Pdx1-/- mice is defective, with gross distortion of the pyloric sphincter and replacement of the villous epithelium and Brunner’s glands of the proximal duodenum by a flat cuboidal lining of a biliary type [23] (Figure 3e, f). Thus, if CDX proteins contribute toward intestinal specification, total absence of PDX1 either overrides this function or prevents expression of Cdx genes. The morphologic defects affecting the gastro-duodenal border in Pdx1-/- mutants are absent in homozygotes carrying a hypomorphic Pdx1 allele deleted for 3 conserved cis-regulatory elements (Area I-II-III) [24], although pancreatic and gut endocrine cell defects are identical to those in Pdx1-/- animals. Thus, various posterior foregut derivatives respond differently to the levels and/or timing of Pdx1 expression. Indeed, expression of a Pdx1 transgene containing Area I-II-III, is sufficient to express Pdx1 in the pancreas and rescues the pancreatic defects in Pdx1-/- mice more fully than it rescues the duodenal anomalies [25]. Furthermore, conditional loss of FOXA1 and FOXA2, transcription factors that bind to Area I-II-III, in Pdx1-expressing tissues, phenocopies the deletion of Area I-II-III [26]. These studies begin to identify the cis-regulatory basis for developmental regulation of a pivotal homeobox gene and trans-acting factors that may control its spatial expression.
Figure 3. Illustrative boundary defects in mutant mice.
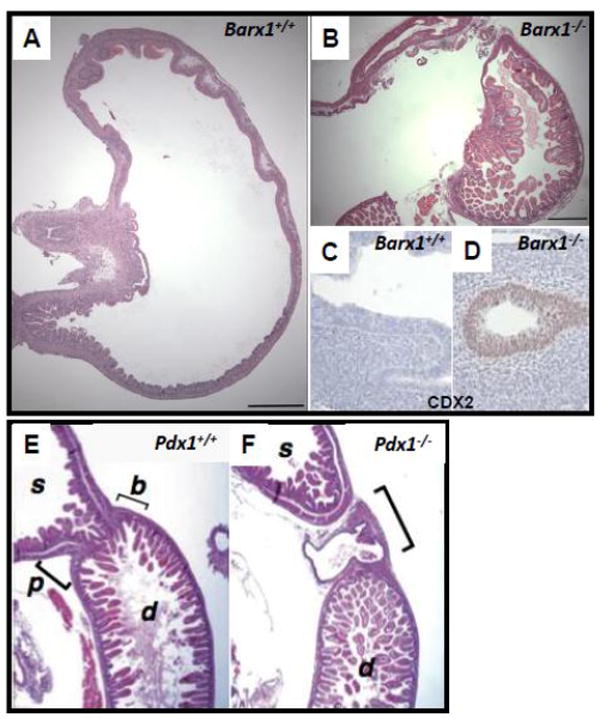
(A-D) Hematoxylin and eosin (H&E)-stained neonatal stomach tissues from wild-type (A) and Barx1-/- (B) mice [52]. The homeotic posteriorization in Barx1-/- stomach is evident from ectopic presence of intestinal villi (B) and expression of the intestinal marker CDX2 (D) as early as E12.5 [27]. (E, F) H&E-stained wild-type (E) and Pdx1-/- (F) tissues at E18.5 reveal defective pylorus development in Pdx1-/- mice. Normally the stomach (s) opens into the duodenum (d) at the pylorus (p), where well-defined Brunner’s glands (b) are found. In Pdx1-/- mutants, a cavity that lacks villi and is lined by a cuboidal epithelium forms in this region [23].
Regional antagonism of cellular pathways as the means to form a boundary
As noted above, depletion of CDX2 induces foregut morphology and gene expression in the distal intestine, without revealing a boundary function per se [16]. Consistent with the instructive role of regional mesenchyme in gastrointestinal patterning, other experiments attribute such a function to the mesenchyme-expressed homeodomain protein BARX1 [27]. Transient Barx1 expression in the mouse stomach mesenchyme coincides with the period of stomach and duodenal specification. Using embryonic tissue explants and Barx1-/- mice, Kim et al. implicated this gene in stomach morphogenesis and, more importantly, in positioning the gastro-duodenal junction. In the absence of Barx1, a CDX2-expressing villous epithelium that carries intestinal goblet cells is present well into the gastric corpus [27] (Figure 3a-d). This abnormality represents a distinctive posterior homeotic transformation and suggests that mesenchymal Barx1 normally represses intestinal differentiation in the overlying endoderm.
