Abstract
In this study, we have developed mineral coatings on polycaprolactone scaffolds to serve as templates for growth factor binding and release. Mineral coatings were formed using a biomimetic approach that consisted in the incubation of scaffolds in modified simulated body fluids (mSBF). To modulate the properties of the mineral coating, which we hypothesized would dictate growth factor release, we used carbonate (HCO3) concentration in mSBF of 4.2 mM, 25mM, and 100mM. Analysis of the mineral coatings formed using scanning electron microscopy indicated growth of a continuous layer of mineral with different morphologies. X-ray diffraction analysis showed peaks associated with hydroxyapatite, the major inorganic constituent of human bone tissue in coatings formed in all HCO3 concentrations. Mineral coatings with increased HCO3 substitution showed more rapid dissolution kinetics in an environment deficient in calcium and phosphate but showed re-precipitation in an environment with the aforementioned ions. The mineral coating provided an effective mechanism for growth factor binding and release. Peptide versions of vascular endothelial growth factor (VEGF) and bone morphogenetic protein 2 (BMP2) were bound with efficiencies up to 90% to mineral mineral-coated PCL scaffolds. We also demonstrated sustained release of all growth factors with release kinetics that were strongly dependent in the solubility of the mineral coating.
Keywords: polycaprolactone, growth factor, bone morphogenetic protein 2, vascular endothelial growth factor, hydroxyapatite, bone tissue engineering
1. Introduction
Regeneration of natural tissue represents a promising approach to replace bone, and could both improve upon many of the current metallic hardware-based bone replacement methods and expand the range of orthopedic conditions that can be effectively treated. Filling of bone voids in non-union fractures or maxillofacial deformities, bridging of gaps in spine fusion surgeries, and stabilization of vertebral compression fractures provide illustrative potential applications of novel bone regeneration approaches. The nature of bone development and repair suggest that soluble growth factors are key regulators of bone formation, and can potentially be exploited to actively promote functional bone formation for repair and replacement[1]. The most common growth factor molecules used in clinical orthopedics to date have been bone morphogenetic proteins (BMPs), most notably BMP-2 and BMP-7 (also known as “OP-1”). Current clinical strategies for delivery of these molecules involve mixing BMPs with a collagen-based carrier, followed by incorporation of the carrier into a non-load-bearing site in vivo, or into a metallic, load-bearing implant. For example, Medtronic’s Infuse® product, which includes recombinant human BMP-2 combined with an absorbable collagen sponge, has been used clinically for alveolar ridge augmentation[2, 3], spine fusion[4, 5], and non-union fracture healing[6], among other applications. These studies and others demonstrate the tremendous promise of growth factor delivery in clinical bone repair.
The importance of growth factor signaling has led to widespread efforts to more effectively deliver bone growth factors. The existing collagen-based BMP carriers use highly supraphysiologic doses (on the order of milligrams) delivered over relatively short timescales, which has recently led to well-documented clinical side effects including edema[7, 8], heterotopic bone formation[9], retrograde ejaculation [10], and potentially increased cancer risk [11]. Emerging approaches have focused on controlling growth factor release kinetics in order to decrease the needed growth factor dose and limit deleterious side effects. Sponges[12], hydrogels[13, 14], pastes[15], putties, particulates, and various micro-/nano-carriers[14] have been used to successfully deliver bone growth factors over longer timescales than the collagen sponges used in current clinical applications[16]. However, these approaches tend to use carrier materials that are not structurally optimized for bone tissue engineering applications, and some strategies may be difficult to translate to clinical applications. Therefore, there is a need for clinically relevant strategies that can deliver bone growth factors over sustained, controllable timeframes from scaffolds with optimized structural properties.
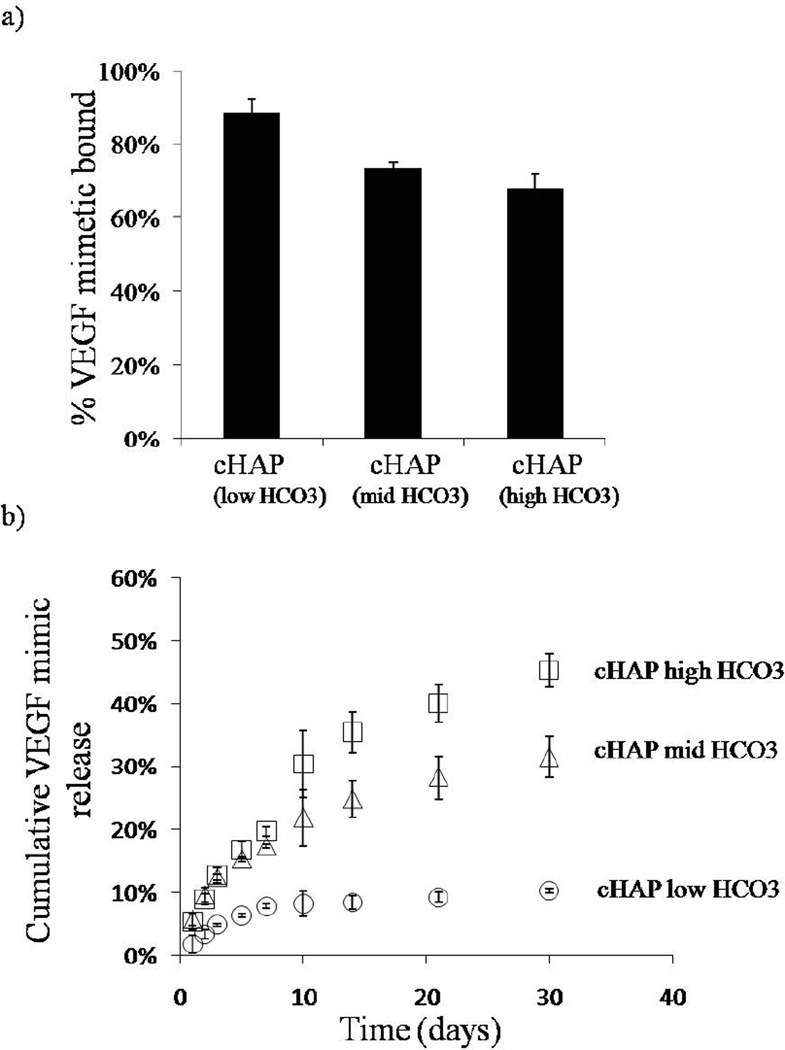
We hypothesized that bioresorbable polymer scaffolds could be coated with mineral layers, which would serve as a controllable platform to bind and release growth factors. Our previous studies have demonstrated that nano-porous mineral coatings can be grown on medical devices (e.g. screws, sutures, microspheres), and these coatings effectively bind and release growth factors. Released growth factors are biologically active, as demonstrated by enhanced stem cell differentiation in vitro, and improved bonetendon healing in vivo. Importantly, these previous studies demonstrate a “modular design” approach, in which the biologics carrier in integrated into the structural device without negatively impacting physical properties. This type of modular design approach may be particularly useful for design of bone tissue engineering scaffolds, in which there is a clear need for optimized physical and biological properties[17]. The focus of our current study was to control growth factor release kinetics from bone tissue engineering scaffolds by varying mineral coating stability. We reasoned that mineral coatings formed in solutions with higher carbonate content would have decreased stability due to carbonate substitution in the mineral, as shown previously. Decreased mineral stability would then lead to increased mineral dissolution rate and, in turn, more rapid growth factor release kinetics.
