Abstract
The intestinal tract, known for its capability for self-renew, represents the first barrier of defense between the organism and its luminal environment. The thiol/disulfide redox systems comprising the glutathione/glutathione disulfide (GSH/GSSG), cysteine/cystine (Cys/CySS) and reduced and oxidized thioredoxin (Trx/TrxSS) redox couples play important roles in preserving tissue redox homeostasis, metabolic functions, and cellular integrity. Control of the thiol-disulfide status at the luminal surface is essential for maintaining mucus fluidity and absorption of nutrients, and protection against chemical-induced oxidant injury. Within intestinal cells, these redox couples preserve an environment that supports physiological processes and orchestrates networks of enzymatic reactions against oxidative stress. In this review, we focus on the intestinal redox and antioxidant systems, their subcellular compartmentation, redox signaling and epithelial turnover, and contribution of luminal microbiota, key aspects that are relevant to understanding redox-dependent processes in gut biology with implications for degenerative digestive disorders, such as inflammation and cancer.
Keywords: Intestinal redox status and control of redox balance, extracellular cysteine/cystine (Cys/CySS) redox state, cellular glutathione/glutathione disulfide (GSH/GSSG) redox state, redox control of intestinal cell phenotypic transitions, mucosal GSH and GSH-dependent enzymes, GSH and intestinal oxidative stress, intestinal disorders and tissue redox state, intestinal microbiota, intestinal NFκB redox signaling
Introduction: Overview of structural and functional organization of the intestinal epithelium
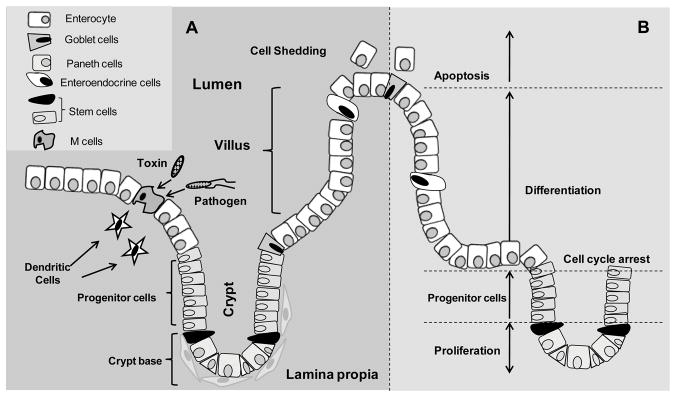
The mammalian gastrointestinal tract is lined with a single layer of epithelial cells that is capable of self-renewal every 4–5 days, and is among the highest proliferative tissue in the organism [1]. The gut is comprised of the small intestine and the colon; the small intestinal epithelium is extensively folded to maximize the absorptive surface area, resulting in distinct villus and crypt (of Lieberkühn) regions (Figure 1A). Within the crypt, stem cells proliferate, and actively dividing progenitors cells differentiate into secretory (Paneth, mucin-secreted goblet, and enteroendocrine cells) or absorptive (enterocyte) lineages. Current paradigm is consistent with the self-renewing and multi-potent intestinal crypt stem cells (ISC) being the source of the four major differentiated epithelial cell types. Cell proliferation begins at the bottom of intestinal crypts where one to six ISC transform into a transient population of rapidly dividing progenitor cells (Figure 1B). During migration towards the villus tip, progenitor cells differentiate into goblet, enteroendocrine cells or enterocytes. Three days after their functional differentiation, intestinal cells undergo apoptosis and are shed into the lumen. Paneth cells differentiate and reside within the crypts and undergo phagocytosis three weeks post differentiation [2]. The surrounding stromal/mesenchymal cells, together with signals generated by stem cells create a complex microenvironment, known as the stem cell niche which regulates stem cell behavior [3]. Unlike the small intestine, the colonic epithelium is devoid of villi and Paneth cells [4], and proliferative and differentiated cells are localized to the bottom of crypt invaginations and to surface epithelium, respectively.
Figure 1. Organization of the small intestinal epithelium.
A. The intestinal epithelium, consisting of a single layer of cells, is extensively folded that results in distinct crypt and villus regions. Four types of cells including enterocytes, Paneth, goblet, and enteroendocrine cells reside in the epithelium and are involved in digestive and immunological functions of the intestine. Particulate antigens (pathogens and toxins) are sampled by the M cells present in the gut-associated lymphoid tissues at the luminal site and presented to dendritic cells or other antigen-presenting cells at their basolateral surface. At the basolateral membrane, cells of the intestinal epithelium associate with and are supported by lamina propria. B. The intestinal epithelium is a highly proliferative tissue. Cell proliferation originates at the base of the crypt where intestinal stem cells reside. Progenitors of the stem cells proliferate and migrate bi-directionally. The precursors that migrate toward the tip of the villus differentiate into one of the following cell types: enterocyte, goblet cell, or enteroendocrine cell; the progenitors that migrate toward the bottom of the crypt differentiate into Paneth cells. At 4–5 days post differentiation, villus tip cells die by apoptosis are shedded into the lumen while Paneth cells remain in the crypt for about 23 days and thereafter are phagocytozed.
The absorptive enterocytes account for over 80% of the intestinal cells [5], and are highly polarized, consisting of an apical surface facing the lumen and a basolateral surface adjacent to the stroma. Adjacent epithelial cells are connected along the cell length from the luminal to lamina propria surfaces via tight junctions, adherens junctions, and desmosomes. These junctional structures provide architectural integrity, maintain an apical-to-basolateral polarity, and regulate paracellular water and electrolyte flux [6,7]. A viscous glycoprotein layer covering the enterocyte brush border membrane functions as a physical barrier that limits the access of luminal proteins and microorganisms. An underlying dense microvilli layer serves as a second barrier that impede intestinal access of most macromolecules, viruses, and bacteria [8]. Goblet cells, representing ~4 to 16% in the duodenum and colon [9] secrete mucins, forming a protective barrier against shear stress and chemical insult. Enteroendocrine cells, which comprise less than 1% of the epithelial cells, release gastrointestinal hormones (serotonin, substance P, secretin), and Paneth cells produces lysosyme, defensin, or antimicrobials that control the luminal flora.