Although such observations help identify key boundary determinants, they inevitably push the problem back, in this case raising the question of what defines the borders of Barx1 expression in prospective stomach mesenchyme in the first place. It is worth noting in this regard that Hoxa5-/- mice, which also have a partially intestinalized hindstomach, show reduced Barx1 expression [28]. Furthermore, selected microRNAs that appear late in stomach development, including miR-7a and miR-203, serve to restrict Barx1 expression temporally and perhaps also spatially [29]. Additional investigation of Barx1 gene regulation will shed useful light. Meanwhile, progress in understanding how BARX1 promotes gastric, and represses intestinal, identity has been more satisfying. Much of the foregut endoderm seems to elicit canonical Wnt signaling early in development, and attenuation of this signal is necessary for proper stomach development. Stomach mesenchymal factors that require BARX1 for their expression include inhibitors of Wnt signaling, the secreted Frizzled-related proteins sFRP1 and sFRP2. Thus, at least one action of BARX1 is to inhibit Wnt signaling in the spatially restricted domain of the prospective stomach [27, 30].
Although the stomach-intestine boundary is indeed sharp in terms of gene expression and mucosal morphology, the antral-pyloric stomach has some properties of a transitional zone. Whereas the gastric corpus carries abundant parietal and chief cells and its LGR5-nonexpressing stem cells reside high in flat glandular units, the antrum and pylorus have a scalloped mucosa with few chief or parietal cells and LGR5-expressing stem cells that lie near the gland base, similar to their counterparts in intestinal villi. Characteristic Brunner’s glands in the duodenal sub-mucosa also resemble mucinous cells found at the base of antral-pyloric glands, and the distal stomach is subject to a distinct patterning mechanism. The rostral boundary of expression of the mesenchymal homeobox gene Bapx1 (Nkx3-2), coincides approximately with the corpus-antrum junction; posteriorly it extends into the full length of the intestine (Figure 2, center). Bapx1 gene disruption in mice causes marked truncation of the antral-pyloric segment; all epithelial borders are preserved, but the pyloric constriction is lost, yielding a wide, valve-less opening from the stomach into the intestine [31]. Importantly, although the posterior Bapx1 expression domain extends well beyond the stomach-restricted Barx1 domain, BARX1 is necessary for Bapx1 expression in the antral-pyloric mesenchyme; conversely, BAPX1 is dispensable for Barx1 expression. These findings speak to a hierarchy of transcriptional control in gastrointestinal patterning and illustrate the idea that individual regions may emerge through the combinatorial actions of spatially restricted transcription factors with nested expression and likely complex interactions.
Cell segregation
Cell segregation is a well-established means for compartmental boundary formation in tissues such as the Drosophila wing and vertebrate rhombomeres (reviewed in [32]) but any role in formation of gut boundaries remains speculative. Two groups recently reached divergent conclusions regarding the time when cells expressing Sox2 or Cdx2 become restricted across the gastro-duodenal boundary. Focusing on whole mouse embryos from e8.25 to e9.5, Sherwood et al. found that many cells initially expressed both factors but progressively lost expression of one before settling on either side of a sharp divide [4]. Such restriction may result from movement of cells that intrinsically express just one factor; alternatively, an extrinsic signal may cause cells in a certain location to retain expression of only one factor or the other. By contrast, Li et al. noted the lack of a sharp Sox2-Cdx2 expression boundary in sectioned mouse embryos five days later in gestation [11]. Although the actual timing of boundary formation hence remains uncertain, further examination of two or more key lineage markers in abutting epithelia might yield clear answers in the future. In particular, a lineage tracing strategy could be applied at various points in development to mark any cell that expresses, for example, Sox2 or Cdx2. By following the marked cells, one could ascertain the earliest time when only CDX2-expressing cells contribute to the intestine and correlate the positions of cells at the boundary with the tissues that the cells mark later.
