Abstract
A liquid chromatography tandem mass spectrometry (LC-MS/MS) method for simultaneous quantification of nicotine (NIC), cotinine (COT), nornicotine (NNIC), norcotinine (NCOT), nicotine-N-β-D-glucuronide (NIC GLUC), cotinine-N-β-D-glucuronide (COT GLUC), nicotine-1′-oxide (NNO), cotinine-N-oxide (CNO), trans-3′-hydroxycotinine (3-HC), anabasine (AB) and anatabine (AT) was modified and validated for quantification of these selected analytes in rat brain tissue. This analytical method provides support for preclinical NIC pharmacokinetic and toxicological studies after controlled dosing protocols. After brain homogenization and solid-phase extraction, target analytes and corresponding deuterated internal standards were chromatographically separated on a Discovery® HS F5 HPLC column with gradient elution and analyzed by LC-MS/MS in positive electrospray ionization (ESI) mode with multiple reaction monitoring (MRM) data acquisition. Method linearity was assessed and calibration curves were determined over the following ranges: 0.1 – 7.5 ng/mg for NIC, COT GLUC and AB; and 0.025 – 7.5 ng/mg for COT, NNIC, NCOT, NIC GLUC, NNO, CNO, 3-HC and AT (R2 ≥ 0.99 for all analytes). Extraction recoveries ranged from 64% - 115%, LC-MS/MS matrix effects were ≤ 21%, and overall process efficiency ranged from 57% - 93% at low and high quality control concentrations. Intra- and inter-assay imprecision and accuracy for all analytes were ≤ 12.9% and ≥ 86%, respectively. The method was successfully applied to quantification of NIC and metabolites in the brain of post-natal day 90 rats that were sacrificed 2-h after a single 0.8 mg/kg s.c. administration of (−) NIC. In these tissues, striatal concentrations were 204.8±49.4, 138.2±14.2 and 36.1±6.1 pg/mg of NIC, COT and NNIC, respectively. Concentrations of NIC, COT and NNIC in the remaining whole brain (RWhB) were 183.3±68.0, 130.0±14.1 and 46.7±10.3 pg/mg, respectively. Quantification of these same analytes in plasma was also performed by a previously validated method. NIC, COT, NNIC, NCOT, NNO and CNO were detected in plasma with concentrations comparable to those reported in previous studies. However, and in contrast to brain tissues, COT concentrations in plasma were significantly higher than were those of NIC (194.6±18.6 ng/mL versus 52.7±12.9 ng/mL). Taken together, these results demonstrate that a sensitive and selective method has been developed for the determination of NIC biomarkers in rat brain.
Keywords: liquid chromatography mass spectrometry, nicotine, metabolites, brain, rat
1. Introduction
Cigarette smoking negatively impacts public health by contributing to massive annual health-related economic losses and many deaths each year. Exposure to tobacco constituents, either via active smoking or passive inhalation of environmental tobacco smoke, harms several organs in the body resulting in an increased risk of heart disease, lung disease and cancers [1, 2]. Extensive research has been conducted to identify which substances in cigarettes are responsible for these increased risks, as well as to identify which constituents contribute to the addictive properties of tobacco [3-6]. As a result, the addictive liability of tobacco smoking has been partly attributed to the presence of NIC [1, 2], and current treatment modalities for addiction often include, but are not limited to, substitution of cigarettes for NIC in the form of approved medications such as transdermal patches, lozenges, nasal sprays, inhalers and gums [7, 8]. Of note, there is an increasing interest in other therapeutic uses of NIC and/or nicotinic agonists beyond substitution therapy, including their use to improve neurological symptoms associated with several disorders such as Alzheimer’s disease, attention deficit hyperactivity disorder and Parkinson’s disease [9-17].
Studies investigating the mechanisms underlying the CNS effects of drugs, such as NIC, in biological tissues, can require sensitive and specific measurement of both the parent compound and its metabolites. To date, there are a limited number of preclinical studies that have used mass spectrometry to report concentrations of NIC and NIC biomarkers in animal brain following controlled dose administration [18-20]. Among these, Deutsch et al. [18] developed a method to quantify NIC and COT from rat brain by gas chromatography/mass spectrometry (GC/MS), and reported the limit of detection (LOD) to be 5 pg/mg for NIC and 15 pg/mg for COT. They also reported that the mean NIC concentration in rats’ brain was 200 pg/mg 2h after a 1 mg/kg intraperitoneal administration [18]. Also, Sarasin et al. [19] quantified NIC and COT by GC/MS in rat fetal brains after 1 week of maternal subcutaneously exposure to 1.9 mg/kg/day NIC with resulting mean concentrations of 520 pg/mg NIC and 1600 pg/mg COT. In addition, Kim et al. [20] measured the concentration of NIC biomarkers in rat brain after 4 weeks of chronic oral administration of 10 mg/kg/day NIC by a previous validated GC/MS plasma method [21], and reported mean concentrations of 83 pg/mg, 830 pg/mg and 13 pg/mg for NIC, COT and NNIC, respectively. For clinical studies, a few additional analytical methods have been validated to quantify NIC biomarkers from human brain. For example, Urakawa et al. [22] validated a GC/MS method for human brain and reported LOD to be 5 pg/mg for NIC and 10 pg/mg for COT. Later, Shakleya and Huestis [23] developed a LC-MS/MS method for quantification of selected NIC biomarkers and other drugs from human brain with a reported LOD of 125 pg/mg, 12.5 pg/mg and 25 pg/mg for NIC, COT and NNIC, respectively. Previously, our laboratory developed and validated an assay for the determination of NIC and several metabolites in plasma [24]. The current study builds upon that work and provides an expanded LC-MS/MS method that simultaneously quantifies several additional NIC metabolites in rat brain tissue, including NNIC, NCOT, NIC GLUC, COT GLUC, NNO and CNO.
Two types of studies contribute to the rationale for the identification and quantification of an expanded panel of NIC metabolites. The first is based on known genetic polymorphisms in NIC metabolizing enzymes, which can lead to differences in the profile of NIC metabolites in biological specimens [25-27]. For example, CYP2A6 is the major enzyme that breaks down NIC in humans, primarily by converting NIC to COT and COT to 3-HC [1]. Polymorphisms in CYP2A6 and other enzymes have been studied and have revealed differences in metabolic pathway among individuals and ethnic groups [25]. Accordingly, slower CYP2A6 metabolism of NIC leads to increased glucuronidation activity, as well as diminished NIC consumption and associated smoking behavior [1, 25]. Polymorphisms have also shown to affect risk of developing cancer and smoking cessation treatment [26, 27]. Thus, these studies illustrate that quantification of metabolites produced via several metabolic routes is necessary to provide accurate results interpretation of pharmacokinetic and toxicological studies of NIC.
Other studies that illustrate the importance of looking at different metabolites involve the pharmacological effect of the metabolites themselves in brain. For example, it has been reported that COT evokes both dopamine release and desensitization of nicotinic acetylcholine receptors [28-30]. Further, NNIC acts as a nicotinic receptor agonist, also evoking dopamine release and receptor desensitization [31, 32]. In behavioral studies, NNIC reportedly maintains self-administration [33]. Still another example suggesting the bioreactivity of NIC metabolites includes findings that NIC GLUC, COT GLUC and 3-HC are all markers for drug clearance and also UGT2B1 enzyme function [34, 35].