2. Methods
2.1 PCL scaffolds and incubation in mSBF with variations in HCO3 concentration
Cylindrical porous PCL scaffolds were fabricated via selective laser sintering as previously reported [18]. The cylindrical porous scaffolds (8mm diameter, 16mm in height) had three-dimensional orthogonal periodic porous architecture. Scaffolds were sectioned into smaller cylinders (8mm diameter, 4mm height) for all experiments. They were incubated at 37 °C in modified simulated body fluids (mSBF) with 4.2 mM HCO3, 25mM or 100mM HCO3 for periods of 7 days under continuous rotation. Each scaffold was incubated in 15 ml of mSBF. The mSBF solution had a similar composition to that of human plasma but with double the concentration of calcium and phosphate to enhance mineral growth, and was prepared as previously reported [19]. Specifically, the following reagents were added to ddH2O heated to 37 °C in the order shown; 141mM NaCl, 4.0 mM KCl, 0.5 mM MgSO4, 1.0 mM MgCl2, 4.2 mM or 100mM NaHCO3, 20.0 mM HEPES, 5.0 mM CaCl2, and 2.0 mM KH2PO4. The solution was then adjusted to a final pH of 6.8 using HCl/NaOH. The mSBF solution was renewed daily in order to maintain a consistent ionic strength throughout the experiment. Prior to mSBF incubation, scaffolds were hydrolyzed in a 0.1 M NaOH solution for 60 min to produce a hydrolyzed, carboxylic acid rich surface. To confirm the need for the hydrolysis pre-treatment, PCL films which were fabricated by solvent casting as previously reported [20], were half exposed to the NaOH solution and the other half covered with tape as a control. After hydrolysis, films were rinsed and incubated in mSBF low HCO3 for 7 days.
2.2 Analysis of mineral growth
In order to assess mineral formation, PCL scaffolds were collected after incubation and the change in mass was recorded. Scaffolds were rinsed at least twice in ddH2O to remove residual salts and then were freeze dried overnight in a lyophilizer at low temperature (−60°C) under vacuum (10μm Hg). The mass was recorded and compared with the initial mass.
The morphology and composition of the mineral coating grown on the scaffolds was investigated by scanning electron microscopy (SEM), and X-ray diffraction (XRD). PCL scaffolds incubated for 7 days were mounted on aluminum stubs and sputter coated with a thin layer of gold. Samples were imaged under high vacuum using a JEOL JSM-6100 scanning electron microscope operating at 10kV. To perform XRD analysis, the mineral coating formed after incubation of PCL scaffolds in mSBF with different HCO3 concentration for 7 days was collected after dissolving PCL scaffolds in chloroform. Mineral coated scaffolds were immersed in chloroform (ACROS Organics) and stirred continuously for 20 minutes or until completely dissolved. The mixture was then centrifuged at 4000rpm for 5 minutes. The mineral was rinsed in fresh chloroform and centrifuged for a second time. After collecting the chloroform, the mineral powder was dried under the hood for 2 days to allow the residual chloroform to evaporate. The mineral powder was then used for XRD analysis. XRD patterns of the mineral coating were recorded using a General Area Detector Diffraction System (GADDS), with a Hi-star 2-D area detector (20° < 2θ < 40°) using Cu Kα radiation. The resulting patterns of the samples were identified by computer matching with an International Centre for Diffraction Data (ICDD) powder diffraction database (ICDD card number for hydroxyapatite: 00-001-1008).
2.3 Mineral stability
The stability of the mineral coating was investigated by measuring the amount of calcium and phosphate released into aqueous buffer and in cell culture medium as a baseline of the stability of the coating in an in vivo environment. In brief, following SBF incubation, scaffolds were incubated in a 24 well plate at 37°C in 1ml of either TRIS/NaCl, pH 7.4 or Dulbecco's Modified Eagle Medium (DMEM). The TRIS/NaCl or DMEM incubating solutions were collected on the following times points; d1, 2, 3, 5, 7, 10, 15, 21, and 30, 40, 50, 60, 75 and 85. Each time that the buffer was collected it was replenished with fresh solution (same volume, 1ml). A total of 4 samples per coating were used for dissolution experiments. To quantify the amount of calcium, 5μl of sample was mixed with 195μl of a 0.4mM Arsenazo III/0.02M tris solution buffer to pH 7.4 as reported before[21]. The amount of calcium was quantitatively detected by measuring the absorbance of the 615nm band on a BioTek Synergy plate reader and comparing it to a set of standards with known calcium concentrations. The amount of phosphate released was measured using a colorimetric assay as previously reported[21]. In brief, 10μl of sample was diluted with 90μl of diH2O. An acetone-acid-molybdate (AAM) solution was prepared immediately prior to performing the assay by mixing 1 part 10mM ammonium molybdate solution with 1 part 5.0N sulfuric acid and 2 parts acetone. Then 100μl of AAM solution was mixed with 100 μl of sample in each well in a 96 well plate. The AAM solution reacts with soluble phosphate ions to form a molydiphosphoric acid complex that has a yellow hue that can be quantified using spectroscopy. The amount of phosphate was quantitatively detected by measuring the absorbance of the 405nm band on a BioTek Synergy plate reader and comparing it to a set of standards with known phosphate concentrations.
2.4 Synthesis of peptide growth factors
All peptides (VEGF mimetic, modular VEGF, and modular BMP2) were synthesized manually by solid phase peptide synthesis on Fmoc-Rink Amide MBHA resin with Fmoc-protected a-amino groups with PyBop/DIPEA/HOBT activation as previously reported[22–24]. To characterize binding and release, 5(6)-FAM (5(6)-carboxyfluorescein, or Rhodamine (Sigma) was conjugated to the N-terminal lysine residue. The resulting peptides were cleaved from resin for 4 h using a TFA:TIS:water (95:2.5:2.5) cocktail solution, filtered to remove resin and precipitated in diethyl ether. Crude peptide mixtures were purified using a Shimadzu Analytical reverse-phase HPLC (Vydac C18 column) with 1% min_1 of 0.1% trifluoroacetic acid in acetonitrile (ACN) for 60 min and analyzed verified using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry (Bruker Reflex II time-of-flight mass spectrometer).
2.5 Binding and release of peptide growth factors (VEGF mimetic peptide, mVEGF, and mBMP2)
Fluorescently tagged peptides were used for release studies. A mineral coated scaffolds was incubated in 500 μL of a solution containing carboxyfluorescein labeled VEGF mimetic peptide (500 μg/mL), mVEGF (500 μg/mL), or rhodamine labeled mBMP2 (500 μg/mL) at 37 °C for 1 hour to allow equilibrium binding. The scaffold was then rinsed, transferred, and incubated in 500μL of Dulbecco’s modified Eagle’s medium (DMEM) at 37 °C. The fluorescence intensity of the solution used for binding was measured to determine the percentage of growth factor bound initially. At the indicated time points, the fluorescence intensity of the release medium was measured using a Packard Cobra II Gamma Counter (Meriden, CT) and a BioTek Synergy plate reader (excitation/emission: 494/519 for carboxyfluorescein labeled VEGF peptides, and excitation/emission: 543/572 for rhodamine labeled mBMP2).
2.6 Statistical analysis
All results are expressed as means ± standard deviations. Differences between data sets were assessed by an analysis of variance (ANOVA). A p value less than 0.05 was considered significant.
