Abstract
Food restriction (FR) decreases BDNF expression in hypothalamic and hindbrain regions that regulate feeding and metabolic efficiency, while increasing expression in hippocampal and neocortical regions. Drugs of abuse alter BDNF expression within the mesocorticolimbic dopamine (DA) pathway, and modifications of BDNF expression within this pathway alter drug-directed behavior. Although FR produces a variety of striatal neuroadaptations and potentiates the rewarding effects of abused drugs, the effects of FR on BDNF expression and function within the DA pathway are unknown. The primary purpose of the present study was to examine the effect of FR on protein levels of BDNF and its TrkB receptor in component structures of the mesocorticolimbic pathway. Three to four weeks of FR, with stabilization of rats at 80% of initial body weight, did not alter BDNF or TrkB levels in nucleus accumbens, caudate-putamen, or medial prefrontal cortex. However, FR decreased TrkB levels in the ventral tegmental area (VTA), without change in levels of BDNF protein or mRNA. The finding that FR also decreased TrkB levels in substantia nigra, with elevation of BDNF protein, suggests that decreased TrkB in VTA could be a residual effect of increased BDNF during an earlier phase of FR. Voltage-clamp recordings in VTA DA neurons indicated decreased glutamate receptor transmission. These data might predict lower average firing rates in FR relative to ad libitum fed subjects, which would be consistent with previous evidence of decreased striatal DA transmission and upregulation of postsynaptic DA receptor signaling. However, FR subjects also displayed elevated VTA levels of phospho-ERK1/2, which is an established mediator of synaptic plasticity. Because VTA neurons are heterogeneous with regard to neurochemistry, function and target projections, the relationship(s) between the three changes observed in VTA, and their involvement in the augmented striatal and behavioral responsiveness of FR subjects to drugs of abuse, remains speculative.
Keywords: food restriction, BDNF, TrkB, ventral tegmental area, reward, ERK 1/2
BDNF belongs to the family of neurotrophins that have well established roles in neurodevelopmental processes. There is increasing evidence that BDNF, acting as a classical neurotrophin and/or excitatory transmitter with rapid synaptic effects (Thoenen, 2000; Rose et al., 2004), also modulates an array of behavioral functions in the mature CNS including mood (Eisch et al, 2003; Nestler and Carlezon, 2006), drug addiction (Horger et al, 1999; Pierce and Bari, 2001; Lu et al., 2004; Graham et al, 2007), learning (Rattiner et al., 2004; Rademacher et al., 2006), and ingestive behavior (Lebrun et al., 2006; Wang et al, 2007a,b). Several of the adaptive functional changes associated with food deprivation are mediated or modulated by decreased BDNF activity. For example, fasting is accompanied by decreases in BDNF protein and mRNA levels in hypothalamic and hindbrain nuclei that regulate energy homeostasis (Xu et al., 2003; Bariohay et al., 2005), and microinjection of BDNF into medial hypothalamic nuclei (Wang et al., 2007a,b) or the dorsal vagal complex (Bariohay et al., 2005) decreases food intake. Complementing these observations, mice that are heterozygous for targeted disruption of BDNF exhibit a chronic hyperphagia and obesity (Fox and Byerly, 2004).
Among the several members of the tyrosine receptor kinase family that bind neurotrophins, BDNF binds with high affinity to TrkB (Squinto et al., 1991; Huang and Reichert, 2003); only the full-length isoform (TrkB-FL) has intracellular tyrosine kinase activity. Mutant mice that express very low levels of TrkB-FL throughout the brain, exhibit hyperphagia and obesity (Xu et al., 2003). Moreover, unlike control mice which decrease food intake when switched from a low fat to moderate fat diet, the TrkB “hypomorphs” increase intake. Overall, BDNF and TrkB signaling appear to be important regulators of ingestive behavior and metabolic function.
In contrast to the food deprivation-induced decreases in BDNF observed in brain regions that regulate energy homeostasis, long-term food restriction increases BDNF protein levels in neocortical, hippocampal and dorsal striatal regions (Lee et al., 2000; Duan et al., 2001). These latter responses have been implicated in the neuroprotective effects of food restriction and are proposed to mediate the cognitive-enhancing effects. It is therefore possible that opposite and neuroanatomically distributed changes in BDNF function during periods of negative energy balance modulate different adaptive functional responses.
Chronic food restriction (FR) is associated with increased behavioral sensitivity to drugs of abuse, as documented in self-administration (Carroll and Meisch, 1984), conditioned place preference (e.g., Bell et al., 1997; Stuber et al., 2002; Cabib et al., 2000; Liu et al., 2011), electrical brain stimulation reward (e.g., Cabeza de Vaca and Carr, 1998; Cabeza de Vaca et al., 2004), and motor activity assays (e.g., Deroche et al., 1993; Carr et al., 2003). These phenomena are likely to reflect neuroadaptations that otherwise facilitate foraging, incentive motivation, and reward-related learning during periods of food scarcity. Ventral tegmental area (VTA) dopamine (DA) neurons that innervate nucleus accumbens (NAc) are fundamentally involved in the unconditioned and conditioned rewarding effects of most abused drugs and express high levels of mRNA for BDNF and TrkB (Merlio et al., 1992; Conner et al., 1997; Furukawa et al., 1998), and both protein products coexpress with tyrosine hydroxylase (Seroogy et al., 1994; Hoover et al, 2007). NAc expresses relatively little BDNF mRNA but has high levels of BDNF protein (Conner et al., 1997), most of which is of VTA and medial prefrontal cortical (mPFC) origin (Altar et al., 1997; McGinty et al., 2010). While microinfusions of BDNF in VTA, NAc, and mPFC have been shown to modify behavioral responsiveness to drugs of abuse (Horger et al., 1999; Lu et al, 2004; Graham et al., 2007; Berglind et al., 2007; McGinty et al., 2010), effects of FR on BDNF and TrkB in this system, and the possible modulatory effects of altered BDNF and TrkB function on drug reward under the condition of FR, have not been investigated.