The two alkaloids AB and AT, commonly found in tobacco products along with NIC, have been shown to be pharmacologically active. AB increases catecholamine secretion from rat adrenomedullary gland [36] and AT increases nicotine self-administration and locomotor activity in rats [37]. Given the potential bioactivity of NIC metabolites, the purpose of this study was to evaluate a comprehensive panel of metabolites in brain when investigating neurological effects and toxicity after NIC exposure.
2. Experimental Methods
2.1 Biological samples for method development and 2-hr drug disposition studies
Male Sprague-Dawley rats (approximately 400 g; Charles River Laboratories; Raleigh, NC) were housed three per cage under conditions of controlled temperature (23°C) and lighting (14:10 light/dark cycle) with food and water provided ad libitum. Animals were sacrificed via decapitation, and brain tissues we collected over ice to obtain whole brain tissue, striatal tissue and RWhB tissue. Whole brains were obtained for method development and brain parts were obtained for the 2-hr drug disposition study. Tissues were immediately frozen on dry ice and then stored at −80°C until thawed for analysis. Trunk blood was collected in sodium heparin-containing tubes and centrifuged at 3000 × g for 15 min to obtain plasma. Plasma was separated, placed in Eppendorf® microcentrifuge tubes (Eppendorf North America, Hauppauge, NY), and stored at −80°C until analyzed. Prior to use as matrix in the development of the analytical method, frozen brain tissues were thawed, homogenized in Milli-Q water (20 mg/mL), extracted, confirmed to be negative for the selected analytes (<LOD), and stored at −4°C. Striata and RWhB sample preparations are described under section 2.4.2. The analytical balance used for tissue weighing was an AG104 Mettler Toledo, readability = 0.1 mg (Columbus, OH).
2.2 Reference Standards, Chemicals and Reagents
(−) Nicotine (NIC) hydrogen tartrate salt (≥ 98 %) was obtained from Sigma (St Louis, MO) and used for preparation of LC-MS/MS calibrators and quality control samples, as well as for animal administration studies. (±)Cotinine-d3 (COT-d3) was obtained from Cerilliant (Austin, TX). The following reference standards and deuterated internal standards were obtained from Toronto Research Chemicals (North York, Canada): trans-3′-hydroxycotinine (3-HC) and trans-3′-hydroxycotinine-d3 (3-HC-d3); (R,S)-anabasine (AB) and (R,S)-anabasine-2,4,5,6-d4 (AB-d4); (R,S)-anatabine (AT) and (R,S)-anatabine-2,4,5,6-d4 (AT-d4); (S)-cotinine-N-oxide (CNO) and (R,S)-cotinine-N-oxide-methyl-d3 (CNO-d3); cotinine N-β-D-glucuronide (COT GLUC) and cotinine-d3 N-β-D-glucuronide (COT GLUC-d3); (R,S)-norcotinine (NCOT) and (R,S)-norcotinine pyridyl-d4 (NCOT-d4); (±)nicotine-d3 (NIC-d3); nicotine-N-(4-deoxy-4,5-didehydro)-β-D glucuronide (NIC GLUC) and nicotine-N-(4-deoxy-4,5-didehydro)-β-D glucuronide-methyl-d3 (NIC GLUC-d3); (R,S)-nornicotine (NNIC) and (R,S)-nornicotine-d4 (NNIC-d4); (1′S, 2′S)-nicotine-1′-oxide and (1′R, 2′S)-nicotine-1′-oxide mixture (NNO) and (±)-trans nicotine-1′-oxide-methyl-d3 (NNO-d3).
Solid phase extraction cartridges (Oasis® MCX (60 mg, 3 mL)) were obtained from Waters (Milford, MA). HPLC grade methanol, dichloromethane (DCM) and isopropyl alcohol (IPA) were obtained from Honeywell Burdick & Jackson (Morristown, NJ). Ammonium acetate and glacial acetic acid were obtained from Spectrum (Gardena, CA). Concentrated hydrochloric acid (HCl), concentrated formic acid and concentrated ammonium hydroxide were obtained from Fisher Scientific (Pittsburgh, PA). All chemicals and reagents were HPLC grade (≥ 99 % purity).
2.3 Calibrator and Quality Control Solutions
Calibrators and quality control (QC) working solutions were prepared in methanol at concentrations of 10 μg/mL, 1 μg/mL, 0.1 μg/mL and 0.01 μg/mL for 3-HC, AB, AT, CNO, COT, COT GLUC, NCOT, NIC hydrogen tartrate salt (weight corrected for nicotine free-base), NIC GLUC, NNIC and NNO. The NIC hydrogen tartrate salt was used as it was determined to be more stable in solution than nicotine free-base. The analytical balance used was XS3DU Mettler Toledo microbalance with readability of 1 μg (Columbus, OH). Due to the unavailability of different commercial lot numbers for some of these reference materials, the same lot numbers were used to prepare both calibrator and QC working solutions, however, two separate analysts prepared them. All working solutions were stored in the freezer at −20 °C and equilibrated to room temperature before each analytical run. Table 1 provides the final calibrator and QC concentrations for each analyte fortified to brain homogenates. Finally, a single combined methanolic deuterated internal standard working solution was prepared at 1 μg/mL and contained: 3-HC-d3, AB-d4, AT-d4, CNO-d3, COT-d3, COT GLUC-d3, NCOT-d4, NIC-d3, NIC GLUC-d3, NNIC-d4 and NNO-d3. The working deuterated internal standard solution was stored in the freezer at −20°C as well.
TABLE 1.
Calibrators and quality control concentrations
| Analyte | Brain calibrators (ng/mg) | Quality control (ng/mg) |
||
|---|---|---|---|---|
| Low | Medium | High | ||
| 3-HC | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.15 | 3.0 | 6.0 |
| AB | 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 1.50 | 3.0 | 6.0 |
| AT | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.15 | 3.0 | 6.0 |
| CNO | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.075 | 3.0 | 6.0 |
| COT | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.30 | 3.0 | 6.0 |
| COT GLUC | 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.30 | 3.0 | 6.0 |
| NCOT | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.30 | 3.0 | 6.0 |
| NIC | 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 1.50 | 3.0 | 6.0 |
| NIC GLUC | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.075 | 3.0 | 6.0 |
| NNIC | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.075 | 3.0 | 6.0 |
| NNO | 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1.0, 2.5, 5.0, 7.5 | 0.075 | 3.0 | 6.0 |
During the initial stage of assay development, the linear range for each analyte was determined (as described below), and target concentrations for routine quality control samples selected. Criteria for selection of the target QC concentrations included: the low QC concentration should be at least three times the lower limit of quantification (LOQ; definition of lower limit of quantification is described below in section 2.7 – method validation); the medium QC concentration should be at the median concentration value of the standard curve; and the high QC concentration should be 80% of the upper limit of quantification. These target concentrations were then utilized for preparation of fortified QC samples for the determinations of assay imprecision, accuracy, extraction recovery, LC-MS/MS matrix effect and overall process efficiency during method validation.
2.4 Sample preparation and extraction
Brain sample preparation was adapted from Shakleya and Huestis 2009 [23] and the extraction was adapted from Miller et al 2010 [24] as described below. Preparation of plasma samples and extraction procedures were as described in Miller et al 2010 [24] and briefly described below.
2.4.1 Preparation of brain tissue for use in method development
Thawed aliquots (1 mL) of (20 mg tissue/mL) analyte-free Sprague-Dawley brain homogenate were transferred to 13×100 glass test tubes and fortified with working solutions to prepare calibrators or QC samples (as described in Table 1). Each homogenate was also fortified with 25 μL of 1 μg/mL deuterated internal standard solution (final concentration of 25 ng/mL). The homogenates were vortexed and centrifuged at 1100 × g for 10 min. The supernatant was transferred to a new test tube and treated with 10 μL of concentrated HCl to adjust the matrix pH to 1. Pellets were discarded. The acidified supernatant samples were loaded onto MCX cartridges as described in solid-phase extraction section (2.4.3).
