Abstract
Aims
Ovarian cancer has the highest mortality of any gynaecological malignancy; this is due to rapid peritoneal spread of tumour cells and neovascularization. Understanding the mechanisms underlying this is critical to developing early diagnostic or treatment strategies. We devised a pilot study to examine the role of γ-SYNUCLEIN (γ-SYN), oestrogen receptor (ER)α, and the splice variant ERαΔ3.
Methodology
With ethical approval, ovarian tissue was collected from patients (n=24) undergoing oopherectomy for non-ovarian pathology or primary surgery for suspected ovarian cancer. Quantitative gene expression analysis was employed for γ-SYN, ERα, and ERαΔ3. To identify the in situ localization, immunofluorescence for γ-syn was carried out.
Results
Ovarian tumour tissue exhibited an elevated expression of γ-SYN and high-grade tumours had an elevated ERαΔ3:ERα ratio compared with benign tissue. The majority of previous studies point to the γ-syn protein being present in epithelial cells of high-grade disease. Our study supports this, but additionally we conclusively identify its presence in the endothelial cells of vasculature surrounding low-grade disease; immunofluorescence was strongest in the apical cells surrounding the lumen.
Conclusion
Our results demonstrate for the first time that there are readily-expressed levels of γ-SYN and ERαΔ3 in normal ovarian tissue and ovarian tumours. In high-grade disease, γ-syn and an elevated ERαΔ3:ERα ratio might confer metastatic potential to the tumourigenic cells and promote neoangiogenesis. Future in vitro studies might be necessary to delineate such a mechanism, which could potentially be the basis of early intervention.
Keywords: Endothelial cells, ovarian cancer, ERα, ERαΔ3, γ-SYNUCLEIN, neoangiogenesis
1. INTRODUCTION
Ovarian cancer has the highest mortality of any gynaecological malignancy. In England the average 5-year survival rate is 38% (Cooper et al., 2008). This poor prognosis is related to the peritoneal spread of ovarian tumour cells leading to a loss of gastrointestinal motility and often resulting in death from bowel obstruction. Malignant cells possess the ability to migrate from the ovary and induce a local vascular supply on peritoneal surfaces. Neoangiogenesis has been shown to play a crucial role in the propagation of tumour growth and metastatic potential of ovarian cancer (Gómez-Raposo et al., 2009).
A large majority (90%) of ovarian cancers arise from the ovarian epithelium. These tumours may be further sub-categorized, depending on histological appearance, into serous, endometrioid, mucinous, clear cell, transitional, mixed and undifferentiated tumours. Borderline ovarian tumours are also epithelial in origin. Not true malignancies, their histological features and behaviour are intermediate between benign and malignant tumours. It is likely that they are a premalignant form of low-grade ovarian cancer (Rosen et al., 1998).
γ-Synuclein (γ-syn) belongs to a family of small highly-charged neuronal proteins, originally studied in neurodegenerative disorders (Lavedan 1998). Its expression outside the nervous system has been associated with cancer, especially of the breast (Ahmad et al., 2007; Bruening et al., 2000). In breast cancer, γ-syn expression is associated with advanced infiltrative, metastatic disease and may show stage-specific elevated expression (Wu et al., 2005). γ-Syn expression is also raised in ovarian tumours in comparison with normal ovary (Bruening et al., 2000). Elevated levels have also been found in non-hormone dependent cancers including pancreatic (Hibi et al., 2009; Li et al., 2004), colorectal (Ye et al., 2008; 2009), bladder (Iwaki et al., 2004) and stomach (Yanagawa et al., 2004). The aberrant expression of γ-syn protein is an indicator of metastasis in a range of cancers (Liu et al., 2005).
γ-SYN expression is raised in ovarian endometriosis (a known risk factor for ovarian cancer) when compared with the eutopic endometrium of the same patients (Singh et al., 2008). Immunohistochemical analysis has shown localisation of γ-syn to the vascular endothelial cells. This suggests that γ-syn may be involved in blood vessel formation in endometriosis, allowing endometriotic implants to embed and grow (Singh et al., 2008), a process analogous to the peritoneal dissemination seen in ovarian cancer. The relationship between γ-syn and hormone-dependent disease has led to its study in association with oestrogen receptors (ER). γ-Syn has been identified as a component of the heat shock protein (Hsp) chaperone complex. It binds Hsp70 and enhances the ligand binding affinity of ERα, thus increasing ERα-modulated oestrogen-dependent transcriptional activity. This mechanism induces a highly proliferative state in mammary epithelial cells and may underlie breast cancer progression (Jiang et al., 2003; 2004; Liu et al., 2007).
ER splice variants may be involved in the regulation of full-length ER function (Hartenbach et al., 1997). However, limited research has been conducted into ER splice variants in ovarian tissues and, of the ERα variants, only ERαΔ4 and ERαΔ5 have been shown to be present (Erenburg et al., 1997; Poola and Speirs 2001). ERαΔ3 lacks exon 3, resulting in a functional receptor with a defective DNA binding domain. When co-expressed at equimolar levels in a transfection system, ERαΔ3 has a dominant-negative effect on the activity of ERα (Poola et al., 2002). However, ERαΔ3 is commonly expressed in breast cancers (Koduri et al., 2006), and is particularly elevated in African-American women, a subgroup with poor prognosis (Taylor et al., 2010). This could be explained by the effect of ERαΔ3 on the expression of vascular endothelial growth factor (VEGF). In breast cancer models, it has three times the stimulatory effect of ERα (Paley et al., 2000). It is therefore plausible that ERαΔ3 contributes to the malignant phenotype in ovarian cancer (Hartenbach et al., 1997). In this small pilot study we have investigated two potential components in the vascularisation of peritoneal deposits in ovarian cancer. The study utilized ovarian tumour tissue to better reflect in vivo gene expression.