In the light of increasing evidence that changes in BDNF function may mediate a variety of adaptive responses to FR, we introduced subjects to a FR regimen that enhances rewarding (Cabeza de Vaca and Carr, 1998; Cabeza de Vaca et al. 2004), place preference conditioning (Liu et al., 2011) and motor-activating effects of abused drugs (Cabeza de Vaca and Carr, 1998; Carr et al., 2003; Liu et al., 2011), then investigated the effects on BDNF and TrkB in several DA-related brain regions. In an initial experiment, an enzyme immunoassay was used to measure BDNF protein levels in mPFC, NAc, caudate-putamen (CPu), VTA and substantia nigra (SN) of FR and ad libitum fed (AL) rats. In a second experiment, Western immunoblotting was used to measure TrkB protein levels in these same forebrain and midbrain regions. In a third experiment, real-time RT PCR was used to measure BDNF mRNA levels in the VTA. In a fourth experiment, and as follow-up to an observation of decreased TrkB protein in the VTA of FR rats, levels of activated signaling molecules downstream of TrkB were measured. Binding of BDNF to TrkB activates several intracellular signaling pathways, with the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt pathways clearly implicated in neurotrophic effects (Numakawa et al., 2010). In a fifth experiment, voltage-clamp recording was used to measure evoked excitatory postsynaptic currents (EPSCs) in VTA neurons in midbrain slices obtained from AL and FR rats.
EXPERIMENTAL PROCEDURES
Subjects and food restriction
All subjects were mature male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 350–400 g at the start of the experiment. Rats were housed, under a 12 h light:dark photoperiod with lights on at 0700 h, in a central animal facility in individual plastic cages with bedding and free access to water and standard lab pellets (Laboratory Rodent Diet #5001, Lab Diet) except when restricted feeding conditions applied (see below). Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York University School of Medicine and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. All efforts were made to minimize the number of animals used and their suffering.
Several days after their arrival in the central animal facility, half of the rats in Experiments 1–4 were switched to a restricted feeding regimen whereby a single 10 g meal of Purina rat chow was delivered at approximately 1700 h each day. These rats continued to have ad libitum access to water. Once body weight had declined by 20% (approximately 15 days) daily food allotments were titrated to maintain body weight at this value for an additional 7–14 days prior to sacrifice. This feeding regimen and time of analysis were the same as used in the prior studies where behavioral, immediate-early gene and striatal cell-signaling responses to psychostimulant and direct DA receptor agonist challenge were found to be augmented in FR relative to AL rats (e.g., Cabeza de Vaca and Carr, 1998; Carr et al., 2003; Haberny et al., 2004). For all experiments, rats were briefly narcotized with CO2, decapitated, and brains were rapidly removed and immediately frozen in powdered dry ice. As in the prior studies, sacrifice occurred during the light phase 4–5 h before the FR subjects were to receive their scheduled daily meal. Subjects were maintained in a quiet location within their home cages in the period preceding sacrifice.
Tissue sampling
Five hundred micrometers sections were cut using an IEC Minotome cryostat. Under an Olympus dissecting microscope, different brain regions (mPFC, NAc, CPu, VTA, and SN) were micropunched from a series of 500 μm frozen coronal sections. For the RT-PCR experiment, all equipment and surfaces were treated with RNase Zap (Ambion, Austin, TX) to minimize RNase contamination.
BDNF immunoassay
Brain-derived neurotrophic factor protein levels were determined using the commercially available BDNF Emax® ImmunoAssay Systems (Promega, Madison, WI). The ELISAs were performed according to the manufacturer’s protocol. Tissue samples were homogenized in lysis buffer, followed by centrifugation and protein determination using BCA reagent kit as described by the manufacturer (Pierce, Rockford, IL). Aliquots of supernatants were stored at −80°C. On the day of the immunoassay, aliquots were thawed, and added to a 96-well immunoplate precoated with human BDNF specific monoclonal antibody. The plate was incubated at room temperature for 2 h with shaking. Anti-BDNF monoclonal antibody was used as the capture Ab and anti-BDNF pAb was used as reporter Ab. After washing, the amount of specifically bound pAb was detected using a species-specific anti-IgY antibody conjugated to horseradish peroxidase as a tertiary reactant. The unbound conjugate was removed by washing followed by incubation with a chromogenic substrate. Absorbance of samples was measured at 450 nm and read using OPTImax microplate reader (Molecular Devices Corp., Sunnyvale, CA). All samples were assayed in duplicate. The readings were normalized to the amount of protein applied. Samples of forebrain and midbrain regions were obtained from 4 and 6 animals per diet group, respectively. Results obtained in each brain region were analyzed as separate experiments using Student’s t-test.