2.4.2 Preparation of animal samples from NIC 2-hr study
Frozen brain tissues from 2-hr NIC administration study were thawed at room temperature before preparation. Rat brain tissues were weighed and homogenized in cold Milli-Q water by using a Wheaton Safe-Grind tissue grinder set, to achieve a final concentration of 20 mg tissue/mL (for RWhB), or the whole left striatum (weight approximately 25 mg) homogenized in 1 mL. Prior to extraction, all brain homogenate samples (1 mL) were fortified with 25 μL of 1 μg/mL deuterated internal standard solution to achieve a final concentration of 25 ng/mL. The homogenates were vortexed and centrifuged at 1100 × g for 10 min. The brain homogenate supernatant was transferred to a new tube and treated with 10 μL of concentrated HCl to adjust the pH to 1. Pellets were discarded. The acidified brain supernatant was loaded onto MCX cartridges as described in solid-phase extraction section (2.4.3). Since striatum weight varies, the final calculation for quantification was performed by multiplying the measured concentration (in ng/mg) by 20 (i.e. 20 mg/mL brain homogenate used for calibration curve) and divided by individual striatum weight (in mg).
Thawed plasma samples were diluted by adding 750 μL of analyte-free Sprague-Dawley plasma (BioChemed, Winchester, VA) to 250 μL of study sample and mixed. Prior to extraction, all plasma samples were fortified with 50 ng/mL deuterated internal standard. Following precipitation of plasma proteins with 1 mL of cold 10% aqueous trichloroacetic acid, the plasma samples were vortexed and centrifuged at 1100 × g for 10 min. The plasma supernatant was then immediately transferred onto MCX and HLB combination cartridges and extracted as described in Miller et al 2010 [24].
2.4.3 Solid-phase extraction of brain samples
The solid-phase extraction was modified from Miller et al 2010 [24]. Briefly, Oasis® MCX (mix-mode cation exchange) cartridges (3cc, 60 mg) were used for extraction of analytes (Waters®, Millford, MA). The cartridges were conditioned with 2 mL HPLC grade methanol followed by 2 mL 2 % aqueous formic acid. The samples were loaded onto the cartridges. The cartridges were washed with 1 mL 2% formic acid and 1 mL HPLC grade methanol. Analytes were eluted with 1.5 mL 5% (v/v) ammoniated methanol and 1.5 mL DCM:IPA:aqueous ammonium hydroxide (78:20:2 v/v). 100 μL of 1% HCl in methanol was added to each sample in order to improve nicotine recovery and vortex before complete evaporation at 40°C (Zymark Turbovap® LV Evaporator). The samples were reconstituted with 130 μL of 10 mM ammonium acetate (pH 5.0) + 0.001 % formic acid:HPLC grade methanol (85:15 v/v). Contents were transferred to plastic autosampler vials and loaded to LC-MS/MS.
2.5 Liquid chromatography tandem-mass spectrometry conditions and criteria for identification of analytes
The LC-MS/MS method was adapted from Miller et al 2010 [24]. Analytes were resolved on a Discovery® HS F5 HPLC column (10 cm × 4 mm × 3 μm, Supelco®, Bellefonte, PA) and Acquity UPLC® system (Waters®, Millford, MA). The mobile phase consisted of a gradient elution of 10 mM ammonium acetate with 0.001% formic acid at pH 5.0 (aqueous) and methanol (organic). Initial conditions were: 85% aqueous and 15% organic phase, increased linearly to 76% organic phase at 11.6 min, and held for 3.4 min to re-equilibrate the HPLC column. The flow rate was set at 0.6 mL/min. The cone voltages, collision energies and MRM transitions are described in Table 2. Methanol in water (5% v/v) was used as a weak wash and acetonitrile in methanol (1% v/v) was used as a strong wash.
TABLE 2.
LC-MS/MS parameter for nicotine biomarkers in rat brain.
| Analyte | MRM transitions (min) |
Cone voltage (V) |
Collision energy (AU) |
|---|---|---|---|
| 3-HC | 193.1>79.9a | 30 | 25 |
| 193.1>85.7 | 30 | 25 | |
| 3-HC-d3 | 196>79.8a | 30 | 25 |
| 196.1>88.8 | 30 | 25 | |
| AB | 163.1>117.9a | 30 | 24 |
| 163.1>130.0 | 30 | 24 | |
| AB-d4 | 167.1>121.9a | 30 | 24 |
| 167.1>134.1 | 30 | 24 | |
| AT | 161.1>144.0a | 25 | 18 |
| 161.1>106.6 | 25 | 18 | |
| AT-d4 | 165.1>148a | 25 | 18 |
| 165.1>111 | 25 | 18 | |
| CNO | 193.1>95.7a | 30 | 25 |
| 193.1>97.9 | 30 | 25 | |
| CNO-d3 | 196.1>95.9a | 30 | 25 |
| 196.1>101.0 | 30 | 25 | |
| COT | 177>79.7a | 30 | 27 |
| 177>97.7 | 30 | 27 | |
| COT-d3 | 180.1>79.6a | 30 | 27 |
| 180.1>100.8 | 30 | 27 | |
| COT GLUC | 353.4>177.0 | 25 | 21 |
| COT GLUC-d3 | 356.2>180.2 | 25 | 21 |
| NCOT | 163.1>79.8a | 30 | 25 |
| 163.1>83.8 | 30 | 25 | |
| NCOT-d4 | 167.1>83.8 | 30 | 25 |
| NIC | 163.1>130.2a | 30 | 20 |
| 163.1>116.7 | 30 | 20 | |
| NIC-d3 | 166.1>130a | 30 | 20 |
| 166.1>116.9 | 30 | 20 | |
| NIC GLUC | 321.2>163a | 15 | 27 |
| 321.2>83.9 | 15 | 27 | |
| NIC GLUC-d3 | 324.3>166.1a | 15 | 27 |
| 324.3>86.9 | 15 | 27 | |
| NNIC | 149.1>79.8a | 30 | 22 |
| 149.1>129.9 | 30 | 22 | |
| NNIC-d4 | 153.1>83.9a | 30 | 22 |
| 153.1>133.9 | 30 | 22 | |
| NNO | 179.1>129.9a | 30 | 24 |
| 179.1>116.9 | 30 | 24 | |
| NNO-d3 | 182.1>130.0 | 30 | 24 |
Most abundant fragment ion only used for quantification.
Mass spectrometric analysis was performed on a Quattro Premier XE™ triple quadruple mass spectrometer (Waters®, Millford, MA) equipped with MassLynx™ v 4.1 software. Mass spectrometer conditions were: electrospray in positive mode with MRM transition acquisition (two transitions were possible for all analytes except for COT GLUC which produced only one fragment ion); capillary voltage 3.25 kV; source temperature 100°C; desolvation temperature 350°C; nitrogen as desolvation gas (600 L/h) and cone gas (50 L/h); argon as collision cell pressure (7.38e-3 mbar); and collision gas flow rate 0.35 mL/min.
2.6 Method Validation
Sensitivity, linearity, selectivity, imprecision, accuracy, matrix effect, analyte recovery and process efficiency were evaluated. Peak area ratios (PAR) of the most abundant ion for both analytes of interest and internal standards for each analyte were automatically calculated with MassLynx ™ v 4.1 software.