2. EXPERIMENTAL DETAILS
2.1 Study Participants, Tissue Retrieval and Storage
Ethical approval was obtained for the study (LREC no. 05/Q1308/3 Preston, Chorley and South Ribble Ethical Committee). Suitable patients were identified in the gynaecology clinic and given an information leaflet detailing the study. Informed consent was obtained pre-operatively. Ovarian tissue was collected prospectively from two groups of patients: those having primary surgery for an ovarian mass/suspected ovarian cancer and those with normal ovaries undergoing oopherectomy in addition to hysterectomy for non-ovarian pathology. None had received chemotherapy prior to surgery. Tissues were prospectively collected from 24 patients, 11 of whom were subsequently found to have ovarian tumours and 13 with apparently normal ovaries (Table 1). Post-surgical resection, the uterus and ovaries were transported to the pathology laboratory and dissected under standard clean conditions by a consultant histopathologist.
Table 1. Details of study participant age, pathology and stage of disease.
| Code | Age 50 | Ovarian histopathology (other relevant information) |
Stage (ovarian) |
|---|---|---|---|
| OV1 | 50 | Mucinous, borderline | 1a |
| OV2 | 58 | Serous borderline, arising in benign cystadenofibroma | 1c |
| OV3 | 62 | Mucinous, borderline | 1a |
| OV4 | 75 | Well-differentiated mucinous arising in borderline | 1a |
| OV5 | 56 | Endometrioid, moderately-differentiated | 1c |
| OV6 | 59 | Mixed, moderately-differentiated | 2a |
| OV7 | 44 | Mucinous, well-differentiated | 1a |
| OV8 | 64 | Serous, high-grade | 3b |
| OV9 | 64 | Serous, high-grade | 3b |
| OV10 | 56 | Serous with clear cell areas, high-grade | 3c |
| OV11 | 44 | Serous, high-grade | 3c |
| B1 | 41 | Normal ovary | n/a |
| B2 | 43 | Normal ovary | n/a |
| B3 | 47 | Normal ovary | n/a |
| B4 | 44 | Normal ovary (history of breast cancer on tamoxifen) | n/a |
| B5 | 59 | Normal ovary (history of breast cancer on tamoxifen) | n/a |
| B6 | 46 | Normal ovary (Progesterone IUS) | n/a |
| B7 | 50 | Normal ovary (on GnRH analogues) | n/a |
| B8 | 51 | Normal ovary (endometriosis) | n/a |
| B9 | 61 | Small simple cyst on left ovary (CIN3) | n/a |
| B10 | 65 | Normal ovary (Grade 2, stage 1a endometrioid endometrial cancer) |
n/a |
| B11 | 57 | Normal ovary (Grade 3, stage 2 undifferentiated endometrial cancer) |
n/a |
| B12 | 62 | Normal ovary (Grade 1, stage 1a mixed endometrioid and serous endometrial cancer) |
n/a |
| B13 | 76 | Microscopic benign Brenner tumour on right ovary (Grade 2, stage 1a endometrioid endometrial cancer) |
n/a |
n/a; not applicable
This was performed within 20 min of removal from the abdomen. Using forceps and scalpel, tissues [1 cm (length) × 0.5 cm (width) × 0.5 cm (depth)] were removed from macroscopically-malignant, non-necrotic portions of the ovarian surface. The tissue blocks obtained were placed in sample tubes and submerged in RNAlater (QIAGEN Ltd, Crawley, West Sussex, UK), refrigerated overnight at 4°C, and then transferred to −85°C until RNA extraction. All sample tubes were labelled with a trial number and this was used in all subsequent analyses to ensure anonymity. Adjacent ovarian tissue was formalin-fixed and wax-embedded for immunohistochemistry and histology.
2.2 Quantitative Real-Time RT-PCR
Tissues were ground under liquid nitrogen and total RNA extraction was performed using the Qiagen RNeasy® Kit in combination with the Qiagen RNase-free DNase kit (QIAGEN Ltd) RNA (0.8 μg) was reverse transcribed in a final volume of 20 μl containing Taqman® reverse transcription reagents (Applied Biosystems, Warrington, Cheshire, UK): 1 × Taqman RT buffer; MgCl2 (5.5 mM); oligo d(T)16 (2.5 μM); dNTP mix (dGTP, dCTP, dATP and dTTP; each at a concentration of 500 μM); RNase inhibitor (0.4 U/μl); reverse transcriptase (Multiscribe™) (1.25 U/μl) and RNase-free water. Reaction mixtures were then incubated at 25°C (10 min), 48°C (30 min), 95°C (5 min). cDNA samples were stored at −20°C prior to use. Primers (Table 2) for γ-SYN (Genbank accession no. NM_000304), ERα (Genbank accession no. NM_000125.3), and the endogenous control β-ACTIN (Genbank accession no. AK222925) were chosen using Primer Express software 2.0 (Applied Biosystems, Warrington, UK) and designed so that one primer spanned an exon boundary. Specificity was confirmed using the NCBI BLAST search tool. The ERαΔ3 primer (Genbank accession no. NM_000125.3) was designed using the splice targeted approach described by Poola et al. (2002), using the Primer-BLAST tool on the NCBI website. All primers were validated.
Table 2. Primers used for quantitative real-time RT-PCR analysis.
| Gene | Primer | Primer Sequence |
|---|---|---|
| β-ACTIN | β-ACTIN-F | CCT GGC ACC CAG CAC AAT |
| β-ACTIN-R | GCC GAT CCA CAC GGA GTA CT | |
| γ-SYNUCLEIN | γ-SYNUCLEIN-F | GTG CGC AAG GAG GAC TTG A |
| γ-SYNUCLEIN-R | CCT CTG CCA CTT CCT CTT TCT TC | |
| ERα | ERα-F | TGG ACA GGA ACC AGG GAA AAT |
| ERα-R | GAG ATG ATG TAG CCA GCA GCA T | |
| ERαΔ3 | ERαΔ3-F | AGA AGT ATT CAA GGG ATA CGA AAA G |
| ERαΔ3-R | ATC ATC TCT CTG GCG CTT GT |
F, Forward primers; R, Reverse primers
Quantitative real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems). Reaction mixtures contained 1 × SYBR® Green PCR master mix (Applied Biosystems); forward and reverse primers (Invitrogen, Paisley, UK) at a concentration of 300 nM; with 20 ng cDNA template; made to a total volume of 25 μl with sterile H2O. Thermal cycling parameters included activation at 95°C (10 min) followed by 60 cycles each of denaturation at 95°C (15 sec) and annealing/extending at 60°C (1 min). Each reaction was performed in triplicate and ‘no-template’ controls were included in each experiment. Dissociation curves were run to eliminate non-specific amplification including primer-dimers.