Boundaries within the small and large intestines
Although the duodenal, jejunal and ileal segments of the small bowel serve some distinctive functions, such as the bulk of nutrient and iron absorption proximally and absorption of bile salts and vitamin B12 exclusively in the ileum, these regions are not separated by mechanical valves, and their boundaries are indistinct (Figure 1). Villus height declines linearly, while the fraction of epithelial goblet and Paneth cells increases, along the intestinal length; functional differences are reflected in the expression of distinctive genes, such as iron transporters in the duodenum and Ilbp and Asbt in the ileum. However, regional genes tend to show graded, imprecise expression domains and gene expression along the small bowel varies less than it does across the stomach-intestine or ileum-cecum boundaries [33]. Certain human intestinal disorders, such as regional ileitis and celiac disease, are largely confined to particular segments. Because alteration of local properties could, in principle, ameliorate such conditions, it is important to understand the basis for intestinal regionalization.
The transcription factor GATA4 is restricted to the duodenum and jejunum, where it seems to regulate manifestation of regional properties (Figure 2, center). In Gata4-/- intestine, ileum-specific genes such as Asbt are expressed ectopically in the jejunum [34], and forced expression of a transcriptionally inactive, dominant-negative form of GATA4 in adult mice induces ileal genes and the ileal proportions of goblet and enteroendocrine cells in the jejunum [34]. Similarly, mice carrying a form of GATA4 that cannot bind Friend of GATA (FOG) cofactors show jejunal expression of transcripts normally restricted to the distal ileum [35]. Thus, GATA4 and its cofactors repress ileal properties in the proximal small bowel. The prospect that graded combinations of various transcriptional regulators constitute a general mechanism for implementing duodenal, jejunal or ileal gene expression programs is fertile for further investigation.
Albeit less well known than the small intestine, the colon also shows regionality, differing between the left and right sides. In humans the superior and inferior mesenteric arteries supply blood to the left and right colon, respectively, with a transition in the mid-transverse colon (Figure 1); the two vascular systems are connected by anastomoses. In another illustration of sub-mucosal heterogeneity, Hirschsprung disease, a congenital segmental aganglionosis that usually results from defective expansion or migration of neural crest-derived enteric neurons, affects the terminal colon far more often than proximal regions [36]. Transcriptional profiling has revealed differences in mucosal gene expression between the left and right colon and the rectum [33], reflecting differences in water absorption, handling of commensal microbes and other functions, but the molecular basis for the transcriptional heterogeneity is unknown. Perhaps reflecting these regional differences, human colon cancers that arise in the right and left colon show some distinctive features; for example, microsatellite instability arising from defective DNA mismatch repair is more common in right-side than in left-side cancers and imparts a favorable prognosis [37].
The role of Hox genes in anterior-posterior gut patterning and boundary formation
Spatio-temporally controlled expression of the clustered Hox genes determines anterior-posterior positional identity in many animal tissues [38]. The same genes are expressed in overlapping patterns along the length of the gut mesenchyme; a few also appear in the endoderm. Some expression boundaries coincide roughly with the borders of specialized regions, but these boundaries tend to be less sharp in the gut than in the developing axial skeleton or neural tube. As in the latter structures, Hox genes show ordered, roughly collinear expression along the alimentary canal in chick and mouse embryos, with 3’ genes expressed rostrally and genes from 5’ groups expressed in caudal regions [39, 40]. For example, 5’-encoded Hoxd transcripts are confined to the extreme caudal end of the mouse gut; however, selected Hox genes show highly restricted, non-collinear expression in the cecum and stomach [39, 41, 42]. Such observations have generated much interest in the idea that a Hox code delineates digestive tract segments and boundaries, much as one specifies embryonic vertebral and rhombomeric identities [41, 42]. However, with the exceptions of Hoxc4 and Hoxa5, targeted disruption of most individual murine Hox genes barely affects gut development. Disruption of Hoxc4 results in additional loss of Hoxc5 and Hoxc6 transcripts and aberrant musculature and luminal obstruction over nearly the whole esophagus [43], without an effect on boundaries per se. Hoxa5 loss induces reciprocal changes in adjoining tissue layers, with submucosal hypertrophy and mucosal thinning in the stomach and colon [28]. The murine Hoxa4 promoter has also been used to drive ectopic expression of two different homoebox genes in transgenic mouse gut mesenchyme. Hoxc8 misexpression induced formation of a duodenal branch anterior to the gastric pylorus [44], whereas misexpression of Pdx1, which is normally restricted to endoderm-derived cells, shifted the ileo-cecal boundary posteriorly, with agenesis of the cecum and emergence of small intestinal villi in the proximal colon [45]. The artificial context of these experiments limits the degree of insight into normal mechanisms of boundary formation. Speaking to the importance of reciprocal tissue interactions in gut development, endodermal Sonic hedgehog (Shh) is sufficient in chick embryos to induce specific Hoxd genes in the adjoining mesoderm [46]. Because Shh is widely expressed along the endoderm, however, this effect is more likely permissive than a source of regional instruction. Indeed, Shh induction of Hoxd13 is confined to the native Hoxd13 domain, indicating facilitation or augmentation of pre-existing patterns [47].
