Abstract
Sexual receptivity, lordosis, can be induced by sequential estradiol and progesterone or extended exposure to high levels of estradiol in the female rat. In both cases estradiol initially inhibits lordosis through activation of β-endorphin (β-END) neurons of the arcuate nucleus of the hypothalamus (ARH) that activate μ-opioid receptors (MOP) in the medial preoptic nucleus (MPN). Subsequent progesterone or extended estradiol exposure deactivate MPN MOP to facilitate lordosis. Opioid receptor-like receptor-1 (ORL-1) is expressed in ARH and ventromedial hypothalamus (VMH). Infusions of its endogenous ligand, orphanin FQ (OFQ/N, aka nociceptin), into VMH-ARH region facilitates lordosis. Whether OFQ/N acts in ARH and/or VMH and whether OFQ/N is necessary for steroid facilitation of lordosis is unclear. In Exp I, OFQ/N infusions in VMH and ARH that facilitated lordosis also deactivated MPN MOP indicating that OFQ/N facilitation of lordosis requires deactivation of ascending ARH-MPN projections by directly inhibiting ARH β-END neurons and/or through inhibition of excitatory VMH-ARH pathways to proopiomelanocortin neurons. It is unclear whether OFQ/N activates the VMH output motor pathways directly or via the deactivation of MPN MOP. In Exp II we tested whether ORL-1 activation is necessary for estradiol-only or estradiol + progesterone lordosis facilitation. Blocking ORL-1 with UFP-101 inhibited estradiol-only lordosis and MPN MOP deactivation but had no effect on estradiol + progesterone facilitation of lordosis and MOP deactivation. In conclusion, steroid facilitation of lordosis inhibits ARH β-END neurons to deactivate MPN MOP, but estradiol-only and estradiol + progesterone treatment appear to use different neurotransmitter systems to inhibit ARH-MPN signaling.
Keywords: ORL-1, orphanin FQ, lordosis, medial preoptic nucleus, μ-opioid receptors, UFP-101, arcuate nucleus of the hypothalamus, ventral medial nucleus of the hypothalamus, estradiol, progesterone
INTRODUCTION
Sexual receptivity (lordosis) can be facilitated in female rats by sequential exposure of neural circuits to a priming dose of estradiol followed by progesterone or by a longer exposure to higher levels of estradiol (Blaustein et al., 1987; Clemens and Weaver, 1985; Pfaff, 1970; Quadagno et al., 1972; Sodersten and Eneroth, 1981). In both cases, estradiol initially inhibits lordosis by signaling rapidly through membrane estrogen receptors complexed to metabotropic glutamate receptors-type 1a (Dewing et al., 2007; Mills et al., 2004). This initiates the activation of a multi-synaptic circuit originating in the arcuate nucleus of the hypothalamus (ARH) to stimulate the release of the opioid β-endorphin (β-END) in the medial preoptic nucleus (MPN; Dewing et al., 2007; Mills et al., 2004; Sanathara et al., 2009), and in turn activates and internalizes μ-opioid receptors (MOP) in the MPN to inhibit lordosis (Eckersell et al., 1998; Sinchak and Micevych, 2001). Lordosis is facilitated when this circuit is deactivated by either subsequent progesterone or after exposure to a high dose of estradiol for 48 hr (Eckersell et al., 1998; Sinchak and Micevych, 2001). Deactivation of MPN MOP is critical for facilitation of lordosis. Infusions of MOP agonists into the MPN dramatically inhibits sexual receptivity in female rats primed with estradiol and progesterone indicating that this region is an important inhibitory output neurocircuit that blocks downstream lordosis motor output pathways (Eckersell et al., 1998; Sinchak and Micevych, 2001). MOP are G protein-coupled receptor that undergo internalization into early endosomes when activated by endogenous opioid ligands and some exogenous ligands (Eckersell et al., 1998; Micevych et al., 2003; von Zastrow et al., 1994). The activation/internalization of MPN MOP has been correlated with an increase in the levels of positive MOP immunoreactivity (Dewing et al., 2007; Eckersell et al., 1998; Mills et al., 2004; Sinchak and Micevych, 2001). This MPN MOP activation/internalization immunohistochemical assay allows us to determine whether hormonal and pharmacological manipulations act through ARH β-END neurons to regulate sexual receptivity (Dewing et al., 2007; Eckersell et al., 1998; Mills et al., 2004; Sinchak and Micevych, 2001).
In previous studies infusions of the opioid, orphanin FQ (OFQ/N; aka nociceptin), into the ventral medial nucleus of the hypothalamus (VMH) facilitated lordosis in estradiol-primed nonreceptive female rats presumably through its cognate receptor, opioid receptor-like receptor-1 (ORL-1; aka NOP; Sinchak et al., 1997). Originally, because of the abundant expression of ORL-1 in the VMH, we concluded that OFQ/N was acting on descending motor output pathways to facilitate lordosis (Calizo and Flanagan-Cato, 2002, 2003; Cottingham et al., 1987; Pfeifle et al., 1980; Sinchak et al., 1997). However, preliminary experiments showed that similar OFQ/N infusions into the VMH deactivated MPN MOP suggesting that OFQ/N activates ORL-1 circuits that inhibit ARH β-END neurons that project to the MPN to facilitate lordosis (Sinchak et al., 2006a; Sinchak et al., 2006b). ORL-1 are expressed in proopiomelanocortin (POMC; putative β-END) neurons that project to the MPN (Sanathara et al., 2009), and electrophysiological studies have demonstrated that OFQ/N hyperpolarizes β-END neurons via G protein-coupled inwardly-rectifying potassium channels (GIRK) in female Guinea pigs and mice (Farhang et al., 2010; Wagner et al., 1998). Therefore, OFQ/N may have diffused into the ARH and is acting directly on the β-END neurons to inhibit β-END release in the MPN. Additionally, OFQ/N may be acting in the VMH to inhibit ARH β-END neuronal activity. Golgi staining indicates that some ARH processes extend into the VMH and some VMH processes extended into the ARH (Millhouse, 1973b; van den Pol and Cassidy, 1982). Although tract tracing studies have produced sparse evidence to support VMH to ARH neurocircuits (Bouret et al., 2008; Canteras et al., 1994; Saper et al., 1976), laser scanning photostimulation studies indicate that VMH has excitatory inputs onto POMC neuron and inhibitory input to NPY ARH neurons (Sternson et al., 2005). In Experiment I (Exp I) we used multiple infusion sites and measured MPN MOP activity to investigate the site(s) of action of OFQ/N for facilitation of lordosis and regulation of MPN MOP activation in the ARH and VMH in estradiol-primed nonreceptive female rats.
The neural pathways for estradiol + progesterone and estradiol-only facilitation of lordosis converge on β-END neurons that project to the MPN (Eckersell et al., 1998; Sinchak and Micevych, 2001). In both cases estradiol initially activates the MPN MOP presumably through the release of β-END from ARH neurons that project to the MPN. Lordosis is facilitated when MPN MOP are deactivated through the actions of progesterone or high levels of estradiol presumably through reduction of β-END release (Eckersell et al., 1998; Sinchak and Micevych, 2001). In Exp I, OFQ/N infusions facilitated lordosis in estradiol-primed nonreceptive rats via deactivation of MPN MOP. Therefore, we hypothesized that steroid facilitation of lordosis is mediated by OFQ/N signaling through the ARH. This idea is supported by progesterone receptor expression in ARH OFQ/N neurons (Haase et al., 2008), ORL-1 expression in POMC neurons that project to the MPN (Sanathara et al., 2009) and VMH to ARH microcircuits that regulate POMC neurons (Sternson et al., 2005). In a previous study, we used OFQ/N immunoneutralization in the VMH/ARH and inhibited lordosis in estradiol-only treated rats but not those primed with estradiol + progesterone (Sinchak et al., 2007). These results suggest that these two steroid priming paradigms potentially act through different pathways to facilitate lordosis. However, since estradiol priming upregulates the OFQ/N-ORL-1 system (Quesada and Micevych, 2008; Sinchak et al., 2006b), it is unclear whether immunoneutralization was able to sufficiently neutralize OFQ/N released by estradiol + progesterone treatment. In Exp II, we tested the hypothesis that estradiol-only and estradiol + progesterone facilitation of lordosis requires activation of ORL-1 to deactivate MPN MOP through the ARH, we used UFP-101, an ORL-1 selective antagonist, and expected to block facilitation of lordosis and maintain MPN MOP activation.
