Abstract
Although the function of adult neurogenesis is still unclear, tools for directly studying the behavioral role of new hippocampal neurons now exist in rodents. Since similar studies are impossible to do in humans, it is important to assess whether the role of new neurons in rodents is likely to be similar to that in humans. One feature of adult neurogenesis that varies tremendously across species is the number of neurons that are generated, so a key question is whether there are enough neurons generated in humans to impact function. In this review we examine neuroanatomy and circuit function in the hippocampus to ask how many granule neurons are needed to impact hippocampal function and then discuss what is known about numbers of new neurons produced in adult rats and humans. We conclude that relatively small numbers of neurons could affect hippocampal circuits and that the magnitude of adult neurogenesis in adult rats and humans is probably larger than generally believed.
1. Introduction
Despite the growing number of studies demonstrating behavioral changes following inhibition of adult neurogenesis, the function of new granule neurons in the dentate gyrus is still far from clear. Logic suggests that any behavioral function of new neurons will reflect some subset of the behavioral roles for the specific regions in which they reside, in this case the hippocampus. Although there is still much to learn about the behavioral function of the hippocampus, it is clear that it plays an important role in spatial and episodic memory [1] and that the ventral hippocampus is important for mediating stress responses [2, 3]. Very little is known about the specific role of the dentate gyrus, but recent studies in animals and humans point to a role in disambiguating related stimuli [4–8], often during emotional learning [9–11]. Potential roles for new granule neurons in emotional or spatial behavior are beginning to emerge, but the data remain very conflicted (see Snyder [12] for a comprehensive list, online). Eventually, the specific function or functions of adult-born neurons will likely become apparent from rodent studies. However, the ultimate goal is to apply the findings on adult neurogenesis in animal models to our understanding of human brain function. Adult neurogenesis does appear to exist in humans, but definitive experiments can be done in humans only in rare circumstances [13]. While the key experiments directly testing whether new neurons are behaviorally significant in humans may never be performed, it is important to consider this possibility since it is the impetus for a great amount of animal research. In this review, rather than interpreting behavioral data from animal neurogenesis-ablation models, we instead focus on what is known about neurogenesis numbers, neuroanatomy, and hippocampal circuitry to assess the likelihood that neurogenesis functions in rodents will be relevant for humans as well.
Before assessing the functional relevance of neurogenesis in rodents it is worth considering whether new neurons would be expected to perform a similar function in humans. One question is whether new neurons are likely to perform the same function in humans as in rodents. Given the comparable anatomy between rodent and human hippocampus [14], it is likely that the computational function is conserved. Whether this translates into similar behavioral functions is unclear, however consistent inter-species roles for the hippocampus in memory [15, 16] suggests this to be the case.
The most common source of skepticism toward a functional role for adult neurogenesis is the perception that too few new neurons are added in adulthood to have a significant impact. Interestingly, this concern, while valid, is usually raised informally and rarely in the scientific literature. Very few studies have addressed this issue [17]. It is difficult to generate even a rough estimate the number of neurons produced in the adult dentate gyrus without cumulative labeling, which has only been feasible in rats and mice and even then is cumbersome and rarely done. More importantly, there is no way to determine how many neurons might be needed to comprise a functional population without a sophisticated understanding of how the neurons function in the circuit. While numbers offer a hint about function, for example loss of cells may suggest loss of function, it is possible that even a small population of neurons could exert a significant impact. This possibility is supported by findings in other neuronal populations – evoked stimulation of individual CA3 interneurons and cortical pyramidal neurons has been shown to alter large-scale network dynamics and even behavior [18–20].
2. How do granule neurons contribute to circuit-level function?
The question of whether there are enough new neurons to affect hippocampal function depends on how granule cells function at the circuit level and how new neurons function within this population. Several studies have linked long-term potentiation of synaptic input to the dentate gyrus with learning and memory [11, 21–24]. Synaptic plasticity has been studied primarily at the medial perforant path input, the primary source of spatial and contextual information to the hippocampus, but other inputs, namely the lateral perforant path and mossy cell inputs are also likely to contribute to hippocampal encoding processes. The lateral perforant pathway, for example, relays nonspatial sensory information and its convergence with the medial perforant path in the hippocampus is thought to underlie the association of the components of episodic memory [25, 26].