2.3 Immunofluorescence Imaging
Formalin-fixed, paraffin-embedded tissues were used for immunofluorescence imaging. Microtomed sections (4 μm) floated onto APES slides were de-waxed and rehydrated prior to labelling. High-temperature antigen retrieval was performed by heating the tissue sections in citrate buffer (pH 6.0) for 20 min at full power (800 W) in a microwave oven. For primary labelling (ABCAM, UK), the polyclonal rabbit γ-syn IgG antibody (ab6169) was used. For negative controls, primary antibodies were replaced with an equivalent non-specific primary antibody at an equivalent dilution. To visualize by fluorescence microscopy, goat anti-rabbit IgG and rabbit anti-mouse IgG secondary antibodies (Sigma-Aldrich, UK) conjugated to fluorescein isothiocyanate (FITC) were used. Slides were placed in 1% Triton X-100 (Fluka) followed by incubation in normal goat or rabbit serum for 20 min to prevent non-specific labelling with the primary antibody. Excess goat/rabbit serum was then removed by blotting with filter paper. Sections were then incubated with the primary antibody at a 1:500 dilution (γ-syn) in phosphate-buffered saline (PBS) buffer. Slides were then incubated overnight at 4°C in a moist chamber to prevent evaporation. Controls consisted of the primary antibody step being replaced by non-specific antibody. Slides were then washed in PBS three times sequentially for 8 min. This was followed by three 8-min washes in d.H20. Slides were then incubated for 3 h at room temperature with secondary antibody at a 1:200 dilution in PBS, after which they were washed in PBS for 8 min three times followed by three 8-min washes in d.H20. After the final wash, sections were dried and sealed using Vectashield containing propidium iodide (Vector Labs Inc, Burlingame, CA) before being examined on a Leica SP2 AOBS Confocal microscope.
3. RESULTS
3.1 Quantitative Real-Time RT-PCR
Quantitative real-time RT-PCR was performed using benign normal ovaries (B1, B2 and B3); each was used as a calibrator control with a different group of ovarian tumour tissue (Table 3A). In order to compare the relative expression levels of the benign controls, and examine the range of expression in normal tissues, two further RT-PCR plates were run with B2 as the calibrator control against B1, B3 and 10 additional benign ovarian tissues. To allow comparison between plates the relative gene expression values obtained with B1 or B3 as the calibrator control were adjusted, using their relative expression levels, to allocate B2 as the universal calibrator control (Table 3B). The ranges of averaged threshold cycle (CT) values of amplified cDNA were 22-32 for γ-SYN, 27-38 for ERα and, 29-44 for ERαΔ3, demonstrating that mRNA transcripts for all three gene targets were readily quantifiable (Table 4).
Table 3A. Original relative gene expression of γ-SYN, ERα and ERαΔ3.
| Sample | γ-SYN | ERαΔ3 | ERα | ERαΔ3:ERα |
|---|---|---|---|---|
| *B1 | 1.00 (0.74-1.35) |
1.00 (0.69-1.46) |
1.00 (0.64-1.56) |
not applicable (n/a) |
| OV1 | 8.52 (1.80-40.19) |
0.11 (0.002-7.24) |
0.20 (0.02-2.24) |
0.53 |
| OV2 | 2.92 (1.15-7.32) |
48.06 (21.93-105.32) |
29.79 (12.51-70.96) |
1.61 |
| OV3 | 5.39 (2.76-10.50) |
13.87 (6.05-31.79) |
2.63 (1.07-6.45) |
5.28 |
|
| ||||
| *B2 | 1.00 (0.86-1.16) |
1.00 (0.25-3.99) |
1.00 (0.71-1.41) |
n/a |
| OV4 | 0.86 (0.53-1.40) |
1.75 (0.15-20.9) |
2.57 (1.52-4.34) |
0.68 |
| OV5 | 0.14 (0.10-0.213) |
159.05 (111.22-227.46) |
128.59 (91.32-181.08) |
1.24 |
| OV6 | 0.60 (0.43-0.84) |
32.9 (17.06-63.45) |
15.96 (11.58-22.01) |
2.1 |
| OV7 | 20.82 (16.99-25.52) |
1.30 (0.12-13.99) |
1.06 (0.97-1.16) |
1.23 |
|
| ||||
| *B3 | 1.00 (0.63-1.59) |
1.00 (0.59-1.69) |
1.00 (0.66-1.52) |
n/a |
| OV8 | 12.01 (7.41-19.49) |
2.4 (1.37-4.21) |
0.63 (0.37-1.07) |
3.82 |
| OV9 | 0.26 (0.14-0.48) |
27.86 (15.67-49.52) |
86 (0.82-4.25) |
14.95 |
| OV10 | 2.36 (0.94-5.93) |
2.38 (0.75-7.59) |
0.76 (0.3-1.92) |
3.15 |
| OV11 | 6.71 (5.68-7.93) |
20.49 (15.08-27.83) |
3.51 (2.61-4.7) |
5.84 |
|
| ||||
| *B2 | 1.0 (0.85-1.18) |
1.0 (0.18-5.54) |
1.0 (0.57-1.75) |
n/a |
| B1 | 0.31 (0.2-0.49) |
3.94 (0.97-15.9) |
2.29 (1.53-3.43) |
1.72 |
| B3 | 3.08 (1.36-6.96) |
42.91 (15.49-118.9) |
9.17 (4.19-20.06) |
4.68 |
| B4 | 2.9 (0.99-8.47) |
195.36 (66.47-574.19) |
69.55 (23.74-203.77) |
2.81 |
| B5 | 5.33 (4.28-6.63) |
11.26 (9.38-13.53) |
4.22 (3.14-5.67) |
2.67 |
| B6 | 3.98 (1.45-10.93) |
49.87 (30.69-81.01) |
6.56 (4.06-10.6) |
7.5 |
| B7 | 3.89 (2.63-5.75) |
42.42 (11.35-158.54) |
14.29 (8.37-24.39) |
2.97 |
|
| ||||
| *B2 | 1.0 (0.79-1.26) |
1.0 (0.3-3.3) |
1.0 (0.79-1.26) |
n/a |
| B8 | 5.39 (4.47-6.5) |
2.42 (0.45-13.06) |
1.41 (1.30-1.53) |
1.72 |
| B9 | 58.15 (46.93-72.05) |
11.43 (4.64-28.19) |
7.29 (5.81-9.13) |
1.57 |
| B10 | 4.68 (4.22-5.18) |
27.44 (20.46-36.8) |
19.01 (16.65-21.7) |
1.44 |
| B11 | 1.88 (0.74-4.79) |
4.15 (1.97-8.75) |
2.60 (1.79-3.77) |
1.6 |
| B12 | 2.86 (2.54-3.21) |
5.30 (4.67-6.02) |
2.05 (1.73-2.43) |
2.59 |
| B13 | 0.74 (0.51-1.06) |
4544.8 3066.4-6736.04) |
198.5 (145.77-270.44) |
22.9 |
Calibrator control (*) with minimum-maximum expression in brackets.
