Abstract
It was previously reported that DHAP-dependent aldolase RhaD selectively chooses L-glyceraldehyde from racemic glyceraldehyde to produce L-fructose exclusively. Contrastingly, we discovered that D-glyceraldehyde is also tolerated as an acceptor and the stereoselectivity of the enzyme is lost in the corresponding aldol addition. Furthermore, we applied this property to efficiently synthesize two rare sugars D-sorbose and D-psicose.
Keywords: Aldolase, rare sugar, synthesis, RhaD
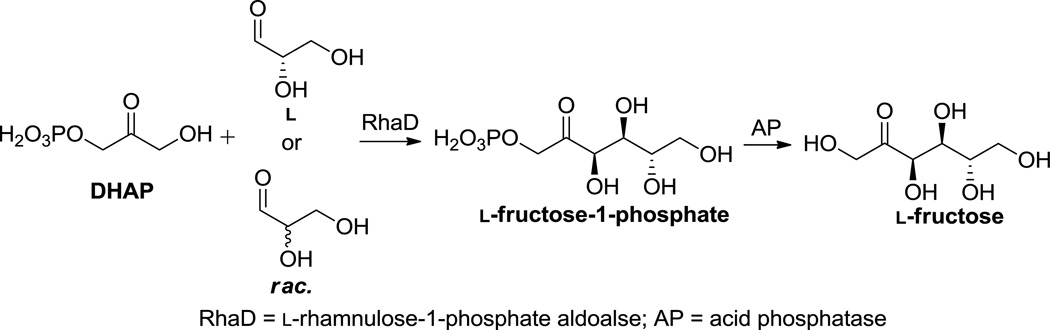
Stereochemically controlled carbon-carbon bond construction by aldolases confers them tremendous utility as synthetic biocatalysts.1–4 Among the aldolase families, dihydroxyacetone phosphate (DHAP)-dependent aldolases are remarkably useful and widely investigated, as the two new stereocenters generated in the aldol additions are predictable.5–6 Four reported DHAP-dependent aldolases are stereocomplementary, so that a complete set of diastereomers of vicinal diols can be achieved.5,7 Over the past two decades, aldolases of this group have been applied successfully in producing many rare monosaccharides and their derivatives.8–10 As a specific example, L-rhamnulose-1-phosphate aldoalse (RhaD) was used to couple DHAP with L-glyceraldehyde or racemic glyceraldehyde to produce L-fructose-1-phosphate. L-Fructose could then be obtained by dephosphorylation with acid phosphatase (AP).9,11 (Scheme 1) Furthermore, Wong and co-workers reported that RhaD preferentially made L-fructose and no diastereomers were found as by-products. Moreover, they concluded that pure L-glyceraldehyde was not necessary since D-glyceraldehyde was not consumed as a substrate by this enzyme. Contrastingly, here we report RhaD also easily tolerates D-glyceraldehyde to produce both D-sorbose and D-psicose, however, the stereoselectivity of this enzyme was totally lost when D-glyceraldehyde was the acceptor.
SCHEME 1.
Enzymatic synthesis of L-fructose with RhaD showing perfect stereoselectivity11
D-sorbose and D-psicose are both rare sugars with many commercial applications, especially in the food industry.12–14 They are low-caloric sweeteners and functional food bulking agents. Additionally, D-psicose has several potential medicinal properties, such as inhibiting the elevation of the blood glucose level. Unfortunately, both of them are quite expensive, and their synthetic routes are limited and costly because they are usually carried out at the expense of other valuable starting materials.14–16