The sensitivity and linearity of the assay were determined by analysis of eight to ten calibrators of increasing concentration as described in Table 1. During initial method development, drug calibrators and deuterated internal standards were fortified to 20 mg tissue/mL of analyte-free brain homogenates, extracted via solid-phase extraction, and analyzed by LC-MS/MS. The lower concentrations of the calibration curve were analyzed for determination of LOD and LOQ. The LOD was defined as the concentration value at which a signal-to-noise ratio (S/N) equal to or greater than 3 was obtained for selected ion transitions. Similarly, the LOQ was defined as the concentration value at which a signal-to-noise ratio (S/N) equal to or greater than 10 was obtained. The highest concentration on the curve was determined based upon previous studies that quantified NIC biomarkers from biological samples and then assigned to be the upper limit of quantification (ULOQ). The LOQ of the assay was confirmed by verifying that the S/N was ≥10:1 for the calibrator fortified at the LOQ, as well as achieving accuracy and imprecision within stated criteria analyzed on 4 distinct batches that were prepared for method validation. Duplicates of double blank samples (i.e. tissue homogenate without target analytes and deuterated internal standard) and negative control samples (i.e. tissue homogenate containing only deuterated internal standard) were analyzed concurrently with every batch. A coefficient of determination (R2) greater than 0.990 was required for determination of linearity. These calibrators were run in all batches in order to provide the standard curve. Results are described in Table 3.
TABLE 3.
Limits of detection (LOD), limits of quantification (LOQ), upper limits of quantification (ULOQ) and calibration curve results (N=11) of nicotine and metabolites in rat brain.
| Analyte | LOD (ng/mg) | LOQ (ng/mg) | ULOQ (ng/mg) | R2 |
|---|---|---|---|---|
| 3-HC | 0.010 | 0.025 | 7.5 | 0.997 |
| AB | 0.010 | 0.100 | 7.5 | 0.995 |
| AT | 0.010 | 0.025 | 7.5 | 0.997 |
| CNO | 0.010 | 0.025 | 7.5 | 0.994 |
| COT | 0.010 | 0.025 | 7.5 | 0.999 |
| COTGLUC | 0.010 | 0.100 | 7.5 | 0.990 |
| NCOT | 0.010 | 0.025 | 7.5 | 0.999 |
| NIC | 0.010 | 0.100 | 7.5 | 0.999 |
| NIC GLUC | 0.010 | 0.025 | 7.5 | 0.998 |
| NNIC | 0.010 | 0.025 | 7.5 | 0.998 |
| NNO | 0.010 | 0.025 | 7.5 | 0.994 |
Signal to noise ratio was used to determine LOD (≥3 and ≤10) and LOQ (>10).
Results come from 4 batches analyzed among 4 different days.
The selectivity and specificity of the assay were assessed by analysis of six distinct analyte-free brain samples (n=3 each) to which deuterated internal standards were fortified. Brain homogenate (20 mg tissue/mL) was fortified with 25 ng/mL deuterated internal standard and subjected to solid-phase extraction and LC-MS/MS analysis. Potential interference from endogenous substances was evaluated by examination of chromatographs for peaks present at the same retention time (±2% range) of deuterated internal standards.
The effect of matrix on analyte ionization response was assessed by analysis of low and high QC samples (n=6 each). Analyte-free brain homogenate (20 mg tissue/mL) were subjected to solid-phase extraction and subsequently fortified with analyte at low and high QC concentrations (specific concentrations are listed in Table 1) and with 25 ng/mL deuterated internal standard. Concentrated HCl (100 μL) was added and samples were dried to completion at 40°C (Zymark Turbovap® LV Evaporator). Unextracted samples, for comparison to extracted samples, were prepared by fortification with the same concentrations of low and high QC calibrators and deuterated internal standard into 100 μL of concentrated HCl. Unextracted samples were dried until completion at 40°C and reconstituted with initial mobile phase condition. The mean PAR of extracted QC samples was compared to the mean PAR of unextracted QC samples for each concentration (± %relative standard deviation (RSD)). A 100% value indicates no LC-MS/MS matrix effect. Values <100% indicate ion suppression and values >100% indicate ion enhancement. Results are shown in Table 4.
TABLE 4.
Matrix effect (N=6), process efficiency (N=5) and extraction recovery (N=5) of nicotine and metabolites in rat brain
| Analyte | Matrix effect (%Mean ± %RSD) |
Process efficiency (%Mean ± %RSD) |
Extraction recovery (%Mean ± %RSD) |
|||
|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | |
| 3-HC | 102 ± 3.6 | 106 ± 4.7 | 91 ± 1.5 | 87 ± 3.5 | 89 ± 2.6 | 82 ± 3.2 |
| AB | 97 ± 5.2 | 98 ± 3.2 | 78 ± 1.5 | 82 ± 1.2 | 82 ± 2.4 | 84 ± 1.1 |
| AT | 92 ± 3.8 | 100 ± 6.4 | 79 ± 0.5 | 81 ± 4.1 | 86 ± 2.9 | 82 ± 0.7 |
| CNO | 90 ± 1.9 | 90 ± 5.6 | 57 ± 5.7 | 73 ± 3.5 | 64 ± 5.5 | 81 ± 3.3 |
| COT | 97 ± 2.9 | 97 ± 4.2 | 82 ± 4.0 | 85 ± 2.0 | 85 ± 4.4 | 89 ± 1.6 |
| COT GLUC | 95 ± 6.4 | 109 ± 7.3 | 33 ± 8.7 | 36 ± 12.2 | 35 ± 24 | 33 ± 12 |
| NCOT | 100 ± 4.6 | 103 ± 3.2 | 80 ± 1.4 | 86 ± 3.3 | 81 ± 3.4 | 84 ± 0.9 |
| NIC | 91 ± 3.0 | 92 ± 3.6 | 69 ± 1.7 | 75 ± 0.7 | 76 ± 0.9 | 75 ± 1.1 |
| NIC GLUC | 102 ± 6.5 | 99 ± 3.2 | 78 ± 3.7 | 81 ± 2.9 | 78 ± 1.9 | 83 ± 2.7 |
| NNIC | 121 ± 8.6 | 105 ± 4.3 | 92 ± 2.5 | 92 ± 4.5 | 115 ± 3.8 | 81 ± 1.6 |
| NNO | 109 ± 9.2 | 113 ± 5.8 | 93 ± 1.9 | 89 ± 4.0 | 87 ± 3.5 | 80 ± 4.0 |
Analyte recovery was obtained by the analysis of five replicates at low and high QC concentrations with deuterated internal standard added before and after solid-phase extraction. First, analyte-free brain homogenates (20 mg tissue/mL) were fortified with low and high QC (concentrations are listed in Table 1) and with 25 ng/mL deuterated internal standard then subjected to solid-phase extraction. Concentrated HCl (100 μL) was added and samples were dried to completion at 40°C (Zymark Turbovap® LV Evaporator). For comparison, analyte-free brain homogenates (20 mg tissue/mL) were fortified at the same concentrations of low and high QC and subjected to solid-phase extraction. Samples were then fortified with 25 ng/mL deuterated internal standard and evaporated until dryness at 40°C. Results were obtained by dividing the mean PAR of samples with internal standards added before extraction by the mean PAR of samples with internal standards added after extraction (± %RSD). Results are shown in Table 4.
