Abstract
To better understand the mechanism of de novo lipid biosynthesis in blood fed Ae. aegypti mosquitoes, we quantitated acetyl-CoA carboxylase (ACC) and fatty acid synthase 1 (FAS1) transcript levels in blood fed mosquitoes, and used RNAi methods to generate ACC and FAS1 deficient mosquitoes. Using the ketogenic amino acid 14C-leucine as a metabolic precursor of 14C-acetyl-CoA, we found that 14C-triacylglycerol and 14C-phospholipid levels were significantly reduced in both ACC and FAS1 deficient mosquitoes, confirming that ACC and FAS1 are required for de novo lipid biosynthesis after blood feeding. Surprisingly however, we also found that ACC deficient mosquitoes, but not FAS1 deficient mosquitoes, produced defective oocytes, which lacked an intact eggshell and gave rise to inviable eggs. This severe phenotype was restricted to the 1st gonotrophic cycle, suggesting that the eggshell defect was due to ACC deficiencies in the follicular epithelial cells, which are replaced after each gonotrophic cycle. Consistent with lower amounts of de novo lipid biosynthesis, both ACC and FAS1 deficient mosquitoes produced significantly fewer eggs than control mosquitoes in both the 1st and 2nd gonotrophic cycles. Lastly, FAS1 deficient mosquitoes, but not ACC deficient mosquitoes, showed delayed blood meal digestion, suggesting that a feedback control mechanism may coordinate rates of fat body lipid biosynthesis and midgut digestion during feeding. We propose that decreased ACC and FAS1 enzyme levels lead to reduced lipid biosynthesis and lower fecundity, whereas altered levels of the regulatory metabolites acetyl-CoA and malonyl-CoA account for the observed defects in eggshell formation and blood meal digestion, respectively.
Keywords: lipogenesis, metabolism, gonotrophic cycle, malonyl-CoA, RNAi
1. INTRODUCTION
Anautogenous mosquito species, including the Dengue vector mosquito, Aedes aegypti, are strictly dependent on a blood meal in order to complete a gonotrophic cycle. In addition to providing metabolic energy to the female mosquito, and amino acids for protein synthesis, blood meal nutrients also provide reduced carbon for fatty acid biosynthesis. Lipids make up approximately 35 percent the dry weight of Ae. aegypti eggs, the majority of which is derived from fat body stores and transported to the ovaries through the hemolymph (Troy et al., 1975; Ziegler and Ibrahim, 2001). Using blood meals containing 14C-labeled amino acids or protein, it was shown that ~65% of blood meal carbon is fully oxidized or excreted, ~15% is converted to maternal and egg lipids, ~10% is found in maternal and egg proteins, and the remaining ~10% is divided amongst glycogen and other metabolites (Zhou et al., 2004a). Most of the accumulated lipid in developing oocytes in the first gonotrophic cycle comes from pre-existing maternal stores in the fat body, which were acquired from larval food and adult nectar meals prior to blood feeding (Briegel et al., 2002; Zhou et al., 2004b). Based on studies showing that urban adult female Ae. aegypti rarely feed on nectar following the first gonotrophic cycles (Edman et al., 1992; Harrington et al., 2001; Scott et al., 1997), egg lipids in subsequent gonotrophic cycles are primarily derived from fatty acids synthesized from blood meal carbon and from the blood meal itself.
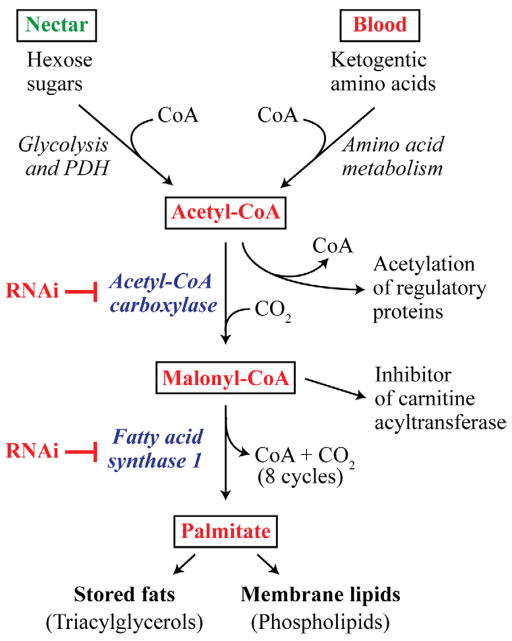
Two key lipid biosynthetic enzymes in eukaryotes are the rate-limiting enzyme acetyl-CoA carboxylase (ACC), and the multifunctional fatty acid synthase (FAS). ACC carboxylates acetyl-CoA to generate malonyl-CoA in a biotin-dependent manner, which is then covalently attached to FAS at the beginning of each elongation cycle to synthesize palmitate, a C16 saturated fatty acid (Wakil et al., 1983). As shown in figure 1, the source of acetyl-CoA for the ACC reaction in adult female mosquitoes is meals consisting of nectar (hexose sugars) and blood (ketogenic amino acids). Palmitate is the building block for stored fats in the form of triacylglycerol (TAG), a neutral lipid used as an energy reserve, and phospholipid (PL), which is an abundant membrane lipid. Acetyl-CoA is a major source of acetate in protein acetyltransferase reactions, which modulate gene expression through chromatin remodeling (Kim and Yang, 2011), whereas malonyl-CoA is an allosteric effector of the mitochondrial transporter protein carnitine acyltransferase I (Saggerson, 2008). It can be seen from the pathway diagram in figure 1 that RNAi-mediated enzyme deficiencies in ACC or FAS1 in blood fed mosquitoes should lead to inhibition of fat storage and membrane biogenesis owing to decreased palmitate synthesis. Moreover, an ACC deficiency in blood fed mosquitoes will increase acetyl-CoA levels due to substrate accumulation and result in decreased levels of malonyl-CoA. However, a deficiency in FAS1 will cause a build-up of malonyl-CoA when ACC is fully active and acetyl-CoA levels are high.
Figure 1.
Overview of the fatty acid biosynthesis pathway in animals showing the predicted functional roles of Ae. aegypti acetyl-CoA carboxylase (ACC) of and fatty acid synthase 1 (FAS1). Hexose sugars from nectar meals, and ketogenic amino acids from blood meals, are converted into the fatty acid substrate acetyl-CoA, which is used to synthesize malonyl-CoA, the precursor to palmitate. RNAi-mediated knock down of ACC and FAS1 expression should result in altered levels of the acetyl-CoA and malonyl-CoA, both of which are regulators of other metabolic and signaling pathways.