MATERIALS AND METHODS
Experiment I
OFQ/N acts within the VMH/ARH region to facilitate lordosis (Sinchak et al., 2006a). However, whether OFQ/N acts through one, or a combination of VMH descending motor output pathways, VMH-ARH-MPN pathways and/or directly through ARH-MPN pathways to induce lordosis was unclear. We tested the ability of OFQ/N to induce lordosis and deactivate MPN MOP neurons by infusing into the ARH and sites that aimed progressively dorsolateral through the VMH and outside the VMH. Thus, if OFQ/N infusions 1) deactivated ascending ARH-MPN MOP neurotransmission and 2) activated VMH descending motor output pathways, we expected that would produce the highest LQ and greatest deactivation of MPN MOP compared to activating individual circuits within the nuclei. We used levels of MPN MOP positive immunoreactivity intensity as a measure of MPN MOP activation/internalization reflective of ARH-MPN activity, and predicted that activation of VMH alone would produce moderate LQ without deactivating MPN MOP to indicate VMH motor output pathway levels of involvement. Briefly, adult ovariectomized (OVX) Long-Evans rats received bilateral guide cannula aimed at one of four regions: 1) the ARH; 2) between the ARH and VMH (ARH-VMH); 3) the VMH; and 4) dorsolateral to the VMH (lateral hypothalamus; OUT-VMH; Figure 2). The animals were primed with 2 μg of 17β-estradiol benzoate (EB) and received site specific bilateral infusions 29.5 hr later with either OFQ/N or saline and tested for sexual receptivity 30 minutes after infusion. On the next EB priming cycle, all the animals received the same drug infusions and 30 minutes after infusion the animals were anesthetized, and perfused through the heart with 4% paraformaldehyde. Their brains were processed for MPN MOP immunohistochemistry to determine the effects of OFQ/N infusions on MPN MOP activation/internalization as measured by MOP immunofluorescence staining intensity levels (Dewing et al., 2007; Mills et al., 2004). Levels of sexual receptivity and OFQ/N MOP immunofluorescence staining intensity were compared to determine regional effects of OFQ/N in the ARH and VMH on lordosis and MPN MOP activity.
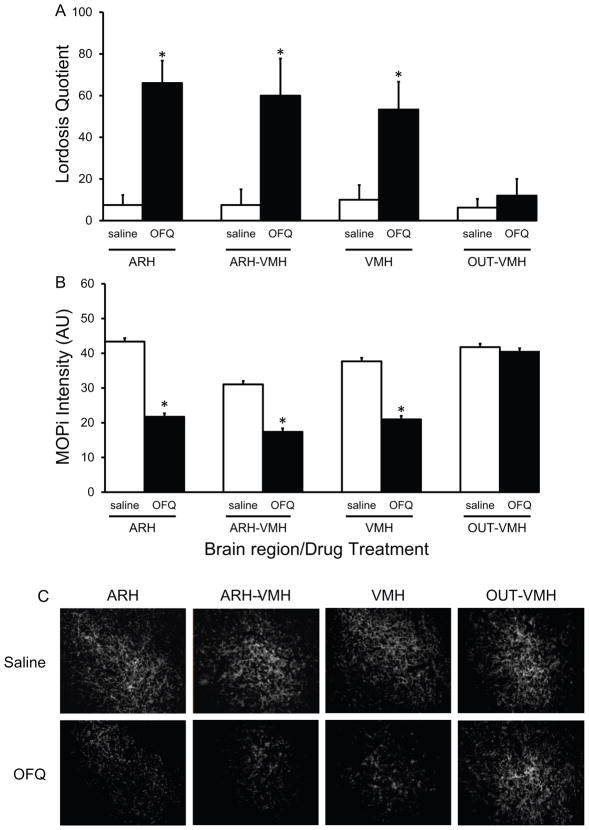
FIGURE 2.
Effects of bilateral infusions of OFQ/N or saline into regions of the ARH and VMH on sexual receptivity and MPN μ-opioid receptor (MOP) immunofluorescence staining intensity levels in EB primed OVX rats. OVX rats were implanted with bilateral cannulae aimed at either 1) the ARH, 2) the ARH-VMH, 3) the VMH, or 4) OUT-VMH (see Figure 1) and treated with 2 μg EB and 29.5 hr later infused bilaterally with either OFQ/N (25 nmol dissolved in 0.5 μl of 0.9% saline/per side) or 0.9% saline. A) Sexual receptivity was measured by lordosis quotient 30 minutes after the OFQ/N or saline infusion. B) On the next steroid cycle animals were given the same steroid and drug infusions and MPN MOP activation/internalization was measured by MOP immunofluorescence staining intensity level in arbitrary units (AU). C) Photomicrographs representative of the MPN region measured for FITC-labeled MOP immunofluorescence staining intensity level in EB primed OVX rats that were infused with either OFQ/N or saline in the four different ARH-VMH regions. * = significantly different than saline treatment group within brain region and compared to OFQ/N treatment in OUT-VMH group (Holm-Sidak; P < 0.05).
Experiment II
In Exp II we tested whether OFQ/N acts through ORL-1 to facilitate lordosis and whether ORL-1 activation is necessary for either estradiol-only or estradiol + progesterone facilitation of lordosis. In Exp IIA, to determine whether OFQ/N acts through ORL-1 to facilitate sexual receptivity, rats were treated with 2 μg EB followed by oil 26 hr later. These animals received 2 sequential third ventricular (3V) infusions starting 29 hr 20 minutes after EB. The first 3V infusion was either UFP-101 or saline followed 10 minutes later by either saline or OFQ/N. In Exp IIB, to determine whether ORL-1 activation was necessary for estradiol + progesterone facilitation of sexual receptivity, 3V cannulated rats were treated with 2 μg EB followed by progesterone 26 hr later (EB + P). These animals received 2 sequential 3V infusions starting 29 hr 20 minutes after EB. The first infusion was either UFP-101 or saline followed 10 minutes later with a saline infusion. In Exp IIC, to determine whether ORL-1 activation was necessary for estradiol-only facilitation of sexual receptivity, rats were made receptive by treating with 5 μg EB. These animals received 2 sequential 3V infusions starting 47 hr 20 minutes after EB. The first infusion was either UFP-101 or saline followed 10 minutes later with a saline infusion saline. In Exp IIA-C sexual receptivity was tested 30 minutes after the second infusion. On the next steroid treatment cycle animals received the same steroid and drug treatments and 30 minutes after the final 3V infusion the animals were anesthetized and brains were processed for MPN MOP immunohistochemistry. The results from the MPN MOP immunofluorescence staining intensity levels were compared with the behavior from the previous week to determine whether blocking progesterone inactivation of MOP is associated with reduced sexual receptivity.
Animals
Adult male and OVX female Long-Evans rats (200–225 g) were purchased from Charles River Laboratory Inc., (Wilmington, MA). The supplier performed the bilateral ovariectomy surgeries. Animals were housed two per cage in a climate and light controlled room (12/12 L/D cycle, lights on 0600 h) with food and water available ad libitum. All procedures were approved by the California State University, Long Beach IACUC Committee.