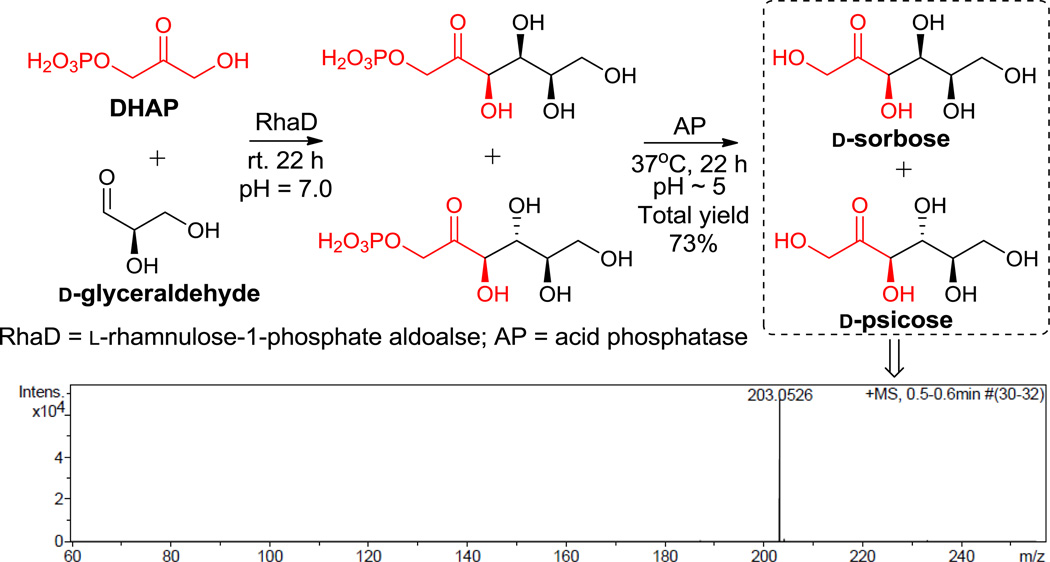
Interestingly and contrasting to Wong’s results, in which D-glyceraldehyde was separated as the remaining starting material, when racemic glyceraldehyde was used, we discovered that D- and L-glyceraldehyde were both consumed in RhaD (from Escherichia coli)17 catalyzed reactions to give a complex mixture of addition products. Thus, we directly examined the RhaD reaction using pure D-glyceraldehyde and found this substrate was mostly consumed within 16 h. Subsequently, after dephosphorylation with AP, examination by TLC revealed that the corresponding product spot had the same Rf value as D-sorbose. A single peak (203.0526, [M + Na+]) on high resolution mass spectrum was observed as well (Scheme 2). However, cation exchange HPLC and NMR revealed that what appeared to be one spot on TLC was actually two different products, D-sorbose and D-psicose (Scheme 2, Figure 1). While L-glyceraldehyde exclusively produced L-fructose (also proven in our one-pot reaction), the production of both D-sorbose and D-psicose interestingly indicated aldolase RhaD had no stereo-preference when D-glyceraldehyde was the acceptor. Additionally, the stereoselectivity of this enzyme was affected by substrate configuration. The product ratio (D-sorbose/D-psicose = ~2/3) was determined by HPLC after calibration with standard curves (see supporting information). After silica gel chromatography and gel filtration separation, the mixture containing only two rare sugars could be well isolated by cation exchange resin (Ca2+ form) chromatography18–19 at 70 °C to provide pure D-sorbose and D-psicose. Figure 2 shows a typical separation profile of the products D-sorbose and D-psicose with the separation mainly resulting from different coordination affinities of the monosaccharides to the Ca2+. Prior temperature studies indicated that the best separation was achieved under 70 °C compared with separation profiles under 50 °C, 60 °C and 80 °C.20
SCHEME 2.
Enzymatic synthesis of D-sorbose and D-psicose with RhaD and AP.
FIGURE 1.
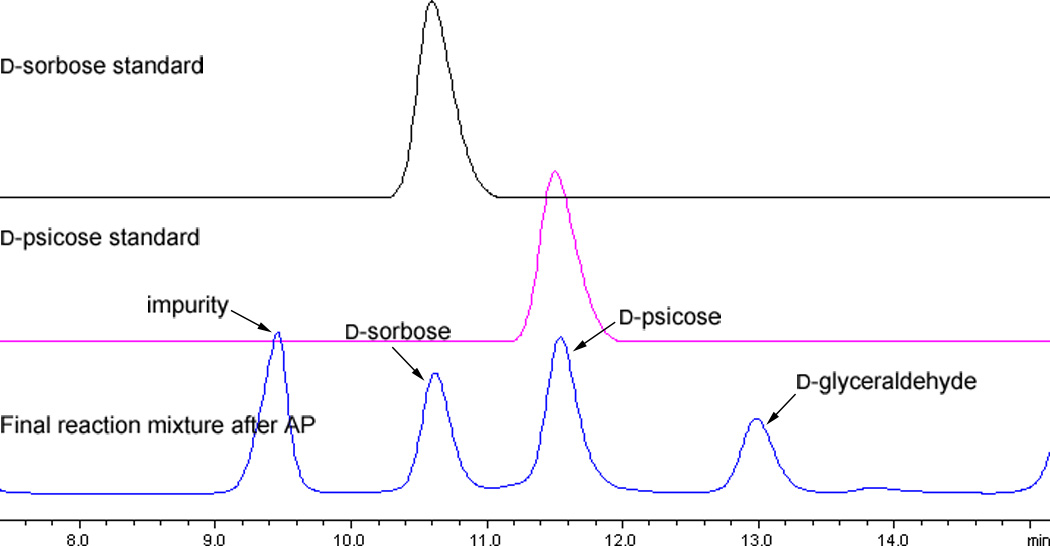
HPLC (Hydrogen form, sulfonated divinyl benzene-styrene copolymer support and eluted with 5 mM H2SO4, detected with refractive index (RI) detector) profile of final reaction (after AP) mixture compared with authentic samples.