Molecular studies investigating the regulation of genes involved in lipid metabolism during diapause in Culex pipiens mosquitoes have recently been reported (Sim and Denlinger, 2009). However, little is known about the expression and biochemical function of ACC and FAS1 in blood feeding Ae. aegypti mosquitoes. Since lipid biosynthesis from blood meal carbon appears to be critical to oocyte maturation in Ae. aegypti (Zhou et al., 2004a; Zhou et al., 2004b), we undertook a series of molecular genetic and biochemical experiments to investigate the function of the Ae. aegypti ACC and FAS1 genes. Our data show that biochemical deficiencies in ACC and FAS1 not only inhibit de novo lipid biosynthesis in blood fed mosquitoes, but also impact eggshell formation and blood digestion.
2. MATERIALS AND METHODS
2.1. Mosquitoes rearing
Ae. aegypti (NIH-Rockefeller strain) were maintained on 10% sucrose and reared at 25°C, 80% relative humidity, and a 16 h light:8 h dark cycle as previously described (Isoe et al., 2009). Blood feeding in the gene expression and metabolic labeling studies used an artificial feeder containing bovine blood purchased from Pel-Freez Arkansas LLC (Rogers, AR), whereas all other experiments were carried out using an artificial feeder containing human blood donated by the American Red Cross (Tucson, AZ). Blood was supplemented with fresh ATP (5.0 mM final concentration) prior to feeding, and a dissecting microscope was used to identify fully engorged females for use in the metabolic studies.
2.2. Bioinformatic analyses
The Ae. aegypti genome and EST sequence databases were queried by a BLAST search using the human ACC1 (AAC50135), ACC2 (NP_001084), and FAS (NP_004095) gene sequences to identify the corresponding ACC and FAS genes in mosquitoes. A summary of the bioinformatic analyses is shown in supplemental table S1 where it can be seen that a single Ae. aegypti ACC gene was identified (XP_001651879), along with six FAS-related genes. Based on percent identities and representation in EST databases, the FAS1 (XP_001658180) and FAS2 (XP_001659008) genes were chosen for further analysis as described in section 3.1.
2.3. Expression Analysis
Quantitative real-time reverse transcriptase polymerase chain reaction (QRT-PCR) was carried out using FastStart Universal SYBR Green Master Mix (Applied Biosystems) using a 7300 Real-Time PCR System (Applied Biosystems). The primer sequences used for these QRTPCR analyses are listed in Table S2. Tissues were collected from three separate cohorts of mosquitoes at 10 discreet time points (0, 3, 6, 12, 24, 36, 48, 72, 96, 120 h post blood meal). cDNA was synthesized using 1.0 μg total RNA isolated from pools of midgut, fat body, and ovary tissue samples as described by Isoe et al (Isoe et al., 2011), and the resulting cDNA was diluted 8-fold. All mRNA transcripts were normalized using the ribosomal protein S7 as an internal control. ACC protein levels were analyzed by Western blotting as described (Isoe et al., 2011). Anti ACC rabbit polyclonal antibody was obtained from Cell Signaling Technology (Danvers, MA), and anti α-tubulin mouse monoclonal antibody was obtained from Developmental Studies Hybridoma Bank (Iowa City, IA). The ACC and α-tubulin primary antibodies were each diluted 1:1,000. The secondary antibodies were diluted 1:10,000 and were either IRDye 800CW goat anti-rabbit secondary antibody (LI-COR Biosciences, Lincoln, NE) or IRDye 800CW goat anti-mouse secondary antibody (LI-COR Biosciences). Immunoreactive proteins were detected with an Odyssey Infrared Imaging System (LI-COR Biosciences).
2.4. RNA interference
Protocols for dsRNA design, synthesis, and injection have been described by Isoe et al (Isoe et al., 2011). The primer sequences for dsRNA production are listed in Table S1. The control dsRNA used in these experiments was derived from the firefly luciferase (Fluc) gene (Isoe et al., 2009). Adult females were injected 2–3 days post-eclosion with of 1.0 μg using a Nanoject II microinjector (Drummond Scientific Company, Broomall, PA) and a MM33 micromanipulator (Märzhäuser Wetzlar, Germany), which was operated hands-free by an electric foot peddle. After dsRNA injection, mosquitoes were fed on only water or blood to ensure that de novo fatty acid synthesis was derived from blood meal proteins. Efficiency of RNAi-mediated knock down was confirmed by quantitative reverse transcriptase polymerase chain reaction (QRT-PCR) and Western blotting as previously described (Isoe et al., 2009). These data are included in supplemental figure S1.
2.5. 14C- labeling and quantification
14C-Leucine was used for radioactive labeling of lipids based on results from Zhou and Miesfeld (Zhou, 2009). In the metabolic labeling experiments described here, 20–50 μCi of 14C-Leu, or 50 μCi of 14C-labeled glucose, were added to 1.0 ml of bovine blood supplemented with 5mM ATP. Each group of dsRNA injected mosquitoes was allowed to feed on the labeled blood for 30 minutes before being moved to the growth chamber. At 48 hours post blood meal (PBM) in the whole body experiments, or 24 and 48 hours PBM in the tissue labeling experiments, mosquito samples were collected and stored in 300 μl chloroform:methanol (2:1 v/v). A standard Folch lipid extraction method was used to isolate lipids from the tissue samples as described by Zhou et al. (Zhou et al., 2004a). The organic phase containing total lipids from the tissue extract was saved and transferred to a silicic column prepared by packing 200 mg of 100-mesh silicic acid (Sigma Chemical Co, MO) in a Pasteur pipette column containing a glass wool plug. The column was washed eight times with 1 ml chloroform and the eluant collected in glass test tubes to extract the triacylglycerol (TAG) content. This was repeated with a methanol wash to collect the phospholipid (PL) fraction. The TAG and PL fractions were then dried completely under nitrogen gas and resuspended in either chloroform (TAG) or methanol (PL). Each sample was mixed with a scintillation cocktail and quantitated by liquid scintillation.
2.6. Measuring mosquito fecundity and egg viability
For each experiment, dsRNA-injected mosquitoes were dissected at 48 hours PBM in 1X PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH of 7.4), and visualized under a dissecting microscope for phenotypic analysis, including visualization of blood digestion in the midgut and follicle size. An additional cohort of mosquitoes was saved for fecundity analysis. At 48 hours PBM, mosquitoes were transferred to individual scintillation vials with 5 ml water and oviposition paper and allowed to lay eggs over the next 72 hrs (up to 5 days PBM). In some experiments, mosquitoes were transferred to clean scintillation vials at 96 hr PBM fecundity was recorded for a second gonotrophic cycle. Mosquitoes were blood fed in the vials and again transferred to clean vials containing oviposition paper at 48 hours PBM. Mosquitoes were allowed to lay eggs as described above. Eggs from individual mosquitoes were counted and recorded for both gonotrophic cycles. Stored eggs were also allowed to hatch to determine egg viability as described by Isoe et al (Isoe et al., 2009).