Table 3B. Adjusted relative gene expression (to B2 as calibrator control) γ-SYN, ERα and ERαΔ3.
| Sample | γ-SYN | ERαΔ3 | ERα | ERαΔ3:ERα |
|---|---|---|---|---|
| OV1 | 2.64 | 0.43 | 0.46 | 0.93 |
| OV2 | 0.91 | 189.36 | 68.22 | 2.78 |
| OV3 | 1.67 | 54.65 | 6.02 | 9.08 |
| OV8 | 36.99 | 102.98 | 5.78 | 17.82 |
| OV9 | 0.8 | 1195.47 | 17.06 | 70.07 |
| OV10 | 7.27 | 102.13 | 6.97 | 14.65 |
| OV11 | 20.67 | 879.23 | 32.19 | 27.31 |
n/a, not applicable
Table 4. CT values for mRNA transcripts in ovarian tissue (mean CT value).
| Tissue sample |
γ-SYN | ERαΔ3 | ERα | β-Actin |
|---|---|---|---|---|
| B1 | 28.3 | 37.2 | 31.0 | 19.9 |
| OV1 | 30.3 | 45.4 | 38.3 | 24.9 |
| OV2 | 29.6 | 34.3 | 28.9 | 22.7 |
| OV3 | 29.8 | 37.3 | 33.5 | 23.8 |
|
| ||||
| B2 | 29.3 | 42.1 | 35.1 | 21.6 |
| OV4 | 31.7 | 43.4 | 35.8 | 23.7 |
| OV5 | 32.3 | 35.0 | 28.3 | 21.8 |
| OV6 | 29.2 | 36.2 | 30.2 | 20.7 |
| OV7 | 25.0 | 41.8 | 35.1 | 21.7 |
|
| ||||
| B3 | 27.3 | 38.0 | 29.9 | 21.4 |
| OV8 | 22.9 | 35.9 | 29.7 | 20.5 |
| OV9 | 28.4 | 32.3 | 28.1 | 20.5 |
| OV10 | 25.4 | 36.0 | 29.6 | 20.7 |
| OV11 | 23.5 | 32.6 | 27.0 | 20.3 |
|
| ||||
| B2 | 31.8 | 43.9 | 36.5 | 23.3 |
| B1 | 30.5 | 38.9 | 32.3 | 20.3 |
| B3 | 29.8 | 38.1 | 32.9 | 22.9 |
| B4 | 30.1 | 36.1 | 30.2 | 23.1 |
| B5 | 28.5 | 39.6 | 33.6 | 22.5 |
| B6 | 29.0 | 37.5 | 33.0 | 22.5 |
| B7 | 29.8 | 38.5 | 32.7 | 23.3 |
|
| ||||
| B2 | 28.7 | 39.5 | 32.7 | 21.0 |
| B8 | 29.2 | 41.2 | 35.1 | 24.0 |
| B9 | 27.4 | 40.5 | 34.3 | 25.6 |
| B10 | 26.6 | 34.9 | 28.6 | 21.2 |
| B11 | 27.1 | 36.8 | 30.6 | 20.4 |
| B12 | 26.5 | 36.4 | 30.9 | 20.3 |
| B13 | 35.4 | 33.7 | 31.3 | 27.3 |
This table presents the raw data required for the relative gene expression analysis shown in Table 3. The mean CT values of each gene investigated and the mean CT value of β-ACTIN (in brackets), is given for each patient. Gene expression analysis is performed by comparing β-ACTIN values with those of the gene of interest, relative to one ‘control‘ patient known as the calibrator in order to determine relative inter-patient differences. In this case benign tissues (patients B1, B2 or B3) were chosen as the calibrator control and consequently assigned a gene expression value of 1 in subsequent analyses (see Table 3A).
3.2 γ-SYN Expression
The level of relative expression in benign tissue ranged from 0.31 to 5.39 with a mean of 3.19; this excludes one benign tissue (B9) which had a much higher relative expression level of 58.15. A small benign serous ovarian cyst was found on histology and may account for this finding. Of the cancer tissues, γ-SYN was raised in three of the four high-grade specimens with results ranging from 7.27 to 36.99. A single well-differentiated mucinous tumour (OV7) also had a high relative expression level of 20.82 with relatively low ERα and ERαΔ3 levels. Four of the seven non-high grade tumours had relatively high ERα and ERαΔ3 levels with low levels of γ-SYN. No such relationship was observed within the high-grade tumours.
3.3 ERα Expression
Relative gene expression levels for ERα are shown in Table 3. The bulk of the relative expression levels in benign tissue lie between 1.41 and 19.0, with a mean of 6.89. Two outliers, B13 and B4, which exhibited relative expression levels of 198.5 and 69.55 respectively, were excluded from this calculation as they had potentially confounding medical conditions (see Table 1).