One of the most unusual components of the hippocampal circuitry is the sparse mossy fiber-CA3 pathway. The granule cell population shows very sparse activity, with only 2–5% of granule cells active during exploration of any environment [27–29]. In addition, each granule cell contacts only ~10–15 CA3 pyramidal neurons [30]. This unique dentate gyrus-CA3 circuitry has led to speculation that this region of the brain performs a pattern separation function, whereby similar inputs arriving via the entorhinal cortex are given more distinct representations, in order to better distinguish related events during memory encoding and retrieval [4, 31]. Importantly, the specialized contacts between granule cells and CA3 pyramidal cells, the giant mossy terminals, are so powerful that a single granule cell is able to trigger firing in downstream CA3 targets [32]. Because of this “detonator” action, and the extensive excitatory collateral connections between CA3 pyramidal neurons [33, 34], a single granule neuron can potentially have a large impact despite representing only a tiny fraction of the population. Consistent with the disproportionate impact of small numbers of granule cells, learning deficits have been observed following overexpression of calbindin in only ~1% of dentate granule neurons [35]. If adult-born granule cells are more likely to be activated than mature granule cells, they could make up a significant proportion of the active granule cells at any given time even if their numbers are small. And in fact, several lines of evidence suggest that adult-born granule cells are reliably activated [36] and more likely to undergo long-term potentiation [37–41] in response to electrophysiological stimulation in vitro. Furthermore, they are up to five times more likely to be activated than older granule cells by hippocampus-dependent behaviors [42–45].
3. Do immature granule neurons have a unique function?
In the past several years, it has become clear that adult-generated granule neurons form synaptic connections and integrate into the hippocampal circuit. In rodents, adult-born neurons receive synaptic inputs from the perforant path that can be detected morphologically [46, 47] and electrophysiologically [41, 48–50]. The new neurons show immediate-early gene (IEG) expression, an indirect indication of synaptic responses, in response to specific aspects of behavioral stimulation [42, 51–54] (see also Snyder et al., this issue) and contribute to long-term potentiation at the population-level [37, 38, 55, 56]. Less is known about the output of new neurons, but retrograde tracer studies [57–59] and morphological investigations at the level of the light [47] and electron microscope [60, 61] suggest that new neurons form functional efferent synapses. More directly, driving channelrhodopsin-expressing new neurons with light generates postsynaptic currents in downstream CA3 neurons [60]. Taken together, these studies clearly demonstrate that individual adult-born granule cells can function as neurons. But they do not address the potential impact of adult-born granule neurons on hippocampal circuits. One key issue for determining whether adult neurogenesis could impact hippocampal circuits is how these late-born granule cells function relative to the rest of the granule cell population. Do they behave just like the granule neurons born during development, and serve to increase the size of the population, or do they have unique properties that confer a function distinct from that of pre-existing granule cells?
Findings from several studies point to similar electrophysiological properties in granule cells born in adulthood once the new granule cells have reached maturity [62–64]. This electrophysiological similarity is consistent with the nearly identical marker expression in mature granule cells regardless of birth date as well as their intermixing within the granule cell layer. Moreover, some studies of IEG activation suggest equivalent rates of behavioral activation in granule neurons born in development and adulthood [65] (but see below), further supporting an equivalent function for these two populations.