2.7. Oocyte staining
Light microscopy was used to examine dissected ovaries that were fixed in 2.5% glutaraldehyde in 0.1 M PIPES buffer (pH 7.4) for 60 min at 4°C and washed in 0.1 M PIPES buffer before imbedding in LX112 epoxy resin and staining with toluidine blue and basic fuchsin. Neutral red (Sigma)and rhodamine B (Sigma) were used to stain oocytes isolated from ovaries of dsRNA Fluc or dsRNA ACC injected mosquitoes at 72 or 96 h PBM. Individual oocytes were separated from the ovaries, transferred to eppendorf tubes, and immediately stained with neutral red (final concentration in H2O of 0.5% w:v) or rhodamine B (final concentration of 1 mM in H2O) for 10 min on a rocking shaker. The stained oocytes were rinsed with ddH2O, and the representative oocytes were photographed under a light microscope.
2.8. BSA protein quantification
Anti-bovine serum albumin (BSA) polyclonal antibody was obtained from Gallus Immunotech (Cary, NC), and BSA western blotting to quantitate blood meal digestion was done as described (Isoe et al., 2011). Briefly, 0.2 midgut equivalents from dsRNA injected mosquitoes were loaded into each well and resolved by 12% acrylamide SDS PAGE prior to transfer to a nitrocellulose membrane (Odyssey Nitrocellulose, LI-COR Biosciences, Lincoln, Nebraska). The nitrocellulose membranes were blocked in a 4% nonfat milk solution before incubation with the primary and secondary antibodies. The BSA primary antibody (Gallus Immunotech, Cary, NC) was diluted 1:1000 and the secondary anti-chicken antibody (LI-COR) diluted 1:10,000.
2.9. Statistical Analysis
Time course data presented in figure 2 were analyzed by one-way ANOVA, and all other statistical analyses used unpaired student’s T-test (GraphPad Software Inc, San Diego, CA). Asterisks indicate significant differences (*P < 0.05; ** P < 0.01; *** P < 0.001).
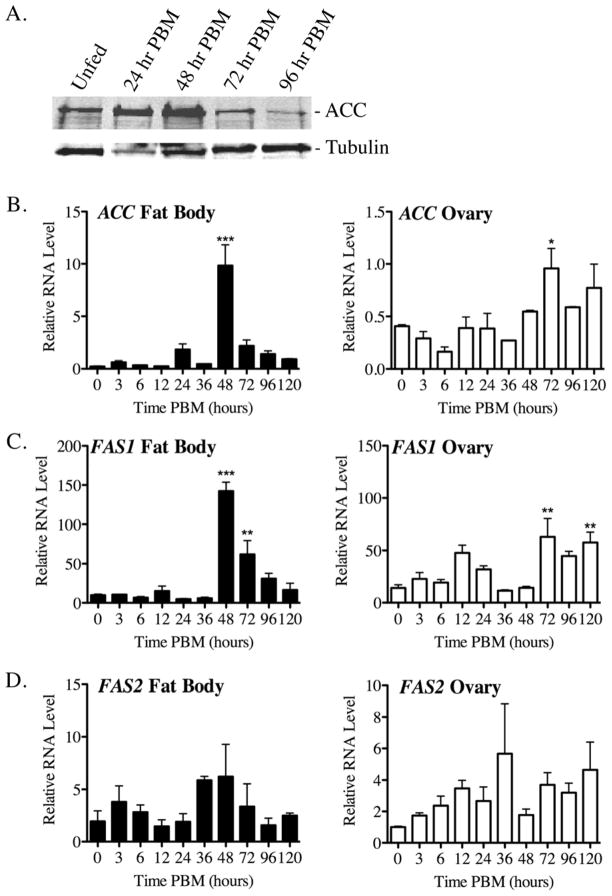
Figure 2.
ACC and FAS1 expression in fat body and ovary tissue of blood fed Ae. aegypti mosquitoes. A) QRT-PCR analysis of transcript levels in blood fed mosquitoes at various times PBM using data collected from three independent biological replicas. Relative values are plotted as the Mean ± SEM using ribosomal S7 transcripts as an internal control across all samples. Asterisks indicate a significant difference between individual time points PBM and unfed mosquitoes (*P<0.05; ** P < 0.01; *** P < 0.001). B) Representative western blot of ACC protein levels in the fat body at various times PBM using a commercial antibody that cross-reacts with the Ae. aegypti ACC protein. The protein loading control is α-tubulin.
3. RESULTS
3.1 ACC and FAS1 transcript levels are regulated in the fat body after blood feeding
Bioinformatic analysis of the Ae. aegypti genome identified a single ACC gene (XP_001651879), and six FAS-related genes, of which FAS1 (XP_001658180) had the highest sequence identity to human FAS (50%), whereas FAS2 (XP_001659008) had the highest number of EST hits in the Ae. aegypti EST database (Table S1). As shown in figure 2, western blotting and QRT-PCR analysis were used to determine if ACC, FAS1, and FAS2 gene expression is regulated by blood feeding in fat body and ovary tissue. As shown in figure 2A, ACC protein levels were found to be maximal in the fat body at 48 hr PBM, and then declined to baseline levels by 96 hr PBM. ACC protein in ovary tissue was not detectable by Western blotting (data not shown). Figure 2B shows results from QRT-PCR studies of ACC expression in fat body and ovary tissue, where it can be seen that ACC transcript levels in the fat body were significantly higher at 48 hr PBM compared to unfed mosquitoes (P < 0.001), whereas 72 hr PBM was the peak expression time point in ovary tissue (P < 0.05). As shown in figure 2C, FAS1 transcript levels also peaked at 48 hr PBM in fat body tissue (P < 0.001), and at 72 hr PBM in ovary tissue (P < 0.01). In contrast to ACC and FAS1, FAS2 transcripts in fat body and ovary tissues were not significantly different at any time points PBM as compared to unfed mosquitoes, suggesting that FAS2 expression is not regulated by blood feeding. In addition, FAS2 transcript levels were 10–20 times lower than FAS1 transcripts in the same RNA samples, which was consistent with our finding that RNAi-mediated knock down of FAS2 expression had no effect on blood meal metabolism (AA and RLM, unpublished data).
Based on these expression data, the ACC and FAS1 genes were chosen for detailed biochemical and molecular genetic analyses as described in the following sections.