Quantitative gene expression was carried out. For inter-individual variations, the mRNA transcript levels derived from the patients B1, B2 or B3 were arbitrarily taken as the calibrator control (*, see Table 3A) and set to 1.Within each experiment, reactions were performed in triplicate and ‘no-template’ controls were included. Averaged CT values for each reaction were normalized to β-ACTIN values thus giving ΔCT values. Alterations in gene expression were determined by comparison with the tissue value assigned as the calibrator, giving ΔΔCT values. Relative gene expression was calculated using the formula 2-ΔΔCT. Adjustment to B2 as universal calibrator control was performed by multiplying the value of the specimen’s original relative gene expression by the value of its calibrator control when analyzed concurrently with B2.
In the ovarian tumour tissue, the ERα expression level was at least twice the mean of the benign tissues in one of the three borderline tumours (OV2, 68.22), in two of the four well/moderately differentiated tumours [(OV5, 128.59) and (OV6, 15.96)] and two of the four high-grade tumours [(OV9, 17.06) and (OV11, 32.19)]. All four of the mucinous tumours (OV1, OV2, OV4 and OV7) had a low relative ERα expression. A previous study has also noted the absence of ERα expression in mucinous ovarian tumours (Czekierdowski et al., 2006).
3.4 ERαΔ3 expression
The benign tissues showed a wide range of relative expression of ERαΔ3 with most between 2.42 and 49.87, giving a mean of 20.1 (outliers excluded as above). The tumour specimens OV2, OV3, OV5 and all of the high-grade tissues had relative expression levels >50-fold higher than the calibrator control. Of the remaining four tumour tissues, three had a low level of expression of <2-times the calibrator control. Finally, one moderately-differentiated mixed tumour (OV6) had a relative expression of 32.9, well within the range for benign tissue.
3.5 Ratio of ERαΔ3 and ERα expression
ERαΔ3:ERα represents the ratio of the relative expression levels of a splice variant form and the standard full length mRNA (i.e., compared with the calibrator). The absolute levels of ERα, whilst not formally quantified, are markedly higher than those of ERαΔ3 in all tissues, as can be judged by the mean CT values (see Table 4). In the benign tissues, the ERαΔ3:ERα ratio was >1 in all instances, demonstrating the low level in the calibrator control. Of the 12 benign tissues, 10 had ratios of <5 and nine had ratios of <3. The remaining two tissues were B13 and B6 with ratios of 22.9 and 7.5 respectively. B13 had previously been noted to have high levels of both ERα and ERαΔ3. In the tumour tissues, there is a striking difference between the high-grade tumours and the non-high-grade tumours. All four of the high-grade specimens exhibited high ERαΔ3:ERα ratios, with values ranging between 14.65 and 70.07. One borderline mucinous tumour (OV3) also had an elevated ratio of 9.08, due to a raised ERαΔ3 with a normal ERα level.
3.6 Immunofluorescence analysis of γ-syn cellular location
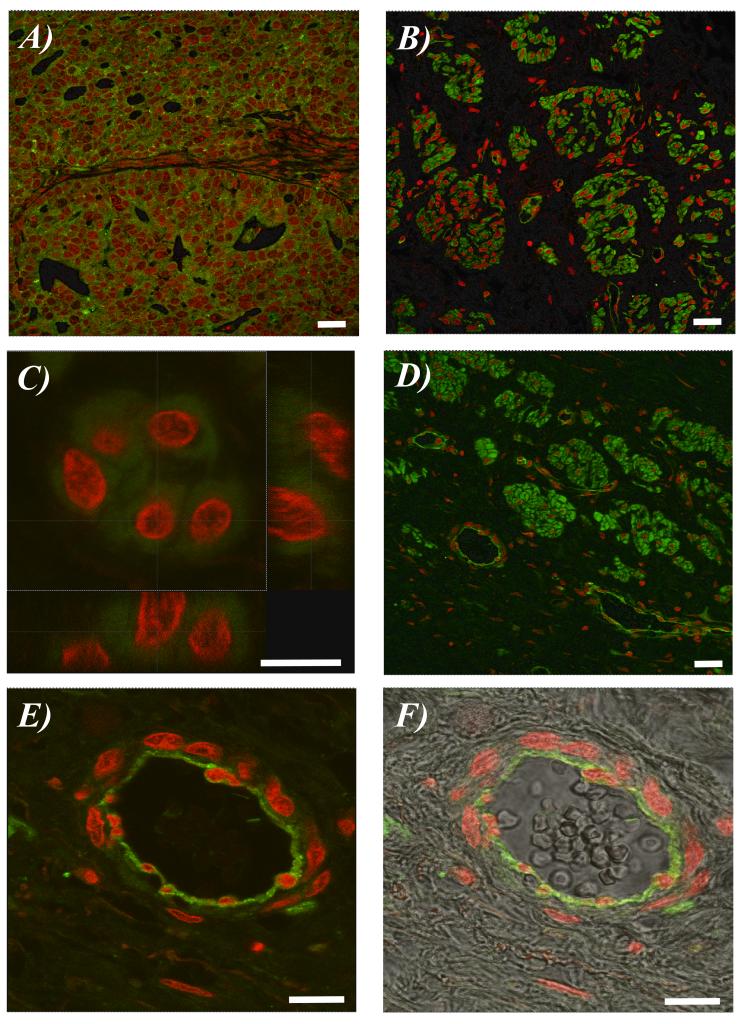
In a high-grade serous carcinoma (OV8), there was clear evidence of fluorescence in the epithelial cell cytoplasm (Figure 1A). Likewise, in a borderline mucinous tumour (OV3), staining again localized to the epithelial cell cytoplasm (Figures 1B and 1C).
Fig. 1. Representative photomicrographs of immunohistochemical staining for γ-synuclein in human ovarian tissue by immunofluorescence over the range 500 nm - 540 nm for fluorescein (green) and 624 nm - 707 nm for PI (red).