3. Results
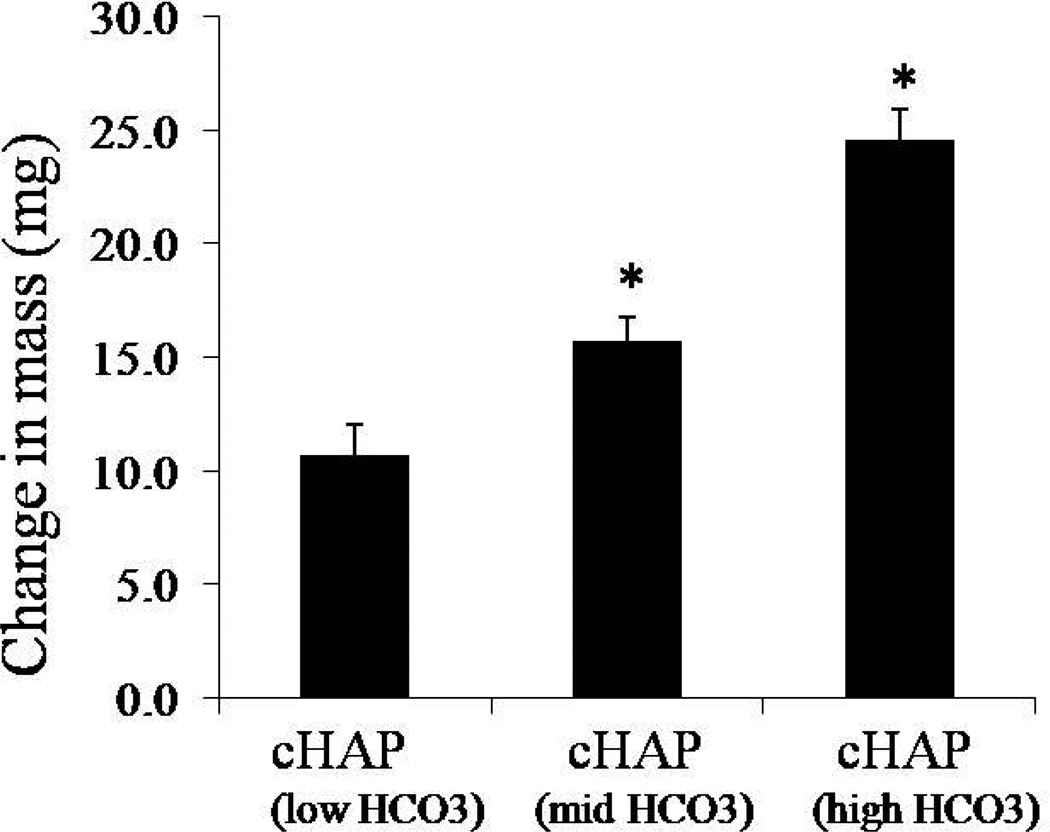
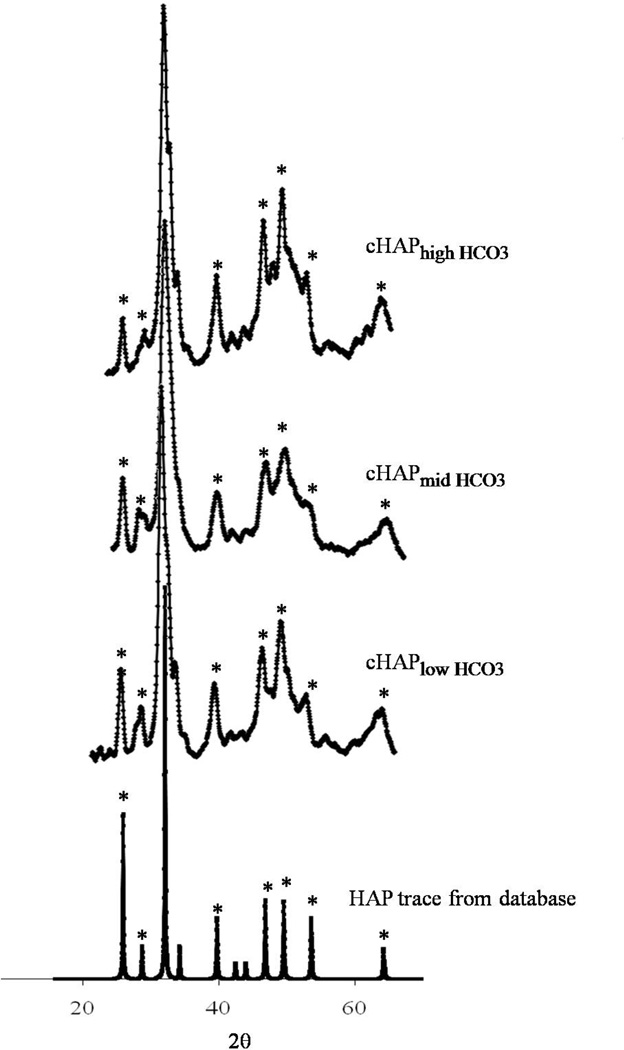
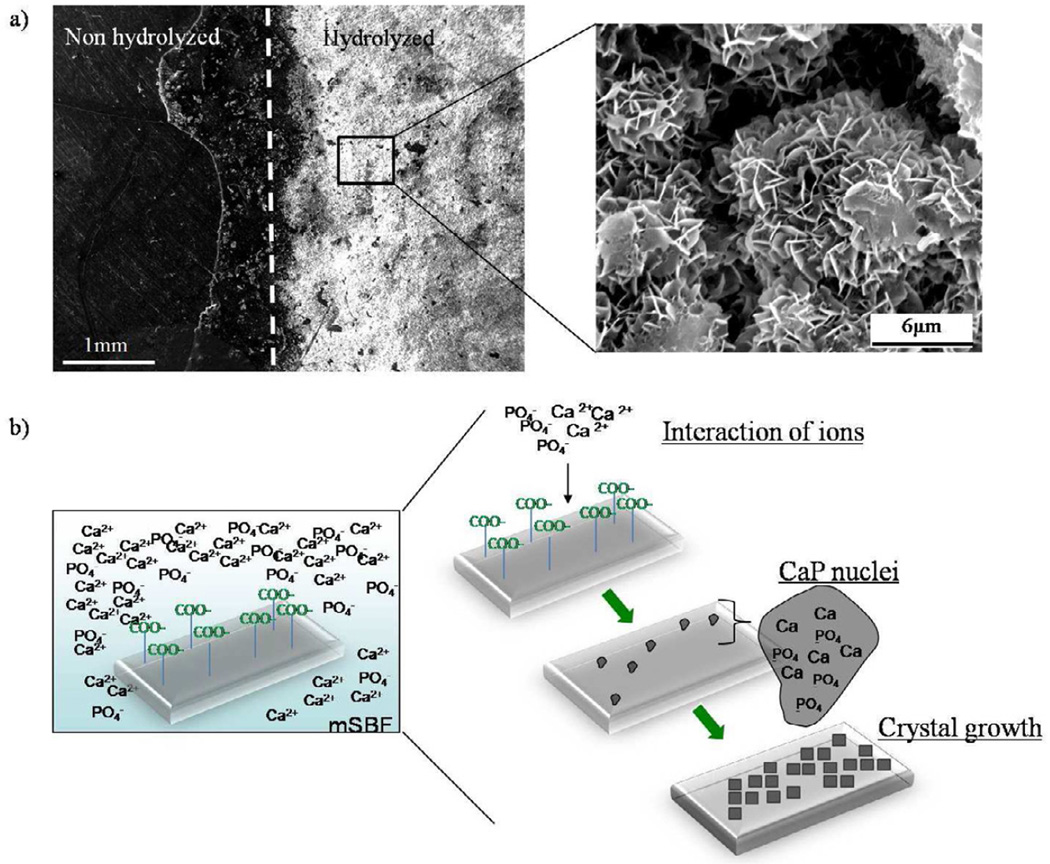
Porous PCL scaffolds were fabricated via selective laser sintering with controlled periodic porous architecture. Incubation of hydrolyzed PCL scaffolds in mSBF resulted in significant increases in the scaffold mass, suggesting the growth of a mineral layer on the material (figure 1). Interestingly, as the HCO3 concentration in mSBF increased, the change in mass significantly increased as well (p<0.05 for all groups). A qualitative analysis of the surface of PCL scaffolds incubated for 7 days in mSBF with varying HCO3 concentration confirmed the formation of continuous mineral coatings in all conditions tested (figure 2a-d). Scanning electron microscopy (SEM) images clearly demonstrated morphological differences resulting from using SBF solutions with HCO3 concentrations of 4.2mM (cHAP low HCO3), 25mM (cHAPmid HCO3), and 100mM (cHAPhigh HCO3) (figure 2e-h). The mineral formed at lower HCO3 concentration, cHAPlow HCO3, displayed primarily a plate-like nanostructure (figure 2f), and the nano-scale plates became smaller as the HCO3 concentration was increased (figure 2g-h). The mineral nucleated on the scaffolds had X-ray diffraction patterns consistent with hydroxyapatite (HAP, Ca10(PO4)6(OH)2) in all groups, regardless of the HCO3 concentration in mSBF (figure 3). The XRD patterns of the mineral layers exhibited broad peaks relative to typical patterns for pure HAP, which likely indicates a small crystal size, a more amorphous mineral and the formation of carbonated HAP, which is typically calcium deficient, poorly crystalline, and not a pure form of HAP. Alkaline pre-treatment of the PCL was necessary for the nucleation of the mineral coating (figure 4). PCL films that were hydrolyzed in spatially defined regions (“patterned” hydrolysis) showed mineral formation only on the region pre-treated with NaOH and no evidence of mineral formation on the region that was protected from the NaOH solution (figure 4a).
Figure 1.
Incubation of PCL scaffolds for 7 days in different mSBF formulations, low (4.2mM) , mid (25mM), and high (100mM) HCO3, resulted in an increase in the change in mass of PCL scaffolds (mass after incubation- mass before incubation). The change in mass significantly increased as the HCO3 concentration in mSBF was increased.
Figure 2.