However, an accumulation of recent evidence indicates that between the time that they acquire synapses and when they are fully mature, young granule neurons in the adult dentate gyrus have very different electrophysiological properties from the granule cell population as a whole, suggesting the possibility that they may serve a unique role in hippocampal signal processing. New granule neurons have a lower threshold for undergoing medial perforant path long-term potentiation [37–41, 55]. This enhanced plasticity at the population level has been consistently observed – in both mice and rats, and using multiple methods for reducing neurogenesis – providing strong evidence that new neurons contribute to hippocampal circuit function. In addition, immature granule cells in the adult, like most developing neurons, are depolarized by GABA [66] and are therefore less likely to be inhibited by the strong GABAergic inhibition in the dentate gyrus [67]. Young neurons also appear to be more excitable than mature neurons [36, 39]. This enhanced excitability is coupled with increased and more variable spike latency [36], suggesting that young neurons may differ from mature neurons in their firing patterns, a feature believed to be critical for encoding of experience by the dentate gyrus [29]. In addition, since low frequency firing of mossy fibers typically recruits inhibitory interneurons, while increased firing rates activate more CA3 pyramidal neurons [68], differential firing of young neurons could therefore introduce a new mechanism by which the balance of CA3 excitation and inhibition is shifted during behavior. A circuit-level role is also supported by recent in vivo data showing that ablation of new neurons with either genetic or radiological approaches reduces the dentate gyrus synaptic response to perforant path stimulation and increases the magnitude and synchrony of spontaneous gamma bursts [69]. Given the role of oscillatory activity in shaping plasticity and behavior-specific neuronal firing [70] these findings suggest adult-born neurons may play a significant role in coordinating hippocampal network function. Finally, some evidence suggests that young granule neurons may be capable of synthesizing and releasing GABA in addition to glutamate [71, 72], suggesting a unique neurotransmitter profile that could provides yet another mechanism through which young granule cells could function very differently from the rest of the granule cell population.
Studies examining IEG expression at a population level, which can bridge the gap between physiological or morphological properties of adult-born neurons at a single-cell level and function in a behaving animal, also suggest distinct activation of young granule cells. IEGs such as Fos, zif268, and Arc are expressed by neurons in an activity-dependent fashion and show population-level patterns of activity similar to measures of in vivo spiking [73, 74], suggesting that IEG analysis and electrophysiology are comparable and complementary in their ability to identify neurons involved in representing or encoding information. Adult-born granule neurons in rats undergo a peak in their zif268 expression in response to spatial water maze training at 3 weeks of age [43], suggesting an enhanced contribution of young neurons to behavior. This peak has not been observed in all studies [65, 75] and could reflect differences between IEGs or functional differences between species. Indeed, in mice, peak responsivity of immature neurons is revealed only after accounting for the fact that not all new neurons are functional at immature stages [43]. In addition, emerging data suggests that young granule neurons and mature granule neurons are maximally activated by different experiences or different aspects of experience. For example, immature neurons, unlike the general population, are primarily activated in the ventral dentate gyrus during water maze learning, consistent with a specific role in regulating the response to stress [42]. Adult-born neurons are also particularly activated by retraining at remote time points [52] and by training in multiple contexts in the spatial water maze (Snyder et al., this issue).
The studies above provide compelling evidence that young, presumably immature, granule neurons have different properties than older granule cells, consistent with the possibility that young granule cells make a unique contribution to hippocampal circuits. Alternatively, it is possible that the unique physiological properties of young granule cells simply reflect synaptic activation that is required for neuronal maturation but does not actually contribute to circuit function. That is, the new granule cells only truly integrate into hippocampal circuits and contribute to network activity once they are fully mature and identical to existing granule cells. However, a third possibility is that granule cells born in adulthood never become truly identical to those born in development. One intriguing study has reported that even 5-month-old adult-born neurons, i.e. assumed to be well beyond their immature plastic stage, are more likely than the general population to express Arc following exploratory behavior [45]. Similarly, granule neurons located in the deepest aspect of the granule cell layer, which are primarily adult-born though not necessarily immature [76, 77], are preferentially activated during water maze learning [42]. Dendritic architecture varies with position in the granule cell layer [78–81], suggesting that granule cells born in adulthood, located deep in the granule cell layer, have a different morphology from those born during development even after they are mature. Since dendritic architecture plays a role in the integration and processing of information [82, 83], these morphological differences could lead to permanent differences in the way adult-born granule cells contribute to behavior.
Future studies will be needed to determine whether adult-born granule neurons do in fact have a function distinct from that of existing granule neurons and whether this role is played by young neurons with immature features or by mature neurons that have different properties from granule cells generated during development. The answers to these questions will influence interpretation of the numbers of neurons necessary to impact function. If new granule cells function just like existing cells, and their role is to increase the population, they need to be added in significant numbers relative to the existing granule cells. If, on the other hand, young granule neurons function differently, as a distinct population, then they could potentially impact function even if they are few in number, like several interneuron subtypes.