Guide cannula stereotaxic surgery
Rats were anesthetized using 2–3% isoflurane in oxygen and injected with Carprofen (s.c. 5 mg/kg). Cannulae were implanted using standard stereotaxic procedures and secured to the skull using dental acrylic and stainless steel bone screws (Sinchak et al., 2007).
In Exp I, OVX Long-Evans rats received bilateral cannula (Plastics One Inc., Roanoke, VA) aimed at one of the four locations within the ARH and VMH region (Fig. 1). The implantation sites from ventromedial to dorsal lateral were directed at 1) the ARH (approximate coordinates beginning at the bregma: anterior −1.56 mm, lateral 0.25 mm, ventral −7.9 mm from the dura; tooth bar at −3.3 mm; OFQ/N, n = 5; saline, n = 4 (Paxinos and Watson, 1986; Swanson, 2004); 2) ARH-VMH (approximate coordinates beginning at the bregma: anterior −1.56 mm, lateral 0.5 mm, ventral −7.5 mm from the dura; tooth bar at −3.3 mm; n = 4 per group); 3) VMH (approximate coordinates beginning at the bregma: anterior −1.56 mm, lateral 0.75 mm, ventral −7.0 mm from the dura; tooth bar at −3.3 mm; OFQ/N, n = 6; saline, n = 4); and 4) OUT-VMH (approximate coordinates beginning at the bregma: anterior −1.56 mm, lateral 1 mm, ventral −7 mm from the dura; tooth bar at −3.3 mm; OFQ/N, n = 5; saline, n = 8).
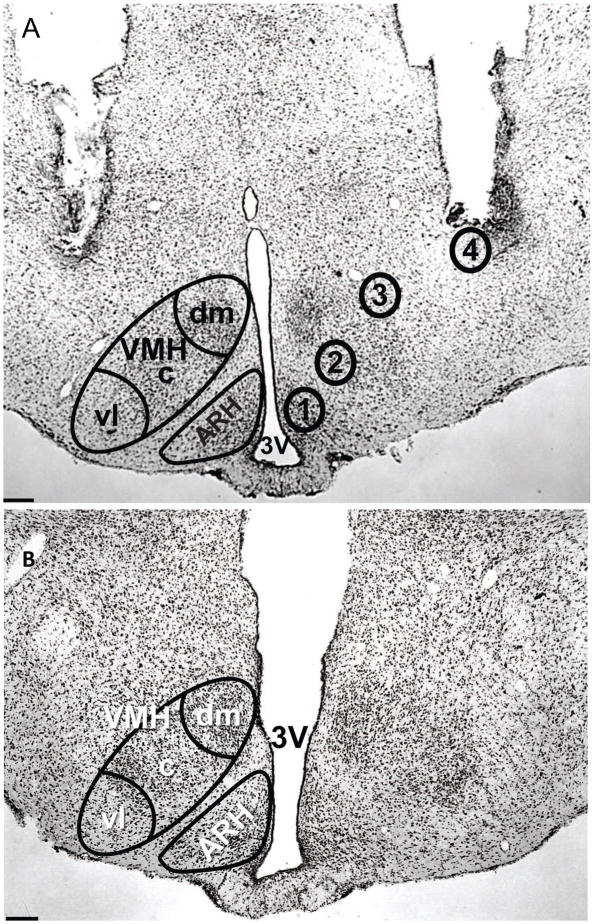
FIGURE 1.
Photomicrographs of representative ARH-VMH and 3V guide cannulae placements in thionin stained sections for Exp I and II. A) Site specific infusions of OFQ/N or saline into the ARH and VMH region. Adult female ovariectomized (OVX) rats were implanted with bilateral guide cannulae to determine whether OFQ/N facilitation of lordosis was through activating both the ARH and VMH behavioral circuits. Four groups of female rats were implanted with bilateral guide cannulae aimed at; 1) the ARH, 2) between the ARH and VMH, 3) the VMH, or 4) dorsolateral to the VMH. Bilateral cannulae allowed for repeated drug infusions into; 1) mainly the ARH; 2) a combination of the ARH and the VMH; 3) mainly the VMH; and 4) outside of both the ARH and the VMH. B) Photomicrograph of a representative 3V guide cannulae placement at the level of the ARH. Adult OVX rats were cannulated in the in the 3V for infusions of drug or saline to examine the role of ORL-1 facilitation of lordosis. 3V = third ventricle; ARH = arcuate nucleus of the hypothalamus; VMH = ventromedial nucleus of the hypothalamus; c = central; dm = dorsomedial; vl = ventrolateral. Scale bar = 100 μm.
In Exp II, OVX Long-Evans rats were implanted with a 21-gauge stainless-steel guide cannula (Plastics One Inc.) directed at the 3V (Figure 1; coordinates from bregma: 4° angle; posterior 2.5 mm, lateral −0.5 mm, ventral −7.3 mm from the dura; tooth bar at −3.3 mm).
Following guide cannula surgery, rats were single housed and given oral antibiotics via drinking water (0.5 mg/ml of sulfamethoxazole and 0.1 mg/ml of trimethoprim; Hi-Tech Pharmacal, Amityvill, NY; Sinchak et al., 2007). Rats were allowed to recover for 1 week after surgery, prior to infusions and behavioral testing.
Steroid and Drug Treatments
All steroids were dissolved in safflower oil. Each steroid dose was delivered subcutaneously in 0.1 ml of oil. Drugs that were infused site specifically or 3V were dissolved in sterile 0.9% saline. Guide cannula drug infusions were performed using a Stoelting microliter syringe pump with a 25 μl Hamilton syringe infusing at the rate of 0.5 μl/minute. The microinjection needles protruded approximately 2 mm beyond the opening of the guide cannula and were left in the guide cannula 1 minute after infusion to allow diffusion of drug from site of infusion (Sinchak et al., 1997).
In Exp I, rats were “cycled” with 2 μg EB once every four days. This treatment primes facilitative neurocircuits but does not produce sexual receptivity without subsequent progesterone (Sinchak and Micevych, 2001). On the third EB cycle after cannula surgery, rats were bilaterally infused with either OFQ/N (25 nmol/side) or sterile 0.9% saline (saline) 29.5 hr after EB (Sinchak et al., 1997). Animals were returned to the home cage and then 30 minutes after infusion animals were placed into the behavioral arena and tested for sexual receptivity. On the following EB cycle animals received the same drug treatments 29.5 hr after EB and 30 minutes later were transcardially perfused with chilled saline followed by 4% paraformaldehyde in Sorenson’s buffer.
In Exp IIA and IIB, steroid priming of the rats was “cycled” once every 4 days as in Exp I. In Exp IIA, OVX rats (n = 7 per group) were treated with 2 μg EB and 26 hr later oil vehicle (2 μg EB). After the third steroid cycle, each animal received 2 sequential 3V drug infusions starting 29 hours 20 minutes after EB treatment. The first was either UFP-101 (50 nmol), an ORL-1 antagonist, or saline. The second drug infusion was given 10 minutes after the first and consisted of either OFQ/N (50 nmol) or saline. In Exp IIB, OVX rats (n = 5 per group) were primed with 2 μg EB and 26 hr later with 500 μg of progesterone (EB + P) to induce maximal sexual receptivity. After the third steroid cycle, each animal received 2 sequential 3V drug infusions starting 29 hours 20 minutes after EB treatment. The first drug infusion was either UFP-101 (50 nmol) or saline. The second drug infusion was saline given 10 minutes after the first. In Exp IIC, OVX rats were made sexually receptive by treating with 5 μg EB (EB-only) once a week. After the third steroid cycle, each animal received 2 sequential 3V drug infusions beginning 47 hours 20 minutes after EB. The first infusion was either UFP-101 (50 nmol; n = 6) or saline vehicle (n = 9) and 10 minutes later all received saline infusions. All animals in Exp IIA-C were tested for sexual receptivity 30 minutes after the second 3V infusion. On the next steroid cycle, all animals in Exp II received the same hormone and drug treatment and 30 minutes after the second 3V infusion were deeply anesthetized with isoflurane and transcardially perfused with chilled 0.9% saline followed by 4% paraformaldehyde in Sorenson’s buffer in preparation for MOP immunocytochemistry and confirmation of cannula placement.