Overall process efficiency was also assessed by the analysis of five replicates at low and high QC concentrations. Analyte-free brain homogenates (20 mg tissue/mL) were fortified with low and high QC (concentrations are listed in Table 1) and subjected to solid-phase extraction. Samples were then fortified with 25 ng/mL deuterated internal standard and evaporated until dryness at 40°C (Zymark Turbovap® LV Evaporator). Unextracted samples, for comparison to extracted samples, were fortified at the same concentrations of low and high QC and deuterated internal standard into 100 μL of concentrated HCl and dried until completion at 40°C. Both extracted and unextracted samples were reconstituted with initial mobile phase condition. Results were obtained by dividing the mean PAR of extracted QC samples by the mean PAR of unextracted QC samples (± %RSD). Results are shown in Table 4.
Imprecision and accuracy of the assay were assessed by the analysis of six replicates of low, medium and high QC samples. Analyte-free brain homogenates (20 mg tissue/mL) were fortified at low, medium and high QC (concentrations listed in Table 1) and with 25 ng/mL deuterated internal standard. Samples were then extracted and analyzed as described above (section 2.4.3 and 2.5). The minimal quantitative acceptance performance criteria for QC samples included: calculated concentrations within ± 20% of theoretical target value; retention time to be within 2% of deuterated internal standard of target analyte; and S/N ratio to be ≥10. Intra-assay imprecision and accuracy results were obtained from one batch analyzed on one day (seen in Table 5); inter-assay results were obtained from 3 to 4 batches analyzed over 3 to 4 distinct days (seen in Table 6). Imprecision was calculated by dividing the standard deviation of the observed concentrations by the mean of the observed concentrations x100 (N=6 for each concentration level) (=%RSD). Accuracy results were obtained by dividing the mean of the observed concentrations by the target concentration and multiplying result by 100 (N=6 for each concentration).
TABLE 5.
Intra-assay imprecision and accuracy (N=5 or 6).
| Analyte | Target concentration (ng/mg) |
Observed concentration (ng/mg ± standard deviation) |
Imprecision (%RSD) |
Accuracy (%) |
|---|---|---|---|---|
| 3-HC | 0.15 | 0.13 ± 0.00 | 0.00 | 87 |
| 3.00 | 3.12 ± 0.20 | 6.27 | 104 | |
| 6.00 | 5.75 ± 0.50 | 8.65 | 96 | |
| AB | 1.50 | 1.39 ± 0.03 | 1.93 | 91 |
| 3.00 | 3.04 ± 0.12 | 4.05 | 101 | |
| 6.00 | 5.69 ± 0.46 | 8.18 | 94 | |
| AT | 0.15 | 0.13 ± 0.00 | 0.00 | 87 |
| 3.00 | 3.05 ± 0.17 | 5.10 | 102 | |
| 6.00 | 5.66 ± 0.46 | 8.17 | 94 | |
| CNO | 0.075 | 0.07 ± 0.01 | 7.04 | 98 |
| 3.00 | 3.24 ± 0.16 | 5.08 | 108 | |
| 6.00 | 6.03 ± 0.47 | 7.82 | 101 | |
| COT | 0.30 | 0.29 ± 0.01 | 3.08 | 97 |
| 3.00 | 3.26 ± 0.15 | 4.69 | 109 | |
| 6.00 | 6.14 ± 0.49 | 7.62 | 102 | |
| COT GLUC * | 0.30 | (0.18 ± 0.02) | 8.21 | (61) |
| 3.00 | (1.89 ± 0.18) | 9.54 | (63) | |
| 6.00 | (3.70 ± 0.32) | 8.71 | (62) | |
| NCOT | 0.30 | 0.28 ± 0.00 | 1.45 | 94 |
| 3.00 | 3.05 ± 0.18 | 6.03 | 102 | |
| 6.00 | 5.60 ± 0.45 | 8.10 | 93 | |
| NIC | 1.50 | 1.37 ± 0.03 | 2.59 | 92 |
| 3.00 | 3.16 ± 0.12 | 3.92 | 105 | |
| 6.00 | 5.95 ± 0.44 | 7.35 | 99 | |
| NIC GLUC | 0.075 | 0.08 ± 0.00 | 5.21 | 104 |
| 3.00 | 3.09 ± 0.21 | 6.86 | 103 | |
| 6.00 | 5.89 ± 0.34 | 5.84 | 98 | |
| NNIC | 0.075 | 0.09 ± 0.00 | 5.08 | 117 |
| 3.00 | 3.36 ± 0.12 | 3.53 | 112 | |
| 6.00 | 6.38 ± 0.46 | 7.30 | 106 | |
| NNO | 0.075 | 0.08 ± 0.00 | 5.00 | 109 |
| 3.00 | 3.38 ± 0.43 | 12.89 | 111 | |
| 6.00 | 6.45 ± 0.28 | 4.35 | 108 |
Acceptable QC criterion was ± 20% of theoretical target concentration.
Numbers in parentheses represent QC that did not meet the requirement criterion for quantitation of ± 20% deviation of theoretical target concentration.
TABLE 6.
Inter-assay imprecision and accuracy (N=16-18).
| Analyte | Target concentration (ng/mg) |
Observed concentration (ng/mg ± standard deviation) |
Imprecision (%RSD) |
Accuracy (%) |
|---|---|---|---|---|
| 3-HC | 0.15 | 0.14 ± 0.01 | 3.81 | 90 |
| 3.00 | 2.95 ± 0.24 | 8.07 | 98 | |
| 6.00 | 5.74 ± 0.56 | 9.81 | 96 | |
| AB | 1.50 | 1.37 ± 0.07 | 4.73 | 91 |
| 3.00 | 3.04 ± 0.22 | 7.09 | 101 | |
| 6.00 | 5.97 ± 0.70 | 11.68 | 100 | |
| AT | 0.15 | 0.13 ± 0.01 | 5.04 | 89 |
| 3.00 | 3.00 ± 0.20 | 6.75 | 100 | |
| 6.00 | 5.88 ± 0.59 | 10.04 | 91 | |
| COT | 0.30 | 0.30 ± 0.02 | 5.39 | 101 |
| 3.00 | 3.23 ± 0.18 | 5.55 | 108 | |
| 6.00 | 6.38 ± 0.46 | 7.27 | 106 | |
| COT GLUC * | 0.30 | (0.18 ± 0.02) | 12.84 | (59) |
| 3.00 | (1.99 ± 0.21) | 10.41 | (67) | |
| 6.00 | (4.05 ± 0.64) | 15.76 | (67) | |
| CNO | 0.075 | 0.07 ± 0.01 | 7.17 | 95 |
| 3.00 | 2.97 ± 0.33 | 11.06 | 99 | |
| 6.00 | 5.93 ± 0.83 | 14.02 | 99 | |
| NCOT | 0.30 | 0.29 ± 0.02 | 5.48 | 96 |
| 3.00 | 3.07 ± 0.19 | 6.07 | 102 | |
| 6.00 | 5.98 ± 0.58 | 9.75 | 100 | |
| NIC | 1.50 | 1.29 ± 0.09 | 6.66 | 86 |
| 3.00 | 3.04 ± 0.29 | 8.85 | 101 | |
| 6.0 | 5.99 ± 0.58 | 9.68 | 100 | |
| NIC GLUC | 0.075 | 0.07 ± 0.01 | 11.57 | 99 |
| 3.00 | 3.08 ± 0.22 | 7.11 | 103 | |
| 6.00 | 6.07 ± 0.64 | 10.49 | 101 | |
| NNO | 0.075 | 0.08 ± 0.01 | 8.62 | 109 |
| 3.00 | 3.32 ± 0.37 | 11.02 | 111 | |
| 6.00 | 6.63 ± 0.36 | 5.46 | 111 | |
| NNIC | 0.075# | 0.09 ± 0.01 | 10.76 | 110 |
| 3.00 | 3.13 ± 0.26 | 8.25 | 104 | |
| 6.00 | 6.12 ± 0.36 | 5.91 | 102 |
Acceptable QC criteria was ± 20% of theoretical target concentration. Maximum number of data points for Between-Run calculations is 18. Batches analyzed among 3 different days (except NNIC and NNO which analysis was done with an extra day).
