Abstract
We examined the relative contributory roles of extracellular vs. intracellular L-arginine (ARG) toward cellular activation of endothelial nitric oxide synthase (eNOS) in human endothelial cells. EA.hy926 human endothelial cells were incubated with different concentrations of 15N4-ARG, ARG, or L-arginine ethyl ester (ARG-EE) for 2 hours. To modulate ARG transport, siRNA for ARG transporter (CAT-1) vs. sham siRNA were transfected into cells. ARG transport activity was assessed by cellular fluxes of ARG, 15N4-ARG, dimethylarginines, and L-citrulline by an LC-MS/MS assay. eNOS activity was determined by nitrite/nitrate accumulation, either via a fluorometric assay or by 15N-nitrite or estimated 15N3-citrulline concentrations when 15N4-ARG was used to challenge the cells. We found that ARG-EE incubation increased cellular ARG concentration but no increase in nitrite/nitrate was observed, while ARG incubation increased both cellular ARG concentration and nitrite accumulation. Cellular nitrite/nitrate production did not correlate with cellular total ARG concentration. Reduced 15N4-ARG cellular uptake in CAT-1 siRNA transfected cells vs. control was accompanied by reduced eNOS activity, as determined by 15N-nitrite, total nitrite and 15N3-citrulline formation. Our data suggest that extracellular ARG, not intracellular ARG, is the major determinant of NO production in endothelial cells. It is likely that once transported inside the cell, ARG can no longer gain access to the membrane-bound eNOS. These observations indicate that the “L-arginine paradox” should not consider intracellular ARG concentration as a reference point.
Keywords: Nitric Oxide, L-arginine, Asymmetric dimethylarginine, L-arginine paradox, L-arginine transport
INTRODUCTION
The “L-arginine paradox” is so termed because the Michaelis-Menten Km for endothelial nitric oxide synthase (eNOS, as the isolated enzyme) to utilize L-arginine (ARG) has been determined to be about 3 μM [1,2] while the intracellular ARG concentration can reach up to 800 μM or higher [3]. Thus, slight increases of circulating ARG concentration after ARG supplementation would not be expected to increase enzyme action to produce additional nitric oxide (NO). However, many studies in diverse patient groups have demonstrated the short-term beneficial effects of ARG supplementation [4,5,6,7]. In order to understand this paradox, it is necessary to delineate the source of ARG that is responsible for the cellular release of NO.
The constitutive endothelial cell transporter that facilitates uptake of ARG is the cationic amino acid transporter 1 (CAT-1) [8]. It has been shown that eNOS and CAT-1 are physically associated in the caveolae of endothelial cells [9], suggesting the delivery of extracellular ARG directly to eNOS. Subsequently, it has also been reported that NOS activity in bovine aortic endothelial cells is associated with extracellular ARG availability and its uptake [10]. In addition, when CAT-1 mediated transport of extracellular ARG into endothelial cells was suppressed by L-lysine, the endothelial NO response was also depressed [11]. These findings suggest that ARG transported via CAT-1 may not be in rapid equilibrium with the bulk intracellular ARG, and that this source of ARG may be critical in mediating NO release from endothelial cells.
While the importance of extracellular ARG in mediating NO release has been emphasized in these studies, the role of intracellular ARG toward cellular activation of eNOS has not been explicitly defined. The objective of the present work, therefore, is to examine the relative contributory roles of extracellular vs. intracellular ARG toward the cellular activation of eNOS in human endothelial cells. Intracellular ARG was manipulated by using L-arginine ethyl ester (ARG-EE), which can increase intracellular ARG through passive diffusion rather than being actively transported. The relationship between cellular ARG and nitrite/nitrate production was examined after cells were exposed to either ARG-EE or ARG. The role of cellular ARG transport in mediating nitrite/nitrate accumulation was further examined by an experiment in which the primary transporter for ARG, the cationic amino acid transporter (CAT-1) was partially silenced.