The mucosal epithelium is supported by the lamina propria which consists of the extracellular matrix, blood and lymphatic vessels, neuronal and smooth muscle cells, and various immune cells (lymphocytes, macrophages), the latter participating in the mucosal immune response [10]. The gut-associated lymphoid tissue (GALT) is one of the largest lymphoid organs in the body and is formed by isolated and aggregated lymphoid follicles known as Peyer’s patches [11] (Figure 1). Peyer’s patches are surrounded by the follicle-associated epithelium (FAE), comprised of specialized, highly invaginated enterocytes named M (for microfold) cells. In contrast to the villus epithelium, the FAE is characterized by poorly organized brush border membrane with short, irregular microvilli, weak mucus production, and a thin glycocalyx that exhibits different glycosylation pattern, a feature that allows for easy access to antigens from the intestinal lumen [12]. Specific luminal antigens (e.g. pathogens, toxins, or commensal bacteria) are sampled by the M cells, phagocytosed, and transported to the underlying immune cells which trigger the immune response [13]. M cell basolateral membranes contain a unique intraepithelial invagination (“pocket”) where antigen-presenting dendritic cells, B-and T-lymphocytes, and macrophages reside [14]. This pocket reduces the distance for the trans-epithelial transport of antigens and enables a faster immune response. Since antigens do not undergo major alterations during transcytosis, M cells are exploited by different pathogens like S. typhimurium, Yersinia enterocolitica, L. monocytogenes and V. cholera as entry sites to the underlying host tissues [15,16]. Oral tolerance against commensal bacteria and proteins is acquired via generation of antigen-specific T lymphocytes that suppress inflammatory immune responses, and thus protects the intestinal mucosa.
General consideration of intracellular redox, antioxidant defense systems and subcellular compartmentation
Concept of redox state and redox environment
The “redox state” of a specific redox couple such as glutathione (GSH), thioredoxin (Trx) or cysteine (Cys) refers to the ratio of their inter-convertible reduced and oxidized forms, i.e., GSH/GSSG, Trx/TrxSS, or Cys/CySS. The term, “redox environment” represents the sum total of the product of the reducing potential and reducing capacity of these linked redox couples [17]. Given the difficulty in quantifying the cellular status of all linked redox couples, and the large cellular GSH pool size, the status of GSH/GSSG is generally considered a good estimate of the intracellular redox environment. The tendency of GSH to accept or donate electrons is determined by its redox potential (electromotive force, Eh), mathematically represented by the Nernst equation: Eh= E0 + (RT/nF) ln([GSSG/GSH]2) [18], where R is the gas constant, T is the absolute temperature, F is Faraday’s constant and n is the number of electrons transferred. E0 represents the standard potential for the redox couple and is calculated at equilibrium conditions relative to a standard hydrogen electrode. Under physiological conditions, Eh for the GSH/GSSG couple is between −260 to −200mV [18].
In biologic systems, the GSH/GSSG, Trx/TrxSS, and Cys/CySS redox couples are found to be far from equilibrium [18], and each redox couple reportedly functions as unique rheostat on/off switches in the redox regulation of cellular proteins [18]. The prevailing argument is that the existence of such distinctive “redox control nodes or circuitry” could afford an elegant mechanism for redox control in optimizing protein function based on the subcellular concentrations and fluxes of the redox couples, thus permitting a mode for independent regulation of the redox status of individual and/or specific protein sets [19]. Accordingly, the disruption of these redox control nodes would dramatically affect activities of various enzymes with redox sensitive catalytic site cysteines or methionines [19,20]. The notion that on/off “sulfur switches” could represent a generalized redox signaling mechanism in the control of redox-sensitive biological processes in normal cell physiology [21] allows redefining cellular oxidative stress as the disruption of physiological redox signaling events [22], beyond the classical definition of oxidative stress as an imbalance between pro- and anti-oxidant systems [23]. This notwithstanding, there remains key unanswered questions; for instance, the degree of cross-talk among redox couples, such as GSH/Trx, within and between compartments, e.g., cytosol, nucleus, or mitochondria, [20], and how changes in the redox state of one or more redox couples in single or multi compartments influence the overall cellular redox environment, cell signaling and, ultimately, cell metabolism or cell fate. Several recent reviews provide excellent discussion of the integration of redox compartments and implications for redox processes in biologic systems [18,24].
Cellular redox systems and their compartmentation
Glutathione/glutathione disulfide (GSH/GSSG) system
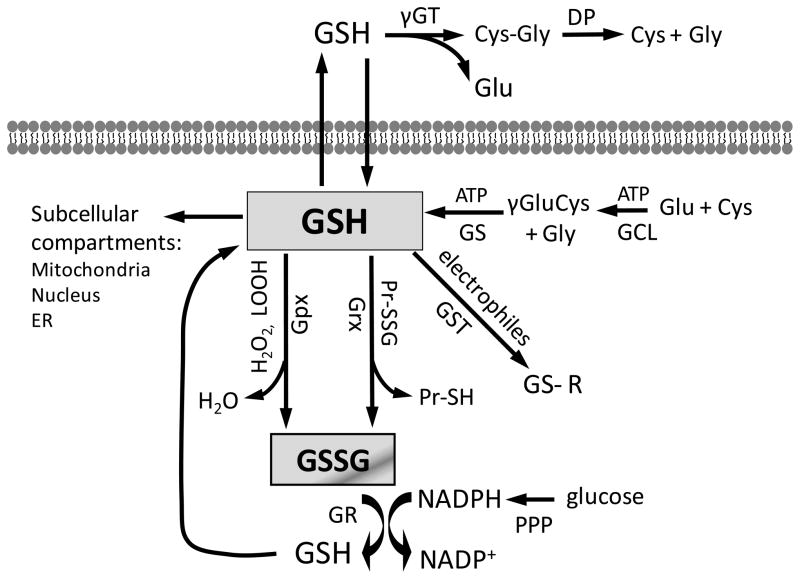
Glutathione (GSH, γ-glutamyl-cysteinyl-glycine) is present in millimolar concentrations (2–10mM) within cells and is a primary determinant of the cellular redox environment [17]. Intracellular GSH exists mainly as the biologically active reduced-thiol form; the oxidation of GSH to GSSG and subsequent decrease in the GSH-to-GSSG ratio is often associated with oxidative stress. Thus, the GSH-to-GSSG ratio is a simple and useful indicator of cellular oxidative stress [17]. Central to the maintenance of cellular redox homeostasis is de novo GSH synthesis, GSSG reduction, and exogenous GSH uptake. De novo GSH synthesis in the cytosol is catalyzed by γ-glutamylcysteine ligase and GSH synthetase [25], while the regeneration of GSH from GSSG is catalyzed by NADPH-dependent GSSG reductase, and GSH transport occurs via plasma membrane carriers [26]. Extracellular GSH uptake has been shown in transport epithelial cells such as enterocytes [27] and proximal tubular cells [28]. Figure 2 illustrates the relationships among these pathways of cellular GSH homeostasis (synthesis, reduction, transport), and GSH consumption in detoxication reactions by GSH-dependent enzymes, such as glutathione reductase (GR), glutaredoxin (Grx), and the family of glutathione peroxidases (GPxs) and glutathione-S-transferases (GSTs). Redox modulation of enzyme function is an important regulatory mechanism in the removal of reactive oxygen or nitrogen species (ROS/RNS), carcinogens, drugs, and xenobiotics.