Lysate preparation and Western blotting
Tissue samples were homogenized in 10 volumes of 50 mM Tris–HCl, pH 7.5 containing 50 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 40 mM β-glycerophosphate, 50 mM NaF and 5 mM Na4P2O7, 1% Tx-100, 0.5 μM okadaic acid, 0.5% sodium deoxycholate, and 0.1% SDS, followed by centrifugation and protein determination using BCA reagent kit as described by the manufacturer (Pierce). Supernatants were mixed with 5× SDS-PAGE sample buffer, boiled for 5 min, cooled on ice, and kept at −80 °C until future use.
Protein (10 μg per lane) was separated by electrophoresis on precast 8% polyacrylamide gels (Bio-Rad). Precision Plus protein standard molecular weight markers (Bio-Rad) were also loaded to assure complete electrophoretic transfer and to estimate the size of bands of interest. The gels were transferred to nitrocellulose membrane (Osmonics, Minnetonka, MN) for 1 h, with a constant voltage of 100 V. Membranes were blocked for 1 h at room temperature with blocking buffer, 5% non-fat dry milk in 50 mM Tris–HCl, pH 7.5 containing 150 mM NaCl and 0.1% Tween 20 (TBS-T), then probed overnight at 4 °C using primary antibodies for target proteins or 1h at room temperature using primary antibody for α-tubulin. Antibodies used included mouse monoclonal anti-TrkB (1:2000; BD Biosciences, San Jose, CA), mouse monoclonal anti-phospho-(Thr202/Tyr204)-p44/42 ERK1/2 (1:1000; Cell Signaling, Beverly, MA), rabbit polyclonal anti-p42/44 ERK1/2 (1:2000; Cell Signaling, Beverly, MA), rabbit polyclonal anti-phospho-Ser473 Akt (1:1000; Cell Signaling, Beverly, MA), rabbit polyclonal anti-Akt (1:10000; Cell Signaling, Beveraly, MA), and mouse monoclonal anti-α-tubulin (1: 5,000; T6199, Sigma-Aldrich, St. Louis, MO).
After probing with primary antibodies and washing with TBS-T buffer (3 × 5 min), membranes were incubated with horseradish peroxidase conjugated anti-mouse IgG (Cell Signaling). Proteins were visualized using a chemiluminescence ECL kit (Pierce). Densitometric analysis of the bands was performed using the MCID imaging system (St. Catherines, Ontario, Canada). Total TrkB values were normalized to tubulin, p-ERK 1 and p-ERK 2 values were normalized to total ERK 1 and ERK 2, and p-Akt was normalized to total Akt. Target protein levels were expressed as percentage of the normalized control, which was the AL group. For measurements of TrkB, samples of striatal and midbrain regions were obtained from 6 animals per diet group, and 4 animals per diet group for mPFC. For measurements of signaling proteins, VTA samples were obtained from 6 animals per diet group. Results of each experiment were analyzed by Student’s t-test.
RNA extraction and quantitative real-time RT PCR
Total RNA was extracted from individual VTA tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Sample RNA concentrations were determined by spectrophotometry.
Specific mRNA levels in each sample were measured on the iCycler™ (Bio-Rad, Hercules, CA) in a final volume of 25 μl. Each reaction was performed using reagents from the one-step SYBR Green Quantitative RT-PCR kit (Sigma-Aldrich, St. Louis, MO) according to the standard protocol provided. Amplification consisted of 40 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 30 s, following one step of activation (pre-soak) at 48 °C for 30 min and initial denaturation at 94°C for 3 min. Fluorescence signals were monitored sequentially for each sample tube once per cycle at the end of extension. An external standard RNA concentration curve for each primer pair was generated using pooled RNA samples and verified by agarose gel electrophoresis. To evaluate RNA purity, each sample was tested without addition of reverse transcriptase and found to contain no genomic DNA above the threshold detection level. For each experiment, specificity of RT-PCR products was confirmed by analysis of melting curves produced by iCycler™ showing the presence of a single species of DNA product per primer pair, and by agarose gel electrophoresis revealing single bands of the predicted molecular weight for each product. Table 1 details the sequence of primer pairs (purchased from GeneLink, Hawthorne, NY) and the sources for their design.
Table 1.
Sequences of primer pairs used for real-time RT-PCR with the sources for their design
| β-actin | 5′ GTC GTA CCA CTG GCA TTG TG 3′ |
| 5′ GCC ATC TCT TGC TCG AAG TC 3′ | |
| (Spangler et al., 2004) | |
| BDNF | 5′ AGC CTC CTC TGC TCT TTC TGC TGG A 3′ |
| 5′ CTT TTG TCT ATG CCC CTG CAG CCT T 3′ | |
| (Fang et al., 2000) |
Sample mRNA levels for the BDNF gene were averaged from two separate experiments, each performed in duplicate. To correct for minor variability among samples, each subject’s average BDNF expression was normalized to its expression level of the housekeeping gene β-actin. VTA samples were obtained from 6 animals per diet group, relative expression levels for each subject were normalized to the corresponding mean of the AL group, and fold changes in induction were calculated and compared between groups using Student’s t-test.