N=12 for NNIC lower QC.
Numbers in parentheses represent QC that did not meet the requirement criterion for quantitation of ± 20% deviation of theoretical target concentration.
3. Results and discussion
3.1 Method validation
NIC, eight metabolites and two alkaloids were fortified to analyte-free rat brain and, after solid-phase extraction, simultaneously resolved in a HPLC column and quantified on a triple quadruple mass spectrometer. The extraction recovery of NIC, COT, NNIC, NCOT, NNO, CNO, NIC GLUC, 3-HC, AT and AB are shown in Table 4.
COT GLUC was the only analyte that did not meet the laboratory’s criteria for acceptable accuracy, however the data are reported in Table 4 for informational purposes. Since its QC samples did not lie within 20% deviation of target concentration, this analyte is considered a qualitative rather than quantitative biomarker using this method. The relatively low accuracy (Table 5 and 6) of COT GLUC can be likely explained by its low process efficiency and extraction recovery (see Table 4).
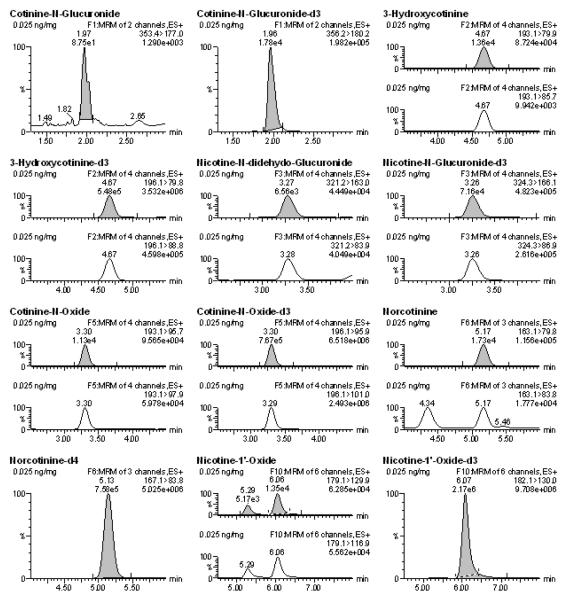
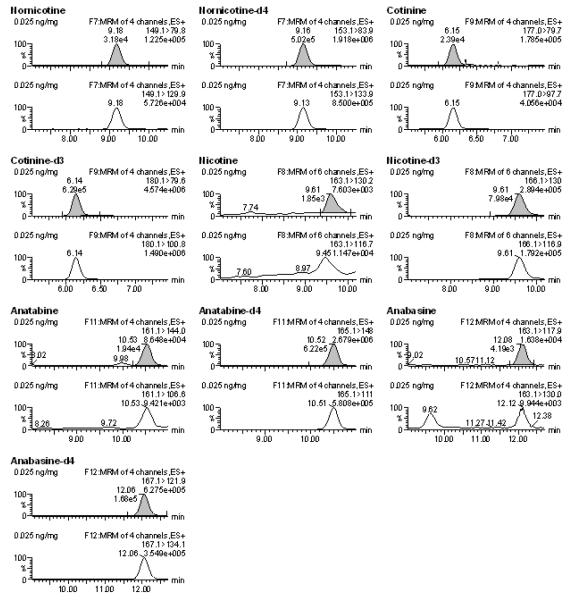
The LOQ for most analytes was determined to be 0.025 ng/mg, with the exception of NIC and AB, for which the LOQ was 0.1 ng/mg (Table 3). Figure 1 shows the chromatogram of all monitored transition ions of the analytes fortified at 0.025 ng/mg. Blank samples and double blank samples were included on each batch and confirmed the lack of endogenous compound interference for any ion transition in analyte-free homogenates (≥LOQ, S/N ≥10:1). With respect to NNIC, however, a small peak above the LOD (i.e. S/N>5) within 2% tR of NNIC internal standard and with ion transitions of 149.1>79.8 was present in most blank samples. Since S/N of these peaks were significantly less than 10:1 and PAR were at least 25% lower than LOQ, they did not interfere with reliable quantification at or above the established LOQ.
Figure 1.
Chromatograms of all analytes at 0.025 ng/mg of calibrator samples that were run on a single batch. The shaded peaks represent the most abundant ions that were used for quantification.
Previous mass spectrometry methods have reported a LOQ for NIC of 0.25 ng/mg, or have reported an LOQ of 0.01 ng/mg using a S/N criterion of 2:1 [23, 18]. In this current study, the LOQ was determined to be 0.1 ng/mg when a S/N criterion of (greater than or equal to) a S/N 10:1 was selected. However, if the laboratory’s S/N criteria for LOQ determination was lowered to 2:1, NIC and all analyzed metabolites and alkaloids in this current study would have a LOQ of 0.01 ng/mg as described in table 3 (LOD values represent S/N ≥3:1). With respect to COT and 3-HC, a previous study reported LOQ to be 0.025 ng/mg and 0.050 ng/mg, respectively [23]. Here COT and 3-HC LOQ were reported to be 0.025 ng/mg for both metabolites and therefore improved. As for our knowledge no other studies have determined LOQ for the other analytes from brain tissue.
The determination of assay linearity was based on PAR of target analyte and corresponding internal standard plotted on a weighted linear regression curve of eight (for AB, NIC and COT GLUC) or ten (for the rest of the metabolites) calibrators (concentrations listed on Table 1). Weighted 1/x linear regression standard curves with excluded calibration origins were automatically generated by the TargetLynx™ feature of MassLynx™ software. Calibration curves were determined to be linear (R2>0.990) for all analytes across the specified concentration ranges (see Tables 1 and 3).
Intra-assay imprecision ranged from 0 - 12.9 %RSD. Quantitative accuracy for these samples was within 87% - 117% of the theoretical target values (Table 5). For determination of inter-assay imprecision and accuracy, three to four unique analytical batches were run over a period of approximately 4 months. Inter-assay imprecision ranged from 3.8 to 14.0 %RSD and quantitative accuracy ranged from 86% to 109% of the theoretical target values (Table 6). COT GLUC was the only metabolite that did not follow the quantification criterion and therefore its results are presented in parenthesis (Table 5 and 6).
Experiments to evaluate specificity of the method demonstrated no significant signal for MRM transitions in analyte-free brains when a S/N threshold criterion of 10:1 was applied. Triplicate analysis of six individual rat brains were used and analysis was based on S/N ratio, tR and PAR calculation for each MRM ion transition. However, it was noted that some samples had small peaks present at 2% tR. Acceptable peak shapes (i.e. S/N > 5 and within 2% tR of deuterated internal standard) were observed for the quantification ion of the following analytes: NIC, COT, NIC GLUC, NNIC and NNO. PAR of these peaks were at least 25% lower than LOQ (10 times lower for NIC, COT, NIC GLUC and NNO, and 25% lower for NNIC) and therefore does not meet criteria for peak identity. The presence of these peaks on these six samples could be due to the animals being handled by smokers and/or interfering unknown endogenous compounds. Blank samples and double blank samples were included on each batch and confirmed the lack of endogenous interfering compounds in analyte-free homogenates (>LOD).