The lack of overt gastrointestinal phenotypes upon loss of single Hox genes is often attributed to genetic redundancy among genes with substantially overlapping expression domains [48]; for example, nearly the entire Hoxd cluster is expressed in the embryonic posterior midgut, including the cecum. However, because disruption of some Hox genes alters others’ expression patterns quite significantly, the simultaneous loss and ectopic gain of gene functions confounds the interpretation of null phenotypes. These challenges and the clear requirements of Hox genes in gut development are illustrated below.
Development of the cecum and intestinal sphincters
The ileo-cecal valve demarcates the boundary between the small and large bowel and opens into the cecum, the first portion of the colon and a region specialized for handling dietary cellulose. The ileo-cecal junction forms the unusually sharp rostral boundary of expression of several Hox genes: Hoxa9, Hoxa10, Hoxd9, and Hoxd10 [39, 49]. The cecum is always missing in mice carrying a deletion of the region encompassing Hoxd1 to Hoxd10, or Hoxd4 to Hoxd11, but not when the deletion is extended to include Hoxd12 [49]. Indeed, absence of the 3’ Hoxd genes allows ectopic Hoxd12 expression, without affecting expression of transcripts encoded in the Hoxa cluster. Thus, in this context the 3’ Hoxd genes serve to restrict the anterior limit of Hoxd12 expression, and by the principle of “posterior prevalence” in Hox gene function, Hoxd12 interferes with “anterior” Hoxa gene functions in specifying the cecum [49]. Ectopic Hoxd12 expression is in turn associated with loss of expression of Fibroblast growth factor (FGF) 10. Mesenchymal expression of Fgf10 thus depends on both the presence of Hoxa genes and absence of Hoxd12. It also requires FGF9 signaling from the cecal endoderm. FGF10 then signals back to this FGF9 source in a reciprocal exchange that is required for genesis of the cecum as a well-delineated pouch [50].
Expression of Hoxd12 and Hoxd13 is normally confined to the most distal end of the gut mesenchyme, and disruption of either gene in mice causes severe hypoplasia of the outer, longitudinal layer of smooth muscle, compromising the anal sphincter [51]. Likewise, mice carrying a deletion that spans Hoxd4 through Hoxd13 show thinning of the fibrous band of ileo-cecal valve smooth muscle. Although these defects are functionally consequential, they are in each case confined to the smooth muscle compartment, without affecting the mucosa, and therefore represent a distinct form of boundary decisions. It is nevertheless notable that the reported defects seem confined to the locations of specific boundaries and of native expression of the targeted genes, and that deletion of multiple Hox genes is often necessary to uncover them. Taken together, these observations imply cell-autonomous, genetically redundant, and region-specific functions for the clustered Hox genes in gut development.