Visualized whole-cell recording
Rats were deeply anesthetized with 50 mg/kg pentobarbital (i.p.) then perfused transcardially with ice-cold (~0 °C) modified artificial (aCSF) (in mM): 225 sucrose; 2.5 KCl; 0.5 CaCl2; 7 MgCl2; 28 NaHCO3; 1.25 NaH2PO4; 7 glucose; 1 ascorbate; and 3 pyruvate (Koós and Tepper, 1999). After perfusion, the brain was removed into ice-cold modified aCSF for 1–2 min, then blocked and coronal midbrain slices (300 μm thickness) prepared using a Leica VT1200S vibrating blade microtome (Leica Microsystems, Bannockburn, IL). Slices were then transferred to a holding chamber for 30 min at 34°C, then slowly returned to room temperature in media containing (in mM): 125 NaCl; 2.5 KCl; 2 CaCl2; 1 MgCl2; 25 NaHCO3; 1.25 NaH2PO4; 25 glucose; 1 ascorbate; and 3 pyruvate; pH 7.3–7.4, equilibrated with 95% O2/5% CO2 (Koós and Tepper, 1999; Avshalumov et al., 2005).
For whole-cell recording, slices were transferred to a submersion chamber (Warner Instruments LLC, Holliston, MA) maintained at 32 °C and superfused at 1.2 mL/min with bicarbonate-buffered aCSF containing (in mM): 124 NaCl; 3.7 KCl; 26 NaHCO3; 1.3 MgSO2; KH2PO4; 10 glucose; 2.4 CaCl2 and saturated with 95% O2/5% CO2 (Avshalumov et al., 2005). Neurons were visualized using infrared differential interference contrast (IR-DIC) Olympus BX51WI microscope equipped with a 40× water-immersion objective (Olympus America, Center Valley, PA). Pipettes were made from 1.5 mm outer diameter borosilicate capillary tubing (World Precision Instruments, Sarasota, FL) using a Sutter P-97 Flaming/Brown micropipette puller (Sutter Instrument Company, Novato, CA). The pipette backfill solution for voltage-clamp recording contained (in mM): 117 Cs-methanesulfonate, 2 MgCl2; 0.2 CaCl2; 2 EGTA; 20 HEPES; 5 TEA-Cl; 3 Na2-ATP; and 0.2 GTP; pH 7.3 (modified from Akopian and Witkovsky 2001; Ungless et al. 2001). Data were acquired using an Axopatch 200B amplifier and Digidata board 1322A controlled by Clampex 9.0; analysis will use ClampFit (Molecular Devices), including correction of liquid junction potential. Recorded cells in the VTA were identified as dopaminergic (DAergic) neurons by the presence of Ih current when membrane voltage was stepped in the hyperpolarizing direction to −140 mV in 10 mV increments (e.g., Avshalumov et al., 2005); this identification procedure was conducted immediately after a cell was patched, before Cs+ and TEA+ in the backfill solution infiltrated the cell.
Components of all physiological solutions were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), as was picrotoxin, a GABAA receptor blocker. All drugs used were water soluble and were prepared as aqueous stock solutions or dissolved directly in aCSF immediately before use.
RESULTS
Brain regional BDNF protein levels
Measurement of BDNF protein levels by ELISA indicated no significant differences between AL and FR rats in NAc (t(6)=0.3, p>.10), CPu (t(6)=1.8, p>.10), mPFC (t(6)=0.6, p>.10) or VTA (t(10)=0.7, p>.10). However, levels were higher in SN of FR relative to AL rats (t(10)=2.47, p<.05; Figure 1).
Figure 1.

Effect of chronic food restriction (FR) on brain regional BDNF protein levels. Levels of BDNF protein (mean ± S.E.M.), as determined by ELISA, expressed as percentage of control ad libitum (AL) fed group, in nucleus accumbens (NAc), caudate-putamen (CPu), medial prefrontal cortex (mPFC), ventral tegmental area (VTA) and substantia nigra (SN). (forebrain: nAL = 4; nFR = 4; midbrain: nAL = 6; nFR = 6), *p<.05
Brain regional TrkB protein levels
Semi-quantitative measurements of TrkB protein levels by Western analysis indicated that in the forebrain regions examined there were no significant differences between AL and FR rats in NAc (t(10)=0.6, p>.10), CPu (t(10)=0.57, p>.10), or mPFC (t(6)=0.27, p>.10; Figure 2). However, in midbrain regions FR subjects displayed decreased levels of TrkB protein in both the VTA (t(10)=2.21, p=.05) and SN (t(10)=3.48, p<.01) relative to AL subjects (Figure 3).
Figure 2.
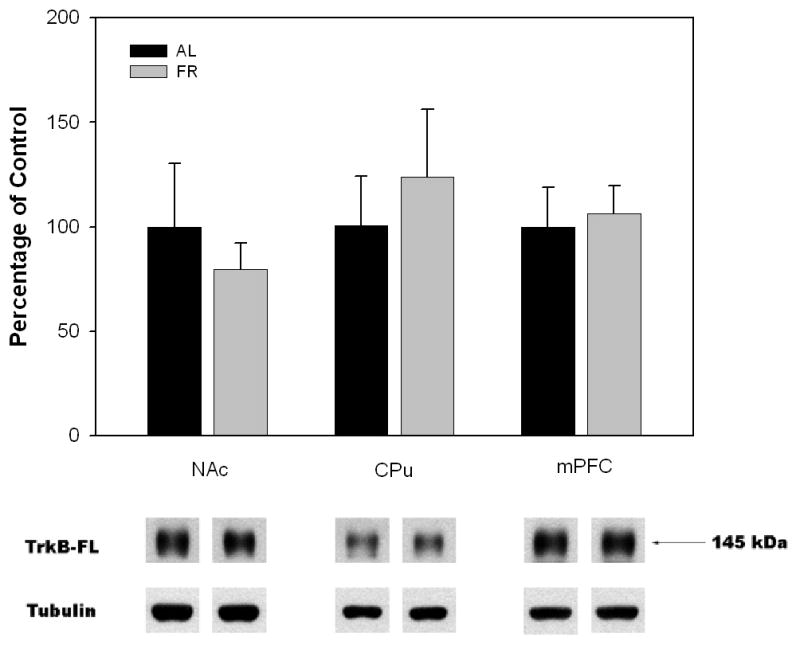
Mean ± SEM ratio of full-length neurotrophin receptor TrkB protein/tubulin in nucleus accumbens (NAc), caudate-putamen (CPu) and medial prefrontal cortex (mPFC) of food-restricted (FR) rats determined by Western blot and expressed in comparison to the normalized control ad libitum (AL) fed group. Graphed results are displayed with representative immunoblots. (NAc and CPu: nAL = 6; nFR = 6; mPFC: nAL = 4; nFR = 4)
Figure 3.