4. How many new neurons are produced in rodents and humans?
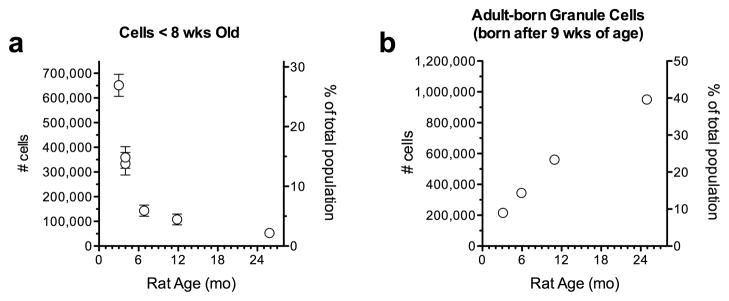
The perception that only a small number of new neurons are added in adulthood likely comes from hypotheses that a stable population of neurons is necessary to preserve memories [84, 85] and is reinforced by immunohistochemical images, which typically show new neurons labeled with a small number of BrdU injections or transiently-expressed endogenous markers of cell proliferation or immaturity. These images therefore only illustrate a snapshot of neurogenesis, labeling only those neurons born within a few hours (with BrdU) or a few weeks (with markers of immature neurons such as doublecortin). Even retroviral and genetic methods for labeling new neurons, which might be expected to cumulatively label newly generated neurons, frequently label only a fraction of the dividing cells and stop labeling additional neurons after a period of time, perhaps due to lingering uncertainty about the identity and targeting of true stem cells [86, 87]. To directly assess the magnitude of adult neurogenesis, we calculated cumulative numbers of neurons born in the adult rat dentate gyrus. We started with cell proliferation rates at different ages based on 2 hour post-BrdU survival data from previous studies in our lab [17, 88, 89], calculated the number of new cells generated per day based on the number generated in young adults [17] and evidence that the cell cycle is unchanged with age [88]. We then integrated across time to obtain total numbers of accumulated new neurons, accounting for the fact that approximately half of all neurons die during the month after their birth [43, 76, 90]. Since adult-generated neurons may play important functional roles when they are young, and have different properties from existing granule cells (see above), we generated a curve that illustrates the number of immature neurons (8 weeks and younger) present at various times throughout the life of the rat (Figure 1a). These calculations reveal that the number of young granule cells in 3-month-old rats is quite large – greater than 650,000 cells. The curve also shows the well-established decrease in adult neurogenesis with age [91, 92], suggesting that if young neurons play a role distinct from that of existing granule neurons, this function may be inhibited with age. However, it has been suggested that the plastic period may be extended in older animals, offsetting this loss to some extent [44]. Moreover, even in rats greater than 2 years old, well past the life expectancy, there are more than 50,000 young granule cells in the dentate gyrus. Since there are approximately 500,000 CA3 pyramidal cells [93], and each granule cell contacts 11–15 pyramidal cells [30], this suggests that even in the oldest animals, each CA3 pyramidal cell could receive a direct contact from a young granule cell.
Fig. 1. Estimates of adult neurogenesis numbers in the rat hippocampus.
a) The estimated number of young (≤8-week-old) granule neurons, at various times throughout adulthood. This number decreases significantly due to the reduction in adult neurogenesis with age. Numbers were calculated using bilateral BrdU+ cell counts 2 hours after injection (proliferating population) at 5-weeks-old [17], 9-weeks-old [17, 88], 5-months-old [89], 10-months-old [88], and 24-months-old [89]. Counts were converted to numbers of cells generated per day by assuming that 4912 BrdU+ cells give rise to 9089 cells/day [17] and that this ratio is constant for all ages because the cell cycle time is constant across age [88]. The number of cells generated each day was multiplied by 7 to calculate the number generated each week. To account for cell death during maturation the number of cells born in one week multiplied by the % surviving for that week [43]. For week 1 it was assumed that all cells survived, since continued proliferation and dilution of BrdU beyond the limit of detection make it difficult to estimate cell death during this period [76]. After 4 weeks, it was assumed that death is complete and that 62% of cells born more than 4 weeks earlier survived [43]. Cells surviving during each of the 8 weeks were summed to estimate the total number of cells ≤8-week-old. b) The estimated cumulative number of granule neurons born in adulthood throughout the lifespan. Numbers were calculated by adding the numbers of <4-week-old (still dying) cells at each age to the number of cells generated/surviving since the previous time point, assuming a linear decline in cell proliferation between adjacent tested ages.