Conclusions
The boundaries between regions and organs in the digestive tract represent fine models to investigate a fundamental process in development. They are also relevant to human disease because certain tissue metaplasias, especially in the distal esophagus and stomach, constitute the ground for lethal malignancies. Although present understanding of how distinctive regions are sharply delineated during embryogenesis is limited, the studies discussed in this review begin to identify some key determinants and to provide useful mechanistic insights.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badreddine RJ, Wang KK. Barrett esophagus: an update. Nat Rev Gastroenterol Hepatol. 2010;7:369–378. doi: 10.1038/nrgastro.2010.78. [DOI] [PubMed] [Google Scholar]
- 3.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Beghtel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Sherwood RI, Chen T-YA, Melton DA. Transcriptional dynamics of endodermal organ formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comelli EM, Lariani S, Zwahlen MC, Fotopoulos G, Holzwarth JA, Cherbut C, Dorta G, Corthesy-Theulaz I, Grigorov M. Biomarkers of human gastrointestinal tract regions. Mamm Genome. 2009;20:516–527. doi: 10.1007/s00335-009-9212-7. [DOI] [PubMed] [Google Scholar]
- 6.Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson KA, Hever AM, Rainger J, Rogers RC, Magee A, Fiedler Z, Keng WT, Sharkey FH, McGill N, Hill CJ, Schneider A, Messina M, Turnpenny PD, Fantes JA, van Heyningen V, FitzPatrick DR. Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum Mol Genet. 2006;15:1413–1422. doi: 10.1093/hmg/ddl064. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez P, Da Silva S, Oxburgh L, Wang F, Hogan BL, Que J. BMP signaling in the development of the mouse esophagus and forestomach. Development. 2010;137:4171–4176. doi: 10.1242/dev.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii Y, Rex M, Scotting PJ, Yasugi S. Region-specific expression of chicken Sox2 in the developing gut and lung epithelium: regulation by epithelial-mesenchymal interactions. Dev Dyn. 1998;213:464–475. doi: 10.1002/(SICI)1097-0177(199812)213:4<464::AID-AJA11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein EM, Qiao XT, Madison BB, Pinson K, Dunbar L, Gumucio DL. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Udager AM, Hu C, Qiao XT, Richards N, Gumucio DL. Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn. 2009;238:3205–3217. doi: 10.1002/dvdy.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray PA, Shivdasani RA. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–4129. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- 13.Smith DM, Nielsen C, Tabin CJ, Roberts DJ. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development. 2000;127:3671–3681. doi: 10.1242/dev.127.17.3671. [DOI] [PubMed] [Google Scholar]
- 14.Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 15.Mutoh H, Sakurai S, Satoh K, Osawa H, Hakamata Y, Takeuchi T, Sugano K. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416–1423. doi: 10.1136/gut.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grainger S, Savory JGA, Lohnes D. Cdx2 regulates patterning of the intestinal epithelium. Dev Biol. 2010;339:155–165. doi: 10.1016/j.ydbio.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Verzi MP, Shin H, Ho LL, Liu XS, Shivdasani RA. Essential and redundant functions of caudal family proteins in activating adult intestinal genes. Mol Cell Biol. 2011;31:2026–2039. doi: 10.1128/MCB.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–971. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- 20.Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 21.Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci U S A. 1999;96:7318–7323. doi: 10.1073/pnas.96.13.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benahmed F, Gross I, Gaunt SJ, Beck F, Jehan F, Domon-Dell C, Martin E, Kedinger M, Freund J-N, Duluc I. Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology. 2008;135:1238–1247. 1247.e1231–1233. doi: 10.1053/j.gastro.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 23.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 24.Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–266. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyer DF, Fujitani Y, Gannon M, Powers AC, Stein RW, Wright CV. Complementation rescue of Pdx1 null phenotype demonstrates distinct roles of proximal and distal cis-regulatory sequences in pancreatic and duodenal expression. Dev Biol. 2006;298:616–631. doi: 10.1016/j.ydbio.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim B-M, Buchner G, Miletich I, Sharpe PT, Shivdasani RA. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Aubin J, Déry U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development. 2002;129:4075–4087. doi: 10.1242/dev.129.17.4075. [DOI] [PubMed] [Google Scholar]