MATERIALS AND METHODS
Chemicals and reagents
ARG [as L-arginine HCl], L-citrulline (CIT), asymmetric dimethylarginine (ADMA) [as NG,NG-dimethylarginine dihydrochloride], symmetric dimethylarginine [as NG,NG′-dimethyl-L-arginine di(p-hydroxyazobenzene-p′-sulfonate) salt], and L-arginine ethyl ester were purchased from Sigma. 15N4-ARG [as its HCl, (U-15N4, 98%)], D7-ADMA [as ADMA:HCl:H2O (2,3,3,4,4,5,5-D7, 98%)], 13C6-ARG [as ARG:HCl (U-13C6, 98%)], and D4-CIT [as L-citrulline (4,4,5,5-D4, 96.5 %)] were obtained from Cambridge Isotope Laboratories, Inc. These compounds were used without further purification. Cell culture reagents were purchased from Invitrogen.
Cell culture
EA.hy926 human vascular endothelial cells [12] were grown in a further modified Dulbecco's modified Eagle's medium (DMEM) containing 0.9 G/L of glucose and 21 mg/L of ARG supplemented with 10 % fetal bovine serum, and 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a 5 % CO2 incubator. The use of a lower concentration of glucose and ARG in the present study was prompted by concurrent work in our laboratory (Mohan and Fung, unpublished data) showing that the high concentrations of glucose (4.5 G/L) and ARG (84 mg/L) in the original DMEM medium inhibited eNOS activity.
CAT-1 siRNA transfection
Stealth Select RNAi ™ siRNA (AGG ACC AGG ACG UUA AUA CAA GUG A, UCA CUU GUA UUA ACG UCC UGG UCC U) for CAT-1 and Stealth RNAi™ siRNA Negative Control Low GC (Invitrogen) were transfected into EA.hy926 cells with Lipofectamine™ RNAiMAX (Invitrogen) following a reverse transfection protocol. The RNAi duplex-Lipofectamine™ RNAiMAX complexes were prepared and cells were added to give a final RNA concentration of 30 nM. After 12 hours of incubation at 37°C in a CO2 incubator, cells were utilized for the experiment.
General protocol for cell studies
Cells were washed twice with phosphate-buffered saline and equilibrated in Locke's solution (LS; 154 mM NaCl, 5.6 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 10 mM HEPES, 3.6 mM NaHCO3 and 5.6 mM glucose) for 1 hour. Then, fresh LS containing various concentrations of 15N4-ARG, ARG or ARG-EE were added. After 2 hours of exposure, the incubation medium was collected. Cells were collected by trypsinization and lysed by lysis buffer. Concentrations of 15N4-ARG, ARG, ADMA, symmetric dimethylarginine (SDMA), CIT, and 15N3-CIT were analyzed by the LC-MS/MS assay [13]. Nitrite and nitrate accumulation was assessed by a fluorometric assay using the Nitrate/Nitrite Fluorometric Assay Kit (Cayman, MI) or by the LC-MS/MS assay when 15N4-ARG was used as a substrate [14]. Protein amounts in the cell lysates were determined by the method of Lowry [15].
Statistical analysis
For comparison of two groups, Student's t-test was used. Comparisons among different exposure groups were made using one-way ANOVA. Statistical significance was then determined by Tukey post-hoc test. Differences with p<0.05 were denoted as statistically significant.
RESULTS
Effects of ARG-EE exposure in the EA.hy926 cells
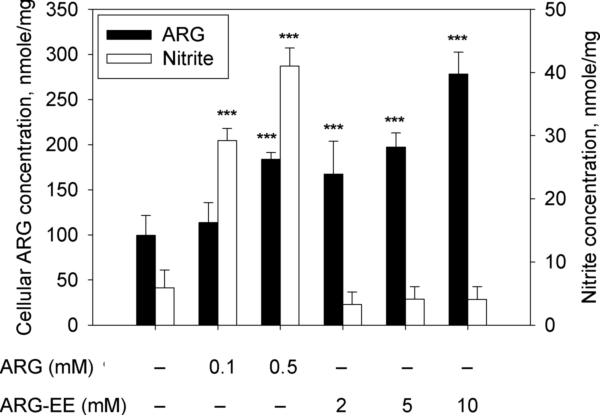
Fig. 1 shows that, similar to ARG exposure, incubation with ARG-EE for 2 hours increased cellular ARG concentration in EA.hy926 cells, although the efficiency of ARGEE was poorer. Compared to control, ARG exposure at 0.5 mM and ARG-EE exposure at 2, 5, and 10 mM significantly elevated mean cellular ARG concentrations by 1.8-, 1.7-, 2.0-, and 2.8-fold, respectively (Fig. 1, filled bars). The exposure concentrations for ARG-EE were selected based on preliminary experiments showing that ARG-EE concentrations <2 mM were not able to increase cellular ARG concentration significantly. The corresponding nitrite accumulations from these exposure conditions to ARG and ARG-EE are also shown in Fig. 1 (open bars). Significant nitrite was observed by ARG exposure at 0.1 and 0.5 mM, in an apparently concentration-dependent manner, whereas ARG-EE exposure did not change nitrite level vs. control. Nitrate concentrations were not different among all the treatment groups, and were marked by high variability (data not shown).