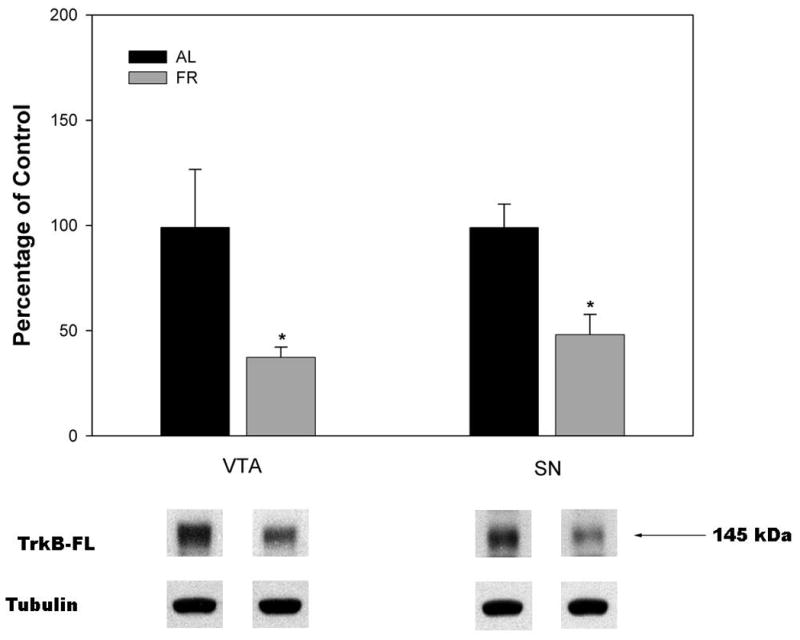
Mean ± SEM ratio of full-length neurotrophin receptor TrkB protein/tubulin in ventral tegmental area (VTA) and substantia nigra (SN) of food-restricted (FR) rats determined by Western blot and expressed in comparison to the normalized control ad libitum (AL) fed group. Graphed results are displayed with representative immunoblots. ( nAL = 6; nFR = 6), *p<.05
BDNF mRNA in VTA
BDNF mRNA as measured in extracts of micropunched VTA using real-time RT PCR revealed no difference between AL and FR subjects (t(10)=0.27; data not shown).
ERK 1/2 and Akt signaling in VTA
Results of Western analyses indicated that under basal conditions, FR subjects displayed increased phosphorylation of ERK 1/2 (t(10)=2.52, p<.05) and a trend toward decreased phosphorylation of Akt(t(10)=1.78, p>.05) in VTA (Figure 4).
Figure 4.

Effect of chronic food restriction on phosphorylation of ERK 1/2 and Akt under basal conditions in ad libitum (AL) fed and food-restricted (FR) rats. Lysates were immunoblotted with anti-phospho p44/42 MAPK, anti-p44/42 MAPK, or anti-phospho-Ser473-Akt antibodies. Following densitometry, intensities of bands corresponding to phospho-proteins for each subject were divided by the intensities of the corresponding total protein (ERK or Akt) bands to correct for small differences in protein loading. Results (mean ± S.E.M.) are expressed in comparison to the normalized control, which was defined as the ad libitum fed group injected with vehicle. Graphed results are displayed with representative immunoblots. (nAL = 6; nFR = 6), *p ≤.05
Voltage-clamp recording of EPSCs in VTA DAergic neurons
Possible alterations in the responsiveness of VTA DAergic neurons to excitatory glutamate input after FR were examined using voltage-clamp recording in midbrain slices. Picrotoxin (100 μM; Johnson and North 1992) was included in all media to block concurrent activation of GABAA receptors. It should be noted that slow GABAB-receptor-and D2 autoreceptor-dependent currents do not interfere with fast EPSCs (Bonci and Malenka, 1999; Beckstead, 2004). In the presence of picrotoxin, local stimulation elicited robust excitatory postsynaptic currents (EPSCs) in FR and control DA cells held at −70 mV (Fig. 5A), at which EPSCs are exclusively AMPAR-dependent (Schilstrom et al., 2006). Under these conditions, EPSCs were lower, albeit not significantly, in VTA neurons from FR vs. AL rats (n = 4–5 neurons from 2–3 rats per group). When the holding potential was raised to +40 mV to reveal NMDA receptor contributions by removing the Mg2+ block of the receptor-gated channel (Bonci and Malenka, 1999), however, EPSC amplitude was a significant 50% lower in DAergic neurons from FR animals than AL controls (p < 0.05) (Fig. 5B), confirming decreased NMDAR and/or AMPAR transmission.