- 29.Kim BM, Woo J, Kanellopoulou C, Shivdasani RA. Regulation of mouse stomach development and Barx1 expression by specific microRNAs. Development. 2011;138:1081–1086. doi: 10.1242/dev.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 31.Verzi MP, Stanfel MN, Moses KA, Kim B-M, Zhang Y, Schwartz RJ, Shivdasani RA, Zimmer WE. Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology. 2009;136:1701–1710. doi: 10.1053/j.gastro.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahmann C, Basler K. Compartment boundaries: at the edge of development. Trends Genet. 1999;15:320–326. doi: 10.1016/s0168-9525(99)01774-6. [DOI] [PubMed] [Google Scholar]
- 33.Bates MD, Erwin CR, Sanford LP, Wiginton D, Bezerra JA, Schatzman LC, Jegga AG, Ley-Ebert C, Williams SS, Steinbrecher KA, Warner BW, Cohen MB, Aronow BJ. Novel genes and functional relationships in the adult mouse gastrointestinal tract identified by microarray analysis. Gastroenterology. 2002;122:1467–1482. doi: 10.1053/gast.2002.32975. [DOI] [PubMed] [Google Scholar]
- 34.Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beuling E, Bosse T, aan de Kerk DJ, Piaseckyj CM, Fujiwara Y, Katz SG, Orkin SH, Grand RJ, Krasinski SD. GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Dev Biol. 2008;322:179–189. doi: 10.1016/j.ydbio.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenny SE, Tam PK, Garcia-Barcelo M. Hirschsprung’s disease. Semin Pediatr Surg. 2010;19:194–200. doi: 10.1053/j.sempedsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087. e2073. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 39.Sekimoto T, Yoshinobu K, Yoshida M, Kuratani S, Fujimoto S, Araki M, Tajima N, Araki K, Yamamura K. Region-specific expression of murine Hox genes implies the Hox code-mediated patterning of the digestive tract. Genes Cells. 1998;3:51–64. doi: 10.1046/j.1365-2443.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- 40.Sakiyama J, Yokouchi Y, Kuroiwa A. HoxA and HoxB cluster genes subdivide the digestive tract into morphological domains during chick development. Mech Dev. 2001;101:233–236. doi: 10.1016/s0925-4773(00)00564-5. [DOI] [PubMed] [Google Scholar]
- 41.Pitera JE, Smith VV, Thorogood P, Milla PJ. Coordinated expression of 3’ hox genes during murine embryonal gut development: an enteric Hox code. Gastroenterology. 1999;117:1339–1351. doi: 10.1016/s0016-5085(99)70284-2. [DOI] [PubMed] [Google Scholar]
- 42.Kawazoe Y, Sekimoto T, Araki M, Takagi K, Araki K, Yamamura K. Region-specific gastrointestinal Hox code during murine embryonal gut development. Dev Growth Differ. 2002;44:77–84. doi: 10.1046/j.1440-169x.2002.00623.x. [DOI] [PubMed] [Google Scholar]
- 43.Boulet AM, Capecchi MR. Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol. 1996;177:232–249. doi: 10.1006/dbio.1996.0159. [DOI] [PubMed] [Google Scholar]
- 44.Pollock RA, Jay G, Bieberich CJ. Altering the boundaries of Hox3.1 expression: evidence for antipodal gene regulation. Cell. 1992;71:911–923. doi: 10.1016/0092-8674(92)90388-s. [DOI] [PubMed] [Google Scholar]
- 45.Heller RS, Stoffers DA, Hussain MA, Miller CP, Habener JF. Misexpression of the pancreatic homeodomain protein IDX-1 by the Hoxa-4 promoter associated with agenesis of the cecum. Gastroenterology. 1998;115:381–387. doi: 10.1016/s0016-5085(98)70204-5. [DOI] [PubMed] [Google Scholar]
- 46.Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 47.Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- 48.Beck F, Tata F, Chawengsaksophak K. Homeobox genes and gut development. Bioessays. 2000;22:431–441. doi: 10.1002/(SICI)1521-1878(200005)22:5<431::AID-BIES5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Zacchetti G, Duboule D, Zakany J. Hox gene function in vertebrate gut morphogenesis: the case of the caecum. Development. 2007;134:3967–3973. doi: 10.1242/dev.010991. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Stappenbeck TS, White AC, Lavine KJ, Gordon JI, Ornitz DM. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development. 2006;133:173–180. doi: 10.1242/dev.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondo T, Dolle P, Zakany J, Duboule D. Function of posterior HoxD genes in the morphogenesis of the anal sphincter. Development. 1996;122:2651–2659. doi: 10.1242/dev.122.9.2651. [DOI] [PubMed] [Google Scholar]
- 52.Kim BM, Miletich I, Mao J, McMahon AP, Sharpe PA, Shivdasani RA. Independent functions and mechanisms for homeobox gene Barx1 in patterning mouse stomach and spleen. Development. 2007;134:3603–3613. doi: 10.1242/dev.009308. [DOI] [PubMed] [Google Scholar]