Sexual receptivity behavioral tests
The female rats were tested for sexual receptive behavior following drug infusions by placing a single female in a behavioral arena with a stimulus male that was sexually experienced. Sexual receptivity was measured by lordosis quotient (LQ) that was determined by dividing the number of times the female displayed lordosis by the number of vigorous mounts by the male rat (10) multiplied by 100 (Sinchak et al., 1997).
Tissue collection and guide cannula placement confirmation
Following perfusion brains were excised from the skull and postfixed overnight in 4% paraformaldehyde and then transferred into 20% sucrose in a 0.1 M phosphate buffer solution (pH 7.5) for cryoprotection and then stored at 4°C until sectioning (Sinchak and Micevych, 2001). Brains were blocked and mounted on the cryostat stage and rapidly frozen by covering in crushed dry ice. Brains were scored with a razor blade on the left cortex to match the cannula placement with MPN MOP immunoreactivity. Tissue was coronally sectioned at 20 μm thickness on a cryostat and collected into wells containing phosphate buffered saline (PBS; pH 7.5). Sections were collected from the level of the anteroventral periventricular nucleus through the mammilary bodies.
To confirm the placement of the guide cannulae after the experiments, every fourth brain section was mounted onto a Superfrost Plus slide, air dried on a 37° C slide warmer, stained with thionin, dehydrated in an alcohol series followed by xylene, and coverslipped with Permount mounting medium (Sinchak et al., 2006b). The injection sites were verified in both experiments using a bright-field microscope to visualize the thionin staining (Figure 1; Sinchak and Micevych, 2001).
MOP immunohistochemistry
To determine the effect of 3V OFQ/N infusions on MPN MOP activation/internalization, MOP were visualized using immunohistochemical techniques on free-floating sections collected through the MPN as described previously (Dewing et al., 2007; Eckersell et al., 1998; Mills et al., 2004; Sinchak and Micevych, 2001). Fluorescein (FITC) staining of MOP antibody signal was amplified using a Tyramide Signal Amplification kit (TSA kit; Perkin Elmer/Life Science Products, Boston, MA). Sections were washed in PBS, removed of endogenous peroxidases and incubated for 2 days at 4°C in MOP primary antibody raised in rabbit (1:5000; Neuromics Antibodies Edina, MN; Dewing et al., 2007; Mills et al., 2004). Sections were washed in Tris-buffered saline (TBS; pH 7.5) and incubated in blocking buffer from the TSA kit. Sections were washed in TBS and incubated with biotin-conjugated goat anti-rabbit IgG (1:200 Vector Laboratories, Burlingame, CA) in TBS, 1% normal goat serum, and 0.17% Triton X-100 for 60 minutes. Following a wash in TBS, sections were incubated in TSA blocking buffer and 1:100 dilution of streptavidin-horseradish peroxidase (Perkin Elmer/Life Sciences Inc., Boston, MA). TBS washed sections were then incubated in Amplification Diluent and FITC conjugated tyramide (1:50) for 4 minutes. The reaction was stopped and excess reagent removed by washing sections in TBS. Sections were then placed in 0.1 M Tris buffer (pH 7.5) and mounted on Fisher Scientific Superfrost Plus slides. Slide mounted sections were dried on a 37°C slide warmer for 15 minutes and immediately coverslipped using Aqua-Poly/Mount anti-fade mounting medium (Polysciences, Inc., Warrington, PA).
MOP image analysis
Relative internalization (activation) of the MPN MOP was estimated in the dorsal region of the MPN by measuring the area of positive MOP immunofluorescence staining in epifluorescent photomicrographs (Dewing et al., 2007). Photographs were captured in grayscale with a Leica DFC360 monochrome fluorescence camera mounted on a 6000B Leica Microscope with Leica software. FITC label was detected using BP 485/20 excitation filter and BP 537/45 suppression filter. Brightness and contrast were adjusted for in Adobe PhotoShop (version 7.0; Adobe Systems Inc., San Jose, CA) and then imported into ImageJ software (version 1.32j; National Institutes of Health, Bethesda, MD).
In ImageJ, the “pixel inverter” function was selected and calibrated. To measure the area of MOP immunofluorescence staining within each photomicrograph, a 60 μm diameter circle was positioned on the dorsal MPN and positive MOP immunofluorescence staining measured. Within the same section an area of background staining was measured. The intensity level of MOP immunofluorescence staining was calculated in arbitrary units (AU) by subtracting the background staining from the positive MPN MOP immunoreactive staining. MOP activation/internalization appears as distinct cytoplasmic MOP immunoreactive puncta of the neuron and processes (Eckersell et al., 1998; Mills et al., 2004; Sinchak and Micevych, 2001). The level of internalized/activated MOP immunostaining has been positively associated with increased MPN MOP immunoreactivity fiber density (Dewing et al., 2007; Dewing et al., 2008; Eckersell et al., 1998; Mills et al., 2004; Sinchak and Micevych, 2001).
In Exp I, the effects of drug treatment on intensity levels of MOP immunofluorescence staining in the animals with different cannula placements was compared between the side with a more ventral medial cannula site versus the more dorsal lateral site by t-test, where P < 0.05 was considered significant (SigmaStat 3.5; Systat Software Inc.). Although MOP intensity did not differ between the sides, only the more ventral medial site side was used in the MOP analysis. The main effects and interactions of drug treatments and infusion site on LQ and MPN MOP intensity were analyzed by two-way ANOVA, followed by Holm-Sidak post-hoc analysis with a significance threshold of P < 0.05 (SigmaStat 3.5, Systat Software Inc.).
In Exp IIA, main effects of drug treatments on LQ and MPN MOP immunofluorescence staining intensity levels were analyzed by one-way ANOVA and Holm-Sidak post hoc with a significance threshold of P < 0.05 (SigmaStat 3.5, Systat Software Inc.). In Exp IIB and IIC LQ and MPN MOP intensities were analyzed by Student’s t-test (Sinchak and Micevych, 2001).