If adult neurogenesis exists to increase the size of the granule cell population, or if adult-born neurons function differently regardless of their level of maturity, then the key question is how many additional neurons they add. To address this question, we calculated the total number of adult-born neurons in rats of different ages (Fig. 1b). Since adult-born granule neurons more than 4 weeks old rarely die [76, 94], the total number of adult-born cells grows with age. By 5 months, the cumulative number of adult-born neurons is equal in size to the 359,000 afferent neurons in layer 2/3 of the entorhinal cortex (using numbers from [95]) and nearly as large as the efferent population of CA3 pyramidal neurons. The population of adult-born neurons, despite the diminishing rate of adult neurogenesis, eventually grows more than twice as large, reaching nearly 1,000,000 cells and 40% of the total population by the end of the animal’s life (Fig. 1b). It is worth noting that, while no studies have cumulatively labeled new neurons for such extended periods, one study has found ~50,000 labeled cells in 4-month-old rats that were injected with a subsaturating dose of BrdU for 12 days, confirming our predictions [96]. These numbers of adult-born neurons clearly seem large enough to have a major impact on the circuitry of the dentate gyrus. It is worth noting that several studies employing genetic labeling techniques have estimated that the cumulative contribution of adult neurogenesis to the granule cell population plateaus earlier (by 4–6 months of age) and is significantly smaller than our estimates (1–10%) [86, 97, 98]. This discrepancy could reflect differences between mice and rats or the labeling of transit amplifying populations that may not generate new neurons indefinitely. Modeling net neurogenesis based on Ki67+ labeling across the lifespan in mice has produced intermediate estimates [99]. Nevertheless, it remains unclear whether adult-born granule cells function primarily during their highly plastic immature period, remain physiologically distinct even in maturity, or become indistinguishable from granule cells born during development.
To extend the rodent data into humans we can assess whether adult neurogenesis occurs in humans, compare its magnitude across species, and determine whether the process is qualitatively similar to that of rodents. For many obvious reasons, the human data is limited. However, a small number of studies have emerged over the last 10 years to enable us to begin to assess whether neurogenesis may contribute to human brain function as it does in rodents. Endogenous markers of immature neurons such as doublecortin and TUC-4 have been used to identify new neurons born in the adult human dentate gyrus and to look for changes in neurogenesis in several pathological conditions [100–102]. Although these endogenous markers appear to label neuronal populations that are not newly-born in some brain regions [103], in the dentate gyrus they are exclusively expressed by newborn neurons in the rodent [96, 104, 105] and show every indication of labeling new neurons in humans based on the similarity of the location and morphology of labeled cells to that in rodents [106] (keeping in mind that morphology can change depending on the fixation delay in post-mortem tissue [107]). The number of doublecortin-expressing cells in the human dentate gyrus shows a dramatic reduction in new neurons with age [106]; this parallel with the age-related decreases seen in rodents and non-human primates [92, 108, 109] provides evidence that the regulation of adult neurogenesis in humans is similar to that in other species. However, since doublecortin and TUC-4 are lost as cells mature or die, they provide no information on the numbers of neurons that survive to maturity. The number of mature neurons produced in the adult human dentate gyrus could, therefore, be much larger than expected based on the relatively few doublecortin-expressing cells observed if maturation and loss of doublecortin is relatively rapid and/or the survival rate of young neurons is relatively high.