(A) A high-grade serous adenocarcinoma (OV8). (B) A borderline mucinous ovarian cancer (OV3). (C) Epithelial cells at high magnification from a borderline mucinous ovarian cancer (OV3). (D) A borderline mucinous ovarian cancer (OV3) showing adjacent blood vessels. (E) Blood vessel at high magnification showing the majority of immunofluorescence was in the apical endothelial cells surrounding the blood vessel lumen. (F) Immunofluorescent image of blood vessel at high magnification superimposed on corresponding image derived using differential interference contrast microscopy. Scale bar: 20 μm.
However, in the same tissue section, there was also vascular-associated immunofluorescence (Figure 1D). At higher magnification, clear endothelial-cell staining was observed (Figure 1E); Figure 1F shows the fluorescent image super-imposed upon the corresponding unstained image, clearly demonstrating the presence of a small blood vessel. Interestingly the majority of the immunofluorescence was in the apical endothelial cells surrounding the blood vessel lumen.
4. DISCUSSION
γ-Syn expression has previously been demonstrated in ovarian endometriosis (Singh et al., 2008), in pre-neoplastic ovarian lesions and in ovarian cancer (Bruening et al., 2000). In ovarian endometriosis immunohistochemical staining is associated with endothelial cells, suggesting possible involvement of γ-syn in blood vessel formation (Singh et al., 2008). It is plausible that γ-syn expression is a factor in endometriosis-associated ovarian cancer. This study demonstrates a similar staining pattern in mucinous borderline ovarian tumours, a tumour type not associated with endometriosis. High-grade, late-stage cancers demonstrated a more intense, diffuse staining pattern with a correspondingly increased level of gene expression in three of the four tissues studied. This is in keeping with a previous study (Bruening et al., 2000). Interestingly γ-SYN expression appears to have an inverse relationship with ERα expression in low-grade and early-stage tumours, as this was seen in three out of four of the well-/moderately-differentiated tumour group (all stage 1a to 2a). The high-grade tumours tend to highly express both and this dual over-expression may permit the growth needed to upstage the tumour.
γ-Syn boosts the metastatic potential of tumour cells in several different ways. Firstly it enhances cell motility and invasiveness (Pan et al., 2006), secondly it increases the ligand affinity of ERα, so amplifying oestrogen-stimulated growth-promoting effects (Jiang et al., 2004), and thirdly it interferes with microtubule function leading to aneuploid cells and enhancing resistance to taxane and vinca alkaloid-induced apoptosis (Pan et al., 2002; Hua et al., 2009; Gupta et al., 2003). We propose that γ-syn may additionally be involved in neoangiogenesis, given its clear localization in endothelial cells in non-cancerous but potentially pre-malignant areas of abnormal cell growth (i.e., borderline tumours and endometriosis).
This study is the first to explore the expression of ERαΔ3 in ovarian tissue. This ER splice variant, lacking a functional DNA binding domain, competitively inhibits ERα function at equimolar levels (Poola et al., 2002), but also interacts with Sp1 proteins to stimulate transcription of VEGF with 3-fold the potency of ERα (Paley et al., 2000). Previous studies have found increased ERαΔ3 levels in subgroups with poor-prognosis breast cancers (Taylor et al., 2010). Measurable levels were found in all benign, borderline and malignant ovarian tissues tested, with universally raised levels in high-grade, high-stage disease. Due to this mixed agonist/antagonist effect, it was of interest to examine the differing proportions of ERαΔ3 to ERα in normal ovaries and ovarian tumours. This revealed a consistently high ERαΔ3 to ERα ratio in late-stage, high-grade disease.
VEGF induces neoangiogenesis by promoting endothelial cell proliferation and increasing vascular permeability. It is expressed in both normal and malignant ovaries, although higher levels are found in cancer cells (Nakanishi et al., 1997). Over-expression of VEGF is associated with an increased microvascular supply, enhanced tumour invasiveness and poor prognosis in epithelial ovarian cancer (Wu et al., 2007; Liu et al., 2008). Various therapeutic strategies targeting VEGF have been investigated in ovarian cancer (Singh et al., 2007), the most promising being the monoclonal antibody, bevacizumab. The ICON 7 and GOG 218 trials recently reported an improved progression-free survival with addition of this drug to the standard chemotherapy regime of carboplatin and paclitaxel; however, the benefits subsided after discontinuing the drug.
Although the CT values strongly suggest that the expression levels of ERαΔ3 are significantly lower than those of ERα, any increase in expression of VEGF is likely to contribute to the malignant phenotype. As the ratio of ERαΔ3:ERα is convincingly raised in all our high-grade/high-stage tumours, but not the low-grade, borderline or benign tissues, we would suggest that this is a factor in the acquisition of metastatic potential in ovarian cancer. γ-Syn is likely to be a prognostic marker of aggressive disease in a number of malignancies (Ye et al., 2008; Singh and Jia 2008). Raised serum levels of γ-syn have been detected in colon cancer (Li et al., 2004; Kumaran et al., 2009); this may also occur in ovarian cancer. This generates the potential for a combination or alternative blood test in addition to CA125. Novel peptide inhibitors of γ-syn protein have shown potential to be developed as a chemotherapeutic adjunct (Sánchez-Muñoz et al., 2009; Suzuki et al., 2008). Further work into γ-syn and ERαΔ3 may reveal therapeutic targets, or prognostic markers of disease severity, progression, relapse and chemo-responsiveness.
5. CONCLUSION
This was a small pilot study conducted with ovarian tissues samples to examine in vivo expression of ERα, its splice variant ERαΔ3 and γ-SYN. It suggests a possible stage-specific role of ERα splice variant ERαΔ3 in the propagation of ovarian cancer, likely through an effect on VEGF and neoangiogenesis. The endothelial localisation of γ-syn suggests it too may play a role in neoangiogenesis in the progression of ovarian cancer. However, γ-syn is known to be involved in multiple complex mechanisms of carcinogenesis and further investigation into its exact role in ovarian cancer is required. Our study suggests it may participate in different stage-related roles in subtypes of epithelial ovarian cancer. Further investigations are required with ovarian cell lines and larger study sample sizes.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank the members of the Pathology laboratory at Lancashire Teaching Hospitals Trust, Preston, UK. This project was supported by the Rosemere Cancer Foundation and the Biotechnology and Biological Sciences Research Council (grant number BB/D010055/1).