Mineral morphology and continuity. Surface of PCL scaffolds before incubation in mSBF at a) low magnification (scale bar-20μm), and e) high magnification (scale bar-3μm). b-d)Incubation for 7 days in all mSBF formulations resulted in the formation of a continuous coating on scaffolds. The morphology of the mineral is affected by the extent of HCO3 substitution. f-h) As the HCO3 substitution increased from e) cHAP low HCO3, to f) cHAP midHCO3, and g) cHAP high HCO3, the plate like nanostructure becomes smaller.
Figure 3.
X-ray diffraction of mineral coating. XRD data confirms the nucleation of mineral that shows hydroxyapatite diffraction peaks. The mineral phase is not significantly affected by increasing the extent of HCO3 substitution. The diffraction patterns were identified by computer matching with an International Centre for Diffraction Data (ICDD) powder diffraction database (ICDD card number for hydroxyapatite: 00-001-1008).
Figure 4.
a)Hydrolysis of PCL. Partial exposure of PCL films to NaOH resulted in the formation of a mineral coating in the region exposed to the hydrolysis pre-treatment. The surface that was protected from the NaOH solution did not show evidence of mineral formation after incubation for 7 days in mSBFlow HCO3. b) Schematic representation of the process of mineral coating formation after incubation in simulated body fluids. Hydrolysis of PCL scaffolds prior mSBF incubation results in an increase in the number of carboxylic acid groups on the surface of the scaffold. These negatively charged groups interact with calcium and phosphate ions in solution to form CaP rich nuclei from which the mineral grows creating a mineral coated surface.
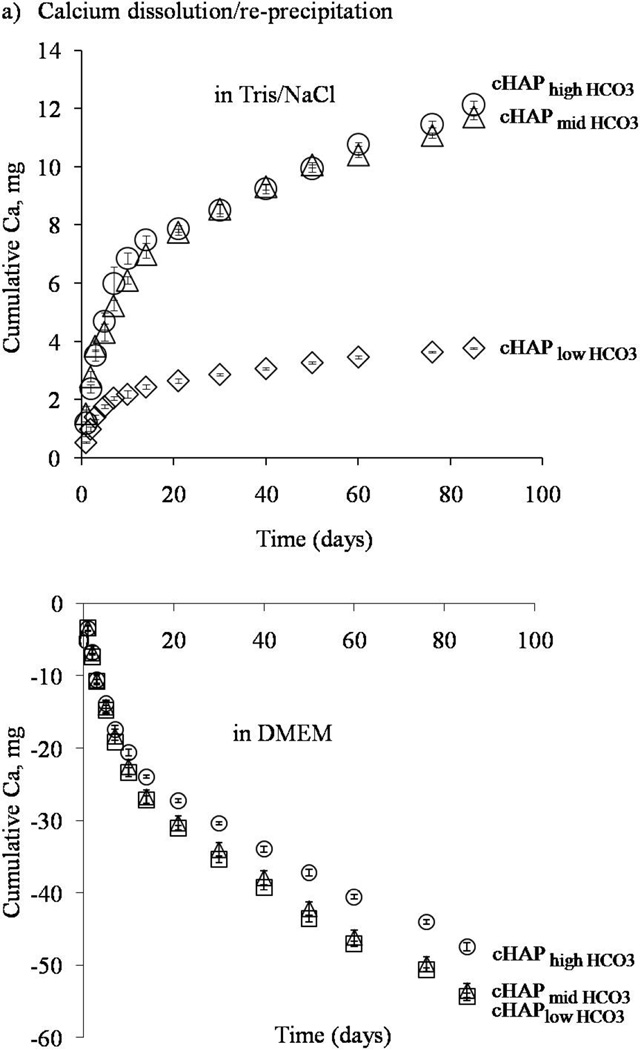
Mineral dissolution involved a balance of dissolution and re-precipitation, which depended on the solution environment in which the scaffolds were placed. Mineral coatings with increased HCO3 substitution showed more rapid dissolution kinetics. The rate of calcium release and the total amount of calcium release – both measures of mineral dissolution- increased for mineral coatings with higher HCO3 content in tris/NaCl buffer (figure 5a). Tris/NaCl buffer does not contain calcium ions, therefore dissolution was favored over re-precipitation. On the other hand, in cell culture medium that contained calcium and phosphate ions, re-precipitation was favored, resulting in a net decrease in calcium and phosphate concentrations (figure 5b).
Figure 5.
Calcium dissolution and re-precipitation in aqueous buffer (upper panel) and cell culture medium (lower panel). The rate of calcium release and the total amount of calcium released increased as the extent of HCO3 substitution increased in aqueous buffer (upper panel). In cell culture medium, as the HCO3 substitution increased, the consumption of calcium ions decreased.