3.2. ACC and FAS1 are required for lipid biosynthesis in sugar and blood fed mosquitoes
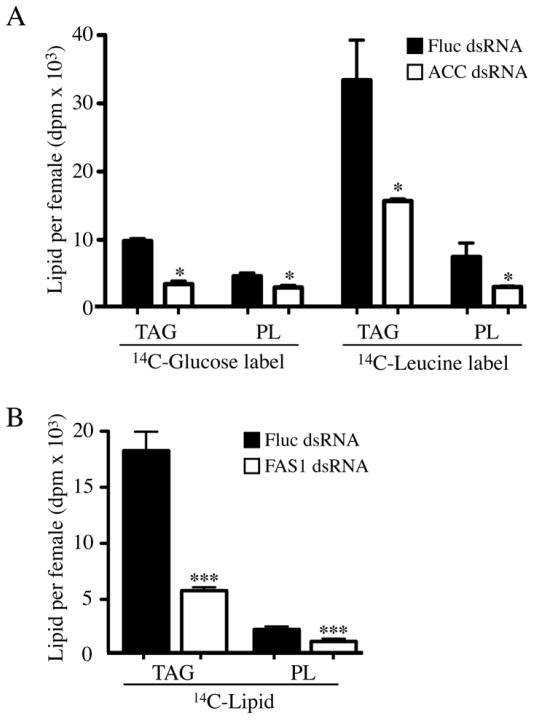
Based on the known enzymatic function of ACC in fatty acid biosynthesis in other organisms (Brownsey et al., 2006), and the fact that the Ae. aegypti genome encodes only a single ACC gene (EAT42106.1), we reasoned that RNAi-mediated knock down of ACC expression should inhibit fatty acid biosynthesis after blood feeding. Using an optimized dsRNA injection protocol we developed for Ae. aegypti (Isoe et al., 2011; Isoe et al., 2009), we injected adult female mosquitoes with 1.0 μg of ACC dsRNA three days prior to blood feeding. As shown in figure S1, ACC transcript and protein levels were reduced by >80% in ACC dsRNA injected mosquitoes based on QRT-PCR analysis and western blotting, respectively. The level of TAG and PL biosynthesis in ACC dsRNA injected blood fed mosquitoes was measured using 14C-glucose or 14C-Leucine as metabolic sources of 14C-acetyl-CoA. The results of these metabolic labeling studies are shown in figure 3A where it can be seen that the amount of 14C-labeled TAG and PL was significantly lower in whole body lipid extracts of ACC dsRNA injected mosquitoes as compared to Fluc dsRNA injected mosquitoes (P<0.05). As shown in Figure 3B, we also examined the effect of FAS1 knock down on the accumulation of 14C-labeled TAG and PL in mosquitoes fed blood containing 14C-leucine (see FAS1 knock down efficiencies in figure S1). In these experiments, the mean level of 14C-labeled TAG was reduced by ~60% in FAS1 dsRNA injected mosquitoes compared to Fluc dsRNA injected mosquitoes (P<0.001), whereas 14C-labeled PL was reduced by ~45% (P<0.001). These data confirm that ACC and FAS1 are required for fatty acid biosynthesis in blood fed mosquitoes, and are consistent with the observed >80% reduction in ACC and FAS1 transcript levels in dsRNA injected mosquitoes based on QRT-PCR (data not shown).
Figure 3.
Metabolic labeling experiments using 14C-Glucose and 14C-Leucine show that de novo lipid biosynthesis is inhibited in blood fed ACC and FAS1 deficient mosquitoes. A) Accumulation of 14C-TAG (triacylglycerol) and 14C-PL (phospholipid) in ACC and Fluc dsRNA injected mosquitoes at 48 hr PBM using blood meals containing 14C-Glucose or 14C-Leucine. Data are shown as Mean ± SEM from three independent biological replicas using whole body lipid extracts. B) Accumulation of 14C-TAG and 14C-PL in FAS1 and Fluc dsRNA injected mosquitoes at 48 hr PBM using blood meals containing 14C-Leucine. Data are shown as Mean ± SEM from three independent biological replicas using whole body lipid extracts. Asterisks indicate a significant difference between 14C-lipid levels in FAS1 and Fluc dsRNA injected mosquitoes (*P<0.05; ***P < 0.001).
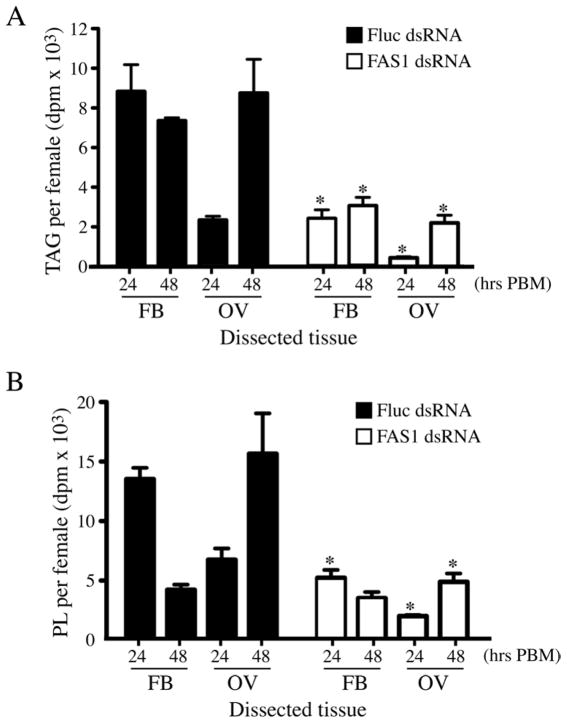
To characterize tissue-specific functions of FAS1 on lipid biosynthesis, we quantitated 14C-labeled TAG and PL accumulation in fat body and ovary tissues of blood fed mosquitoes at 24 hr and 48 hr PBM using 14C-leucine as the metabolic label. As shown in Figure 4A, the level of 14C-labeled TAG in the control Fluc dsRNA injected mosquitoes was significantly higher than in FAS1 dsRNA injected mosquitoes in both fat body and ovary tissues at 24 hr and 48 hr PBM, indicating that FAS1 has a central role in TAG synthesis after blood feeding. A similar pattern of decreased 14C-labeled PL accumulation in FAS1 dsRNA injected mosquitoes, compared to Fluc dsRNA injected mosquitoes, is seen in figure 4B, with the exception of the 48 hr PBM time point in fat body samples, which were not significantly different. Since lipids are transported from the fat body to the ovaries by lipoproteins during the gonotrophic cycle (Cheon et al., 2006), it is likely that some portion of the 14C-labeled TAG and PL accumulated in the ovaries at 48 hr PBM was derived from fatty acids synthesized in the fat body.
Figure 4.
Effect of FAS1 deficiency on 14C-lipid levels in fat body and ovary tissue of blood fed mosquitoes. A) Accumulation of 14C-TAG in fat body and ovary tissue of FAS1 and Fluc dsRNA injected mosquitoes at 24 hr and 48 hr PBM using blood meals containing 14C-Leucine. Data are shown as Mean ± SEM from three independent biological replicas. Asterisks indicate a significant difference between 14C-TAG levels in FAS1 and Fluc dsRNA injected mosquitoes (*P <0.05). B) Same experimental procedure in A showing 14C-PL levels in fat body and ovary tissues. Asterisks indicate a significant difference between 14C-PL levels in FAS1 and Fluc dsRNA injected mosquitoes (*P <0.05).