Figure 2. Cellular GSH homeostasis and GSH-dependent reactions.
Intracellular GSH balance is maintained by de novo synthesis, regeneration from GSSG, and extracellular GSH uptake. In transport epithelial cells, such as enterocytes, γ-glutamyl transferase (γ-GT) and dipeptidase (DP) catalyzed the hydrolysis of extracellular GSH to its constituent amino acids, glutamate, cysteine and glycine. Additionally, intestinal epithelial cells can import intact GSH from the lumen via specific plasma membrane transporters. Cytosolic synthesis of GSH takes place in two ATP-dependent reactions catalyzed by glutamate-cysteine ligase (GCL) and glutathione synthase (GS). The intracellular GSH pool, present in millimolar concentrations, is involved in various GSH-dependent reactions. Compartmentation of GSH within the mitochondria, nucleus, or endoplasmic reticulum creates distinct and independently regulated subcellular redox pools. As part of the antioxidant defense system, GSH participates in conjugation reactions catalyzed by glutathione-S-transferases (GSTs), in the reduction of hydrogen peroxide (H2O2) and lipid hydroperoxides (LOOH) catalyzed by glutathione peroxidases (Gpxs), and the reduction of protein-disulfides (PrSSG) catalyzed by glutaredoxins (Grxs). The reduction of glutathione disulfide (GSSG) by glutathione reductase (GR) in the GSH redox cycle regenerates GSH. GSSG reduction occurs at the expense of NADPH, produced from the pentose phosphate pathway (PPP) from glucose oxidation.
Cytosolic GSH is highly reduced (Eh for GSH/GSSG of −260mV) [18], and is the source of distinct GSH redox pools within the cellular compartments of mitochondria, endoplasmic reticulum (ER) and nucleus. Mitochondrial GSH, at concentrations similar to those in the cytosol, can account for 15% to 30% of total GSH [29,30]. The inner mitochondrial membrane GSH transporters, dicarboxylate (DIC) and 2-oxoglutarate (OGC) carriers [31] maintain the matrix GSH/GSSG Eh at −300mV [32]. Interestingly, the GSH/GSSG redox state of the intermembrane space (IMS) is more oxidized despite free access of cytosolic GSH through porin channels [33]. A redox potential of Eh −255mV in the IMS is believed to be an ideal redox environment for disulfide bond formation of imported cytosolic proteins [34]. Similarly, the highly oxidized GSH redox milieu of the ER matrix (GSH:GSSG Eh between −170mV and −185mV [35]) ensures proper folding of nascent proteins, and maintains the redox buffering capacity in this compartment [36]. Cytosol-to-nuclear GSH distribution occurs through passive diffusion via nuclear pores [37]. While the size of the nuclear GSH pool is unclear, it is reportedly higher than that of the cytosol, suggesting that the two pools are independently maintained [19]. Additionally, a higher ratio of reduced- to-glutathionylated nuclear proteins [38] is consistent with a more negative GSH/GSSG redox potential in the nucleus [21]. The interaction of nuclear and cytosolic GSH is dynamic; during cell cycle, cytosolic GSH is distributed to the nucleus [38] (4 times higher) while confluent cells exhibited equal nuclear-to-cytosol GSH distribution [38].
Thioredoxin (Trx) and Trx-dependent redox system
Reduced and oxidized Trx (Trx-S/Trx-SS) maintain intracellular redox homeostasis in conjunction with GSH/GSSG [20]. Trx are small ubiquitous proteins that catalyze the reversible reduction of disulfide bonds resulting from oxidation of active site cysteines, Cys-XX-Cys (CGPC) in proteins. Reduction of the CGPC motif is mediated by NADPH-dependent Trx reductase (TrxR). The mammalian Trx1 and Trx2 are distinct cytosolic/nuclear and mitochondrial isoforms, respectively that exhibit redox potential of −280mV and −300mV, and −330mV [18]. Trx1/2 are regulated independently and are differentially sensitive to various stimuli [20,39]. TrxR isoenzymes are ubiquitous selenoproteins that catalyze the NADPH-dependent reduction of oxidized Trx. TrxR1 and TrxR2 are respectively cytosolic and mitochondrial enzymes while TGR is exclusively found in the testis [40]. The C-terminal motif contains a selenium-Cys (Sec) residue that is essential for catalytic activity; the loss of Sec is associated with enzyme inactivation. Deficiency inTrxR1 or TrxR2 genes results in embryonic lethality [41].
Peroxiredoxins (Prxs) are non-seleno thiol-specific peroxidases that catalyze the reduction of H2O2 and organic hydroperoxides wherein the peroxidatic Cys is oxidized to sulfenic acid (Cys-SOH) that forms a disulfide bond with a C-terminal Cys residue. Regeneration of active cysteines is catalyzed by Trx and TrxR. To date, six Prx isoforms (Prx 1–6) are described and divided into typical 2-Cys Prxs, atypical 2-Cys Prxs, and 1-Cys Prx based on the number of active cysteines [40]. Among these, Prx 1–4 are typical 2-Cys Prxs with different subcellular localizations: cytosol, Prx 1 and 2; extracellular space, Prx 4; and mitochondria, Prx3. Prx 5 is the only atypical 2-Cys enzyme widely distributed in the mitochondria, cytosol, nucleus, and peroxisomes [42] while Prx 6 is a cytosolic 1-Cys Prx [43]. High Prx expression (up to 1% of the cellular proteins) and catalytic rates (~107M−1s−1) suggests that Prxs may be responsible for the bulk of intracellular H2O2 reduction under physiological conditions [44]. However, it remains to be resolved as to whether Prxs are more significant than GPxs in the quantitative elimination of H2O2 generated within the various subcellular organelles. Localized H2O2 accumulation and the inactivation of Prx via peroxidatic Cys-to-sulfinic acid (-SO2H) oxidation, a process that was reversed by sulfiredoxins [45], was implicated in cell signaling [46]. More recent evidence show that plasma membrane signaling was linked to specific inactivation of membrane-associated Prx1 through receptor engagement and tyr194 phosphorylation, a novel mechanism that allowed for H2O2 accumulation and signal propagation initiated at the receptor site [47].