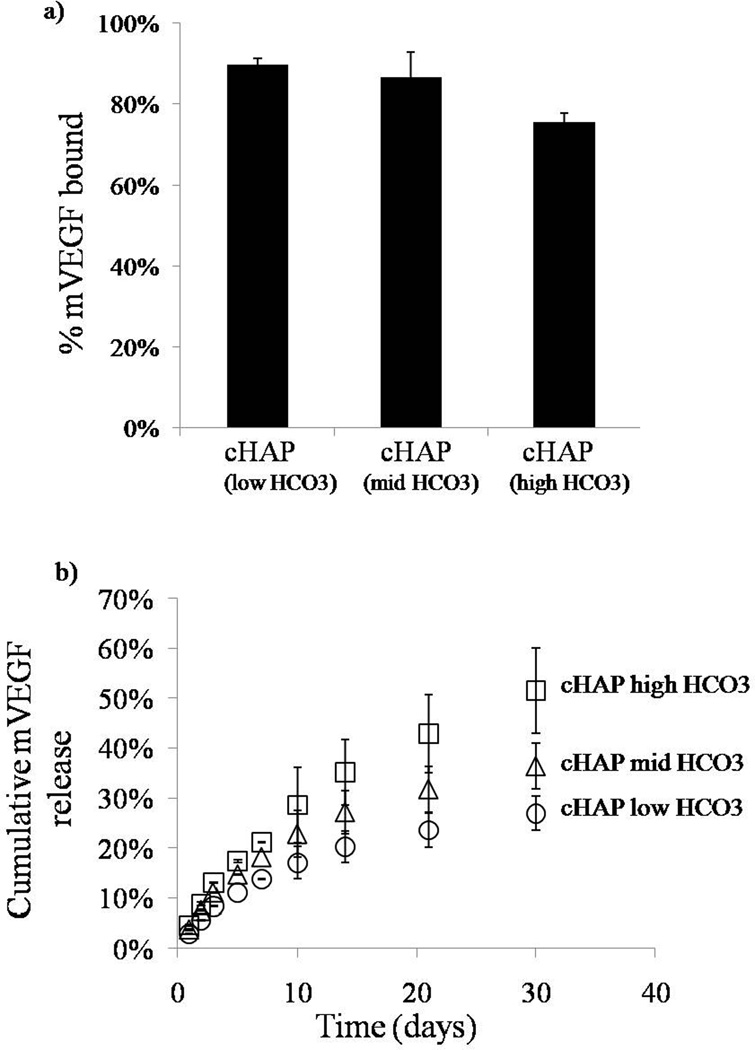
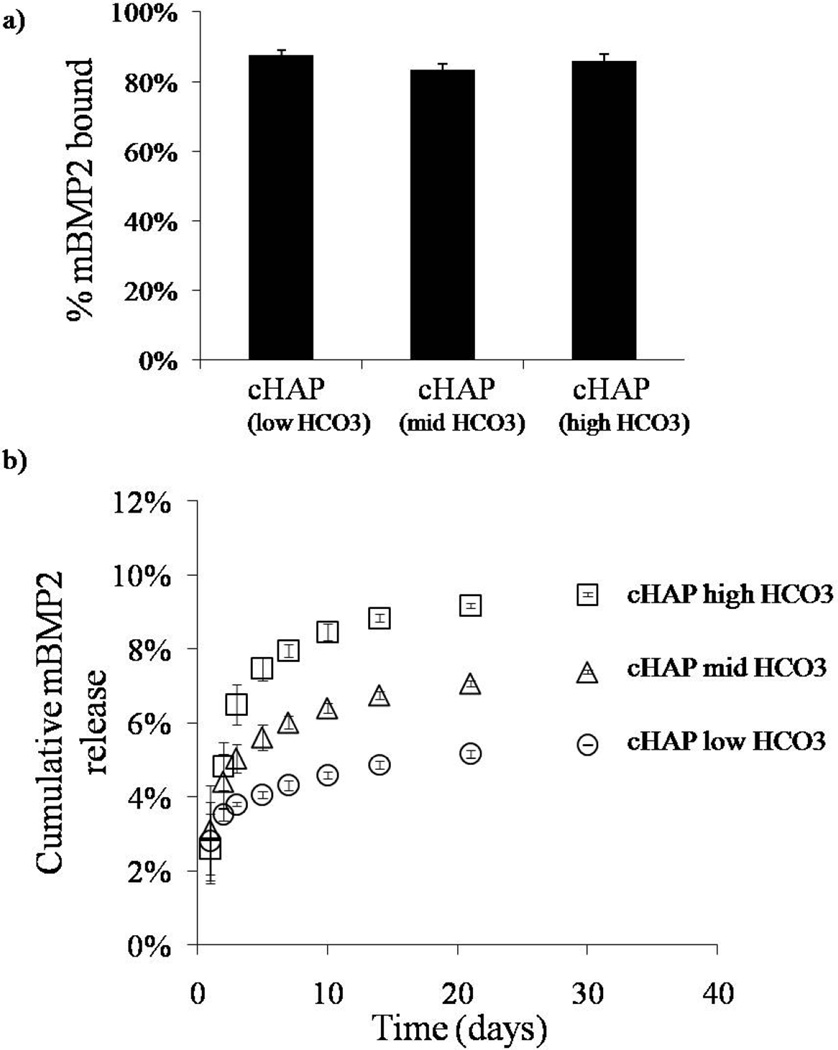
Distinct growth factors bound with high efficiency to mineral-coated PCL scaffolds. The percentage of VEGF mimetic peptide bound varied depending on the mineral coating (figure 6a). In the scaffolds with cHAP low HCO3 coating, the percentage of VEGF mimetic peptide bound was 89% (±4%). The percentage of VEGF mimetic bound decreased to 74% (± 1%) and 68% (±4%) for the cHAPmid-HCO3 and cHAP high HCO3 coatings respectively (figure 6a). We next explored binding of modular, mineral-binding peptides mVEGF and mBMP2 to address the hypothesis that mineral-binding peptides would bind to mineral coatings with increased efficiency. The percentage of mVEGF bound to the mineral coatings formed after incubation in cHAPlow HCO3, cHAPmid HCO3, and cHAPhigh HCO3, were 90% (±2%), 87% (±6), and 76% (±2%) respectively (figure 7a), while the percentages of mBMP2 bound to the same mineral coating types were 88% (±1%), 83% (±2), and 86% (±2%) (figure 8a). Thus, the modular, mineral-binding growth factors each bound with significantly higher efficiency than the VEGF mimetic peptide, to the cHAPmid HCO3 and cHAPhigh HCO3 coatings.
Figure 6.
Binding and release of VEGF mimic. a) VEGF mimic peptide bound with high affinity to the different mineral coatings formed on PCL. b) The release kinetics changed based on the extent of HCO3 substitution in the mineral coating. The release rate of cHAP low HCO3 was lower and as the HCO3 substitution increased, the rate of VEGF mimic release increased as well.
Figure 7.
Binding and release of mVEGF. a) mVEGF bound with high affinity to the different mineral coatings. b) Sustained release of mVEGF in DMEM was observed in all conditions. The release kinetics changed based on the extent of HCO3 substitution in the mineral coating. At lower HCO3 substitution, the release rate was lower and as the HCO3 substitution increased, the rate of mVEGF release increased as well.
Figure 8.
Binding and release mBMP2. a) mBMP2 bound with high affinity to the different mineral coatings. b) Sustained release of mBMP2 in DMEM was observed in all conditions. The release kinetics changed based on the extent of HCO3 substitution in the mineral coating.