FIGURE 2.
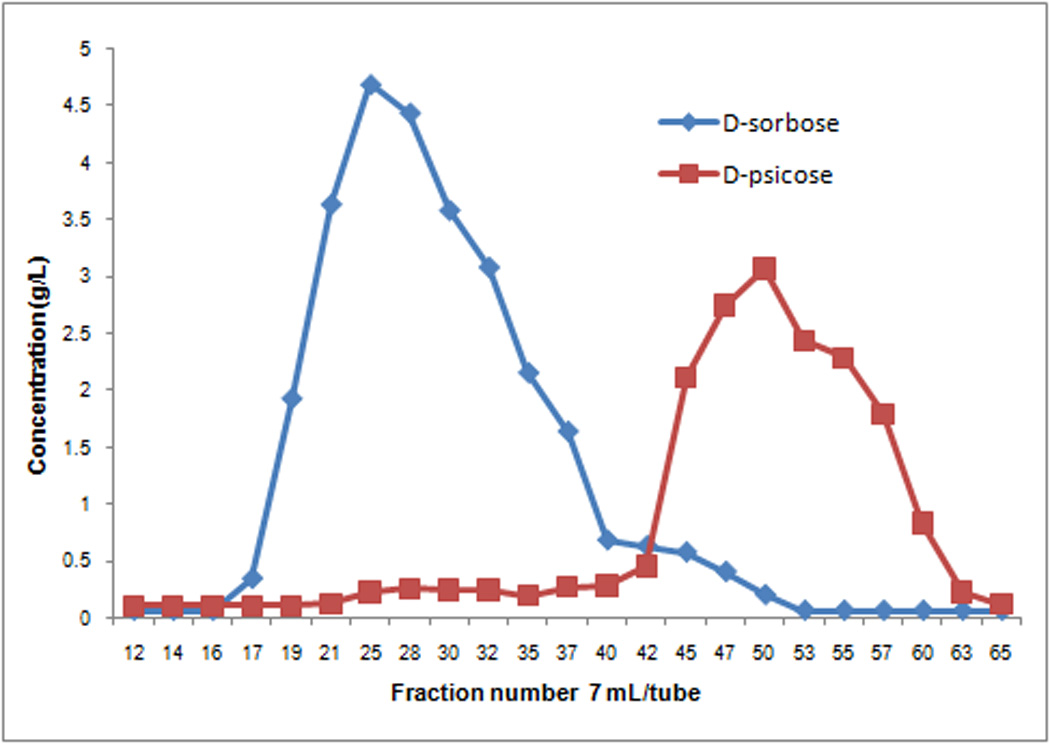
Separation profile of D-sorbose and D-psicose with cation exchange resin (Ca2+ form) eluted with ddH2O under 70 °C.