RESULTS
Experiment I
To test the hypothesis that OFQ/N acts in both the ARH and VMH to facilitate lordosis bilateral guide cannulae were aimed at 1) ARH; 2) ARH-VMH; 3) VMH; and 4) OUT-VMH (outside both the ARH and VMH) and OFQ/N or saline was infused into estradiol-primed nonreceptive animals, as illustrated in Figure 1. There were main effects of site of infusion (ANOVA, df = 3,39; F = 3.716, P = 0.021), drug treatment (ANOVA, df = 1,39; F = 31.744, P < 0.001) and an interaction (ANOVA, df = 3,39; F = 3.219, P = 0.036) on sexual receptivity (LQ). OFQ/N infusions into either ARH, ARH-VMH or VMH regions increased LQ compared to saline controls within infusion site (Holm-Sidak P < 0.05; Figure 2A). In contrast, OFQ/N infusions into the OUT-VMH region did not facilitate sexual receptivity compared to saline controls (Holm-Sidak P > 0.05; Figure 2A). The LQ did not differ among rats that received infusions of OFQ/N into the ARH, ARH-VMH and VMH regions (Holm-Sidak P > 0.05; Figure 2A), but were all greater than VMH-OUT (Holm-Sidak P < 0.05; Figure 3A). LQ in saline infused animals did not differ among the four brain regions (Holm-Sidak P > 0.05; Figure 2A). Similar to the behavior, there was a main effect of site of infusion (ANOVA df = 3,39; F = 7.996; P < 0.001), drug treatment (ANOVA df = 1,39; F = 25.998; P < 0.05) and an interaction (ANOVA df = 3,39; F = 3.161; P = 0.038) on the MPN MOP immunofluorescence staining intensity levels. OFQ/N infusions reduced MPN MOP immunofluorescence staining intensity levels in the ARH, ARH-VMH, and VMH compared to within group saline controls (Holm-Sidak P < 0.01; Figure 2B & C) but not in the OUT-VMH group (Holm-Sidak P > 0.05; Figure 3B). MOP immunofluorescence staining intensity levels did not differ among the ARH, ARH-VMH, and VMH sites (Holm-Sidak P > 0.05; Figure 2B & C), but all were significantly reduced compared to VMH-OUT (Holm-Sidak P < 0.05; Figure 2B & C). Infusion of OFQ/N into OUT-VMH neither increased LQ nor altered MPN MOP immunofluorescence staining intensity levels compared to saline treated control animals (Holm-Sidak P > 0.05; Figure 2B & C). Regardless of infusion region, OFQ/N infusions that facilitated lordosis was correlated with a reduction in MPN MOP activation (Pearson’s Correlation n = 40, cc = −0.595; P < 0.001).
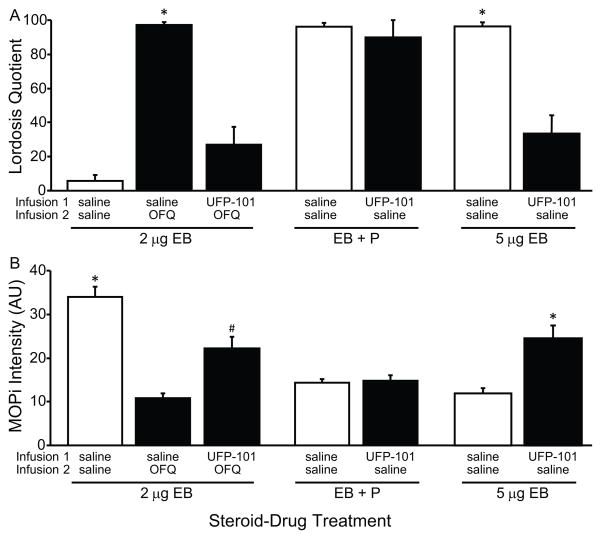
FIGURE 3.
Role of ORL-1 activation in OFQ/N, estradiol-only and estradiol + progesterone facilitation of A) sexual receptivity and regulation of B) MPN MOP activation. Rats were steroid primed with 2 μg EB and 26 hr later received either oil (2 μg EB) or 500 μg progesterone (EB + P), and a third set of rats received 5 μg EB (5 μg EB). All rats received two sequential 3V infusions at the level of the ARH. The first was 40 minutes prior to behavioral testing and the second 10 minutes later. The 2 μg EB group of animals first received either saline or 50 nmol UFP-101 and then either saline or 50 nmol OFQ/N. EB + P animals received first either saline or 50 nmol UFP-101 and then saline. A) The 2 μg EB and EB + P groups were tested for sexual receptivity as measured by lordosis quotient 30 hr after EB priming. The 5 μg EB animals were first infused with either saline or 50 nmol UFP-101 and then saline. They were tested for sexual receptivity 48 hours after EB. B) Using the same steroid and drug treatments on the next steroid priming cycle, brains were processed MOP immunohistochemistry to measure MPN MOP activation by immunofluorescence staining intensity level in arbitrary units (AU). * = significantly greater than other groups within steroid treatment group, P < 0.05; # = significantly greater than saline-OFQ drug group within steroid treatment, P < 0.05.
Experiment II
In general, an increase in LQ was correlated with decreased MPN MOP activation/internalization as measured by MOP immunofluorescence staining intensity levels. In Exp IIA, OFQ/N significantly increased LQ in EB + oil primed rats compared to saline controls (Figure 3A; ANOVA df = 2,20; F = 56.966, P = 0.002: Tukey Test, P < 0.001). OFQ/N infusions that increased LQ in EB-primed rats significantly decreased MPN MOP staining intensity compared to the saline infused EB-primed nonreceptive females (Figure 3B; ANOVA df = 2,20; F = 29.958, P < 0.001; Holm-Sidak: P < 0.05). Pretreatment with the ORL-1 selective antagonist, UFP-101, blocked OFQ/N facilitation of lordosis and blocked OFQ/N reduction of MPN MOP activation compared to saline-OFQ/N (Holm-Sidak: P < 0.05) indicating that OFQ/N facilitation is acting through ORL-1 to inhibit ARH β-END release in the MPN. The increased LQ was correlated with a decrease in MPN MOP intensity (Pearson’s Correlation n=21, cc = -0.898; P < 0.001).
In Exp IIB, EB + P primed animals that received saline infusions displayed maximal sexual receptivity and low levels of MPN MOP immunofluorescence staining intensity levels. Antagonism of ORL-1 with UFP-101 3V infusions neither reduced sexual receptivity (t - test, t = 0.478, df = 8, P = 0.654; Figure 3A) nor increased MPN MOP immunofluorescence staining intensity levels in comparison to control (t - test, t = 0.369, df = 8, P = 0.972; Figure 3B).
In contrast, in Exp IIC, where females were made sexually receptive with EB-only, antagonism of ORL-1 significantly decreased LQ (t-test, df = 13, t = 7.348, P < 0.001) and increased MPN MOP immunofluorescence staining intensity levels (t-test, df = 14, t = 7.372, P < 0.001) compared to saline infused EB-only controls (Figure 3B). The increased LQ was correlated to the decrease in MPN MOP intensity (Pearson’s Correlation n = 15, cc = −0.872; P < 0.001).
DISCUSSION
The ARH and VMH are important nuclei in the steroid regulation of sexual receptivity (Dewing et al., 2007; Dewing et al., 2008; Eckersell et al., 1998; Mills et al., 2004; Pfaff and Sakuma, 1979a, b; Sinchak and Micevych, 2001). Previous studies indicated that OFQ/N may act in both nuclei to facilitate lordosis (Sinchak et al., 2006a; Sinchak et al., 1997). These experiments were designed to determine the roles of the opioid-peptide OFQ/N in the ARH and the VMH to facilitate lordosis and what role its receptor, ORL-1, plays in the estradiol-only and estradiol + progesterone facilitation of lordosis. The results from Exp I demonstrate that in the region of the VMH/ARH, OFQ/N infusions that facilitate lordosis reduced the activity of the ARH-MPN β-END pathway as measured by MPN MOP immunoreactive staining intensity. These data support the idea that OFQ/N may act directly on β-END-MPN projecting neurons (Mills et al., 2004) or indirectly regulate ARH-MPN projections through VMH-ARH microcircuits that regulate POMC neuronal output (Sternson et al., 2005). Although it is likely that OFQ/N is acting directly through ORL-1 on the descending motor pathway from the VMH because muscle tone was reduced, the exact level of involvement remains unclear since the deactivation of MPN MOP occurred whenever lordosis was facilitated. Results from Exp II indicate that in the VMH/ARH region ORL-1 activation is necessary for estradiol-only facilitation of lordosis, but not for estradiol + progesterone facilitation of lordosis. Based on these results, estradiol-only and estradiol + progesterone signal through different neurotransmitter systems that converge on the ARH-MPN β-END neurocircuits to reduce MPN MOP activity for facilitation of lordosis.