Release of growth factors was sustained for over 30 days in cell culture medium, and release kinetics were strongly dependent on the HCO3 content in the mineral. In all studies, as the HCO3 substitution was enhanced, the percentage of growth factor release was greater. By day 30, the percentages of VEGF mimetic peptide released were 45 ± 3%, 31 ±3%, and 10±0.1% for scaffolds with cHAPhigh HCO3,cHAPmid HCO3, and cHAPlow HCO3 coatings, respectively (figure 6b). Similar trends were observed for mVEGF and mBMP2. The percentages of mVEGF released by day 30 were 51±9%, 36±5%, and 27 ±3% for scaffolds coated with cHAPhigh HCO3, cHAPmid HCO3, and cHAPlow HCO3, respectively (figure 7b). The percentage of release of mVEGF was significantly higher that the release of VEGF mimetic peptide from the same coating for all coating conditions. Lastly, the percentages of mBMP2 released by day 30 were 10 ±0.1%, 7 ±0.1%, and 5 ±0.2% from scaffolds coated with cHAPhigh HCO3, cHAPmid HCO3, and cHAPlow HCO3, respectively (figure 8b). Thus, modular BMP2 release was significantly slower than mVEGF and VEGF mimetic peptide.
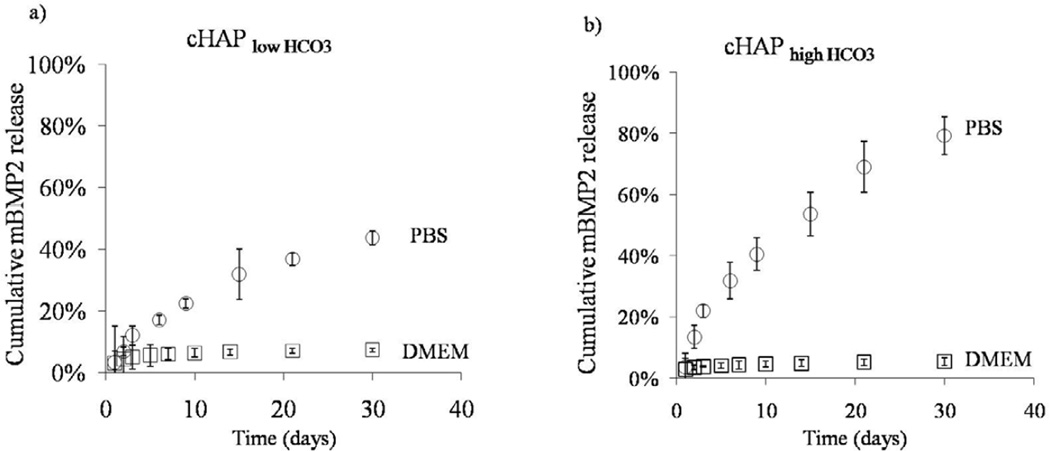
Release of growth factors also depended on the solution environment used for the release studies. We evaluated the release of mBMP2 in aqueous buffer and compared with the release in cell culture medium (figure 9). By day 30, the release of mBMP2 from cHAPlow HCO3 coatings with differed from 10% ±0.1% in cell culture medium to 80 ±6% in phosphate buffer saline (PBS) (figure 9). The release of mBMP2 from cHAPlow HCO3 coatings also showed the same trend of lower release in cell culture medium 5 ±0.2% and significantly higher release in PBS 44 ±2%. Finally, in PBS, the release of mBMP2 from cHAPlow HCO3 coatings released significantly slower compared to the cHAPhighHCO3 coatings, similar to the studies described in the previous paragraph and in Fig. 9.
Figure 9.
Release of mBMP from different buffer systems. A) Release of mBMP2 into PBS or cell culture medium from mineral coated PCL from cHAPlowHCO3 coatings. Release of mBMP2 was significantly lower in DMEM compared to PBS. B) Higher percentage of mBMP2 was released from cHAP highHCO3 coatings compared to (a) in either buffer system but a similar trend was observed. Release of mBMP2 was higher in PBS compared to DMEM.
4. Discussion
Our results demonstrate that mineral coatings provide an effective platform for efficient growth factor binding. Our fundamental approach to growth factor incorporation into a biomaterial is based on a phenomenon that is well known in protein chromatography and bone biology – the ability of proteins to bind efficiently to calcium phosphate biomineral surfaces [25–27]. In particular, calcium ions in HAP can interact with carboxylate residues at the protein surface and phosphate ions in HAP can interact with basic protein residues. In the current study we modulated peptide binding efficiency to HAP by synthesizing peptides with or without mineral-binding domains, and the peptide identity clearly influenced binding efficiency. The VEGF mimic peptide bound with efficiencies ranging from 68%–89% (figure 6a), whereas the peptides with mineral binding domain, mBMP2 and mVEGF, bound with efficiencies from 76% to 90% (figures 7a and figure 8a). The generally high binding efficiency of all of these peptides to biomineral coatings (>68% in all cases) may be due to the the the high surface area of the nano-porous mineral coating (figure 2). The observation that mVEGF and mBMP2 bind with enhanced efficiency when compared to the VEGF mimic peptide can be attributed to the presence of a mineral binding domain in these peptides. The mineral binding domain contains three Gla residues, which we have previously shown to play an important role in peptide-HAP binding . Specifically, Lee et al. systematically substituted Gla residues with Ala residues in the mineral binding domain of mBMP2 and observed significant decreases in binding to both pure HAP surfaces [28] and HAP coatings[29]. High efficiency of growth factor binding may be critically needed for biomedical applications, as most clinical growth factor delivery devices developed to date have required high concentrations of growth factors, in some cases on the order of milligram quantities[30]. High efficiency of growth factor incorporation has been explored in previous studies by encapsulating growth factors in beads[31], microspheres[32–34], or hydrogel coatings[34, 35]. For example, Gu et al. encapsulated 67% of VEGF into alginate beads[31], and Kempsen et al encapsulated 82% of BMP2 into poly(lactic-co-glycolic acid) microspheres[36]. Relative to these other studies the mechanism of growth factor binding described in the current study may represent a particularly simple and adaptable approach for efficient binding of growth factors, since the process consists of simply dipping a mineral coated material into a growth factor solution. Additionally, since the mineral is nucleated from an aqueous solution, this technique can be applied to scaffolds with complex 3D structures and turn complicated structures into efficient growth factor delivery vehicles.