Fig. 1.
Effects of ARG or ARG-EE exposure on cellular concentrations of ARG (filled bars) and inorganic nitrite (open bars) after EA.hy926 cells were incubated with different concentrations of ARG or ARG-EE for 2 hours. Nitrite contents in cell lysates and in the incubation medium were combined and converted to an estimated concentration in the whole system based on the observed protein content. Data are presented mean ± SD (n=6). ***, p<0.001 vs. control
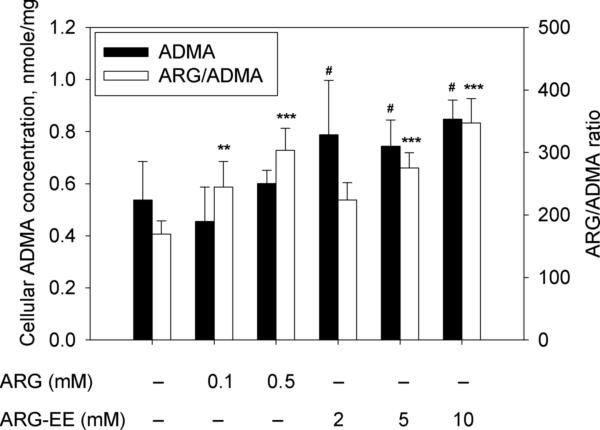
Under the study conditions, cellular concentrations of ADMA, an endogenous inhibitor of eNOS, were largely unaltered either by ARG or by ARG-EE exposures, when compared to control. ARG exposure at 0.1 mM showed a trend of decreased ADMA level, which was not statistically different from control but significantly lower than cells treated with ARG-EE (Fig. 2, filled bars). The ARG/ADMA ratio, calculated from the observed cellular ARG and ADMA concentrations, was increased 1.4- and 1.8-fold by ARG exposure at 0.1 and 0.5 mM, respectively. ARG-EE exposure also enhanced the cellular ARG/ADMA ratio to a similar extent, by 1.6- and 2.0-fold at 5 and 10 mM, respectively (Fig. 2, open bars). No substantial change was observed in the concentrations of SDMA and CIT after ARG or ARG-EE incubation (data not shown).
Fig. 2.
Effects of ARG or ARG-EE exposure on cellular ADMA concentrations (filled bars) and ARG/ADMA ratio (open bars) after EA.hy926 cells were exposed to ARG or ARG-EE for 2 hours. Data are presented mean ± SD (n=6). **, p<0.01; ***, p<0.001 vs. Control; #, p<0.05 vs. ARG 0.1 mM.
Effects of CAT-1 siRNA transfection on cellular fluxes and NO production
Table 1 shows that CAT-1 siRNA transfection in EA.hy926 cells significantly impaired 15N4-ARG uptake upon exposure to 100 μM 15N4-ARG. However, this change in uptake was small in comparison to the endogenous ARG concentration, which was not altered. Cellular ADMA, SDMA, and CIT concentrations were not different between sham vs. CAT-1 siRNA transfected cells. A significant decrease in cellular 15N3-CIT, an index of NO generation from 15N4-ARG [16] was observed.
Table 1.