BrdU and its analogues are the only markers of new neurons that can be followed for weeks, allowing conclusive identification of newborn neurons that survive and mature. These markers are very difficult to use in human studies, however, since they must be injected into living subjects. There is to date only one study of BrdU-labeled cells in the adult hippocampus, by Eriksson et al. [13], who examined brains containing BrdU used for diagnostic purposes in non-brain cancer patients. This study provides solid evidence that adult neurogenesis occurs in humans by demonstrating that BrdU+ cells are located in the subgranular zone of the dentate gyrus, the same region in which new neurons reside in rodents and non-human primates, 6 months to 2 years after BrdU administration in adults. In addition, the quantitative data presented in the study suggest that adult neurogenesis may occur at rates at or above those in rodents. The human subjects received a very small dose of BrdU compared to typical rodent studies; each subject was given 250 mg, or roughly 3.3 mg/kg (assuming average human body weight of 75 kg). This dose is 15–60 times lower than the 50–200 mg/kg generally used in rodent studies, but simple conversions adjusting only for weight differences do not usually provide equivalent doses in different species. One rule for translating pharmacological doses across species, based on normalizing body surface area, divides mouse doses by 12 and rat doses by 6 to give an equivalent human dose [110]. According to this general rule, the human dose in the Eriksson study may be equivalent to 40 mg/kg in mice or 20 mg/kg in rats – doses that fail to detectably label 40% and 90% of S-phase cells, respectively, using the immunohistochemical methods employed in these studies [17, 111]. However, the pharmacokinetics of a thymidine analogue that has been extensively studied, azidothymidine, show that similar plasma levels are achieved at doses only 50% higher in mice than in humans [112–114], suggesting that the BrdU dose used in the Eriksson study may be equivalent to even lower doses in rodents. Given this very low dose of BrdU it is remarkable that ~25–325 BrdU+ cells/mm3 were found in the in the human subgranular zone and granule cell layer. Moreover, the subjects in this human study were terminally ill, which is likely to be stressful and therefore inhibit adult neurogenesis, and were advanced in age (mean = 64 years). Densities of labeled cells in middle-aged rats fall within this range after adjusting for multiple BrdU injections [44, 92], suggesting that the true number of new neurons in humans may be significantly higher than in rats. Eriksson et al. also co-immunostained BrdU+ cells for phenotypic markers, and found that approximately 25% of BrdU+ cells expressed neuronal markers. This is lower than the proportion of BrdU+ cells that label with NeuN in many young adult rodent studies but agrees with findings showing reduced NeuN expression in older animals [115–119], perhaps due to prolonged maturation with age [47, 120, 121]. Alternatively, it could reflect conservative labeling cutoffs [43], low signal to noise in NeuN staining due to species differences in expression [43] or use of formamide in staining [122], and/or species-dependent differences in maturation [43]. It is worth noting that other studies have quantified aspects of neurogenesis in non-human primates and humans and, collectively, have reported tremendous variation in the magnitude of neurogenesis (comprehensive lists compiled online, primates: [123], humans: [124]). While important to consider, the numerous methodological differences between these studies make comparisons with the rodent literature and the Eriksson study very challenging, and will have to be the topic of future discussion.
5. Are these numbers potentially sufficient to exert a functional impact in humans?
We feel that the answer to this question is an overwhelming “yes”. The number of new neurons added to the adult dentate gyrus is likely to be large enough to make an impact on hippocampal function both in rodents and in humans. While it is not entirely clear what behavioral role new neurons play, the unique properties of adult-born neurons may enable them to process information in novel ways or serve as naïve substrates for information storage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal M, Richter-Levin G, Maggio N. Stress-induced dynamic routing of hippocampal connectivity: A hypothesis. Hippocampus. 2010;20:1332–8. doi: 10.1002/hipo.20751. [DOI] [PubMed] [Google Scholar]
- 3.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–59. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 4.Rolls ET. A computational theory of episodic memory formation in the hippocampus. Behav Brain Res. 2010;215:180–96. doi: 10.1016/j.bbr.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 6.Hunsaker M, Rosenberg J, Kesner R. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008 doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 7.Hunsaker M, Kesner R. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008 doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–2. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsetsenis T, Ma X-H, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–61. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–9. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 12.Snyder JS. A list of experiments that relate adult hippocampal neurogenesis to behavior. ( http://www.functionalneurogenesis.com/blog/2010/01/a-list-of-studies-that-relate-adult-hippocampal-neurogenesis-to-behavior/)