REFERENCES
- Ahmad M, Attoub S, Singh MN, Martin FL, El-Agnaf OMA. γ-Synuclein and the progression of cancer. FASEB J. 2007;21:3419–3430. doi: 10.1096/fj.07-8379rev. [DOI] [PubMed] [Google Scholar]
- Bruening W, Giasson BI, Klein-Szanto AJ, Lee VM, Trojanowski JQ, Godwin AK. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000;88:2154–2163. [PubMed] [Google Scholar]
- Cooper BC, Ritchie JM, Broghammer CL, Coffin J, Sorosky JI, Buller RE, Hendrix MJ, Sood AK. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clin. Cancer Res. 2002;8:3193–3197. [PubMed] [Google Scholar]
- Cooper N, Quinn MJ, Rachet B, Mitry E, Coleman MP. Survival from cancer of the ovary in England and Wales up to 2001. Br. J. Cancer. 2008;99:S70–S72. doi: 10.1038/sj.bjc.6604593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekierdowski A, Czekierdowska S, Wielgos M, Smolen A, Kaminski P, Kotarski J. The role of CpG islands hypomethylation and abnormal expression of neuronal protein synuclein-γ (SNCG) in ovarian cancer. Neuro. Endocrinol. Lett. 2006;27:381–386. [PubMed] [Google Scholar]
- Erenburg I, Schachter B, Mira y Lopez R, Ossowski L. Loss of an estrogen receptor isoform (ERαΔ3) in breast cancer and the consequences of its reexpression: interference with estrogen-stimulated properties of malignant transformation. Mol. Endocrinol. 1997;11:2004–2015. doi: 10.1210/mend.11.13.0031. [DOI] [PubMed] [Google Scholar]
- Gómez-Raposo C, Mendiola M, Barriuso J, Casado E, Hardisson D, Redondo A. Angiogenesis and ovarian cancer. Clin. Transl. Oncol. 2009;11:564–571. doi: 10.1007/s12094-009-0406-y. [DOI] [PubMed] [Google Scholar]
- Gupta A, Godwin AK, Vanderveer L, Lu A, Liu J. Hypomethylation of the synuclein γ gene CpG island promotes its aberrant expression in breast carcinoma and ovarian carcinoma. Cancer Res. 2003;63:664–673. [PubMed] [Google Scholar]
- Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, Carson LF, Ramakrishnan S. Vascular endothelial growth factor expression and survival in human epithelial ovarian cancer. Cancer Lett. 1997;121:169–175. doi: 10.1016/s0304-3835(97)00350-9. [DOI] [PubMed] [Google Scholar]
- Hibi T, Mori T, Fukuma M, Yamazaki K, Hashiguchi A, Yamada T, Tanabe M, Aiura K, Kawakami T, Ogiwara A, Kosuge T, Kitajima M, Kitagawa Y, Sakamoto M. Synuclein-γ is closely involved in perineural invasion and distant metastasis in mouse models and is a novel prognostic factor in pancreatic cancer. Clin. Cancer Res. 2009;15:2864–2871. doi: 10.1158/1078-0432.CCR-08-2946. [DOI] [PubMed] [Google Scholar]
- Hua H, Xu L, Wang J, Jing J, Luo T, Jiang Y. Up-regulation of γ-synuclein contributes to cancer cell survival under endoplasmic reticulum stress. J. Pathol. 2009;217:507–515. doi: 10.1002/path.2465. [DOI] [PubMed] [Google Scholar]
- Iwaki H, Kageyama S, Isono T, Wakabayashi Y, Okada Y, Yoshimura K, Terai A, Arai Y, Iwamura H, Kawakita M, Yoshiki T. Diagnostic potential in bladder cancer of a panel of tumor markers (calreticulin, γ-synuclein, and catechol-o-methyltransferase) identified by proteomic analysis. Cancer Sci. 2004;95:955–961. doi: 10.1111/j.1349-7006.2004.tb03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu YE, Lu A, Gupta A, Golberg ID, Liu J, Shi YE. Stimulation of estrogen receptor signalling by γ-synuclein. Cancer Res. 2003;63:3899–3903. [PubMed] [Google Scholar]
- Jiang Y, Liu YE, Goldberg ID, Shi YE. γ-Synuclein, a novel heat-shock protein-associated chaperone, stimulates ligand dependent estrogen receptor α signalling and mammary tumorigenesis. Cancer Res. 2004;64:4539–4546. doi: 10.1158/0008-5472.CAN-03-3650. [DOI] [PubMed] [Google Scholar]
- Koduri S, Goldhar AS, Vonderhaar BK. Activation of vascular endothelial growth factor (VEGF) by the ER-α variant, ERΔ3. Breast Cancer Res. Treat. 2006;95:37–43. doi: 10.1007/s10549-005-9028-4. [DOI] [PubMed] [Google Scholar]
- Kumaran GC, Jayson GC, Clamp AR. Antiangiogenic drugs in ovarian cancer. Br. J. Cancer. 2009;100:1–7. doi: 10.1038/sj.bjc.6604767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavedan C. The synuclein family. Genome Res. 1998;8:871–880. doi: 10.1101/gr.8.9.871. [DOI] [PubMed] [Google Scholar]
- Li Z, Sclabas GM, Peng B, Hess KR, Abbruzzese JL, Evans DB, Chiao PJ. Overexpression of synuclein-γ in pancreatic adenocarcinoma. Cancer. 2004;101:58–65. doi: 10.1002/cncr.20321. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C, Wang L, Zhao W, Jiang JD, Liu J. Loss of epigenetic control of synuclein-γ gene as a molecular indicator of metastasis in a wide range of human cancers. Cancer Res. 2005;65:7635–7643. doi: 10.1158/0008-5472.CAN-05-1089. [DOI] [PubMed] [Google Scholar]
- Liu YE, Pu W, Jiang Y, Shi D, Dackour R, Shi YE. Chaperoning of estrogen receptor and induction of mammary gland proliferation by neuronal protein synuclein γ. Oncogene. 2007;26:2115–2125. doi: 10.1038/sj.onc.1210000. [DOI] [PubMed] [Google Scholar]
