Introduction
With each heartbeat, gap junctions provide direct electrical coupling between cardiomyocytes, thereby permitting rapid and coordinated spread of cardiac excitation. The result of normal gap junction communication is near simultaneous initiation of all cardiomyocyte action potentials and an organized contraction. Alterations in gap junction coupling occur with many forms of heart disease. These coupling alterations lead to defects in electrical excitation which, in the ventricle, can result in malignant arrhythmias and sudden cardiac death1, 2
At the molecular level, gap junction hemichannels, which are called ‘connexons’, are integral membrane channels comprised of six connexin43 molecules. The connexons converge at intercalated discs in clusters of hundreds to thousands, and bind end-to-end with connexonhemichannels of apposing cells to form dense arrays of gap junction plaques. The plaques function as continuous conduits allowing intracellular ions and other small molecules to pass freely between ventricular cardiomyocytes. Biochemical and imaging studies have measured the half-life of connexin43 to be within one and five hours, and of that, less than two hours at the plasma membrane3, 4 In support of this time scale, remodeling of gap junction coupling occurs rapidly after insult, indicating the highly dynamic and active turnover of these channels. The rapid movement of connexons to, within, and from, the plasma membrane is critical to their function. We believe that understanding the regulation of these movements will identify pharmacologic targets to modify gap junction localization. Thus, the basic biology of gap junction trafficking will be used to develop therapeutic interventions to prevent or reverse altered gap junction coupling, and restore more normal electrical function to diseased hearts.
All electrophysiologists understand that at any given point in time the net transmembrane current during a cardiac action potential is the difference between inward current and outward current. Similarly, the number of connexons at the intercalated disc is the balance between connexon delivery to the cell plasma membrane (forward/anterograde trafficking), connexonmovement within the plasma membrane (lateral diffusion), and connexon internalization from the plasma membrane (retrograde trafficking). Forward trafficking, lateral diffusion, and retrograde trafficking combined comprise the connexin43 gap junction life cycle (Figure 1). Opportunities for cells to regulate this life cycle occur directly through posttranslational modification of residues on the connexin molecule, and indirectly through effects on the molecular machinery and cytoskeleton responsible for taking connexons to the plasma membrane, maintaining them there, and bringing about their internalization. In this viewpoint article, we will define the commencement of the gap junction life cycle at the endoplasmic reticulum (ER), where the connexin43 protein has already been transcribed and translated, and begins its journey to the cardiomyocyte plasma membrane. With limited referencing allowed in this viewpoint format, we refer readers to an earlier reviews5 for detailed citations.
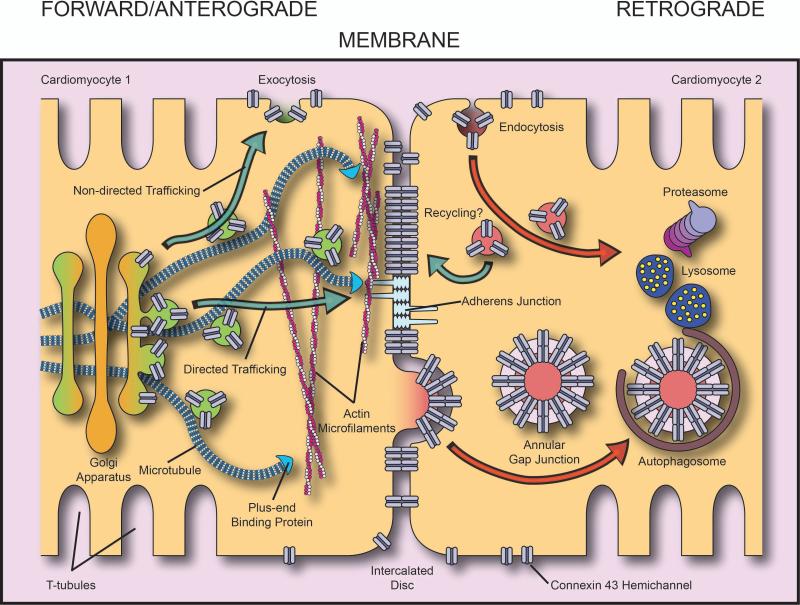
Figure 1. The Gap Junction Life cycle.
Schematic representation of the life cycle of connexin43 gap junctions. Connexin43 hemichannels are packaged into vesicles at the TGN from where they traffic along microtubules that can use specific plus-end binding proteins and adherens junctions to directly localize delivery of connexons to the intercalated disc. Once in the plasma membrane, connexons bind to hemichannels of apposing cells and form gap junctions. Connexons are endocytosed and degraded and gap junctions can be internalized as double-membrane ‘annular’ gap junction structures destined for degradation.