3.3. Blood fed ACC deficient mosquitoes fail to synthesize an intact eggshell
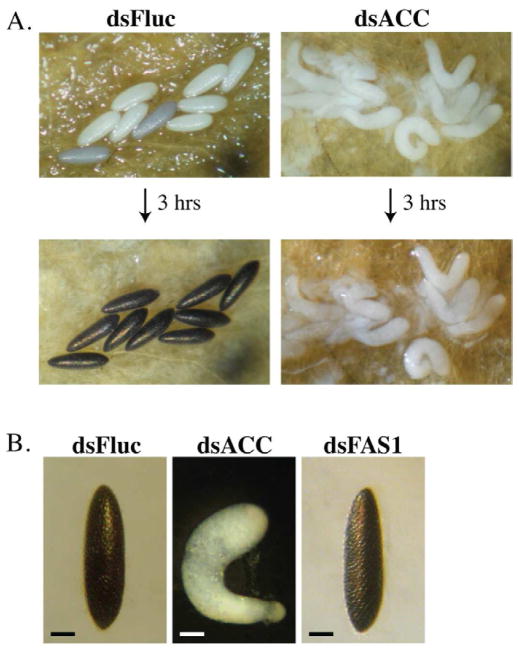
Female Ae. aegypti mosquitoes depend primarily on maternal stores of fat body lipids to complete the 1st gonotrophic cycle, whereas lipids derived from blood meal proteins are used for the subsequent gonotrophic cycle (Briegel et al., 2002; Cheon et al., 2006; Zhou et al., 2004b). Based on this observation, we expected to find that ACC deficient female mosquitoes would have only a minimal reduction in egg production during the 1st gonotrophic cycle, whereas egg production in the 2nd gonotrophic cycle would be more severe due to decreased lipid biosynthesis after the first blood feeding. However, as shown in figure 5A, mosquitoes injected with ACC dsRNA two days prior to feeding oviposited defective eggs that failed to tan, and were found to be inviable (data not shown). In all cases, the untanned eggs from ACC deficient mosquitoes were curved and elongated. This was not the case with FAS1 deficient mosquitoes, which oviposited viable eggs that tanned normally and were indistinguishable from eggs oviposited by Fluc dsRNA control mosquitoes (figure 5B).
Figure 5.
A deficiency in ACC disrupts egg development in the 1st gonotrophic cycle. A) Representative photographs of oviposited eggs from blood fed mosquitoes injected 3 days prior to feeding with dsRNA encoding Fluc (dsFluc) or ACC (dsACC) sequences. Top panel shows eggs collected within 1 hr of oviposition and bottom panels shows the same eggs 3 hrs later. Untanned eggs oviposited by ACC deficient mosquitoes were found to be inviable (data not shown). B) High magnification photograph of representative eggs from mosquitoes injected with 1.0 μg of dsFluc, dsACC, and dsFAS1 RNA at 3 days prior to blood feeding. The eggs were photographed 4 hrs after oviposition. Bar scale is equal to 0.2 mm.
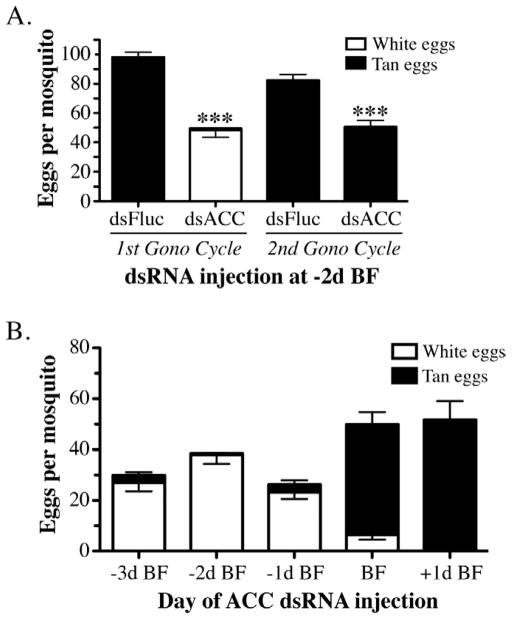
To determine if an ACC deficiency in the 1st gonotrophic cycle affected egg production during the 2nd gonotrophic cycle, we followed batches of ACC dsRNA injected mosquitoes through two blood feedings. As shown in figure 6A, ACC deficient mosquitoes oviposited 50% fewer eggs in the 1st gonotrophic cycle than Fluc dsRNA injected mosquitoes, and moreover, >95% of these eggs failed to tan and were inviable. However, during the 2nd gonotrophic cycle, all of the eggs from ACC dsRNA injected mosquitoes tanned normally and were viable, even though there was a significant reduction in the total number of eggs compared to the Fluc dsRNA control mosquitoes (P≤0.001). We extended these experiments to determine if the defective egg phenotype in the 1st gonotrophic cycle was dependent on the time of ACC dsRNA injection relative to blood feeding. For these experiments, we injected female mosquitoes at 1, 2, or 3 days prior to blood feeding, 1 hr after feeding, or at 24 hr after feeding (figure 6B). The results of this time course experiment revealed that ACC dsRNA injection prior to blood feeding resulted in >85% defective eggs, whereas, the majority of eggs were normal when the ACC dsRNA was injected at any time after blood feeding. The mean number of normal and defective eggs oviposited by the ACC dsRNA injected mosquitoes under all conditions was ~30–55/mosquito, as compared to ~100 eggs/mosquito in Fluc dsRNA injected mosquitoes (figure 6A).
Figure 6.
Quantitation of egg production in ACC deficient mosquitoes. A) Data collected from mosquitoes after the 1st and 2nd gonotrophic cycle that had been injected with 1.0 μg of dsFluc or dsACC RNA at 2 days prior to the first blood feeding. Each bar represents the total number of eggs laid per mosquito (Mean ± SEM), which were scored at 4 hrs post-oviposition as inviable white eggs (white fill) or viable tan eggs (black fill). Asterisks indicate a significant difference between the total number of eggs oviposited per ACC deficient mosquito compared to the control Fluc dsRNA injected mosquitoes (***P <0.001). B) Number of white and tan eggs oviposited per dsACC RNA injected mosquito at various times before and after the blood meal plotted as Mean ± SEM. Data in A and B were derived from three independent biological replicas.