We wanted to determine the relative importance of OFQ/N-ORL-1 opioid peptide system in the facilitation of lordosis in the ARH and the VMH. Since preliminary studies showed that OFQ/N infusions into the VMH/ARH deactivated MPN MOP (Sinchak et al., 2006a), in Exp I we hypothesized that OFQ/N facilitation of lordosis was due to a combination of 1) deactivating MPN MOP neurons through OFQ/N inhibition of ascending ARH β-END neurons either directly (Mills et al., 2004; Sanathara et al., 2009) and/or indirectly via VMH-ARH microcircuits (Sternson et al., 2005) and 2) the simultaneous activation of descending lordosis motor output pathways originating in the VMH (Calizo and Flanagan-Cato, 2002, 2003; Pfeifle et al., 1980; Sinchak et al., 1997). Thus, expectations were that facilitation of lordosis would be greatest and MPN MOP activation lowest when OFQ/N acted in ARH and VMH simultaneously. Additionally, as the infusion sites moved dorsolaterally away from the ARH, the expectations were that the VMH descending motor pathway would be mainly activated, and the deactivation of MPN MOP would be reduced producing moderate levels of sexual receptivity. As expected, OFQ/N infusion into the ARH and the ARH-VMH region facilitated lordosis and decreased MPN MOP activation. However, OFQ/N infusions that were aimed at the dorsolateral region of the VMH, facilitated lordosis and deactivated MPN MOP to the same degree as infusions into the ARH, and ARH-VMH. OFQ/N infusions in OUT-VMH neither induced lordosis nor decreased MPN MOP activation indicating that OFQ/N did not diffuse into the ARH or VMH regions. These results indicate that OFQ/N facilitation of lordosis requires the deactivation of MPN MOP via ARH β-END neurons that project to the MPN and support the idea that OFQ/N infusions can act in the ARH directly and/or through VMH-ARH microcircuits to inhibit β-END release in the MPN (Mills et al., 2004; Sanathara et al., 2009; Sternson et al., 2005). The infusions of OFQ/N aimed at the lateral side of the VMH (VMH) were not expected to diffuse into the ARH and directly inhibit β-END release (Myers, 1966) and therefore be a measure of OFQ/N actions mainly in the VMH. If little or no diffusion of OFQ/N into the ARH occurred that would indicate that mechanism(s) exist for OFQ/N released in the VMH to inhibit ARH β-END neurons. One possible mechanism is β-END neurons may have dendritic projections extending dorsolateral beyond the boundaries of the ARH into portions of the central VMH (VMHc) and ventrolateral VMH (VMHvl) that may be regulated by steroid exposure (Millhouse, 1973a, b; van den Pol and Cassidy, 1982). This possibility is supported by estradiol priming increasing the area of ORL-1 immunoreactivity in the VMH (Sinchak et al., 2007) and the ability of steroid priming to rapidly alter dendritic length in the VMH (Flanagan-Cato et al., 2006; Griffin et al., 2010; Griffin and Flanagan-Cato, 2008, 2009, 2011). The second viable mechanism for OFQ/N acting in the VMH to regulate ARH POMC neurons is through VMH-ARH microcircuits (Sternson et al., 2005). Using laser scanning photostimulation, Sternson et. al., (2005) demonstrated that the VMH has direct excitatory inputs onto ARH POMC neurons and Neuropeptide Y neurons as well as other excitatory inputs onto unknown ARH neurons. Since activation of ORL-1 induces inhibitory K+ currents, OFQ/N in the VMH may reduce β-END release by inhibiting these VMH-ARH excitatory inputs to POMC neurons. Fasting alters the strength of these microcircuits, therefore it is possible that neurotransmission within these microcircuits may be also regulated by steroid levels across the estrous cycle (Sternson et al., 2005). These data support the possibility of an indirect OFQ/N responsive multisynaptic pathway originating in the VMH that ultimately converges on and regulates ARH β-END neurons that project to the MPN.
Although MPN MOP activity must be reduced for facilitation of lordosis in estradiol + progesterone primed female rats (Sinchak and Micevych, 2001), we were somewhat surprised by the complete inverse correlation of MPN MOP activity and sexual receptivity. We expected to dissect out some of the actions of OFQ/N on the motor output pathways from the VMH. The OFQ/N infusions into the ARH, ARH-VMH and VMH most likely stimulated ORL-1 located in the VMHvl; a region associated with lordosis facilitative descending circuits to the PAG (Calizo and Flanagan-Cato, 2002, 2003; Cottingham et al., 1987; Pfeifle et al., 1980; Sinchak et al., 1997), since some muscle tone was lost as seen previously (Sinchak et al., 2007; Sinchak et al., 1997). The OFQ/N infusions into the OUT-VMH region did not diffuse into the delineated boundaries of the ARH (Paxinos and Watson, 1986; Swanson, 2004) or regions that may contain potential ORL-1 pathways that regulate the ARH, since MPN MOP activation was not affected. If the area of OFQ/N diffusion was larger than expected OFQ/N would have diffused into regions of the VMH that contain ARH projections and/or into the VMHvl and acted on motor output pathways. The results from Exp I indicate that OFQ/N may facilitate lordosis through inhibition of ARH MPN projecting β-END neurons directly via ORL-1 and indirectly by inhibition of VMH excitatory inputs to ARH POMC neurons. This deactivation of MPN MOP appears to be important for OFQ/N to act on descending lordosis motor output pathways of the VMH to facilitate lordosis, since activation of MPN MOP can rapidly and completely inhibit lordosis in estradiol + progesterone treated rats (Sinchak and Micevych, 2001).
To investigate whether steroid priming signals through the OFQ/N-ORL-1 system to mediate sexual receptivity, the selective ORL-1 antagonist UFP-101 was used to inhibit sexual receptivity. First, we demonstrated that 3V infusions of OFQ/N facilitated lordosis and reduced MPN MOP activation in estradiol-primed females indicating OFQ/N is acting through similar pathways as observed in Exp I. Pretreatment with UFP-101 blocked OFQ/N facilitation of lordosis and blocked OFQ/N deactivation of MPN MOP. These results demonstrate that exogenous OFQ/N acts through ORL-1 to inhibit release of β-END in the MPN and facilitate lordosis.
Since OFQ/N facilitates lordosis in estradiol-primed nonreceptive rats in a similar manner to steroid priming by deactivation of MPN MOP, we hypothesized that activation of the OFQ/N-ORL-1 system is required for steroid facilitation of lordosis. UFP-101 3V infusions were expected to block estradiol-only and estradiol + progesterone facilitation of lordosis and prevent deactivation of MPN MOP as seen with the OFQ/N infusions. In OVX rats that were made sexually receptive by treating with 5 μg EB and testing 48 hr later, 3V infusions of UFP-101 inhibited sexual receptivity and blocked the deactivation of MPN MOP, as expected. Previously, we showed that immunoneutralization of OFQ/N in the VMH/ARH region was capable of blocking estradiol-only facilitation of lordosis (Sinchak et al., 2007). However, MPN MOP activity was not measured in that study, and OFQ/N was thought to be acting on VMH descending motor output pathways. The present results indicate that estradiol-only facilitation of lordosis requires the release of OFQ/N and activation of ORL-1 in the ARH/VMH region to inhibit the release of β-END in the MPN to facilitate lordosis.