Cysteine/cystine (Cys/CySS) redox system
Cysteine (Cys) and cystine (CySS) constitute the most abundant low-molecular thiols in extracellular fluids with concentrations reaching 40 μM and 8–10 μM, respectively. The status of Cys and CySS contributes to maintaining the extracellular redox environment [48]. The measured Cys/CySS redox potential, Eh, in cell culture media or in plasma of healthy human subjects is around −80mV, a value that is more oxidized than that of GSH/GSSG and Trx/TrxSS redox systems [18]. Plasma Cys/CySS homeostasis is highly regulated by dietary Cys/CySS [49], hydrolysis of exported GSH [50], thiol-disulfide exchange between CySS and plasma GSH or homocysteine [51], and intracellular/extracellular Cys/CySS shuttle [52]. An oxidized Cys/CySS redox state has been associated with age-related diseases like diabetes, cardiovascular disease, and atherosclerosis [53]. Since the plasma Cys/CySS as well as GSH/GSSG redox states serve as quantitative measures of oxidative stress, a broader question is whether the redox states of these couples in the plasma could function as predictive markers of health and disease, a notion championed by Jones [48].
Redox balance in the intestine: physiological and pathological significance
Intestinal redox systems and related enzymes
Mucosal GSH and GSH-dependent enzymes
Like most tissues, the intestinal epithelium contain millimolar concentrations of GSH [54–56] that is maintained by de novo synthesis [57], regeneration from GSSG [58], and GSH uptake at the apical membrane [55,59]. Induction of GSH deficiency by inhibition of synthesis caused severe epithelial degeneration in vivo [60] while oral GSH administration reversed jejunal and colonic degeneration in accordance with increased tissue GSH [27], consistent with a role of GSH in intestinal integrity. Uptake of intact GSH across the brush-border membrane is stimulated by cations (Na+, K+, Li+) [59,61] that was not linked to GSH synthesis [57], indicating that GSH transport and intracellular GSH synthesis are independently regulated [62].
Exogenously administered GSH is efficiently taken up and excreted across the intestinal basal membrane, thereby increasing plasma GSH levels and GSH bioavailability [63]; however, uptake of precursor amino acids did not alter plasma GSH, consistent with local consumption of newly synthesized GSH within intestinal cells [63]. In vascularly perfused rat small intestine, milimolar concentrations of luminal GSH was absorbed intact across intestinal apical membranes; ~70–80% of [3H]GSH (1mM) was transported as intact GSH into blood with half-maximal transport at 1mM and saturation at 3mM [59]. Interestingly, at 0.3mM GSH ([35S]-labeled), abluminal recovery of GSH consisted of intact GSH (60%) and cysteine/cystine products (30 and 10%). At 0.1mM, the percent of intact GSH absorbed (45%) equaled that of cysteine and cystine (45% and 10%), indicating that substantial hydrolysis of luminal GSH occurred at submillimolar levels [59]. Intestinal GSH uptake and GSH hydrolysis are thus independent processes that occur concurrently, and the final metabolic fate of GSH is a function of its luminal concentration. In animal studies, dietary intake and biliary output are major contributors to luminal GSH homeostasis [54,64]. Additionally, intestinal GSH export into the lumen can occur during periods of fasting [65]. Liver is the major source of biliary GSH, and duodenal luminal concentrations can account for 50% of the hepatic GSH pool [54,64]. GSH contents in the human diet is highly variable; GSH-rich sources include fresh fruits, vegetables, and lean meat, while processed foods, grains, or dairy products generally have low GSH [66,67]. Therefore, dietary habit would contribute to the variability of sulfur amino acid and GSH levels and varied degree of oxidative stress in the lumen and plasma [68].
The luminal GSH pool is important in intestinal absorptive and detoxication functions and in protecting the mucus layer [65]. In this regard luminal GSH participates in dietary disulfide reduction and uptake [69] and in maintenance of mucus fluidity [70]. Mucins, which comprised the matrix/mucus gel layer, are glycosylated glycoproteins that assemble into homo-oligomeric structures via disulfide bonds formation between cysteine-rich domains. Thus, mucus viscosity can be modulated by GSH [71,72] and NAC [73] through reduction of disulfide bonds and disassembly of mucin oligomers. In addition, the hydration of the mucus layer can indirectly be influenced by transepithelial GSH transport via CFTR (cystic fibrosis transmembrane conductance regulator) channel and altered electrolyte (chloride and bicarbonate) fluxes [74,75]. Mucus-associated GST [76] catalyzed the conjugation-detoxication of luminal reactive electrophiles, carcinogens, drugs or food preservatives and prevent damage to the intestinal epithelium [77]; luminal GSH levels (250 μM) can stimulate GST-dependent conjugation reactions by 300% [76]. However, GSH can bind divalent metals like Cu, Se, Cr or Zn, and non-enzymatic oxidative damage to the intestinal epithelium can occur as a consequence of metal-mediated ROS production [78]. Of significance, therapeutic use of iron complexes could result in increased availability of free iron in the intestinal lumen [79]. Since ascorbic acid is typically administered in combination to increase iron bioavailability from storage ferritin [80], the potential for H2O2 generation is enhanced through Fe-catalyzed two-electron oxidation of ascorbate [81]. During iron/ascorbate supplementation, an elevated consumption of the luminal GSH pool for ascorbate reduction and ROS scavenging could impose a significant stress on the redox buffering capacity of the intestinal lumen.
Mucosal antioxidant defense are mediated by GSH-dependent enzymes including GR, Gpx, Grx, and GST that are ubiquitously present along the intestinal tract. Among these, GR and GRx (thiol-disulfide oxidoreductases) reduce GSSG or GSH-mixed disulfides in targeted proteins through thiol-disulfide exchanges [82]. Mammalian Grx isoenzymes exhibit different catalytic properties and are localized to various subcellular compartments of cytosol (Grx1) and mitochondrion (Grx2(a)); however, two Grx2 variants, Grx2b and Grx2c, were identified in the cytosol and nucleus. The monothiol Grxs (cytosolic Grx3, mitochondrial Grx5) lack thiol-disulfide oxidoreductase activities; Grx3, also called PICOT or TXNL-2, contains two [2Fe-2S]2+ clusters and reportedly functions as redox sensors for ROS/RNS signals rather than participate in redox reactions [83]. All Grx isoforms have been detected in mouse intestine with high expression of Grx2 in duodenal enterocytes and preferred localization of Grx5 and Grx3 to apical and apical/lateral surfaces, respectively [84]. Similarly, Grx2 is highly expressed in jejunal enterocytes, Paneth and certain lamina propria cells [84]. The location of nuclear Grx5 in enterocytes, and its presence in Paneth cells and colonic epithelium [84] suggest a role for this isoform in intestinal/colonic redox reactions. The pathophysiolgical or clinical significance of the existence of defined intestinal Grx isoenzymes and their subcellular distribution remains to be investigated.