Figure 5.
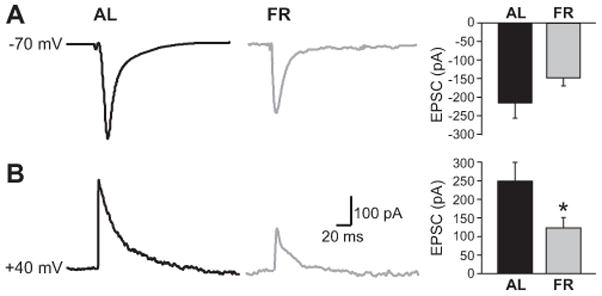
Decreased glutamatergic excitability of VTA DA neurons in FR (food-restricted) vs. AL (ad libitum fed) controls. A) Representative voltage-clamp EPSC records and mean EPSC amplitude in presumed VTA DA neurons at a holding potential of −70 mV, reflecting primarily AMPA receptor-mediated current (negative deflection indicates inward current). B) Representative EPSCs in VTA DA neurons with +40 mV holding potential to remove Mg2+ block of NMDARs (positive deflection is outward current); mean AMPA + NMDA EPSCs were lower in FR than AL (n = 4–5 cells from 2–3 rats per group; *p<.05).
DISCUSSION
Recent findings indicating that changes in brain regional BDNF mediate adaptive responses to negative energy balance and also regulate behavioral responsiveness to drugs of abuse provided the rationale for investigating BDNF and its TrkB receptor in reward-related brain regions of rats that were food-restricted in a manner that potentiates drug reward. A spatial component determining distinct consequences of altered BDNF levels on behavioral responses to abused drugs is apparent, with opponent effects being exerted by mPFC and the mesoaccumbens DA pathway. Chronic cocaine decreases levels of BDNF protein in PFC (Fumagalli et al., 2007), which likely facilitates drug-directed behavior, given that decreased BDNF protein expression in mPFC increases cocaine self-administration (Sadri-Vakili et al., 2008) and microinjection of BDNF decreases cocaine-seeking (Berglind et al., 2007). On the other hand, though basal levels of BDNF mRNA in NAc are extremely low, acute and chronic cocaine increase BDNF mRNA and protein in NAc (Narita et al., 2003; Filip et al., 2006; Graham et al., 2007), with a significant portion of the latter attributed to transport from VTA and activity-dependent release (Altar and DiStefano, 1998; Krishnan et al., 2007). The involvement of NAc BDNF/TrkB function in drug abuse and addiction is further supported by findings that microinjection of BDNF into NAc increases cocaine self-administration (Graham et al., 2007) while microinjection of BDNF or TrkB antiserum into NAc decreases self-administration, cocaine-seeking during withdrawal, and methamphetamine-induced hyperlocomotion (Graham et al., 2007; Narita et al., 2003).
Thus, although alterations of BDNF/TrkB in mPFC and NAc during FR would have provided a tractable new line of approach to illuminate the effect of FR on drug-directed behavior, the present results indicate that FR did not alter levels of BDNF or TrkB protein in mPFC, NAc, or CPu, despite the efficacy of the FR regimen to enhance drug-induced behavioral responses linked to numerous pre- and postsynaptic neuroadaptations in striatum (Carr et al., 2003; Haberny et al., 2004; Zhen et al., 2006; Carr et al., 2010). The lack of effect of FR on BDNF/TrkB in these forebrain regions contrasts with the prior finding that FR increased BDNF protein levels in neocortex and striatum (Lee et al. 2000; Duan et al., 2001). The discrepant observations can most likely be attributed to differences in the FR regimens employed. In the prior studies, subjects were maintained on an every other day (EOD) protocol, which interdigitated days of free feeding with days of total food deprivation for a period of 3 months. In the present study, subjects were fed a limited amount of food every day and assays were conducted after 3–4 weeks of FR. The ability of this FR protocol to increase the reward magnitude and reinforcing efficacy of abused drugs without upregulation of BDNF in key forebrain regions, casts doubt on involvement of forebrain BDNF in the augmentation of drug reward and drug-seeking by FR. Of course, the possibility of altered responsiveness of BDNF/TrkB to acute or repeated drug challenge in FR subjects cannot be ruled out.
Additionally, FR caused a decrease in TrkB protein levels in VTA, which would in fact imply decreased BDNF signaling. The high expression of TrkB in basal midbrain DA cells makes it likely that this reflects decreased TrkB in DA neurons, with the caveat that the methods used cannot distinguish cell type. The source of BDNF that normally interacts with VTA TrkB is believed to be primarily the VTA DA neurons themselves, which synthesize and locally release BDNF (Pu et al., 2006). In cultured cortical and hippocampal neurons, BDNF increases the synthesis and synaptic delivery of NMDA and AMPA receptors (Caldeira et al., 2007; Nakata and Nakamura, 2007). Consequently, we assessed BDNF mRNA and excitatory synaptic transmission in VTA DA neurons to provide evidence consistent with TrkB downregulation during FR. The RT PCR experiment revealed no alteration in BDNF synthetic activity; however, consistent with TrkB downregulation, voltage-clamp data yielded evidence of decreased glutamate receptor transmission in the DA neurons of FR subjects. These data might predict lower average firing rates in FR than AL, which would contribute to the low basal extracellular concentrations of DA observed in NAc of FR subjects (Pothos et al., 1995), the compensatory decrease in Vmax of the striatal DA transporter (Zhen et al., 2006), and upregulation of cellular and behavioral responses downstream of NAc DA receptor stimulation (Carr, 2007).