In contrast, UFP-101 3V infusions in estradiol + progesterone primed animals neither reduced sexual receptivity nor blocked progesterone deactivation of MPN MOP. Likewise, immunoneutralization of OFQ/N in the VMH/ARH region did not block progesterone facilitation of lordosis either (Sinchak et al., 2007). These results suggest the possibility that estradiol + progesterone induces the release of another neurotransmitter that acts like OFQ/N to inhibit directly the activity of the ARH β-END neurons that project to the MPN to facilitate lordosis. One such mechanism may be through the release of γ-aminobutyric acid (GABA) that primarily serves to inhibit neuronal activity. Like OFQ/N, GABA induces inhibitory K+ currents in ARH POMC neurons that are modulated by estradiol (Nguyen and Wagner, 2006). Further, 3V bicuculline treatments that block GABAA receptors inhibited lordosis (Luine et al., 1999), and progesterone increases GABA turnover in the VMH region indicating progesterone may enhance GABA activity (Luine et al., 1997; McCarthy et al., 1990). The estradiol-only and estradiol + progesterone facilitation of sexual receptivity using different neurotransmitter systems may reflect multiple reproductive mechanisms that are present but used at different reproductive stages in the rat to coordinate behavior with the luteinizing hormone surge and ovulation. The young intact rat is a spontaneous ovulator, and her neural circuits are exposed to sequential estradiol and progesterone. The estradiol + progesterone induced release of another neurotransmitter may be part of a mechanism that initiates the termination of sexual receptivity after spontaneous ovulation to prevent her from seeking copulation after the window of opportunity that would result in pregnancy (Georgescu et al., 2009; Pfaus et al., 1999). Thus, progesterone released from the ovary signals ovulation, augments sexual receptivity and initiates termination of sexual receptivity through release of a specific neurotransmitter(s). Also present in the young female are behavioral circuits that respond to prolonged high doses of estradiol that induce a later onset and longer window of sexual receptivity compared to estradiol + progesterone (reviewed in Clemens and Weaver, 1985). In the intact rat, sexual receptivity induced by prolonged, high circulating levels of estradiol is associated with the onset of menopause (Lu et al., 1979). At this reproductive stage the female rat appears to be a reflex ovulator and responds to male sexual stimulation with a luteinizing hormone surge and ovulation (Day et al., 1988), and it is to her advantage to delay ovulation and prolong sexual receptivity until she finds a suitable mate. These actions of estradiol on behavior during these reproductive stages appear to coordinate the induction of the luteinizing hormone surge and ovulation through regulating neuroprogesterone synthesis (Micevych et al., 2008).
However, the priming and activational effects that steroid hormones have on the OFQ/N-ORL-1 system in the VMH/ARH may also account for the observation that neither UFP-101 antagonism of ORL-1 nor OFQ/N immunoneutralization blocked progesterone facilitation of lordosis and deactivation of MPN MOP. Estradiol upregulates the mRNA expression of OFQ/N and ORL-1, as well as increases the functional coupling of ORL-1 to its G-protein, in regions of the hypothalamus that control lordosis (Quesada and Micevych, 2008; Sinchak et al., 2006b). Further, estradiol also increases the number of neurons that express OFQ/N in the ARH as well as its colocalization with progesterone receptors in ARH neurons suggesting that progesterone may induce the release of the increased stores of OFQ/N (Haase et al., 2008). In addition, estradiol increases the ORL-1 mRNA expression in POMC neurons that project to the MPN (Sanathara et al., 2009). Thus, following estradiol priming, ORL-1 are more abundant and more efficient in the VMH/ARH region. Subsequently, progesterone may augment OFQ/N release in the VMH/ARH region that may increase the area and amount of OFQ/N in the region. This suggests that UFP-101 administration and OFQ/N immunoneutralization may not have been able to block the activation of ORL-1 because of increased ORL-1 expression and efficiency as well as OFQ/N release throughout the VMH/ARH region (Sinchak et al., 2007). The results from Exp I indicate that the area of OFQ/N influence includes the ARH and VMH to facilitate lordosis but still signals through the ARH, since VMH OFQ/N infusions were as effective as ARH infusions in facilitating lordosis and deactivating MPN MOP.
Ovarian steroids induce rapid dendritic remodeling of the processes that extend dorsolateral into areas that surround the ARH as seen in the ARH/VMH region (reviewed in Garcia-Segura et al., 1986; Griffin and Flanagan-Cato, 2011; Perez et al., 1993; Rethelyi, 1985; van den Pol and Cassidy, 1982). Thus, estradiol + progesterone may induce synaptic remodeling of the VMH to ARH microcircuits to reduce excitatory inputs onto POMC neurons to decrease MPN MOP activation and facilitate lordosis (Griffin and Flanagan-Cato, 2011; Rethelyi, 1985; Sternson et al., 2005; van den Pol and Cassidy, 1982). This may cause the larger area of the VMH to be responsive to OFQ/N and reduce the effectiveness of 3V UFP-101 infusions if they do not diffuse into these regions of the VMH. However, we do not think that this is the case. We used a high dose of inhibitor compared to other studies (Duzzioni et al., 2011; Goeldner et al., 2010), which was enough to inhibit lordosis and block MPN MOP deactivation in the estradiol-only animals, and we observed similar effects with OFQ/N immunoneutralization bilateral infusions into the VMH/ARH region (Sinchak et al., 2007). Even with greater OFQ release and ORL-1 responsiveness in the VMH/ARH region, we would expect some antagonism of ORL-1 to induce a moderate inhibition of lordosis and partial attenuation of MPN MOP deactivation, but observed none. In addition, the total lack of effect by UFP-101 and OFQ/N immunoneutralization on lordosis and MPN MOP activation in the estradiol + progesterone primed animals compared to the estradiol-only animals strongly suggests that estradiol + progesterone signals through a neurotransmitter system other than ORL-1 that converges on the β-END ARH neurons that project to the MPN to facilitate lordosis. In general these data also indicate that some actions of hormones, neurotransmitters and neuropeptides that regulate other behavioral and physiological functions that have been ascribed to the VMH may have to be reevaluated as to their potential for acting through ARH pathways either directly or through VMH-ARH circuits.
CONCLUSIONS
Estradiol-only and estradiol + progesterone priming initially inhibits lordosis by activating ARH β-END neurons that project to MPN. Lordosis is then facilitated when MPN MOP are deactivated by either extended exposure to estradiol or subsequent progesterone. OFQ/N acts through ORL-1 in the ARH and/or VMH to facilitate lordosis in estradiol-primed female rats by inhibiting ARH β-END neurons to deactivate MPN MOP. Estradiol-only facilitation of lordosis requires endogenous OFQ/N activation of ORL-1 to deactivate MPN MOP. In contrast, estradiol + progesterone appears not to require ORL-1 activation to facilitate lordosis and deactivate MPN MOP. Although both steroid treatments facilitate lordosis by inhibition of ARH β-END neurons to deactivate MPN MOP, it appears that different neurotransmitter systems are used to directly inhibit ARH neurons.