- 13.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 14.Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Prog Brain Res. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- 15.Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–7. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 16.Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48:2339–56. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 18.Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–24. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- 19.Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–10. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- 20.Li CY, Poo MM, Dan Y. Burst spiking of a single cortical neuron modifies global brain state. Science. 2009;324:643–6. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–42. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 22.Niewoehner B, Single FN, Hvalby O, Jensen V, Meyer zum Alten Borgloh S, Seeburg PH, et al. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur J Neurosci. 2007;25:837–46. doi: 10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro CA, Silbert LH, McNaughton BL, Barnes CA. Recovery of spatial learning deficits after decay of electrically induced synaptic enhancement in the hippocampus. Nature. 1989;342:545–8. doi: 10.1038/342545a0. [DOI] [PubMed] [Google Scholar]
- 24.Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–74. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Si B, Treves A. The role of competitive learning in the generation of DG fields from EC inputs. Cogn Neurodyn. 2009;3:177–87. doi: 10.1007/s11571-009-9079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–24. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treves A, Tashiro A, Witter ME, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–72. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 28.Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–86. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- 29.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 30.Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerasti E, Treves A. How informative are spatial CA3 representations established by the dentate gyrus? PLoS Comput Biol. 2010;6:e1000759. doi: 10.1371/journal.pcbi.1000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–5. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- 33.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 34.Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 35.Dumas TC, Powers EC, Tarapore PE, Sapolsky RM. Overexpression of calbindin D(28k) in dentate gyrus granule cells alters mossy fiber presynaptic function and impairs hippocampal-dependent memory. Hippocampus. 2004;14:701–9. doi: 10.1002/hipo.10210. [DOI] [PubMed] [Google Scholar]
- 36.Mongiat LA, Esposito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS ONE. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 38.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–7. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–57. [PubMed] [Google Scholar]
- 41.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19:360–70. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–95. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder JS, Ramchand P, Rabbett S, Radik R, Wojtowicz JM, Cameron HA. Septo-temporal gradients of neurogenesis and activity in 13-month-old rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.022. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–41. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci U S A. 107:7963–8. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambrogini P, Cuppini R, Lattanzi D, Ciuffoli S, Frontini A, Fanelli M. Synaptogenesis in adult-generated hippocampal granule cells is affected by behavioral experiences. Hippocampus. 2009 doi: 10.1002/hipo.20679. [DOI] [PubMed] [Google Scholar]
- 49.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–62. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 52.Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106:5919–24. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epp JR, Haack AK, Galea LA. Activation and survival of immature neurons in the dentate gyrus with spatial memory is dependent on time of exposure to spatial learning and age of cells at examination. Neurobiol Learn Mem. 2011;95:316–25. doi: 10.1016/j.nlm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–12. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 55.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 58.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–54. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 59.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–60. [PubMed] [Google Scholar]
- 60.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–7. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105:14157–62. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laplagne DA, Kamienkowski JE, Esposito MS, Piatti VC, Zhao C, Gage FH, et al. Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis. Eur J Neurosci. 2007;25:2973–81. doi: 10.1111/j.1460-9568.2007.05549.x. [DOI] [PubMed] [Google Scholar]
- 64.Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS biology. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone SSD, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, et al. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus. 2010 doi: 10.1002/hipo.20845. [DOI] [PubMed] [Google Scholar]
- 66.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–43. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- 68.McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res. 2008;169:225–40. doi: 10.1016/S0079-6123(07)00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2010 doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 71.Mody I. The GAD-given Right of Dentate Gyrus Granule Cells to Become GABAergic. Epilepsy Curr. 2002;2:143–5. doi: 10.1046/j.1535-7597.2002.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao S, Zhou Y, Gross J, Miao P, Qiu L, Wang D, et al. Fluorescent labeling of newborn dentate granule cells in GAD67-GFP transgenic mice: a genetic tool for the study of adult neurogenesis. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, et al. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–82. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 75.Sandoval CJ, Martinez-Claros M, Bello-Medina PC, Perez O, Ramirez-Amaya V. When are new hippocampal neurons, born in the adult brain, integrated into the network that processes spatial information? PLoS ONE. 2011;6:e17689. doi: 10.1371/journal.pone.0017689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 77.Crespo D, Stanfield BB, Cowan WM. Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Exp Brain Res. 1986;62:541–8. doi: 10.1007/BF00236032. [DOI] [PubMed] [Google Scholar]