Synthesis and transport to the plasma membrane: Anterograde/Forward Trafficking
Transcribed mRNA of the connexin43 gene (Gja1) is translated to the connexin43 protein by ribosomes and translocated through the membrane of the rough ER, the trafficking start point for denovointegral membrane proteins. Oligomerization of ion channel subunits into channels is usually a rate-limiting step upon exit from the ER to the Golgi apparatus, although this may not to be the case for connexin43, which can progress as a monomer until exiting the Golgi apparatus at the Trans-Golgi network (TGN)6. Chaperone proteins may also bind to ion channels to both facilitate and filter out proteins as they progress from the ER to the TGN. Six connexin43 molecules oligomerize to form connexonhemichannels at the TGN, where they are packaged into vesicles and loaded onto microtubules to be transported via motor proteins to their destination at the cell surface3.
We have previously identified specificity in this trafficking process through microtubule-based targeted delivery of connexon to the intercalated disc. Microtubules are polarized structures with a dynamic growing plus-end and a more stable minus-end which is usually anchored at the centrosomal microtubule organizing center (MTOC). The microtubule plus-end constantly undergoes polymerization (growth) and depolymerization (catastrophe). This process of growth and catastrophe is regulated, in part, by microtubule plus-end binding proteins such as EB1, p150GLUED, CLASPs, CLIPs, and APC. Of these, we have found that EB1 and p150GLUED cooperate in the targeting of connexin43 vesicles to specific membrane subdomains through interaction with the adherens junction complex3. Interestingly, we also found that EB1 is displaced from microtubules in stressed human and mouse myocardium, as well as isolated cardiomyocytes and cell lines, limiting delivery of de novo connexons to the plasma membrane5, 7.
Directed targeting of connexons to intercalated discs places the hemichannels in, or in the immediate vicinity of, existing gap junction plaques. The adherens junction structure is the scaffolding complex that allows connexons to arrive in plasma membrane regions coincident with the regions where gap junctions need to occur. It has also been reported that connexin43 hemichannels are randomly inserted into the plasma membrane and subsequently diffuse freely in the lipid bilayer to gap junctions8. Both targeted delivery and random diffusion paradigms quite possibly are true, especially given the increasing number of reports on the function of non-junctionalconnexons in the plasma membrane. The architecture of the cardiomyocyte sarcolemma, however, with its many subdomains, and the sheer size of these cells, makes non-specific delivery to lateral membrane seem a highly inefficient method of delivering such an essential, and short lived, component of the intercalated disc.
In addition, there is evidence emerging in other cell types that a microtubule based pathway to the cell membrane may involve a complex interplay between microtubule- and actin-based transport. Myosin-based vesicular transport on actin is generally slower than microtubule transport, and vesicles have been observed switching from microtubules to actin filaments9. Conceptually, vesicles loaded with connexin43 hemichannels on a microtubule could be unloaded to the actin cytoskeleton from where they may be transported at a slower rate elsewhere, or await collection at a later time by the appropriate microtubule transporter for selective membrane targeting. Cardiomyocytes may therefore rapidly redirect connexin43 cargo from a particular microtubule's course without having to reorient the original microtubule. Vesicles residing on actin could be sent for degradation, directed to other plasma membrane subdomains than the intercalated disc, or may be maintained as pools of channel ready to be rapidly transported to the plasma membrane once an appropriate signal is received. Such processes would permit more rapid gap junction remodeling than having to await de novo channel arrival from the TGN.
Behavior in the Plasma Membrane
The properties of the connexon within the plasma membrane, such as membrane diffusion coefficients, and the existence of intracellular binding partners that affect hemichannel mobility, are still poorly understood. Above we discussed two complementary models of connexon arrival to the intercalated disc, random placement with lateral diffusion of hemichannels to plaque regions8, and targeted delivery of hemichannels to the appropriate membrane subdomain3. The techniques do not yet exist to label connexin43 with a high quantum efficiency fluorophore such as quantum dots, and thus perform single particle tracking to directly observe individual connexon behavior once in the plasma membrane. Once such labeling is possible, then plasma membrane based connexon movements will be elucidated and mechanisms of connexon delivery to plaques better understood.