Of the eight characterized mammalian Gpxs (Gpx1–8), most are selenoproteins (Gpx1, Gpx2, Gpx3, Gpx4, and Gpx6) with different subcellular locations [85]. Gpx-catalyzed removal of H2O2 is kinetically fast (~ 5×107 M−1 s−1), thus rendering the elimination of H2O2 an efficient process even at high hydroperoxide concentrations. Gpx1 and Gpx2 are the major H2O2 reducing enzymes in the intestinal epithelium [86]. Although these isoenzymes have similar substrate specificity and cytosolic location, Gpx1 is uniformly distributed along the crypt-villus axis, while Gpx2 predominates in the crypt [86,87]. In addition, Gpx2, previously known as GSHPx-GI [88], is an intestinal specific GSH peroxidase that is highly expressed in the ileum and cecum [89]. A notable distinction between Gpx1 and Gpx2 is their sensitivity to selenium (Se) deprivation. Unlike Gpx2, Gpx1 protein and mRNA expression are highly vulnerable to Se deficiency and is rapidly degraded; the relative resistance of Gpx2 to Se variation [90] suggests the importance of this isoenzyme in intestinal cell survival. Gpx3, an extracellular Gpx isoform secreted by intestinal cells into the lumen, reportedly participates in protecting the intestinal mucosa against oxidant injury [91]. Gpx4, the only Se-Gpx protein known to reduce phospholipid hydroperoxides, is present in the cytoplasmic and nuclear compartments of human small intestine and colon [92]; interestingly, Se deficiency has no effect on Gpx4 message level or its stability [90]. Pprotein expression and enzymatic activity of Gpx4 are notably increased in differentiated colonocytes in association with enhanced antioxidant protection, especially in the nuclear compartment [92].
GST proteins are abundant in the gastrointestinal tract. However, GST distribution varies considerably along the digestive tract and their activity and expression is influenced by diet, drug exposure, and clinical conditions [93]. Human GST multigenic family of isoenzymes is divided into the Alpha, Mu, Pi, Omega, Theta, Zeta, Sigma, Kappa, and microsomal GSTs [94]. Typically, GST activity and the expression of GST isoforms, such as Pi and Mu are lower in the colon as compared to the small intestine [93], a fact that may explain the rare incidence of neoplasia in the small intestine given that GST catalyzes GSH-dependent detoxication of luminal electrophiles and carcinogens. In human colon, the highest expressed isoform is GST Pi [95]; GST Mu is also abundant in primary colonocytes [96] while GST alpha expression is low. The decrease in GST activity from proximal to distal colon [93] is consistent with decreased colonic xenobiotic detoxication and increased cancer risk.
Luminal Cys/CySS redox status
Aside from GSH, the Cys/CySS pool plays an important role in regulating the luminal thiol-disulfide redox state [65] and preserving the redox status of extracellular proteins [97]. In rat intestine, GSH hydrolysis, catalyzed by γ-glutamyl transpeptidase and dipeptidase (Figure 2) accounted for ~40% of luminal Cys. Luminal Cys exhibit dual functions in facilitating the absorption of diverse redox sensitive nutrients [98], and in maintaining the fluidity and integrity of the intestinal mucus layer [70]. Redox control of extracellular surface proteins by luminal Cys/CySS is believed to regulate signaling processes at the apical plasma membrane [99]. Cys/CySS homeostasis is maintained by dietary sources, the Cys/CySS shuttle, and the degradation of luminal GSH (Figure 3). A recent study with minipigs found that ~60% of dietary cysteine was captured and sequestered by the small intestine, and only a small portion was released into the blood [100]. Dietary methionine, another sulfur amino acid, can be taken up by enterocytes at the brush border membrane, and through intracellular trans-sulfuration, yields Cys that supports the synthesis of mucosal GSH and extracellular mucin secreted by goblet cells [23]. It is suggested that the requirements for these processes govern the rate of methionine trans-sulfuration [101,102].
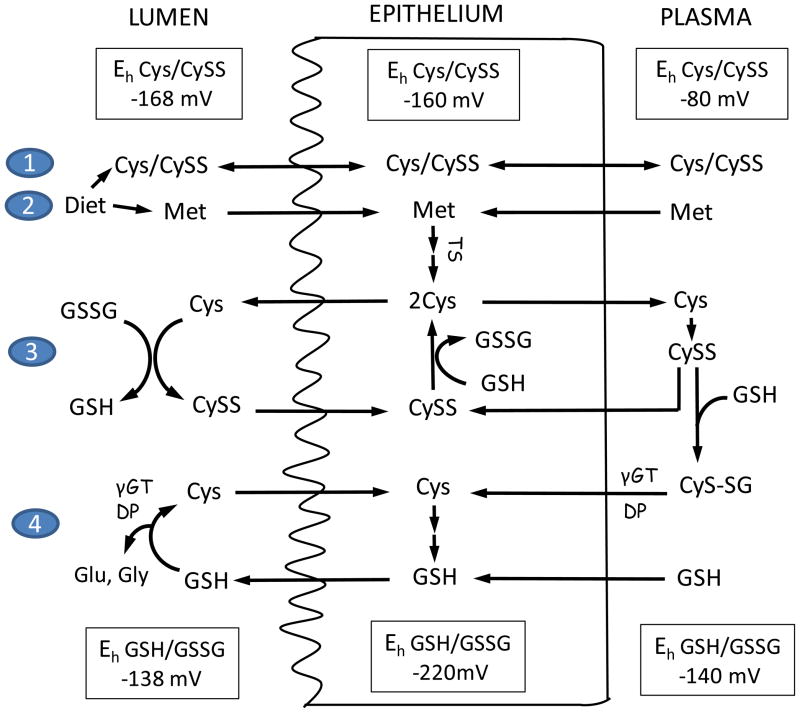
Figure 3. Homeostatic control of Cys/CySS redox status in intestinal lumen, intestinal epithelium, and plasma.