- 78.Desmond NL, Levy WB. A quantitative anatomical study of the granule cell dendritic fields of the rat dentate gyrus using a novel probabilistic method. J Comp Neurol. 1982;212:131–45. doi: 10.1002/cne.902120204. [DOI] [PubMed] [Google Scholar]
- 79.Green EJ, Greenough WT. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: evidence from hippocampal slices maintained in vitro. J Neurophysiol. 1986;55:739–50. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- 80.Claiborne BJ, Amaral DG, Cowan WM. Quantitative, three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J Comp Neurol. 1990;302:206–19. doi: 10.1002/cne.903020203. [DOI] [PubMed] [Google Scholar]
- 81.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 82.Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- 83.Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–6. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 85.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752–7. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 87.DeCarolis NA, Mechanic M, Petrik D, Malhotra S, Ables JL, Lagace DC, et al. Subpopulations of radial-glia like cells may differentially contribute to adult neurogenesis in the hippocampus in vivo. 2010 Neuroscience Meeting Planner. 2010 Online. [Google Scholar]
- 88.Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–67. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- 89.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 90.McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–5. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 91.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–82. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 92.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 94.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 95.Mulders WH, West MJ, Slomianka L. Neuron numbers in the presubiculum, parasubiculum, and entorhinal area of the rat. J Comp Neurol. 1997;385:83–94. [PubMed] [Google Scholar]
- 96.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–46. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 97.Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–11. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–9. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lazic SE. Modelling hippocampal neurogenesis across the lifespan in seven species. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2011.03.008. In press. [DOI] [PubMed] [Google Scholar]
- 100.Gerber J, Tauber SC, Armbrecht I, Schmidt H, Bruck W, Nau R. Increased neuronal proliferation in human bacterial meningitis. Neurology. 2009;73:1026–32. doi: 10.1212/WNL.0b013e3181b9c892. [DOI] [PubMed] [Google Scholar]
- 101.Mattiesen WR, Tauber SC, Gerber J, Bunkowski S, Bruck W, Nau R. Increased neurogenesis after hypoxic-ischemic encephalopathy in humans is age related. Acta Neuropathol. 2009;117:525–34. doi: 10.1007/s00401-009-0509-0. [DOI] [PubMed] [Google Scholar]
- 102.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–20. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 103.Gómez-Climent M, Castillo-Gómez E, Varea E, Guirado R, Blasco-Ibáñez J, Crespo C, et al. A Population of Prenatally Generated Cells in the Rat Paleocortex Maintains an Immature Neuronal Phenotype into Adulthood. Cereb Cortex. 2008 doi: 10.1093/cercor/bhm255. [DOI] [PubMed] [Google Scholar]
- 104.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 105.Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 106.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 108.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104:17169–73. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96:5263–7. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 111.Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–22. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Falcone A, Danesi R, Dargenio F, Pfanner E, Brunetti I, Del Tacca M, et al. Intravenous azidothymidine with fluorouracil and leucovorin: a phase I-II study in previously untreated metastatic colorectal cancer patients. J Clin Oncol. 1996;14:729–36. doi: 10.1200/JCO.1996.14.3.729. [DOI] [PubMed] [Google Scholar]
- 113.Brunetti I, Falcone A, Calabresi P, Goulette FA, Darnowski JW. 5-Fluorouracil enhances azidothymidine cytotoxicity: in vitro, in vivo, and biochemical studies. Cancer Res. 1990;50:4026–31. [PubMed] [Google Scholar]
- 114.Tosi P, Calabresi P, Goulette FA, Renaud CA, Darnowski JW. Azidothymidine-induced cytotoxicity and incorporation into DNA in the human colon tumor cell line HCT-8 is enhanced by methotrexate in vitro and in vivo. Cancer Res. 1992;52:4069–73. [PubMed] [Google Scholar]
- 115.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–13. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 116.Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–54. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 117.Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–75. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 118.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–43. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 119.Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–40. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 120.Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–76. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- 121.Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–34. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J Comp Neurol. 2009;517:123–33. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Snyder JS. Studies of adult hippocampal neurogenesis in primates. ( http://www.functionalneurogenesis.com/blog/2011/05/studies-of-adult-hippocampal-neurogenesis-in-primates/)
- 124.Snyder JS. Studies of adult hippocampal neurogenesis in humans. ( http://www.functionalneurogenesis.com/blog/2011/01/studies-of-adult-hippocampal-neurogenesis-inhumans/)