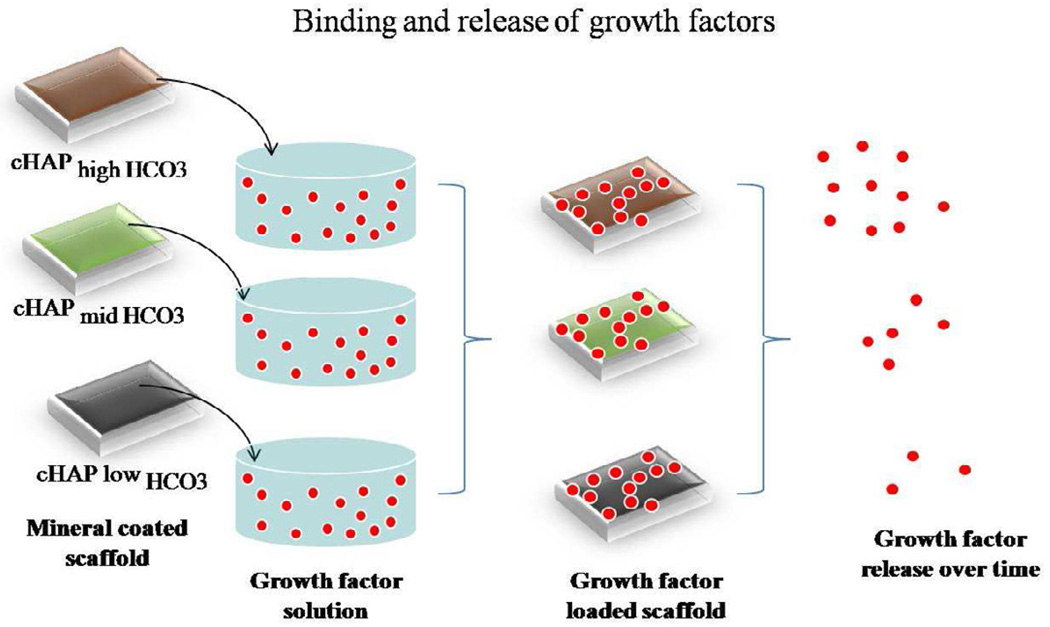
Growth factors bound to mineral coated PCL scaffolds released in a sustained and controllable manner over time, which is likely to be an important property in future bone tissue engineering applications. Bone repair and regeneration involve a complex cascade of biological events. During bone repair, BMP-2 is expressed early during the early inflammatory phase, and later in the process during osteogenesis[37]. Thus, it is not surprising that many reports have shown that bone regeneration can be enhanced by sustained release of BMP-2[38, 39]. The data presented here showed sustained release of each growth factor for over 30 days (figures 6b, 7b, and 8b), and the ability to modulate the release profiles based on the properties of the mineral coating. The morphological features and stability of the biomineral were modulated by changing the HCO3 concentration in mSBF. We took advantage of changes in solubility of a mineral coating as a way of modulating growth factor release. SEM observations showed that the plate like nanostructure in the coating became smaller as the carbonate substitution increased and more spheroidal shaped crystals were formed (figure 2f-h). These results are consistent with previous literature, which has shown that increased carbonate substitution in hydroxyapatite leads to decreased crystallinity, smaller crystal size, and faster dissolution [40, 41]. Mineral dissolution correlated with growth factor release kinetics. In the biomimetic mineralization process, mineral coating formation and dissolution are reversible, and their equilibrium is detemnined by the degree of saturation of the surrounding solution. In general, mineral coatings can be formed when the surrounding environment is supersaturated with calcium and phosphate ions, whereas mineral coatings are dissolved in undersaturated conditions (i.e., lower [calcium] and [phosphate] in the solution). The mechanism of mineral dissolution/re-precipitation strongly influenced growth factor release as the release profile of mBMP2 was significantly slower in cell culture medium- in which there is a net reprecipitation of ions, when compared to PBS in which dissolution is the dominant mechanism (figure 9). We hypothesize that during the dissolution process, growth factors are released, however, growth factors that remain bound can become blocked after reprecipitation of ions which slows down the release kinetics (figures 6b, 7b, 8b, and 10).
Figure 10.
Growth factor binding and release to mineral coating. Mineral coated scaffolds are incubated in a solution with the growth factor of interest for 1 hour. After binding, the release profile strongly depends on the solubility of the coatings which is varied by the extent of HCO3 substitution. At higher HCO3 substitution, the amount of growth factor released over time is greater.
Importantly, mineral coatings can be grown on structurally optimized bone tissue engineering scaffolds allowing for “modular design”[17]. The concept of modular design here is meant to indicate that the physical scaffold properties (e.g. porosity, pore size, geometry, permeability, stiffness, degradation rate) [18] can be designed somewhat independently from the biological proerties (osteoconductivity, growth factor incorporation, growth factor release), since the former are optimized in the base scaffold, and the latter are designed into the surface coatings. This design approach could lead to unique applications. For example, PCL scaffolds fabricated via SLS and coated via the approach described here could prove useful for the construction of scaffolds with patient-specific anatomies and interior porous architectures derived from computational design optimizations. Control over the physical characteristics of these scaffolds may enhance cell infiltration and mass transport of nutrients and metabolic waste throughout the scaffold, while surface coatings can be designed to promote osteoconductivity and deliver key growth factors to enhance healing. It is also noteworthy that PCL is an attractive polymer in bone tissue engineering since it can be used in a wide range of scaffold fabrication technologies, it is relatively inexpensive, and is FDA approved in multiple medical device formulations[42].
The mechanism for mineral nucleation and growth presented here is consistent with previous studies on other bioresorbable polymer substrates[19, 43–45]. The biomimetic process used for the formation of the mineral coating consists of a simple incubation in a solution that approximates the ionic constituents, pH, and temperature of blood plasma – termed mSBF. The mineral coating was continuous in all mSBF conditions after incubation for 7 days (figure 2b-d) and an alkaline pre-treatment was necessary to initiate the biomineral coating process (figure 4a), similar to previous studies[43, 45]. The pre-treatment likely increased the number of primary carboxylic acid and hydroxyl groups revealed upon surface hydrolysis. Following the alkaline pre-treatment, the formation of the biomineral coating involved the interaction of calcium- and phosphate-rich pre-nuclei in solution with the polar carboxylic acid and hydroxyl groups on the surface of the scaffold. Those interactions lead to heterogeneous formation of HAP nuclei and subsequent growth of these nuclei to form the continuous coating (figure 4b). X-ray diffraction analysis of mineral coatings with different carbonate content on PCL revealed characteristic peaks of HAP(figure 3).These finding are consistent with previous studies, as the apatite structure (Ca10(PO4)6(OH)2) allows varied substitutions to take place without significant alteration in its fundamental structure [40, 41]. Because the mineral is nucleated from an aqueous solution, this technique can be applied to scaffolds and devices with varying geometry, and on various base materials. The current study, when combined with previous studies from our group and others, shows that hydroxyapatite mineral coatings can be formed on scaffolds[19], screws[36], sutures[46], and microparticles[47], composed of alginate [19], poly(D,L-lactide-co-glycolide) (PLG)[43, 44, 47, 48], poly(L-lactide), poly(dioxanone), and poly (ethylene) PE [46]. Thus, the current approach for controllable growth factor binding and release could be broadly applicable in medical device applications.
Finally, peptide based growth factors may be advantageous in some ways over full length recombinant protein growth factors. Over 60 peptide drugs sold worldwide had combined sales approaching $13 billion in 2010[49], and this market is growing. The advantages they can offer as drugs include specificity, potency, and low toxicity. The fact that peptides can be manufactured at large scales under regulatory compliance has helped advance peptides as drugs, and ease of manufacturing leads to low cost and high reproducibility. Importantly, peptides also simplify modular design approaches, as shown here with the mineral-binding, modular growth factors mVEGF and mBMP2. This type of modular design approach is easily adaptable in standard peptide design and synthesis, but more complex in full-length proteins with substantial secondary and tertiary structure considerations.
5. Conclusions
In current bone tissue engineering approaches there is still a need for simple and adaptable mechanisms for growth factor delivery that offer controllable and sustained release kinetics of growth factors. In the present study, we demonstrated the potential of using scaffolds for multiple purposes- as a structurally optimized scaffold and as an intraoperative drug loading device. PCL scaffolds fabricated by SLS can be manufactured to fit complex anatomic locations and mineral coated to serve as a localized delivery platform. Properties of the mineral coating such as the morphology and dissolution rate were modulated by varying the degree of carbonate substitution during mineral growth. Coated scaffolds efficiently bound mimicking peptides of VEGF and BMP2 and allowed for sustained and controllable growth factor release.