- Liu C, Guo J, Qu L, Bing D, Meng L, Wu J, Shou C. Applications of novel monoclonal antibodies specific for synuclein-γ in evaluating its levels in sera and cancer tissues from colorectal cancer patients. Cancer Lett. 2008;269:148–158. doi: 10.1016/j.canlet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Kodama J, Yoshinouchi M, Tokumo K, Kamimura S, Okuda H, Kudo T. The expression of vascular endothelial growth factor and transforming growth factor-β associates with angiogenesis in epithelial ovarian cancer. Int. J. Gynecol. Pathol. 1997;16:256–262. doi: 10.1097/00004347-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Paley PJ, Goff BA, Gown AM, Greer BE, Sage EH. Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol. Oncol. 2000;78:336–341. doi: 10.1006/gyno.2000.5894. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Bruening W, Giasson BI, Lee VM, Godwin AK. γ-Synuclein promotes cancer cell survival and inhibits stress- and chemotherapy drug-induced apoptosis by modulating MAPK pathways. J. Biol. Chem. 2002;277:35050–35060. doi: 10.1074/jbc.M201650200. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Bruening W, Godwin AK. Involvement of RHO GTPases and ERK in synuclein-γ enhanced cancer cell motility. Int. J. Oncol. 2006;29:1201–1205. [PubMed] [Google Scholar]
- Poola I, Speirs V. Expression of alternatively spliced estrogen receptor α mRNAs is increased in breast cancer tissues. J. Steroid Biochem. Mol. Biol. 2001;78:459–469. doi: 10.1016/s0960-0760(01)00118-2. [DOI] [PubMed] [Google Scholar]
- Poola I, Clarke R, DeWitty R, Leffall LD. Functionally active estrogen receptor isoform profiles in the breast tumors of African American women are different from the profiles in breast tumors of Caucasian women. Cancer. 2002;94:615–623. doi: 10.1002/cncr.10274. [DOI] [PubMed] [Google Scholar]
- Rosen DG, Yang G, Liu G, Mercado-Uribe I, Chang B, Xiao XS, Zheng J, Xue FX, Liu J. Ovarian cancer: pathology, biology and disease models. Front. Biosci. 2009;14:2089–2102. doi: 10.2741/3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Muñoz A, Pérez-Ruiz E, Mendiola Fernández C, Alba Conejo E, González-Martín A. Current status of anti-angiogenic agents in the treatment of ovarian carcinoma. Clin. Transl. Oncol. 2009;11:589–595. doi: 10.1007/s12094-009-0409-8. [DOI] [PubMed] [Google Scholar]
- Singh VK, Zhou Y, Marsh JA, Uversky VN, Forman-Kay JD, Liu J, Jia Z. Synuclein-γ targeting peptide inhibitor that enhances sensitivity of breast cancer cells to antimicrotubule drugs. Cancer Res. 2007;67:626–633. doi: 10.1158/0008-5472.CAN-06-1820. [DOI] [PubMed] [Google Scholar]
- Singh MN, Stringfellow HF, Taylor SE, Ashton KM, Ahmad M, Abdo KR, El-Agnaf OMA, Martin-Hirsch PL, Martin FL. Elevated expression of CYP1A1 and γ-SYNUCLEIN in human ectopic (ovarian) endometriosis compared with eutopic endometrium. Mol. Hum. Reprod. 2008;14:655–663. doi: 10.1093/molehr/gan056. [DOI] [PubMed] [Google Scholar]
- Singh VK, Jia Z. Targeting synuclein-γ to counteract drug resistance in cancer. Expert Opin. Ther. Targets. 2008;12:59–68. doi: 10.1517/14728222.12.1.59. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, Sasano H, Yaegashi N. Loss of estrogen receptor β isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Sci. 2008;99:2365–2372. doi: 10.1111/j.1349-7006.2008.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Martin-Hirsch PL, Martin FL. Oestrogen receptor splice variants in the pathogenesis of disease. Cancer Lett. 2010;288:133–148. doi: 10.1016/j.canlet.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Wu K, Weng Z, Tao Q, Lin G, Wu X, Qian H, Zhang Y, Ding X, Jiang Y, Shi YE. Stage-specific expression of breast cancer-specific gene γ-synuclein. Cancer Epidemiol. Biomarkers Prev. 2003;12:920–925. [PubMed] [Google Scholar]
- Wu K, Quan Z, Weng Z, Li F, Zhang Y, Yao X, Chen Y, Budman D, Goldberg ID, Shi YE. Expression of neuronal protein synuclein γ gene as a novel marker for breast cancer prognosis. Breast Cancer Res. Treat. 2007;101:259–267. doi: 10.1007/s10549-006-9296-7. [DOI] [PubMed] [Google Scholar]
- Yanagawa N, Tamura G, Honda T, Endoh M, Nishizuka S, Motoyama T. Demethylation of the synuclein γ gene CpG island in primary gastric cancers and gastric cancer cell lines. Clin. Cancer Res. 2004;10:2447–2451. doi: 10.1158/1078-0432.ccr-03-0107. [DOI] [PubMed] [Google Scholar]
- Ye Q, Zheng MH, Cai Q, Feng B, Chen XH, Yu BQ, Gao YB, Ji J, Lu AG, Li JW, Wang ML, Liu BY. Aberrant expression and demethylation of γ-synuclein in colorectal cancer, correlated with progression of the disease. Cancer Sci. 2008;99:1924–1932. doi: 10.1111/j.1349-7006.2008.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Feng B, Peng YF, Chen XH, Cai Q, Yu BQ, Li LH, Qiu MY, Liu BY, Zheng MH. Expression of γ-synuclein in colorectal cancer tissues and its role on colorectal cancer cell line HCT116. World J. Gastroenterol. 2009;15:5035–5043. doi: 10.3748/wjg.15.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.