At the brush-border membrane, uptake of dietary Cys and methionine (1 & 2); Cys/CySS shuttle (3), and γ-glutamyl transferase (γGT) and dipeptidase (DP)-catalyzed hydrolysis of extracellular GSH (4) participate in Cys homeostasis. The Cys/CySS shuttle and luminal GSH hydrolysis maintains the Eh for luminal Cys/CySS at −168mV and that of GSH/GSSG at −138mV. Within the intestinal epithelium, CySS is reduced by GSH, the resultant Cys is exported or utilized in GSH synthesis. Additionally, intracellular Cys is increased through the trans-sulfuration (TS) of imported dietary or circulatory methionine (Met). Plasma Cys exists mainly as CySS, and the redox state of plasma Cys is controlled by thiol/disulfide exchange with liver-derived GSH. The hydrolysis of Cys-GSH mixed disulfide (CyS-SG) releases Cys which is taken into enterocytes by basolateral membrane associated transporters. The Eh for Cys/CySS and GSH/GSSG redox couples are tightly regulated at values of −80mV and −140mV, respectively. Luminal values of Eh for Cys/CySS and GSH/GSSG redox couples were taken from [69] and the plasma values were from [53,106].
Cys was found to be released at a high rate into the lumen of the vascularly perfused rat small intestine in situ [65] where it reduced GSSG to GSH with resultant formation of CySS. A more reduced luminal Eh for Cys/CySS than that for GSH/GSSG is consistent with the function of extracellular Cys in luminal GSSG reduction [69]. CySS uptake across the brush border membrane [103] was reduced intracellularly by GSH and Cys is released back into the lumen, thus constituting a Cys/CySS shuttle (Figure 3), a pivotal mechanism in controlling the luminal thiol-disulfide redox status [69]. The transport of Cys and CySS at the apical and basal membranes [104,105] is facilitated by sodium-dependent and independent carrier systems. These include the apical amino acid transporters: b0,+, X−AG, ASC, respectively, neutral and cationic amino acid transporter, aspartate/glutamate anionic amino acid transporter, and alanine/serine/cysteine carrier system, as well as basal amino acid transporters: L, y+L, ASC, respectively, leucine and large hydrophobic neutral amino acid carrier system, cationic amino acid transporter and alanine/serine/cysteine carrier system [104,105]. Characterization of the apical and basal thiol-disulfide redox control in polarized Caco-2 epithelial cells revealed that the regulation of Eh for Cys/CySS at the basal and apical surfaces occurred at different rates [105]. Moreover, the regulation of the extracellular Cys/CySS redox status on the basal surface was mediated by the y+L and x−c system [105]. What this means is that basal surface proteins, such as receptors or transporters could exhibit greater redox sensitivity than proteins situated at the apical surface and that redox signaling events at these opposite polar membrane surfaces would be independently controlled.
Previous studies have shown that the redox couples of Cys/CySS and GSH/GSSG in the plasma are considerably displaced from equilibrium and their Eh are tightly regulated to the values of −80mV and −140mV, respectively [19,53]. Cys in plasma predominantly exists as CySS which undergoes thiol-disulfide exchange with GSH released from the liver, a process that is important for maintaining the extracellular/plasma Eh for the Cys/CySS redox couple [106]. γ-glutamyl transferase- and dipeptidase- catalyzed hydrolysis of CySSG [107] and intestinal methionine uptake from the arterial blood [108] have been described as mechanisms that contribute to the homeostasis of plasma and intestinal Cys/CySS redox status (Figure 3).
Intestinal Trx redox system
The presence of a full complement of Trx proteins suggests their function in antioxidant defense and redox regulation in the intestinal tract [84]. A notable role for mucosal Trx is in gut immune response. Trx expression is particularly high in the intestinal mucosa [109], and is reportedly involved in redox regulation of human β-defensin 1 (hBD-1) which possesses antimicrobial activity [110]. Trx-catalyzed reduction of disulfide bridges in hBD-1 enhances hBD-1 killing activity against commensal bacteria and opportunistic fungi, consistent with Trx function in innate immunity [110]. Unstimulated lamina propria T lymphocytes (LP-T) similarly exhibit high expression of Trx which contributed to the regulation of intracellular redox homeostasis in these cells [111]. In stimulated LP-T cells, constitutive as well as inducible Trx function as amplifiers of the inflammatory response by promoting and sustaining T-cell cytokine production [111]. Potential Trx/TrxSS involvement in the etiology of intestinal disorders is a relevant topic in the redox pathobiology of the intestine that warrants further study.
Luminal commensal bacteria and intestinal redox state
A vast consortium of bacteria comprising 500–1,000 species and an estimated 108 to 1011–12 bacteria per ml of luminal content in the distal small intestine and the colon, forms a complex luminal microenvironment within the human intestinal tract [112,113]. The microflora of facultative bacteria (Enterobacteriacae, Enteroccocus, and Streptococcus) expands during infancy and creates a reducing environment that supports population of the gut by anaerobic strains (Bifidobacterium, Bacteroides, and Clostridium). By year 1, the gut microflora assumes the adult pattern of 100–1000 times greater anaerobes to aerobes [114,115]. The microbiota prevents colonization by exogenous pathogens, provides nutrients for the intestinal cells from undigested food [113] or regulates the mucosal immune system [116]. A “dysbiotic” flora characterized by an abundance of pathogenic species leads to aberrant mucosal immune responses that contribute to chronic inflammatory intestinal disorders [116]. The diverse microbiota metabolic profiles under various nutrient conditions directly affect functions of the host intestinal tract such as lipid, carbohydrate, and amino acid metabolism [117] at different anatomical locations [113]. For instance, elevated threonine, a mucin constituent is cytoprotective against gastric acid injury in the duodenum while elevated phosphocholines, GSH, taurine and betaine support bile acid and lipid metabolism in the ileum [118]. Jejunal GSH levels of germ-free mice inoculated with human infant microbiota, were similar to those of conventional mice despite decreased constituent amino acid levels, suggesting that microbiota can modulate jejunal GSH synthesis [118].
The relationship between bacteria-host interaction and intestinal redox biology is not completely understood and is of considerable research interest. Much attention is focused on hydrogen sulfide (H2S), a product of methionine and cysteine transsulfuration [119]. While bacteria production of H2S are in millimolar range [119], H2S is largely catabolized within the lumen [120,121]. H2S at high concentrations inhibits mitochondrial cytochrome oxidase [122], a process that would trigger ROS production and intracellular redox changes. However, in HT-29 cells, mitochondrial sulfide quinone reductase (SQR) catalyzed the oxidation of H2S to sulfide, a substrate of similar effectiveness as succinate in stimulating mitochondrial respiratory rate [123]. Moreover, unique to colonic cells, reverse electron flow between SQR and complex I yields NADH [124]. Thus, the removal of H2S/sulfide is a priority for colonic cells to prevent mitochondrial inhibition. As yet to be demonstrated in intestinal cells, H2S was shown to scavenge ROS in neuronal cells [125], promote intraneuronal GSH production through stimulation of cystine/cysteine transport, and protect against H2O2-induced oxidative stress via redistribution of newly synthesized GSH to the mitochondria [126]. The question of how the microbiota controls intestinal redox signaling is unresolved; emerging evidence implicates a role for bacteria-induced mucosal ROS generation (see section on NF-κB signaling).