Intracellular scaffolding proteins, such as ZO-1, are understood to anchor connexin43 via it's c-terminus, and regulate gap junction plaque size10. However, the mechanisms of pathologic remodeling of gap junctions during disease states remain poorly understood. One possibility is the membrane signals that permit directed targeting of connexons to intercalated discs may themselves be relocated to lateral membrane during disease, thus attracting hemichannel delivery. Another more traditional suggestion is that connexons can become untethered from plaques during disease, and diffuse within the membrane to lateral regions11. As discussed, the high rate of connexin43 turnover, rapid rate of connexon delivery to plasma membrane, and lack of direct visualization of connexon movements once in the plasma membrane, together severely limit the ability for us to understand the mechanisms by which remodeled and poorly localized connexons arrived at their new destination. It will be necessary to temporally label connexons, and record their real-time movements to determine their cellular fate in the normal or stressed myocardium, and thus solve this conundrum.
Internalization : Retrograde trafficking
The half-life of connexin43 in the plasma membrane is measured on the order of minutes to hours. Therefore, just as with forward trafficking, internalization from the plasma membrane represents an important regulatory step in determining the level of gap junction coupling. Posttranslational modification of plasma membrane proteins is well established in endocytic trafficking, and the c-terminus of connexin43 has many residues known to be subject to ubiquitin and ubiquitin-like, acetylation, and phosphorylation based modifications, of which phosphorylation has been most intensively studied. The importance of phosphorylation of connexin is highlighted by recent findings that casein kinase-dependent phosphorylation alters gap junction remodeling and decreases arrhythmic susceptibility12. The specifics of the kinases acting on other residues, and the outcome of specific residues being phosphorylated, remain unclear and in some cases even contradictory. It is useful to consider that, with 22 serines, 5 tyrosines, and 4 threonines, a high number of residues on the c-terminus of connexin43 are potentially subject to phosphorylation. Phosphorylation based modification of the c-terminus is likely complex, with upstream sites that, once modified, cause structural changes to the c-terminus, eliciting gain or loss of binding partners. An added level of complexity is encountered by the fact that connexin43 exists as a hexamer on the plasma membrane, and it is currently not known how phosphorylation differs between individual connexinprotomers of the same connexon.
Ubiquitination of connexin43 is understood to occur following stimulation of protein kinase C, and presumed to result in channel internalization13. Indeed, mono-ubiquitination is a well-studied phenomenon in endosomal trafficking of membrane proteins. Endocytosis of connexin43 can occur either through internalization of uncoupled hemichannels or entire gap junctions, which entails engulfment of the opposing cells plasma membrane, and generation of double-membrane intracellular structures termed annular gap junctions. Both the lysosome and the proteasome have been implicated in degradation of connexin43 and interestingly, autophagy is now known to be involved in degradation of annular gap junctions in failing hearts14. Recent studies have shown recycling of gap junctions to occur during cell cycle progression in cell lines15, but whether gap junctions are recycled in cardiomyocytes remains a controversial issue. It is exciting to consider the possibility that posttranslational modifications of connexin43 may be acting as checkpoints within the same connexin molecule, or connexonhemichannel, requiring a specific ‘code’ of events to permit ubiquitination and internalization of a hemichannel, or annular gap junction. The increasing accessibility of mass spectrometry-based proteomic approaches should assist in the elucidation of this complex process.
Concluding remarks
Following translation at the ER, the gap junction life cycle encompasses many processes including vesicular transport to the plasma membrane, lateral movement within that membrane, internalization, and eventual degradation or possible recycling. By elucidating the molecular mechanisms of these processes, we can determine how gap junction coupling is perturbed in the stressed myocardium, and exploit this knowledge to reverse altered pathologic localization of connexin 43 based gap junctions in damaged hearts.
Acknowledgments
The authors express their gratitude to SamyLamouille Ph.D. (UCSF) for critical review of this manuscript.
This work was supported in part by funding from the National Institutes of Health HL094414 (RMS) and the American Heart Association SDG3420042 (JWS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- 1.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and l-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 3.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- 5.Smyth JW, Shaw RM. Forward trafficking of ion channels: What the clinician needs to know. Heart Rhythm. 2010;7:1135–1140. doi: 10.1016/j.hrthm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the er. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 7.Smyth JW, Hong TT, Gao D, et al. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J. Clin. Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross JL, Ali MY, Warshaw DM. Cargo transport: Molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 2008;20:41–47. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieken F, Mutsaers N, Dolmatova E, et al. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–1112. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remo BF, Qu J, Volpicelli FM, et al. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-o-tetradecanoylphorbol 13-acetate treatment. J. Biol. Chem. 2004;279:50089–50096. doi: 10.1074/jbc.M402006200. [DOI] [PubMed] [Google Scholar]
- 14.Hesketh GG, Shah MH, Halperin VL, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 2010;11:1471–1486. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]