Ion suppression and ion enhancement are shown in Table 4. The high ion enhancement for NNIC at the low QC could be explained by the fact that peaks of S/N>5 were present on samples in which no drug was added and although these peaks were substantially lower than PAR of LOQ calibrator (25% lower), they potentially contributed to the measured values for matrix effect. NNIC matrix effect analysis at high QC samples as well as its sensitivity, imprecision, accuracy, recovery and process efficiency throughout the experiments were acceptable (Tables 3 – 6 and Figure 1).
The overall process efficiency of the method was assessed at a low and a high QC concentration and as shown in Table 4, the results for most analytes ranged from 70-93% with exception of CNO and COT GLUC. The relatively low process efficiency of CNO at low QC and COT GLUC were likely due to the low extraction recovery of these analytes (seen in Table 4).
3.2 Application to biological samples obtained ex vivo from NIC- treated rats
Striatal tissue and RWhB were collected from nicotine treated rats 2h after single injection. The 2-h time point was chosen based on a previous study by Ghosheh et al 1999 [38] in which NIC, COT, and NNIC were demonstrated in whole rat brain at 60 and 240 minutes after an acute s.c. administration of NIC. Based on data from that study, we anticipated that expected brain concentrations in our experiments (at 2 h) would be higher than the LOQ validated for NIC in this current method.
Consistent with previous studies, the analytes quantified in rat brain were NIC, COT and NNIC [39]. The mean concentration of analytes in striatal tissue and RWhB from the present study are shown in Figures 2A and 2B, respectively. NNO was also detected in striata and RWhB but concentrations were lower than LOQ. Although NCOT has been previously reported to be present in rat brain at 4-hr after NIC s.c. administration [39], the reported concentrations were lower than LOD defined in this current study. Furthermore, if NCOT originates in the brain from NNIC oxidation [39], it is possible that NCOT might not yet have been produced at detectable amounts at the 2 h time point. Ghosheh et al also reported NCOT in rat brain but with a delay in its appearance, reaching only small amount of approximately 1 pg/mg at 2h time point [38]. The mean concentration of NIC in the present study was comparable to the previous study by Deutsch et al [18] in which an average of 200 pg/mg of NIC was quantified from rat brain 2-h after a single 1.0 mg/kg administration as assessed by GC/MS analysis. However, COT was not detected in brain in that study [18]. In contrast, Ghosheh et al 1999 [38] detected NIC, COT and NNIC in rat brain at several different time points. In that study, NIC concentrations in whole brain at 1 h after a single 0.8 mg/kg s.c. administration were 122 pg/mg, 39 pg/mg and 10 pg/mg of NIC, COT and NNIC, respectively [38]. Differences in the concentrations found in that study and our study might be attributed to distinct time points analyzed, weight of the rats, analytical technique and analyte recovery.
Figure 2.
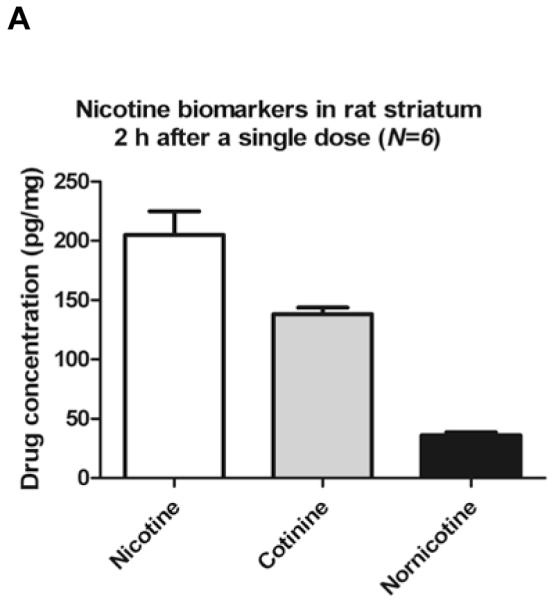
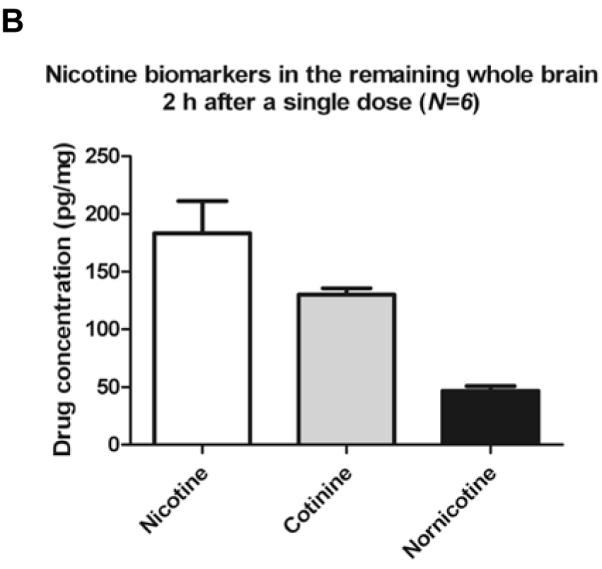
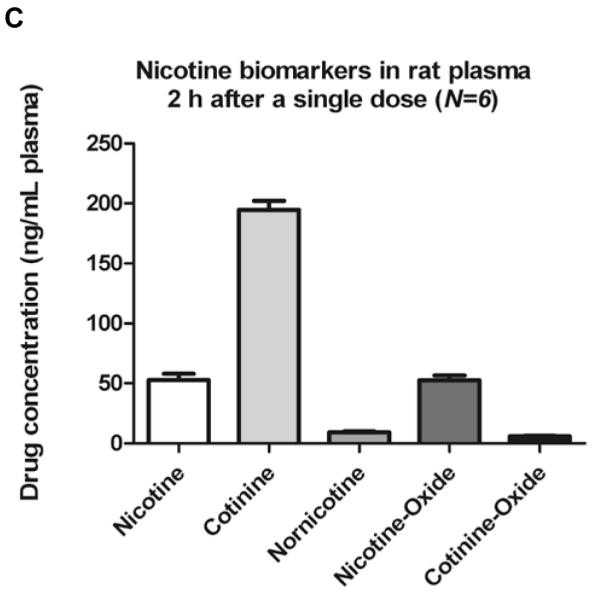
Nicotine and metabolites found in rats brain 2 h after single administration.
Six PND90 male Sprague-Dawley rats received a single s.c. injection of (−)nicotine (0.8mg/kg). Animals were sacrificed by decapitation 2 hours later. Data are expressed as mean value ± S.E.M. of drug concentration in pg of the analyte per mg of striatum (A) and RWhB (B) or in ng of the analyte per mL of plasma (C).
The concentration of nicotine and metabolites were also measured in plasma and compared with previous studies. Plasma analysis revealed the presence of several other metabolites than those found in brain. Average metabolites concentrations were: 52.7 ng/mL for NIC, 194.6 ng/mL for COT, 52.4 ng/mL for NNO, 9.3 ng/mL for NNIC and 6.0 ng/mL for CNO. The concentration of NIC and metabolites in rat plasma found in this study are comparable to previously published studies. Deutsch et al [18] reported rat NIC concentration to be approximately 50 ng/mL 2h after a 1 mg/kg intraperitoneal injection. Jung et al reported an average of 80 ng/mL for NIC and 200 ng/mlL of COT [21]. Although concentrations of NIC in this current study are slightly lower, the administered dose was lower (0.8 mg/kg vs. 1 mg/kg) and a different analytical technique was used (GC-MS) [21]. Rowell and Li reported 50 ng/mL NIC 2h after 0.6 mg/kg s.c NIC injection by GC analysis [40]. Therefore, the measured plasma concentrations of NIC and COT in the present study are consistent with those of previous publications.