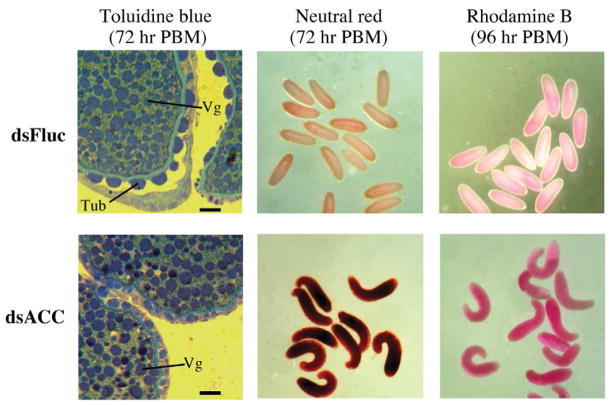
To better understand what might be causing the defective egg phenotype in ACC dsRNA injected mosquitoes, we examined oocytes at 72 hr and 96 hr PBM in females withheld from oviposition substrate. As shown in figure 7, the density of vitellogenin granules in Fluc and ACC dsRNA injected mosquitoes at 72 hr PBM was similar based on toluidine blue staining, however oocytes from ACC dsRNA injected mosquitoes lacked tubercles, which are normally seen on Ae. aegypti eggs (Linley, 1989). The porosity of the oocytes was tested using two different dyes, neutral red and rhodamine B, which should be excluded from the oocytes if the eggshell is intact. It can be seen that oocytes isolated from ACC deficient mosquitoes at 72 hr and 96 hr PBM (oviposition substrate was not available) accumulated both dyes to a much greater extent than oocytes from the control Fluc dsRNA injected mosquitoes, indicating that the eggshell is porous. Moreover, oocytes from ACC deficient mosquitoes were misshapen similar to the oviposited eggs (see figure 5B).
Figure 7.
Oocytes in ACC deficient mosquitoes lack an intact eggshell layer. Oocytes were collected from Fluc and ACC dsRNA injected mosquitoes, and either fixed with glutaraldehyde and stained with toluidine blue to visualize eggshell structure by high magnification light microscopy, or stained in water with neutral red or rhodamine B. Tubercle structures (Tub) and vitellogenin granules (Vg) are labeled. Bar scale is equal to 20 μm.
Taken together, these data suggest that defective eggshells are due to ACC deficiencies in the follicular epithelial cells, since this ovarian cell type is responsible for eggshell production during oocyte maturation (Raikhel and Lea, 1991). In contrast, the decreased total number of oviposited normal (tanned) and defective (white) eggs in ACC deficient mosquitoes (see figure 6), is more likely the result of ACC deficiencies in the fat body, which results in lower amounts of de novo lipid biosynthesis and reduced egg production.
3.4. Delayed blood meal digestion and reduced fecundity in FAS1-deficient mosquitoes
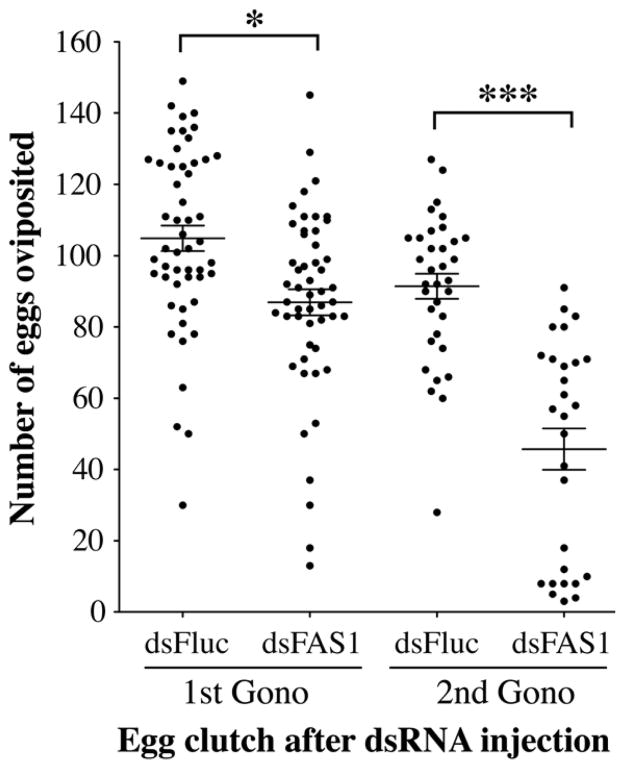
To determine the effect of a FAS1 deficiency on egg production, we injected female mosquitoes three days prior to blood feeding and counted the number of oviposited eggs after the 1st and 2nd gonotrophic cycles (96 hr PBM). The data in figure 8 show ~20% fewer oviposited eggs/female after the 1st gonotrophic cycle in the FAS1 dsRNA injected mosquitoes compared to the Fluc controls (P<0.05). The decrease in egg production is more pronounced in the 2nd gonotrophic cycle, in which ~50% fewer eggs are oviposited by the FAS1 dsRNA injected mosquitoes compared to the Fluc controls (P<0.001). These results are consistent with metabolic labeling studies in blood fed Ae. aegypti showing that egg development in the 2nd gonotrophic cycle is dependent on de novo lipid biosynthesis in the 1st gonotrophic cycle (Zhou et al., 2004b)
Figure 8.
FAS1 deficient mosquitoes produce significantly fewer eggs during the 1st and 2nd gonotrophic cycle. The total number of eggs oviposited by individual Fluc or FAS1 dsRNA injected mosquitoes was determined from three independent biological replicas. The Mean ± SEM are shown for each data set as horizontal lines. Asterisks indicate a significant difference between dsFluc and dsFAS1 RNA injected mosquitoes (*P <0.05; ***P < 0.001).
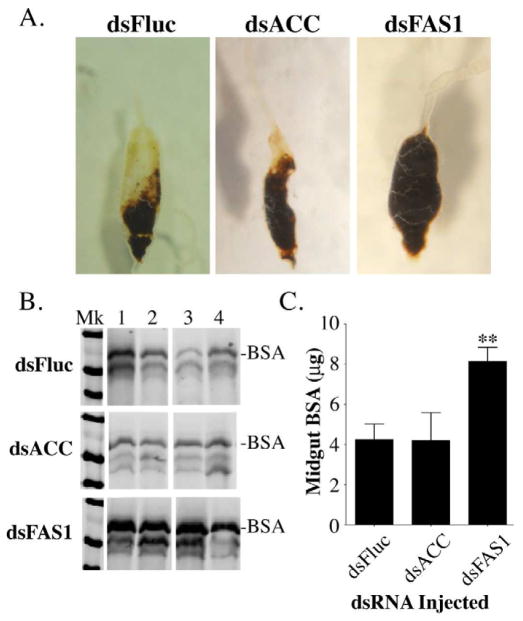
While the reduced fecundity in the 1st gonotrophic cycle of FAS1 deficient mosquitoes could be due to decreased de novo lipid biosynthesis alone, we observed that dissected midguts isolated from dsFAS1, dsACC, and dsFluc RNA injected mosquitoes were quite different, suggesting that a FAS1 deficiency may affect midgut digestion. As seen in figure 9A, midguts from ACC dsRNA injected mosquitoes at 48 hr PBM were similar in size to that of Fluc dsRNA injected mosquitoes, and considerably smaller than midguts from FAS1 dsRNA injected mosquitoes. In order to quantitate this apparent difference in digestion rates, we measured the amount of undigested bovine serum albumin (BSA) in the midguts of individual dsRNA injected mosquitoes at 24 hr PBM using a BSA Western blot assay (Isoe et al., 2009). Figure 9B is a BSA Western blot showing representative midgut protein samples from four individual blood fed Fluc, ACC, and FAS1 dsRNA injected mosquitoes, and figure 9C contains data collected from three independent biological experiments. It can be seen from these data that BSA digestion at 24 hr PBM in Fluc and ACC dsRNA injected mosquitoes was similar, however BSA digestion in FAS1 dsRNA injected mosquitoes was significantly reduced (P<0.01).