Highlights.
lordosis requires deactivation of medial preoptic nucleus μ-opioid receptors (MOP)
Estradiol-only (E) acts through orphanin FQ activation of ORL-1 to deactivate MOP
Estradiol + progesterone (E + P) MOP deactivation does not require orphanin FQ
E and E + P signaling converges on arcuate nucleus β-endorphin neurons
E and E + P use different neurotransmitters to inhibit MOP and facilitate lordosis
Acknowledgments
This research was supported by NIH Award Number RO1HD058638, CSUPERB Grant, CSULB SCAC, CSULB Start-up. We thank Robyn Bowlby, Pamela Charukulvanich, Kirstin Fuentes, Bertha Garcia, Asma Mana, Kathy Noboru, Jessica Phillips, and Martin Vignovich for technical assistance, and Dr. Paul Popper for his insightful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blaustein JD, Finkbohner R, Delville Y. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinology. 1987;45:152–159. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Hormonal-neural integration in the female rat ventromedial hypothalamus: triple labeling for estrogen receptor-alpha, retrograde tract tracing from the periaqueductal gray, and mating-induced Fos expression. Endocrinology. 2003;144:5430–5440. doi: 10.1210/en.2003-0331. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Weaver DR. The role of gonadal hormone in the activation of feminine sexual behavior. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York: 1985. pp. 183–227. [Google Scholar]
- Cottingham SL, Femano PA, Pfaff DW. Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp Neurol. 1987;97:704–724. doi: 10.1016/0014-4886(87)90127-0. [DOI] [PubMed] [Google Scholar]
- Day JR, Morales TH, Lu JK. Male stimulation of luteinizing hormone surge, progesterone secretion and ovulation in spontaneously persistent-estrous, aging rats. Biol Reprod. 1988;38:1019–1026. doi: 10.1095/biolreprod38.5.1019. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzzioni M, Duarte FS, Leme LR, Gavioli EC, De Lima TC. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behavioural brain research. 2011;222:206–211. doi: 10.1016/j.bbr.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59:190–200. doi: 10.1016/j.neuropharm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Calizo LH, Griffin GD, Lee BJ, Whisner SY. Sexual behaviour induces the expression of activity-regulated cytoskeletal protein and modifies neuronal morphology in the female rat ventromedial hypothalamus. Journal of neuroendocrinology. 2006;18:857–864. doi: 10.1111/j.1365-2826.2006.01483.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Baetens D, Naftolin F. Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain research. 1986;366:131–136. doi: 10.1016/0006-8993(86)91287-4. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Sabongui C, Del Corpo A, Marsan L, Pfaus JG. Vaginocervical stimulation induces Fos in glutamate neurons in the ventromedial hypothalamus: attenuation by estrogen and progesterone. Hormones and behavior. 2009;56:450–456. doi: 10.1016/j.yhbeh.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Kieffer BL, Ouagazzal AM. Endogenous nociceptin/orphanin-FQ in the dorsal hippocampus facilitates despair-related behavior. Hippocampus. 2010;20:911–916. doi: 10.1002/hipo.20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Ferri-Kolwicz SL, Reyes BA, Van Bockstaele EJ, Flanagan-Cato LM. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. The Journal of comparative neurology. 2010;518:4531–4545. doi: 10.1002/cne.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Flanagan-Cato LM. Estradiol and progesterone differentially regulate the dendritic arbor of neurons in the hypothalamic ventromedial nucleus of the female rat (Rattus norvegicus) The Journal of comparative neurology. 2008;510:631–640. doi: 10.1002/cne.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Flanagan-Cato LM. Sex differences in the dendritic arbor of hypothalamic ventromedial nucleus neurons. Physiology & behavior. 2009;97:151–156. doi: 10.1016/j.physbeh.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin GD, Flanagan-Cato LM. Ovarian hormone action in the hypothalamic ventromedial nucleus: remodelling to regulate reproduction. Journal of neuroendocrinology. 2011 doi: 10.1111/j.1365-2826.2011.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J, Mana A, Chinn E, Sinchak K. Abstract Viewer/Itinerary Planner. Vol. 2008. Washington, DC: Society for Neuroscience; Washington, DC: 2008. Estradiol upregulates expression and colocalization progesterone receptor and orphanin FQ/nociceptin immunopositive neurons in the arcuate nucleus of the hypothalamus, Society for Neuroscience; pp. 781–789. [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. BiolReprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Luine VN, Grattan DR, Selmanoff M. Gonadal hormones alter hypothalamic GABA and glutamate levels. Brain research. 1997;747:165–168. doi: 10.1016/s0006-8993(96)01255-3. [DOI] [PubMed] [Google Scholar]
- Luine VN, Wu V, Hoffman CS, Renner KJ. GABAergic regulation of lordosis: influence of gonadal hormones on turnover of GABA and interaction of GABA with 5-HT. Neuroendocrinology. 1999;69:438–445. doi: 10.1159/000054447. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Malik KF, Feder HH. Increased GABAergic transmission in medial hypothalamus facilitates lordosis but has the opposite effect in preoptic area. Brain Res. 1990;507:40–44. doi: 10.1016/0006-8993(90)90519-h. [DOI] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res Rev. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. Certain ventromedial hypothalamic afferents. Brain research. 1973a;55:89–105. doi: 10.1016/0006-8993(73)90490-3. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The organization of the ventromedial hypothalamic nucleus. Brain research. 1973b;55:71–87. [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RD. Injection of solutions into cerebral tissue: Relation between volume and diffusion. Physiology & Behavior. 1966;1:171–174. IN179. [Google Scholar]
- Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid- induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology. 2006;84:123–137. doi: 10.1159/000096996. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; Orlando: 1986. [DOI] [PubMed] [Google Scholar]
- Perez J, Luquin S, Naftolin F, Garcia-Segura LM. The role of estradiol and progesterone in phased synaptic remodelling of the rat arcuate nucleus. Brain research. 1993;608:38–44. doi: 10.1016/0006-8993(93)90771-e. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Nature of sex hormone effects on rat sex behavior: Specificity of effects and individual patterns of response. J Comp Physiol Psychol. 1970;73:349–358. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979a;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979b;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Smith WJ, Coopersmith CB. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. I. A correlational and factor analysis and the effects of ovarian hormones. Horm Behav. 1999;35:224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- Pfeifle JK, Shivers M, Edwards DA. Parasagittal hypothalamic knife cuts and sexual receptivity in the female rat. Physiol Behav. 1980;24:145–150. doi: 10.1016/0031-9384(80)90026-8. [DOI] [PubMed] [Google Scholar]
- Quadagno DM, McCullough J, Langan R. The effect of varying amounts of exogenous estradiol benzoate on estrous behavior in the rat. Horm Behav. 1972;3:175–179. doi: 10.1016/0018-506x(72)90029-3. [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych P. Estrogen and progesterone modulate [35S]GTPgammaS binding to nociceptin receptors. Neuroendocrinology. 2008;88:35–42. doi: 10.1159/000XXXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethelyi M. Dendritic arborization and axon trajectory of neurons in the hypothalamic arcuate nucleus of the rat--updated. Neuroscience. 1985;16:323–331. doi: 10.1016/0306-4522(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Sanathara NM, Vignovich M, Sinchak K. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, Washington, DC; 2009. Proopiomelanocortin neurons in the arcuate nucleus project to the medial preoptic nucleus and express opioid receptor-like receptor-1 mRNA, Society for Neuroscience. [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Nguyen YV, Micevych P. Program No. 258.5. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006a. Orphanin FQ/Nociceptin in the mediobasal hypothalamus reverses the estradiol induced activation of μ-opioid receptors in the medial preoptic nucleus, Society for Neuroscience; p. 2006. Online. [Google Scholar]
- Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport. 1997;8:3857–3860. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006b;496:252–268. doi: 10.1002/cne.20949. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Eneroth P. Serum levels of oestradiol-17 beta and progesterone in relation to receptivity in intact and ovariectomized rats. J Endocrinol. 1981;89:45–54. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. 3. Elsevier; San Diego: 2004. [Google Scholar]
- van den Pol AN, Cassidy JR. The hypothalamic arcuate nucleus of rat--a quantitative Golgi analysis. The Journal of comparative neurology. 1982;204:65–98. doi: 10.1002/cne.902040108. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Keith D, Zaki P, Evans C. Intracellular trafficking of epitope-tagged opioid receptors different effects of morphine and enkephalin. Regul Pept. 1994;54:315–316. [Google Scholar]
- Wagner EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]