Effects of 100 μM 15N4-ARG exposure for 2 hours after CAT-1 siRNA or sham transfection in EA.hy926 cells.
| Sham transfected | CAT-1 siRNA transfected | ||
|---|---|---|---|
| Cellular (nmole/mg) | 15N4-ARG | 4.87 ± 0.62 | 2.81 ± 0.59*** |
| ARG | 120 ± 11 | 106 ± 15 | |
| ADMA | 0.362 ± 0.080 | 0.319 ± 0.029 | |
| SDMA | 0.035 ± 0.009 | 0.027 ± 0.007 | |
| CIT | 0.035 ± 0.022 | 0.033 ± 0.018 | |
| 15N3-CIT | 0.068 ± 0.010 | 0.041 ± 0.007*** | |
| Extracellular (nmole/L) | 15N4-ARG | 59.6 ± 4.5 (×103) | 62.4 ± 5.2 (×103) |
| ARG | 7.22 ± 0.70 (×103) | 6.34 ± 0.50 (×103)* | |
| ADMA | 49.6 ± 8 | 40.4 ± 10 | |
| SDMA | ND | ND | |
| CIT | ND | ND | |
| 15N3-CIT | 5.63 ± 1.51 | 4.15 ± 2.49 | |
| Total (nmole/mg) | Nitrite | 0.658 ± 0.162 | 0.374 ± 0.073* |
| Nitrate | 5.51 ± 1.54 | 4.61 ± 0.79 | |
| Nitrite+Nitrate | 6.17 ± 1.41 | 5.01 ± 0.81 | |
| 14N-nitrite | 0.670 ± 0.377 | 0.563 ± 0.175 | |
| 15N-nitrite | 0.085 ± 0.011 | 0.062 ± 0.005* | |
| 14N+15N-nitrite | 0.755 ± 0.384 | 0.623 ± 0.177 | |
Data are presented mean ± SD (n=4-6).
p<0.001
p<0.05 vs. sham transfected.
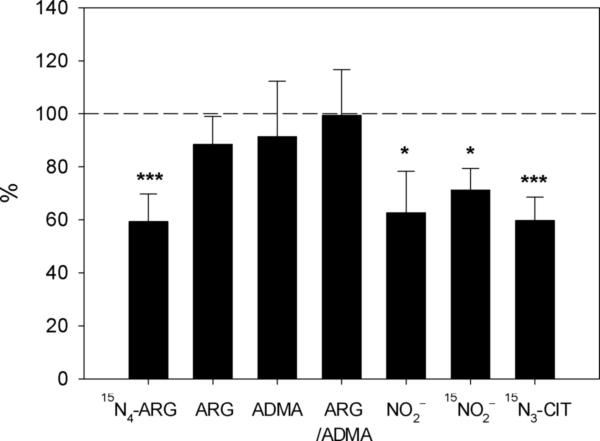
In the incubation medium, only ARG concentration was decreased as a result of CAT-1 siRNA transfection and 15N4-ARG exposure while no other changes in the other amino acids were observed. Examination of the total nitrite and nitrate concentrations in this experiment showed that CAT-1 siRNA transfection and subsequent 15N4-ARG exposure led to significant decreases in the accumulation of nitrite as determined by the fluorescence assay. However, LC-MS/MS assay only showed a significant decrease in 15N-nitrite accumulation in CAT-1 siRNA transfected cells. Fig. 3 summarized these findings by expressing the various parameters observed in CAT-1 silenced cells vs. sham-transfected cells.
Fig. 3.
Cellular concentrations of various amino acids and nitrite in CAT-1 siRNA transfected cells, expressed as a % of the corresponding values observed in sham-transfected cells. Data for NO2– indicates values obtained by fluorometry. Data are presented as mean ± SD (n=4-6). ***, p<0.001; *, p<0.05 vs. sham transfection.
DISCUSSION
In this work, we examined the relative roles of intracellular ARG and extracellular ARG as a substrate source for NOS in human endothelial cells. Several reports have suggested an important role for extracellular ARG uptake as a determinant for cellular NOS activity. For example, it has been shown that NOS activity in bovine aortic endothelial cells was related to extracellular ARG concentration and that L-lysine suppressed ARG uptake and NO production [10]. Inhibition of CAT-1 mediated ARG transport was accompanied by suppressed NO-dependent vasodilation in vivo [11]. Insulin-induced endothelium-dependent vessel relaxation was also suggested to be a result from stimulation of ARG transport by insulin [17]. In these studies, however, the role of intracellular ARG was not independently examined. Here, we used ARG-EE to increase intracellular ARG without exposing cells to extracellular ARG. Our results clearly showed that when ARG is delivered inside the cell without transporter involvement, little eNOS activity could be detected. Thus, the intracellular concentration of ARG may be irrelevant to the cellular production of NO through eNOS.