Redox modulation of intestinal cell fate: proliferation, differentiation, and apoptosis
Cellular redox potential and normal phenotypic cell transition
The intestinal epithelium turns over continuously and the canonical Wnt/β-catenin and Notch signaling pathways have been identified as major determinants of intestinal stem cell commitment to one of the four specialized intestinal epithelial cell types (for review [4,127]). Major functions of the canonical Wnt/β-catenin pathway include maintenance of crypt stem/progenitors proliferation via cell cycle control and inhibition of differentiation, migration of epithelial cells along the crypt-villus axis, and development of secretory lineage and terminal differentiation of Paneth cells in the crypt. Thus, an active Wnt pathway is consistent with intestinal stem cell proliferation, a pre-requisite for cell differentiation and migration toward the villus tip. Furthermore, the Notch signaling cascade controls cell fate evolution to absorptive or secretory lineages. In addition, cell proliferation and the determination of cell fate are also controlled by bone morphogenic protein (BMP) and PI3K/Akt signaling [128,129]. The mechanism of how these signaling pathways interact to maintain intestinal homeostasis and the implication of dysfunctional signaling for intestinal disorders is not completely understood. Recent evidence suggest that ROS and redox mechanisms could coordinate the interplay of Wnt and Notch signaling in controlling the balance between goblet and absorptive cell phenotypes and the proliferative responses in the colon [130]. Direct relationships among NADPH oxidase 1 (NOX-1)-dependent ROS generation, differential activation of Wnt/β-catenin or Notch signaling pathways, and intestinal proliferation and lineage commitment were demonstrated [130].
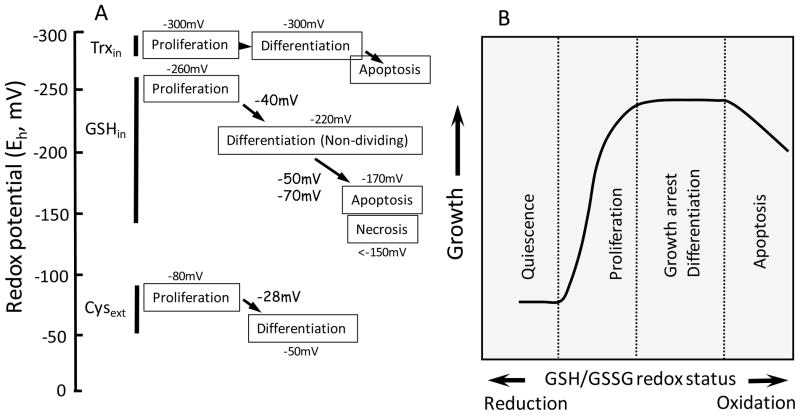
Quantitative changes in GSH/GSSG and Cys/CySS redox potentials have been correlated with normal intestinal phenotypic cell transitions; a reducing redox environment favors proliferation, whereas an oxidized milieu favors growth arrest and differentiation. A highly oxidized redox environment associates with cells undergoing apoptosis or necrosis. Specifically, Eh for GSH/GSSG varies from −260mV to −240mV at proliferation, switches towards more oxidizing conditions (−220mV to −200mV) at differentiation and growth arrest, and is typically −170mV or more oxidized (−150mV) at apoptosis or necrosis (Figure 4A) [131,132]. Similar changes in Eh of extracellular Cys/CySS redox couple from −80mV to −50mV are associated with the transition of cells from proliferation to a growth-arrested, non-dividing or differentiated state [99] (Figure 4A). In contrast, intestinal cell transition is associated with little or no change in the Eh of the Trx/TrxS redox couple [133]. While the progression of Caco-2 cells from a proliferative to a differentiated state was accompanied by 40mV and 28mV oxidation of intracellular GSH/GSSG and extracellular Cys/CySS redox status, respectively, the Eh of the Trx/TrxSS redox system remained essentially unchanged [133], at a constant reduced potential of −300mV (Figure 4A) [18,21,133].
Figure 4. Redox potential (Eh) of thiol redox couples during normal phenotypic transition of intestinal cell (A) and cellular responses to changes in the cellular GSH/GSSG redox status (B).
A. Normal intestinal cell transitions from proliferation to differentiation or growth arrest, and apoptosis are associated with increased oxidation of the redox potentials (Eh) of the intracellular GSH/GSSG (GSHin) or extracellular Cys/CySS (Cysext) redox couples. A 40mV oxidation (from −260mV to −220mV) in Eh for cellular GSH/GSSG is associated with cell transition from proliferation to differentiation. An additional 50mV or 70mV oxidation characterizes apoptotic or necrotic cells, respectively [18]. Similarly, an increase in 28mV oxidation of Eh for extracellular Cys/CySS (from −80mV to −50mV) accompanies intestinal cell progression from proliferation to differentiation [99]. In contrast, the Eh for intracellular Trx/TrxSS (Trxin) remains unchanged at −300mV through cell proliferation and differentiation [99], but significant Trx 1 oxidation results in cell apoptosis. B. Fully differentiated intestinal cells typically exhibit a biological constraint to proliferate due to a mitotic block and are arrested in a quiescent state. An imposed change in the status of the cellular GSH/GSSG redox couple during oxidative challenge can initiate entry into cell cycle, differentiation/growth arrest, or apoptosis, depending on the duration and severity of the redox shift.