Figure 9.
Blood meal digestion is delayed in FAS1 deficient mosquitoes. A) Representative photos of midgut tissues from dsFluc, dsACC, and dsFAS1 RNA injected mosquitoes dissected 48 hours after blood feeding. B) Representative western blot of midgut extracts prepared from four individual dsFluc, dsACC, and dsFAS1 RNA injected mosquitoes showing the extent of BSA degradation at 48 hr PBM. C) Quantitative analysis of BSA degradation data using pooled mosquito midguts isolated at 48 hr PBM from dsFluc, dsACC, and dsFAS1 RNA injected mosquitoes. Data were collected from three independent biological replicas using image analysis of BSA western blots and plotted as Mean ± SEM as previously described (Isoe et al., 2009). Asterisks indicate a significant difference between the amount of BSA remaining in midguts from dsFAS1 and dsFluc RNA injected mosquitoes (**P <0.01). In contrast, there was no significant difference between the amount of BSA remaining in midguts from dsACC and dsFluc RNA injected mosquitoes.
4. DISCUSSION
We found that inhibiting fatty acid biosynthesis in Ae. aegypti mosquitoes by RNAi-mediated knock down of the ACC and FAS1 gene expression reduced conversion of blood meal derived carbon into TAG and PL. Indeed, fecundity was significantly reduced in both ACC and FAS1 deficient mosquitoes during the 1st and 2nd gonotrophic cycles, indicating that de novo fatty acid biosynthesis contributes to egg production. In addition, data collected from metabolic labeling studies using the ketogenic amino acid 14C-leucine as a source of 14C-acetyl-CoA, provided experimental evidence that fatty acids synthesized in the fat body of blood fed mosquitoes are transported to the ovaries. This can be seen in figure 4, which shows a decrease in TAG and PL in the fat body from 24 hr to 48 hr, which is coincident with an increase in TAG and PL levels in the ovaries over this same time period. An apparent redistribution of lipids from the fat body to the ovaries was seen in both the Fluc and FAS1 dsRNA injected mosquitoes, although the total lipid content in the FAS1 deficient mosquitoes was significantly lower than in the control mosquitoes owing to decreased fatty acid synthesis.
A significant reduction in de novo lipid biosynthesis and egg production in blood fed ACC and FAS1 deficient mosquitoes was predicted based on what is known about the fatty acid biosynthetic pathway in insects (Arrese and Soulages, 2010). However, we were surprised to find that decreased ACC expression led to defects in eggshell formation, and moreover, that a deficiency in FAS1 caused a delay in blood meal digestion. One explanation for the observed defect in eggshell formation in ACC deficient mosquitoes is that de novo fatty acid biosynthesis in ovary tissues is required to generate lipid components of the eggshell (Urbanski et al, 2010), i.e., fat body lipid biosynthesis is not the only source of lipids for egg production. However, this would not explain why FAS1 dsRNA injected mosquitoes produced normal eggs, given that ACC and FAS are both required to synthesize fatty acids and FAS1 transcripts are present at high levels in ovary tissue (figure 2).
A second explanation is that increased levels of acetyl-CoA and decreased levels of malonyl-CoA, both of which would occur in ACC deficient mosquitoes (see figure 1), could alter metabolic flux through biosynthetic pathways involved in eggshell formation. For example, acetyl-CoA is not only the primary substrate for the citrate cycle, but is also a major source of acetyl groups in protein acetyltransferase reactions (Kim and Yang, 2011), some of which modulate metabolic flux through gene regulation (Jeninga et al., 2010). Therefore, ACC deficient mosquitoes may have an altered transcriptome due to increased acetylation of gene regulatory proteins in response to elevated acetyl-CoA, which could affect eggshell formation independent of decreased lipid biosynthesis. Similarly, it is possible that decreased malonyl-CoA levels in ACC deficient mosquitoes specifically inhibits fatty acid elongation reactions, which might be required to produce specialized eggshell lipids using maternal stores of palmitate as a precursor.
What explains the delay in blood meal digestion in FAS1 deficient mosquitoes? The primary site of fatty acid biosynthesis in insects is the fat body (Arrese and Soulages, 2010), and indeed we observed high levels of TAG and PL biosynthesis in the fat body tissue of blood fed mosquitoes. Moreover, since ACC and FAS1 expression in the midgut is very low in mosquitoes maintained on water prior to blood feeding (AA and AM, unpublished), it seems likely that the observed delay in blood meal digestion in FAS1 deficient mosquitoes results from indirect physiological affects. For example, fatty acid biosynthesis in the fat body within 24 hr PBM may provide TAG for β-oxidation in the midgut to support energy needs during digestion. Since FAS1 deficient mosquitoes accumulate ~70% less 14C-TAG than control mosquitoes at 24 hr PBM (see figure 3), the observed delay in digestion could result from insufficient metabolic energy in midgut epithelial cells. Another possibility is that midgut digestion rates may be regulated by a feedback signaling mechanism initiated in the fat body which serves to coordinate blood meal digestion in multiple tissues. Indeed, such a fat body signal may be a fatty acid derivative, or some intermediate metabolite in the fatty acid synthesis pathway.
Future studies will be necessary to uncover the mechanisms behind ACC and FAS1 control of eggshell formation and digestion, respectively. As we learn more about the metabolic processes unique to blood feeding mosquitoes, it may be possible to develop mosquito specific control methods to slow the spread of mosquito borne diseases.