This conclusion was strongly supported by comparing ARG-EE vs. ARG as a substrate source for eNOS. ARG-EE does not enter the cell via the cationic transport system, but by passive diffusion. Once inside the cell, ARG-EE can release free ARG via the action of cellular esterases. In follow-up studies, we observed a linear increase of ARG concentration when cell lysate was incubated with ARG-EE over time, and about 60 % of ARG-EE was found to be converted to ARG after 2 hour. Our results showed that when EA.hy926 cells were exposed to ARG-EE at 2 – 10 mM, cellular ARG concentration was increased to a similar extent when compared to ARG incubation at much lower concentrations, i.e., 0.1 and 0.5 mM. However, the increase in cellular ARG concentration by ARG-EE was not associated with enhanced nitrite production (Fig. 1). In contrast, at similar increases in cellular ARG, cells incubated with ARG induced significant increases in nitrite/nitrate accumulation. Additional analysis of these data showed no correlation between nitrite production and cellular ARG concentration, indicating that enhanced NO production by ARG exposure was not related to intracellular ARG (Supplementary Fig. 1A).
Changes in ADMA concentration were relatively marginal in this study and the cellular ARG/ADMA ratio was significantly enhanced due primarily to the increase in ARG uptake. However, no correlation was observed between nitrite production and ARG/ADMA ratio (Supplementary Fig. 1B). These results are inconsistent with the conclusion of Cardounel et al. [1] who showed that ADMA/ARG ratio was correlated with cellular NO production in bovine aortic endothelial cells. However, these investigators manipulated the ADMA/ARG ratio by exposure to 0.5 – 50 μM ADMA. As we have reported elsewhere [18], extracellular ADMA exposure induces a variety of interactions between ARG and ADMA, including trans-stimulated efflux and metabolic inhibition of ARG, as well as competitive inhibition of eNOS action. Here, we used ARGEE to alter the cellular ADMA/ARG ratio, mainly through increasing ARG supply while bypassing the responsible transporter system. Thus, interactions between ARG and ADMA at the transporter level could be avoided.
The critical role of transporter-supplied ARG in mediating cellular NO release was further supported by proof-of-concept experiments in which the major ARG transporter CAT-1 was partially silenced. Transfection with CAT-1 siRNA was associated with reduced 15N4-ARG uptake, and suppressed NO production, as monitored by the production of total nitrite (measured by fluorescence), 15N-nitrite, and 15N3-CIT. These results support the view that CAT-1 mediated ARG transport plays a critical role to elicit cellular eNOS activity and NO generation.
Our results may help to further refine, at least partially, the conundrum of the “L-arginine paradox”, which indicates that the intracellular ARG concentration (about 1 mM) was already much higher than the Km of NOS (of about 3 μM) to permit any additional production of NO from ARG supplementation, a deduction that is contrary to experimental observations. Our results showed that this comparison is not appropriate because the intracellular ARG concentration is not relevant for cellular NO production. Rather, it is the extracellular ARG concentration that ought to be compared to the Km. Thus, the “L-arginine paradox”, as it is presently formulated, should be re-structured to remove intracellular ARG as a point of comparison.
The circulating ARG plasma concentration is about 50 – 100 μM [19], an order of magnitude lower than its intracellular concentration of about 1 mM. This value is still about 20-fold higher than that of the Km of the isolated eNOS enzyme (about 3 μM). However, Hardy et al. have reported an effective Km of 29 μM for bovine endothelial cells when exposed radioactive ARG [10]. We showed elsewhere that the apparent Km of NOS activity for EA.hy926 cells (in contrast to the purified enzyme), when exposed to extracellular 15N4-ARG, was 36.2 ± 9.8 μM [20]. The higher Km value for cells (as opposed to the purified enzyme) probably is the result of incorporation of one or other additional rate-limiting steps, including that of cellular transport. Since the baseline plasma ARG concentration (50 – 100 μM) is in the same range as the cellular Km for NO production, ARG supplementation that produces a peak ARG plasma concentration of 200 – 300 μM, e.g., after an oral dose of 10 g [21], is expected to increase NO production in vivo. Viewed in this manner, the “L-arginine paradox” may cease to be a paradox after all. Our results indicate that intracellular ARG is dissociated with cellular NOS activity, while extracellular ARG transport is the major determinant of NO production in endothelial cells. This observation can lead to a better understanding of the “L-arginine paradox”.