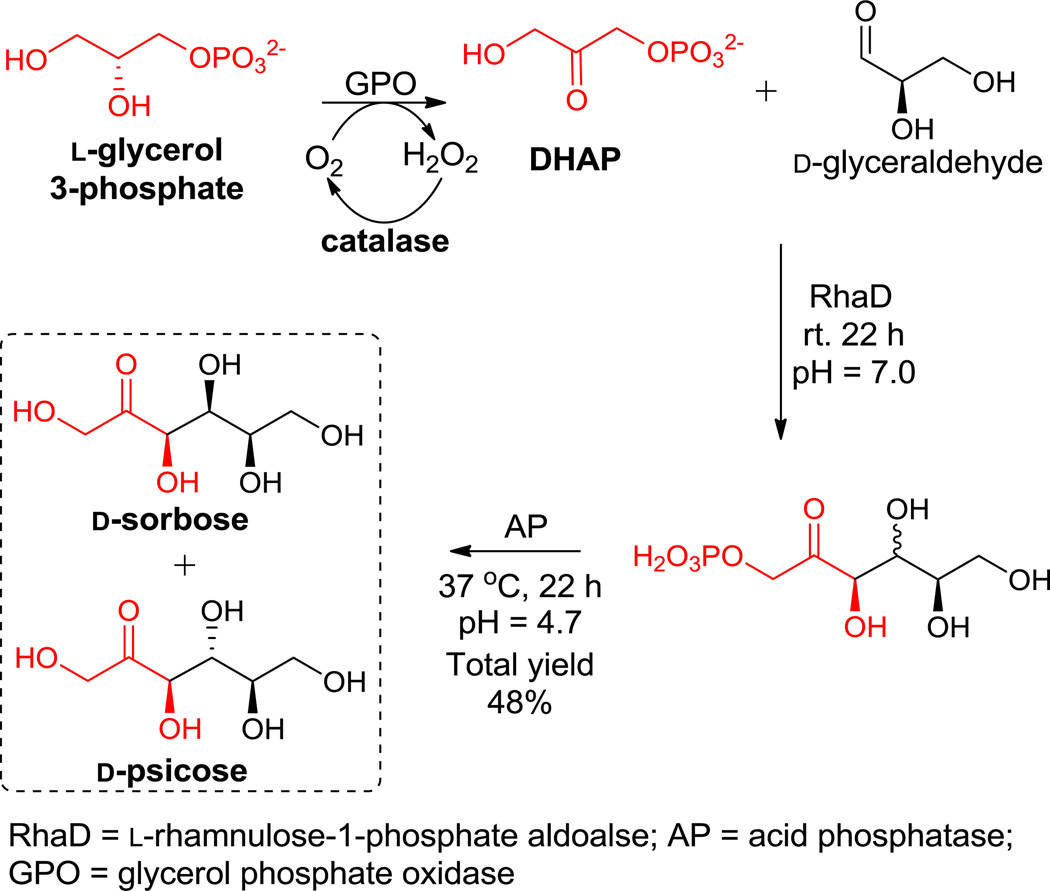
Subsequently, to improve the practicality of the reaction by avoiding the use of DHAP, a rather unstable and expensive substrate,21 we optimized our experiment to provide DHAP in situ from cheaper starting materials. A one-pot four enzyme system was used in which DHAP was generated from oxidation of L-glycerol 3-phosphate by glycerol phosphate oxidase (GPO).22 The by-product of this oxidation, H2O2, being harmful for GPO activity was thus selectively degraded by adding catalase. The DHAP generated was coupled with D-glyceraldehyde in situ by RhaD and following AP catalyzed dephosphorylation furnished D-sorbose and D-psicose in moderate yields in a one-pot fashion. (Scheme 3) The product ratio (D-sorbose/D-psicose = ~1/1) was determined by HPLC. Under the same conditions, when L-glyceraldehyde was used instead of the D-glyceraldehyde, L-fructose was produced exclusively with 66% overall yield (see supporting information).
SCHEME 3.
One-pot four enzymes synthesis of D-sorbose and D-psicose
In summary, we report that L-rhamnulose-1-phosphate aldoalse (RhaD) loses its stereoselectivity when accepting D-glyceraldehyde for aldol addition. A possible explanation could be the different enzyme concentrations used. While Fessner and co-workers previously reported a diastereomeric ratio of 97:3 (syn:anti) of RhaD,23 our observed results indicated that the anti product D-psicose may be thermodynamically favored and is accumulated during reaction time. Nonetheless, we utilized this property to synthesize two rare sugars D-sorbose and D-psicose simultaneously. It is worth noting that similar acceptor-controlled stereo preference was also observed when another DHAP-dependent aldolase l-fuculose-1-phosphate aldolase from Thermus thermophilus HB8 (FucAT.HB8) was used: FucAT.HB8 seems to lose its stereoselectivity when accepting l-glyceraldehyde.24 The enzymatically synthesized diastereomers could then be easily isolated with cation exchange resin (Ca2+ form) under elevated temperature. Lastly, with the discovery of these features, the large scale production of D-sorbose and D-psicose using fermentation are currently underway.