Supplementary Material
RNAi-mediated knock down of ACC and FAS1 expression during the 1st and 2nd gonotrophic cycles. A) QRT-PCR analysis of ACC transcript levels at 48 hr PBM in mosquitoes injected with 1.0 μg of ACC or Fluc dsRNA 3 days prior to blood feeding. ACC transcript levels were normalized to S7 ribosomal protein transcript levels in the same RNA samples. Asterisks indicate a significant difference in the level of ACC transcripts between dsFluc and dsACC RNA injected mosquitoes (*P <0.05). B) Western blot of ACC protein levels in uninjected, dsFluc, and dsACC injected mosquitoes at 48 hr PBM during the 1st and 2nd gonotrophic cycles. Mosquitoes were injected with 1.0 μg of dsRNA at 3 days prior to the first blood feeding. C) QRT-PCR analysis of FAS1 transcript levels in dsFluc and dsFAS1 injected mosquitoes at 48 hr PBM during the 1st and 2nd gonotrophic cycles. Mosquitoes were injected with 1.0 μg of dsRNA at 3 days prior to the first blood feeding. FAS1 transcript levels were normalized to S7 ribosomal protein transcript levels in the same RNA samples. Asterisks indicate a significant difference in the level of FAS1 transcripts between dsFluc and dsFAS1 RNA injected mosquitoes (** < 0.01).
HIGHLIGHTS.
Deficiencies in ACC and FAS1 led to decreased lipid biosynthesis and fecundity.
Mosquitoes deficient in ACC, but not FAS1, produced defective eggshells.
Mosquitoes deficient in FAS1, but not ACC, had delayed blood meal digestion.
Data suggest altered acetyl-CoA or malonyl-CoA may mediate these effects.
Acknowledgments
We thank Mary Hernandez for rearing mosquitoes, Drs. Rolf Ziegler and Jorge Zamora for sharing reagents and experimental insights during the initial phases of these studies, and Drs. Mike Riehle and Tsu-Shuen Tsao for help with experimental design and data analysis. AA thanks James Pennington and Michelle Brandon for training in mosquito rearing and dissecting techniques. This work was supported by NIH Grant R01AI046451 to RLM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel H, Hefti M, DiMarco E. Lipid metabolism during sequential gonotrophic cycles in large and small female Aedes aegypti. J Insect Physiol. 2002;48:547–554. doi: 10.1016/s0022-1910(02)00072-0. [DOI] [PubMed] [Google Scholar]
- Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- Cheon HM, Shin SW, Bian G, Park JH, Raikhel AS. Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti. J Biol Chem. 2006;281:8426–8435. doi: 10.1074/jbc.M510957200. [DOI] [PubMed] [Google Scholar]
- Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J Med Entomol. 1992;29:1035–1038. doi: 10.1093/jmedent/29.6.1035. [DOI] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Isoe J, Collins J, Badgandi H, Day WA, Miesfeld RL. Defects in coatomer protein I (COPI) transport cause blood feeding-induced mortality in Yellow Fever mosquitoes. Proc Nat Acad Sci USA. 2011;108:E211–217. doi: 10.1073/pnas.1102637108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoe J, Rascon AA, Jr, Kunz S, Miesfeld RL. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Bioc Mol Biol. 2009;39:903–912. doi: 10.1016/j.ibmb.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29:4617–4624. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Yang XJ. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Bioc Sci. 2011;36:211–220. doi: 10.1016/j.tibs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Linley JR. Comparative fine structure of the eggs of Aedes albopictus, Ae. aegypti, and Ae. bahamensis (Diptera: Culicidae) J Med Entomol. 1989;26:510–521. doi: 10.1093/jmedent/26.6.510. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Control of follicular epithelium development and vitelline envelope formation in the mosquito; role of juvenile hormone and 20-hydroxyecdysone. Tissue Cell. 1991;23:577–591. doi: 10.1016/0040-8166(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr. 2008;28:253–272. doi: 10.1146/annurev.nutr.28.061807.155434. [DOI] [PubMed] [Google Scholar]
- Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am J Trop Med Hyg. 1997;57:235–239. doi: 10.4269/ajtmh.1997.57.235. [DOI] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol Genomics. 2009;39:202–209. doi: 10.1152/physiolgenomics.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy S, Anderson WA, Spielman A. Lipid content of maturing ovaries of Aedes aegypti mosquitoes. Comp Biochem Physiol B. 1975;50:457–461. doi: 10.1016/0305-0491(75)90258-8. [DOI] [PubMed] [Google Scholar]
- Urbanski JM, Benoit JB, Michaud MR, Denlinger DL, Armbruster P. The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc Biol Sci. 2010;277:2683–2692. doi: 10.1098/rspb.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WA, Spielman A. Lipid content of maturing ovaries of Aedes aegypti mosquitoes. Comp Biochem Physiol B. 1975;50:457–461. doi: 10.1016/0305-0491(75)90258-8. [DOI] [PubMed] [Google Scholar]
- Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Zhou G, Flowers M, Friedrich K, Horton J, Pennington J, Wells MA. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J Insect Physiol. 2004a;50:337–349. doi: 10.1016/j.jinsphys.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Zhou G, Miesfeld RL. Differential utilization of blood meal amino acids in mosquitoes. Open Access Insect Physiol. 2009;1:1–12. [Google Scholar]
- Zhou G, Pennington JE, Wells MA. Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Bioc Mol Biol. 2004b;34:919–925. doi: 10.1016/j.ibmb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Ibrahim MM. Formation of lipid reserves in fat body and eggs of the yellow fever mosquito, Aedes aegypti. J Insect Physiol. 2001;47:623–627. doi: 10.1016/s0022-1910(00)00158-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNAi-mediated knock down of ACC and FAS1 expression during the 1st and 2nd gonotrophic cycles. A) QRT-PCR analysis of ACC transcript levels at 48 hr PBM in mosquitoes injected with 1.0 μg of ACC or Fluc dsRNA 3 days prior to blood feeding. ACC transcript levels were normalized to S7 ribosomal protein transcript levels in the same RNA samples. Asterisks indicate a significant difference in the level of ACC transcripts between dsFluc and dsACC RNA injected mosquitoes (*P <0.05). B) Western blot of ACC protein levels in uninjected, dsFluc, and dsACC injected mosquitoes at 48 hr PBM during the 1st and 2nd gonotrophic cycles. Mosquitoes were injected with 1.0 μg of dsRNA at 3 days prior to the first blood feeding. C) QRT-PCR analysis of FAS1 transcript levels in dsFluc and dsFAS1 injected mosquitoes at 48 hr PBM during the 1st and 2nd gonotrophic cycles. Mosquitoes were injected with 1.0 μg of dsRNA at 3 days prior to the first blood feeding. FAS1 transcript levels were normalized to S7 ribosomal protein transcript levels in the same RNA samples. Asterisks indicate a significant difference in the level of FAS1 transcripts between dsFluc and dsFAS1 RNA injected mosquitoes (** < 0.01).