Supplementary Material
Highlights.
Our findings provide a possible solution to the “L-arginine paradox”
Extracellular L-arginine concentration is the major determinant of NO production
Cellular L-arginine action is limited by cellular ARG transport, not the Km of NOS
We explain how L-arginine supplementation can work to increase endothelial function
Acknowledgement
This work was supported in part by NIH grant HL081580.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 3.Baydoun AR, Emery PW, Pearson JD, Mann GE. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 4.Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, Pala MG, Formica F, Paolini G, Catapano AL, Bosi E, Alfieri O, Piatti P. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism. 2009;58:1270–1276. doi: 10.1016/j.metabol.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Ruel M, Beanlands RS, Lortie M, Chan V, Camack N, deKemp RA, Suuronen EJ, Rubens FD, DaSilva JN, Sellke FW, Stewart DJ, Mesana TG. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the Endothelial Modulation in Angiogenic Therapy randomized controlled trial. J Thorac Cardiovasc Surg. 2008;135:762–770. 770, e761. doi: 10.1016/j.jtcvs.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 6.Kernohan AF, McIntyre M, Hughes DM, Tam SW, Worcel M, Reid JL. An oral yohimbine/L-arginine combination (NMI 861) for the treatment of male erectile dysfunction: a pharmacokinetic, pharmacodynamic and interaction study with intravenous nitroglycerine in healthy male subjects. Br J Clin Pharmacol. 2005;59:85–93. doi: 10.1111/j.1365-2125.2004.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bednarz B, Jaxa-Chamiec T, Gebalska J, Herbaczynska-Cedro K, Ceremuzynski L. L-arginine supplementation prolongs exercise capacity in congestive heart failure. Kardiol Pol. 2004;60:348–353. [PubMed] [Google Scholar]
- 8.Zharikov SI, Block ER. Characterization of L-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. Biochim Biophys Acta. 1998;1369:173–183. doi: 10.1016/s0005-2736(97)00191-0. [DOI] [PubMed] [Google Scholar]
- 9.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 10.Hardy TA, May JM. Coordinate regulation of L-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med. 2002;32:122–131. doi: 10.1016/s0891-5849(01)00781-x. [DOI] [PubMed] [Google Scholar]
- 11.Zani BG, Bohlen HG. Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol. 2005;289:H1381–1390. doi: 10.1152/ajpheart.01231.2004. [DOI] [PubMed] [Google Scholar]
- 12.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin S, Fung SM, Mohan S, Fung HL. Simultaneous bioanalysis of L-arginine, L-citrulline, and dimethylarginines by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:467–474. doi: 10.1016/j.jchromb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin S, Fung HL. Evaluation of an LC-MS/MS assay for 15N-nitrite for cellular studies of l-arginine action. J Pharm Biomed Anal. 2011 doi: 10.1016/j.jpba.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Shin S. University at Buffalo; The State University of New York, Buffalo: 2011. L-Arginine Transport, Metabolism and Action, Pharmaceutical Sciences. [Google Scholar]
- 17.Gonzalez M, Gallardo V, Rodriguez N, Salomon C, Westermeier F, Guzman-Gutierrez E, Abarzua F, Leiva A, Casanello P, Sobrevia L. Insulin-stimulated L-arginine transport requires SLC7A1 gene expression and is associated with human umbilical vein relaxation. J Cell Physiol. 2011 doi: 10.1002/jcp.22635. [DOI] [PubMed] [Google Scholar]
- 18.Shin S, Fung H-L. Role of Asymmetric Dimethylarginine in L-Arginine Paradox: Transport and Metabolism Considerations, AAPS Annual Meeting and Exposition. The AAPS Journal. 2009 Abstract #1103. [Google Scholar]
- 19.Bode-Boger SM, Scalera F, Ignarro LJ. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114:295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Shin S, Fung H-L. Extracellular L-arginine transport, not its intracellular concentration, determines L-arginine action in endothelial cells, 2010 FIP Pharmaceutical Sciences World Congress. The AAPS Journal. 2010:T3392. [Google Scholar]
- 21.Tangphao O, Grossmann M, Chalon S, Hoffman BB, Blaschke TF. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47:261–266. doi: 10.1046/j.1365-2125.1999.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.