Although a neurophysiological change consistent with TrkB downregulation was observed, the downregulating stimulus is unknown. One speculative scheme is that FR leads to an initial increase in midbrain BDNF that leads to a downregulation of TrkB, which is still seen after 3 weeks of FR as a residual compensatory response. The plausibility of this explanation gains some support from the finding that in SN, TrkB was downregulated and BDNF protein levels were (still) elevated. A short time course of increased BDNF activity in VTA may be adaptive because increased excitatory synaptic transmission would facilitate behavioral activation, which during early FR could promote foraging and food procurement but after a certain duration of FR, particularly in an unchanging environment, may increase counterproductive energy expenditure. Moreover, analogous to the case of chronic drug treatment, the neuroadaptations that underlie enduring changes in goal-directed behavior and reward may be based primarily in NAc, though dependent upon antecedent events in VTA (Kauer and Malenka, 2007). Among other possible mechanisms of TrkB downregulation it is important to consider that VTA DA neurons express receptors for endocrine hormones that are affected by FR, including leptin and insulin (Figlewicz et al., 2003) and insulin-like growth factor (IGF-1; Bondy et al., 1992; Kar et al., 1993), as well as feeding-related peptides including ghrelin (Abizaid et al., 2006) and orexin (Malherbe et al., 2009). All of these signals are known to modify DA neuronal function and may be involved in regulatory cross-talk.
Of particular interest is IGF-1. FR decreases expression and secretion of hepatic IGF-1 and decreases serum and brain concentrations (Olchovsky et al., 1993; LaPaglia et al., 1998; Sohlstrom et al., 1998; Bondy et al., 2004). The FR protocol used in the present set of experiments decreased serum IGF-1 concentrations by more than 50% (Liu and Carr, unpublished). In some cell types, IGF-1 activates the PI3K-Akt pathway, transiently activates the Ras-Raf-MEK-ERK 1/2 pathway, but then exerts a prolonged inhibition of ERK 1/2 signaling via Akt-mediated phosphorylation of Raf-1 on Ser259 (Moelling et al., 2002). Consequently, a sustained decrease in physiological IGF-1 stimulation may decrease phospho-Akt and secondarily increase phospho-ERK. Our finding of a trend toward decreased phospho-Akt and a significant increase in phospho-ERK in VTA of FR subjects may be consistent with a role of low IGF-1 tone in VTA. Importantly, IGF-1 has a strong positive interaction with BDNF/TrkB at the cellular level (Ding et al., 2006; Johnson-Farley et al., 2006; Chen et al., 2007), and can regulate TrkB expression (McCusker et al., 2006). Thus, IGF-1 deficiency in VTA could be involved in the downregulation of TrkB and the upregulation of ERK phosphorylation.
The findings obtained in FR subjects strongly suggest altered functional properties of VTA DA neurons. However, the relationship(s) between any two or all three of the observed changes remains speculative, in part because VTA is a heterogeneous tissue with up to 35% GABAergic neurons and DA neuronal subpopulations that differ in function (Matsumoto and Hikosaka, 2009) and projection patterns (Ikemoto, 2006; Lammel et al., 2008). Yet, the plausible involvement of TrkB downregulation in the enhanced behavioral and striatal responses of FR subjects to drugs of abuse receives additional support from the recent demonstration that 30-min after ingestion of a palatable high fat meal, mice displayed elevation of TrkB mRNA in VTA (Cordeira et al., 2010). In addition, central BDNF depletion decreased evoked DA release in NAc shell and CPu. These results suggest reciprocal effects of FR and palatable meal ingestion on VTA DA neuronal TrkB, with corresponding modulatory effects on excitatory synaptic transmission and striatal DA release.
Highlights.
Food restriction did not alter BDNF or TrkB protein levels in dopaminergic forebrain regions
Food restriction decreased TrkB levels in ventral midbrain
Food restriction decreased glutamate receptor transmission in dopamine neurons
Food restriction increased levels of pERK 1/2 in ventral tegmental area
Observed changes in midbrain may enhance drug reward in food-restricted rats
Acknowledgments
We thank Dr. Ipe Ninan for helpful comments. This research was supported by DA003956 (KDC), NS036362 (MER), and T32 DA007254 from NIDA/NIH.