4. Conclusion
A simultaneous extraction and quantification of 3-HC, AB, AT, COT, CNO, NIC, NIC GLUC, NNO, NCOT and NNIC in brain tissue was successfully validated. Solid-phase extraction and liquid chromatography tandem mass spectrometry were the methods used. The method was used to identify and quantify the concentration of selected nicotine biomarkers in the brain of rats 2h after administration of a single 0.8 mg/kg s.c. Future studies will treat rats in which nicotine and metabolites have been investigated for potential use in neurological diseases as well as for pharmacokinetics studies.
Highlights.
We validated a LC-MS/MS method for quantification of nicotine, eight metabolites and two alkaloids in brain. > We evaluated and reported imprecision, accuracy, matrix effect, extraction recovery and process efficiency. > We utilized this method to measure concentration of nicotine and metabolites in rat brain. > This method supports research of nicotine and its pharmacokinetics in the brain.
Acknowledgements
We acknowledge the National Institutes of Health grants 019447, 013367, 00869, 11389 and 04222 for financially supporting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Benowitz NL. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benowitz NL. The New England Journal of Medicine. 2010;362:2294–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li C, Schuetz JD, Naren AP. Cancer letters. 2010;292(2):246–53. doi: 10.1016/j.canlet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weems JM, Yost GS. Chem. Res. Toxicol. 2010;23:696–704. doi: 10.1021/tx9004506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alexandrov K, Rojas M, Satarug S. Toxicology letters. 2010;198:63–68. doi: 10.1016/j.toxlet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [6].Chiba M, Masironi R. Bulletin of the World Health Organization. 1992;70(2):269–275. [PMC free article] [PubMed] [Google Scholar]
- [7].Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. J Consult Clin Psychol. 2011;79(1):34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kralikova E, Kozak JT, Rasmussen T, Gustavsson G, Le Houezec J. BMC Public Health. 2009;9:433. doi: 10.1186/1471-2458-9-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bordia T, Campos C, Huang L, Quik M. JPET. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- [10].Huang L, Paramenswaran N, Bordia T, McIntosh M, Quik M. Journal of Neurochemistry. 2009;109:826–837. doi: 10.1111/j.1471-4159.2009.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Akaike A, Takada-Takatori Y, Kume T, Izumi Y. J Mol Neuroscience. 2010;40:211–216. doi: 10.1007/s12031-009-9236-1. [DOI] [PubMed] [Google Scholar]
- [12].Buckingham SD, Jones AK, Brown LA, Sattelle DB. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Levin ED, Conners CK, Silva D, Canu W, March J. Experimental and Clinical Psycopharmacology. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- [14].Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Biochem Pharmacol. 2009;78:677–85. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Neurology. 2001;57:1032–35. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- [16].Villafane G, Cesaro P, Rialland A, Baloul S, Azimi S, Bourdet C, HouezecLe J, Macquin-Mavier I, Maison P. European Journal of Neurology. 2007;14:1313–1316. doi: 10.1111/j.1468-1331.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- [17].Ebersbach G, Stock M, Muller J, Wenning G, Wissel J, Poewe W. Movement Disorders. 1999;14:1011–1013. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [18].Deutsch J, Hegedus L, Greig NH, Rapoport SI, Soncrant TT. J Chromatography. 1992;579(1):93–8. doi: 10.1016/0378-4347(92)80366-x. [DOI] [PubMed] [Google Scholar]
- [19].Sarasin A, Schlumpf M, Muller M, Fleischmann I, Lauber ME, Lichtensteiger W. Reproductive Toxicology. 2003;17:153–162. doi: 10.1016/s0890-6238(02)00119-3. [DOI] [PubMed] [Google Scholar]
- [20].Kim KY, Lee Y-J, Chung BC, Hong J, Jung BH. Drug and Chemical Toxicology. 2010;33(2):166–72. doi: 10.3109/01480540903196832. [DOI] [PubMed] [Google Scholar]
- [21].Jung BH, Chung BC, Chung S-J, Lee M-H, Shim C-K. Journal of Pharmaceutical and Biomedical Analysis. 1999;20:195–202. doi: 10.1016/s0731-7085(99)00020-5. [DOI] [PubMed] [Google Scholar]
- [22].Urakawa N, Nagata T, Kudo K, Kimura K, Imamura T. Int J Legal Med. 1994;106:232–6. doi: 10.1007/BF01225411. [DOI] [PubMed] [Google Scholar]
- [23].Shakleya DM, Huestis MA. Anal Bioanal Chem. 2009;393:1957–65. doi: 10.1007/s00216-009-2661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miller EI, Norris HK, Rollins DE, Tiffany ST, Wilkins DG. Journal of Chromatography B. 2010;878:725–37. doi: 10.1016/j.jchromb.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mwenifumbo JC, Tyndale RF. Nicotine psycopharmacology review. 2009;192:235–259. doi: 10.1007/978-3-540-69248-5_9. [DOI] [PubMed] [Google Scholar]
- [26].Rossini A, de Almeida Simão T, Albano RM, Pinto LFR. Pharmacogenomics. 2008;9:1737–1752. doi: 10.2217/14622416.9.11.1737. [DOI] [PubMed] [Google Scholar]
- [27].Ho MK, Mwenifumbo JC, Koudsi AI, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. Clinical Pharmacology and Therapeutics. 2009;85(6):635–43. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dwoskin LP, Teng L, Buxton ST, Crooks PA. JPET. 1999;288:905–911. [PubMed] [Google Scholar]
- [29].Crooks PA. Chemical properties of nicotine and other tobacco related compounds. In: Gorrod JW, Jacob P III, editors. Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Elsevier; Oxford: 1999. pp. 69–147. [Google Scholar]
- [30].Crooks PA, Dwoskin LP. Biochemical Pharmacology. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- [31].Teng L, Crooks PA, Buxton ST, Dwoskin LP. JPET. 1997;283:778–787. [PubMed] [Google Scholar]
- [32].Dwoskin LP, Teng L, Crooks PA. European Journal of Pharmacology. 2001;428:69–79. doi: 10.1016/s0014-2999(01)01283-3. [DOI] [PubMed] [Google Scholar]
- [33].Bardo MT, Green TA, Crooks PA, Dwoskin LP. Psychopharmacology. 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- [34].Guo J, Zhou D, Grimm SW. J Pharm Biomed Anal. 2011;55:964–71. doi: 10.1016/j.jpba.2011.03.034. [DOI] [PubMed] [Google Scholar]
- [35].Chen G, Giambrone NE, Jr, Dluzen DF, Muscat JE, Berg A, Gallagher CJ, Lazarus P. Cancer Res. 2010;70:7543–52. doi: 10.1158/0008-5472.CAN-09-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hong SP, Jeong MG, Lim DY. Journal of Cardiology. 2007;50:351–62. [PubMed] [Google Scholar]
- [37].Clemens KJ, Caille S, Stinus L, Cador M. Int J Neuropsychopharmacology. 2009;12:1355–56. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- [38].Gosheh O, Dwoskin LP, Li Wen-Kui, Crooks PA. Drug Metabolism and Disposition. 1999;27:1448–55. [PubMed] [Google Scholar]
- [39].Crooks PA, Li M, Dwoskin LP. Drug Metabolism and Disposition. 1997;25:47–54. [PubMed] [Google Scholar]
- [40].Rowell PP, Li M. Journal of Neurochemistry. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]