Supplementary Material
Acknowledgements
P.G.W. acknowledges support from the NIH (R01 HD061935 and R01 GM085267) and financial support from Georgia State University. L.C. acknowledges support from University of South Carolina Salkehatchie.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data: Supplementary data (experimental procedures and characterization of compounds) associated with this article can be found, in the online version, at doi:
References and notes
- 1.Samland AK, Sprenger GA. Appl. Microbiol. Biotechnol. 2006;71:253. doi: 10.1007/s00253-006-0422-6. [DOI] [PubMed] [Google Scholar]
- 2.Castillo JA, Calveras J, Casas J, Mitjans M, Vinardell MP, Parella T, Inoue T, Sprenger GA, Joglar J, Clapes P. Org. Lett. 2006;8:6067. doi: 10.1021/ol0625482. [DOI] [PubMed] [Google Scholar]
- 3.Fessner W-D. Curr. Opin. Chem. Biol. 1998;2:85. doi: 10.1016/s1367-5931(98)80040-9. [DOI] [PubMed] [Google Scholar]
- 4.Fessner W-D, Helaine V. Curr. Opin. Biotechnol. 2001;12:574. doi: 10.1016/s0958-1669(01)00265-8. [DOI] [PubMed] [Google Scholar]
- 5.Clapes P, Fessner W-D, Sprenger GA, Samland AK. Curr. Opin. Chem. Biol. 2010;14:154. doi: 10.1016/j.cbpa.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Machajewski TD, Wong C-H. Angew. Chem. Int. Ed. 2000;39:1352. doi: 10.1002/(sici)1521-3773(20000417)39:8<1352::aid-anie1352>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Breuer M, Hauer B. Curr. Opin. Biotechnol. 2003;14:570. doi: 10.1016/j.copbio.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama M, Hong Z, Greenberg WA, Wong C-H. Bioorg. Med. Chem. 2007;15:5905. doi: 10.1016/j.bmc.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alajarin R, Garcia-Junceda E, Wong C-H. J. Org. Chem. 1995;60:4294. doi: 10.1016/0968-0896(95)00119-2. [DOI] [PubMed] [Google Scholar]
- 10.Wong C-H, Alajarin R, Moris-Varas F, Blanco O, Garcia-Junceda E. J. Org. Chem. 1995;60:7360. [Google Scholar]
- 11.Franke D, Machajewski T, Hsu C-C, Wong C-H. J. Org. Chem. 2003;68:6828. doi: 10.1021/jo030021m. [DOI] [PubMed] [Google Scholar]
- 12.Noma A, Sato M, Tsuzuki Y. Comp. Biochem. Physiol. A: Physiol. 1974;48:249. doi: 10.1016/0300-9629(74)90706-3. [DOI] [PubMed] [Google Scholar]
- 13.Dhawale MR, Szarek WA, Hay GW, Kropinski AMB. Carbohydr. Res. 1986;155:262. [Google Scholar]
- 14.Kim N-H, Kim H-J, Kang D-I, Jeong K-W, Lee J-K, Kim Y, Oh D-K. Appl. Environ. Microbiol. 2008;74:3008. doi: 10.1128/AEM.00249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huwig A, Emmel S, Giffhorn F. Carbohydr. Res. 1996;281:183. doi: 10.1016/0008-6215(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 16.Oh D-K, Kim N-H, Kim H-J, Park C-S, Kim SW, Ko M, Park BW, Jung MH, Yoon K-H. World J. Microbiol. Biotechnol. 2007;23:559. [Google Scholar]
- 17.Garcia-Junceda E, Shen G-J, Sugai T, Wong C-H. Bioorg. Med. Chem. 1995;3:945. doi: 10.1016/0968-0896(95)00077-t. [DOI] [PubMed] [Google Scholar]
- 18.Doten RC, Mortlock RP. Appl. Environ. Microbiol. 1985;49:158. doi: 10.1128/aem.49.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodyer RD, Wymer NJ, Racine FM, Khan SN, Saha BC. Appl. Environ. Microbiol. 2008;74:2967. doi: 10.1128/AEM.02768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Mu W, Jiang B. Food and Fermentation Industries. 2008;34:168. [Google Scholar]
- 21.Schumperli M, Pellaux R, Panke S. Appl. Microbiol. Biotechnol. 2007;75:33. doi: 10.1007/s00253-007-0882-3. [DOI] [PubMed] [Google Scholar]
- 22.Fessner W-D, Sinerius G. Angew. Chem., Int. Ed. Engl. 1994;33:209. [Google Scholar]
- 23.Fessner W-D, Sinerius G, Schneider A, Dreyer M, Schulz GE, Badia J, Aguilar J. Angew. Chem., Int. Ed. Engl. 1991;30:555. [Google Scholar]
- 24.Li Z, Cai L, Qi Q, Styslinger TJ, Zhao G, Wang PG. Bioorg. Med. Chem. Lett. 2011;21:5084. doi: 10.1016/j.bmcl.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.