ABBREVIATIONS
- DA
dopamine
- BDNF
brain-derived neurotrophic factor
- TrkB
tropomyosin receptor kinase B
- ERK
extracellular signal-related kinase
- NAc
nucleus accumbens
- mPFC
medial prefrontal cortex
- FR
food-restriction, food-restricted
- AL
ad libitum fed
- VTA
ventral tegmental area
- EPSC
excitatory postsynaptic current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Intracellular calcium reduces light-induced excitatory post-synaptic responses in salamander retinal ganglion cells. J Physiol. 2001;532:43–53. doi: 10.1111/j.1469-7793.2001.0043g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. doi: 10.1016/s0166-2236(98)01273-9. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Marshall SP, Peña DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci. 2003;23:2744–2750. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Koós T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as ananorexigenic factor in the dorsal agal complex. Endocrinol. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacol. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy CA, Bach MA, Lee W-H. Mapping of brain insulin and insulin-like growth factor receptor gene expression by in situ hybridization. Neuroprotocols. 1992;1:240–249. [Google Scholar]
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Krahne L, Carr KD. A progressive ratio schedule of self-stimulation testing reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a “sensitizing” regimen of d-amphetamine. Psychopharmacol. 2004;175:106–113. doi: 10.1007/s00213-003-1768-4. [DOI] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Carr KD. Chronic food restriction: Enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91:459–472. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Carr KD. Reward-related neuroadaptations induced by food restriction: pathogenic potential of a survival mechanism. In: Dube L, Bechara A, Dagher A, Drewnowski A, Lebel J, James P, Richard D, Yada R, editors. Obesity Prevention: The Role of Society and Brain on Individual Behavior; A handbook for integrative science, policy and action to stop the progression of the obesity pandemic. Elsevier; 2010. [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey D, Restituito S, Ziff E. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neurosci. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol. 1984;4:47–88. [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007;25:118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Le Moal M, Simon Sensitization to the psychomotor effects of amphetamine and morphine induced by food restriction depends on corticosterone secretion. Brain Res. 1993;611:352–356. doi: 10.1016/0006-8993(93)90526-s. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neurosci. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16:1–12. doi: 10.1385/JMN:16:1:1. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiat. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Fang M, Wang Y, Liu HX, Liu XS, Han JS. Decreased GDNF mRNA expression in dorsal spinal cord of unilateral arthritic rat. Neuroreport. 2000;11:737–741. doi: 10.1097/00001756-200003200-00017. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Myers M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Filip M, Faron-Górecka A, Kuśmider M, Gołda A, Frankowska M, Dziedzicka-Wasylewska M. Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Res. 2006;1071:218–225. doi: 10.1016/j.brainres.2005.11.099. [DOI] [PubMed] [Google Scholar]
- Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol. 2004;286:R994–R1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Sugihara Y, Iwasaki F, Fukumitsu H, Nita A, Nomoto H, Furukawa Y. Brain-derived neurotrophic factor-like immunoreactivity in the adult rat central nervous system predominantly distributed in neurons with substantial amounts of brain-derived neurotrophic factor messenger RNA or responsiveness to brain-derived neurotrophic factor. Neurosci. 1998;82:653–670. doi: 10.1016/s0306-4522(97)00315-1. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Haberny S, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP Kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Horger BA, Lyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Ann Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Farley NN, Travkina T, Cowen DS. Cumulative activation of Akt and consequent inhibition of glycogen synthase kinase-3, by brain-derived neurotrophic factor and insulin-like growth factor-1 in cultured hippocampal neurons. J Pharmacol Exp Ther. 2006;316:1062–1069. doi: 10.1124/jpet.105.094433. [DOI] [PubMed] [Google Scholar]
- Kar S, Chabot J-G, Quirion R. Quantitative autoradiographic localization of [125I]insulin-like growth factor I, [125I]insulin-like growth factor II, and [125I]insulin receptor binding sites in developing and adult rat brain. J Comp Neurol. 1993;333:375–397. doi: 10.1002/cne.903330306. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolaños CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatr 2008. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jonew I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- LaPaglia N, Steiner J, Kirsteins L, Emanuele M, Emanuele N. Leptin alters the response of the growth hormone releasing factor-growth hormone—insulin-like growth factor-I axis to fasting. J Endocrinol. 1998;159:79–83. doi: 10.1677/joe.0.1590079. [DOI] [PubMed] [Google Scholar]
- Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci. 2006;126–127:30–38. doi: 10.1016/j.autneu.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases survival of newly-generated neural cells and induces BDNF expression in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Liu S, Zheng D, Peng X-X, Cabeza de Vaca S, Carr KD. Enhanced cocaine-reinforced conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluR1 in food-restricted rats. Brain Res. 2011;1400:31–41. doi: 10.1016/j.brainres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker RH, McCrea K, Zunich S, Dantzer R, Broussard SR, Johnson RW, Kelley KW. Insulin-like growth factor-I enhances the biological activity of brain-derived neurotrophic factor on cerebrocortical neurons. J Neuroimmunol. 2006;179:186–190. doi: 10.1016/j.jneuroim.2006.06.014. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol. 2009;76:618–631. doi: 10.1124/mol.109.055152. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;495:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;581:2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neurosci. 2003;119:767–775. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiat. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Olchovsky D, Song J, Gelato MC, Sherwood J, Spatola E, Bruno JF, Berelowitz M. Pituitary and hypothalamic insulin-like growth factor I (IGF-I) and IGF-I receptor expression in food-deprived rats. Mol Cell Endocrinol. 1993;93:193–198. doi: 10.1016/0303-7207(93)90123-2. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu L, Liu Q-S, Poo M-M. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nature Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. BioEssays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sohlstrom A, Katsman A, Kind KL, Grant PA, Owens PC, Robinson JS, Owens JA. Effects of acute and chronic food restriction on the insulin-like growth factor axis in the guinea pig. J Endocrinol. 1998;157:107–114. doi: 10.1677/joe.0.1570107. [DOI] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Molec Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, Distefano PS, Yancopoulos GD. TrkB enclodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R1003–1012. doi: 10.1152/ajpregu.00011.2007. [DOI] [PubMed] [Google Scholar]
- Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R1037–1045. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith MEA, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]