Acknowledgements
The authors acknowledge funding from the National Institutes of Health (R01 HL093282), the National Science Foundation (0745563), the AO Research Foundation (Focus Grant F-07-65M), and a pre doctoral fellowship from the Harriet Jenkins Pre-Doctoral Fellowship Program (DS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosure statement
Authors W.L. Murphy and S.J. Hollister are co-founders and shareholders in a company, Tissue Regeneration Systems Inc, that develops scaffold technology.
References
- 1.Murphy WL, Grorud K, Vanderby R. Healing of bone and connective tissue. In: Wnek G, editor. Encyclopedia of Biomaterials and Biomedical Engineering. Informa HealthcareUSA, Inc.; 2006. pp. 1–14. [Google Scholar]
- 2.Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11–25. [PubMed] [Google Scholar]
- 3.Cochran DL, Jones AA, Lilly LC, Fiorellini JP, Howell H. Evaluation of recombinant human bone morphogenetic protein-2 in oral applications including the use of endosseous implants: 3-year results of a pilot study in humans. J Periodontol. 2000;71:1241–1257. doi: 10.1902/jop.2000.71.8.1241. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Zdeblick TA, Sandhu HS, Stephen E. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 5.Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Govender S, Csimma C, Genant HK, Valentin-Opran A. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 8.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 9.Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009;467:3257–3262. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011;11:511–516. doi: 10.1016/j.spinee.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.United States Food and Drug Administration. Executive Summary for P050036 Medtronic’s AMPLIFY™ rhBMP-2 Matrix. 2010 [Google Scholar]
- 12.Takahashi Y, Yamamoto M, Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26:4856–4865. doi: 10.1016/j.biomaterials.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375–4383. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 15.Kempen DH, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li RH, Bouxsein ML, Blake CA, D'Augusta D, Kim H, Li XJ, et al. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. J Orthop Res. 2003;21:997–1004. doi: 10.1016/S0736-0266(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 17.Glassman SD, Carreon L, Djurasovic M, Campbell MJ, Puno RM, Johnson JR, et al. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007;7:44–49. doi: 10.1016/j.spinee.2006.06.381. [DOI] [PubMed] [Google Scholar]
- 18.Hollister SJ, Murphy WL. Scaffold Translation: Barriers between concept and clinic. Tissue Eng Part B. doi: 10.1089/ten.teb.2011.0251. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 20.Suarez-Gonzalez D, Barnhart K, Saito E, Vanderby R, Hollister SJ, Murphy WL. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J Biomed Mater Res A. 2010;95:222–234. doi: 10.1002/jbm.a.32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang ZG, Black RA, Curran JM, Hunt JA, Rhodes NP, Williams DF. Surface properties and biocompatibility of solvent-cast poly[-caprolactone] films. Biomaterials. 2004;25:4741–4748. doi: 10.1016/j.biomaterials.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Murphy WL, Messersmith PB. Compartmental control of mineral formation: adaptation of a biomineralization strategy for biomedical use. Polyhedron. 2000;19:357–363. [Google Scholar]
- 23.D'Andrea LD, Iaccarino G, Fattorusso R, Sorriento D, Carannante C, Capasso D, et al. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci U S A. 2005;102:14215–14220. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JS, Wagoner Johnson AJ, Murphy WL. A modular, hydroxyapatite-binding version of vascular endothelial growth factor. Adv Mater. 2010;22:5494–5498. doi: 10.1002/adma.201002970. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorbunoff MJ. The interaction of proteins with hydroxyapatite. I. Role of protein charge and structure. Anal Biochem. 1984;136:425–432. doi: 10.1016/0003-2697(84)90239-2. [DOI] [PubMed] [Google Scholar]
- 27.Gorbunoff MJ. The interaction of proteins with hydroxyapatite. II. Role of acidic and basic groups. Anal Biochem. 1984;136:433–439. doi: 10.1016/0003-2697(84)90240-9. [DOI] [PubMed] [Google Scholar]
- 28.Schroder E, Jonsson T, Poole L. Hydroxyapatite chromatography: altering the phosphatedependent elution profile of protein as a function of pH. Anal Biochem. 2003;313:176–178. doi: 10.1016/s0003-2697(02)00567-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Wagoner-Johnson A, Murphy WL. Modular peptide growth factors for substrate-mediated stem cell differentiation. Angew Chem Int Ed Engl. 2009;48:6266–6269. doi: 10.1002/anie.200901618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6:21–28. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release. 2004;96:463–472. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 33.King TW, Patrick CW. Development and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) microspheres using a solid encapsulation/single emulsion/solvent extraction technique. J Biomed Mater Res. 2000;51:383–390. doi: 10.1002/1097-4636(20000905)51:3<383::aid-jbm12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 35.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81:858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempen DH, Kruyt MC, Lu L, Wilson CE, Florschutz AV, Creemers LB, et al. Effect of autologous bone marrow stromal cell seeding and bone morphogenetic protein-2 delivery on ectopic bone formation in a microsphere/poly(propylene fumarate) composite. Tissue Eng Part A. 2009;15:587–594. doi: 10.1089/ten.tea.2007.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998:26–37. [PubMed] [Google Scholar]
- 39.Woo BH, Fink BF, Page R, Schrier JA, Jo YW, Jiang G, et al. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm Res. 2001;18:1747–1753. doi: 10.1023/a:1013382832091. [DOI] [PubMed] [Google Scholar]
- 40.Fu YC, Nie H, Ho ML, Wang CK, Wang CH. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng. 2008;99:996–1006. doi: 10.1002/bit.21648. [DOI] [PubMed] [Google Scholar]
- 41.Barralet J, Best S, Bonfield W. Carbonate substitution in precipitated hydroxyapatite: an investigation into the effects of reaction temperature and bicarbonate ion concentration. J Biomed Mater Res. 1998;41:79–86. doi: 10.1002/(sici)1097-4636(199807)41:1<79::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal NC, Betts F, Posner AS. Effect of carbonate and biological macromolecules on formation and properties of hydroxyapatite. Calcif Tissue Res. 1975;18:81–90. doi: 10.1007/BF02546228. [DOI] [PubMed] [Google Scholar]
- 43.Woodruff MA, Hutmacher DW. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 44.Murphy WL, Kohn DH, Mooney DJ. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res. 2000;50:50–58. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–2527. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 46.Murphy WL, Mooney DJ. Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. J Am Chem Soc. 2002;124:1910–1917. doi: 10.1021/ja012433n. [DOI] [PubMed] [Google Scholar]
- 47.Lee JS, Lu Y, Baer GS, Markel MD, Murphy WL. Controllable protein delivery from coated surgical sutures. J Mater Chem. 2010;20:8894–8903. [Google Scholar]
- 48.Jongpaiboonkit L, Franklin-Ford T, Murphy WL. Growth of hydroxyapatite coatings on biodegradable polymer microspheres. ACS Appl Mater Interfaces. 2009;1:1504–1511. doi: 10.1021/am9001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi S, Murphy WL. Sustained plasmid DNA release from dissolving mineral coatings. Acta Biomater. 2010;6:3426–3435. doi: 10.1016/j.actbio.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thayer AM. Chemical & Engineering News. American Chemical Society Publications; 2011. Small firms develop better peptide drug candidates to expand this pharmaceutical class and attract big pharma partners; pp. 13–20. [Google Scholar]