Our laboratory has established that the cellular GSH/GSSG redox status governs cell transitions from quiescence to that of a proliferative, growth arrested, differentiated or apoptotic states in intestinal and other cell types [131,134] (Figure 4B). Fully differentiated epithelial cells are typically arrested in the quiescent state, but mitotic-competent enterocytes maintain a genetic program that signals proliferation in response to an altered redox stimulus. For instance, an increase in GSH oxidation or lower GSH/GSSG ratio allows entry into cell cycle likely through overcoming regulatory checkpoints (G0/G1 transition) and mitotic block; depending on the severity of the redox shift, proliferation, growth arrest, differentiation, or apoptosis may predominate (Figure 4B). Similarly, cell transitions are modulated by the extracellular Cys/CySS redox status [99,131,134,135]. Culturing Caco-2 cells in media with variable Eh for Cys/CySS (0 to −150mV), elicited proliferation rates (measured by DNA synthesis) that were 2-fold higher in reduced (−150mV) as compared to oxidized conditions (0mV) [99]. The interaction between the GSH/GSSG and Cys/CySS redox systems in the redox control of the intestinal cell’s phenotypic fate is poorly understood; importantly, these two redox systems are displaced from equilibrium and each functions independently in redox regulation [99,136,137]. Interestingly, we demonstrated that exogenous cysteine promoted CaCo-2 cell proliferation only under conditions of GSH depletion, but not GSH repletion, yet the proliferative response is independent of intracellular GSH concentration or of cysteine-mediated de novo GSH synthesis [136]. Moreover, oxidation of the extracellular Cys/CySS pool, without a change in the cellular GSH/GSSG redox status, was sufficient to elicit CaCo-2 proliferation via redox activation of growth receptors at the plasma membrane [99]. Although the Eh of Trx/TrxSS remained essentially unchanged through cell proliferation and differentiation [133] (Figure 4A), oxidation of Trx1 (by ROS/RNS) resulted in activation of MAP kinase signaling and colonic cell apoptosis [138].
Manipulation of intracellular and extracellular redox environment induces intestinal cell transition
An imposed shift in the redox environment, such as occurs during oxidative stress, can induce the exit of cells from normal quiescence into cell cycle or cell apoptosis [131,134]. In early studies, we validated the notion that for a given oxidant, such as lipid hydroperoxide (LOOH), a different phenotypic outcome can result from titrating the oxidant (LOOH) load and the associated extent of GSH/GSSG redox disruption [139]. At low levels of LOOH (1–5 μM) an early loss of GSH-to-GSSG redox balance triggered a proliferative response in Caco-2 cells as evidenced by increases in ornithine decarboxylase activity, DNA synthesis, and expression of cyclin D1/cyclin-dependent kinase 4, consistent with transition from G0/G1 to S phase [139]. Higher LOOH doses (10–50 μM) resulted in severe GSH redox imbalance that mediated Caco-2 cell apoptosis as evidenced by mitochondrial dysfunction, caspase 3 activation, and DNA fragmentation [140]. Interestingly, induction of sustained GSH/GSSG redox imbalance (24h) induced Caco-2 growth arrest, specifically the blockade of cells at the G1-to-S transition and G2/M phases of the cell cycle [139,141]. Parallel studies in rat intestine in vivo supported our hypothesis that intestinal epithelial cell transition is modulated by mucosal GSH/GSSG redox status. The proliferative and apoptotic phases of intestinal epithelial cells follow a circadian rhythm under physiological conditions that was correlated with the feeding and post-prandial periods [142,143] wherein feeding stimulated ornithine decarboxylase activity and epithelial cell growth [142] while apoptosis was initiated post-prandially upon cessation of feeding [143]. Chronic administration of a peroxidized lipid diet for 2–8 weeks significantly disrupted mucosal GSH-to-GSSG redox status, and induced a cytostatic state in the intestine, indicating that impairment of normal epithelial turnover occurred in conjunction with the loss of basal mucosal GSH/GSSG balance [144,145]. LOOH-mediated disruption of normal intestinal turnover activity was abrogated by restoration of mucosal GSH/GSSG redox status through exogenous GSH supplementation [145]. Collectively, our studies underscore the centrality of mucosal GSH/GSSG redox status in intestinal cell responses; the final phenotypic outcome is a function of the magnitude and the duration of GSH redox disruption.
The addition of exogenous growth factors like insulin-like growth factor-I, epidermal growth factor (EGF), or the amino acid glutamine contributed to a more reduced extracellular Cys/CySS Eh which stimulated CaCo-2 cell growth [99,135]. This finding suggests that changes in extracellular redox environment induced by the Cys/CySS redox couple was connected with activation of redox signaling cascade at the plasma membrane [99,135]. Indeed, the most reduced Eh for Cys/CySS couple (−150mV) has been shown to increase EGFR phosphorylation which triggered p44/42 MAPK signaling and cell growth [146]. We similarly demonstrated a potentiation of ornithine decarboxylase activity by EGF administration in rat small intestine and colon previously challenged by chronic lipid hydroperoxide exposure [99,135]. The suggestion that mild oxidative stress, through modulating the extracellular Cys/Cyss redox state, could prime the intestine for EGF-induced EGFR phosphorylation and hyperproliferation is an intriguing notion that requires experimental validation.
Under certain conditions, such as massive small bowel resection, a more oxidized colonic GSH/GSSG and Cys/CySS redox status has been shown to promote mucosal growth in rat colon [147]. Moreover, BSO-induced decreases in colonic GSH and Cys significantly stimulated colonic mucosal growth as evidenced by crypt depth, cell/crypt and increased DNA synthesis without an effect on cell apoptosis [147]. Despite massive depletion of colonic tissue GSH and Cys and an oxidized Eh of plasma GSH/GSSG, these rats exhibited increased plasma Cys and CySS levels, suggesting that elevated plasma Cys/CySS redox status may be responsible for the stimulation of mucosal proliferation [147]. This finding raises the interesting, heretofore untested notion that the initiation of redox signaling of proliferative cascades originates from the basal polar of the intestinal epithelium, possibly by altering the plasma Cys/CySS redox status (see Figure 3). Subsequent studies revealed that local GSH redox status did not influence the adaptive growth of the remnant intestine following small bowel resection [148], and that the stimulation of ileal mucosal growth in rats supplemented with dietary sulfur amino acid was through reduction of the mucosal thiol/disulfide redox state [149].
Intestinal oxidative challenge and role of GSH
The intestinal epithelial barrier is constantly challenged by diet- and endogenous-derived oxidants that can accumulate and disrupt mucosal redox control with deleterious consequences. Examples of luminal oxidants include products of lipid peroxidation and organic hydroperoxides [150], toxic metals like aluminium [151], and redox cycling compounds like menadione [152]. Enterocytes are replete with GSH- and Trx- dependent systems in all segments of the intestinal tract that function to preserve cellular redox homeostasis and intestinal cell integrity [84,93]. Using the conscious, lymph and bile fistula rat model, we demonstrated that mucosal GSH is crucial in the intestinal elimination of luminal peroxidized lipids and the prevention of lymphatic peroxide transport [55,56]. Enhanced accumulation of lipid peroxides [56] in enterocytes and in intestinal lymph was associated with decreased mucosal GSH concentrations, consistent with attenuated Gpx-catalyzed peroxide catabolism [56,131]. Elevated luminal GSH, either through exogenous GSH supplementation or from biliary GSH output, promoted luminal lipid peroxide uptake and reduced lymphatic peroxide transport, the result of increased intracellular GSH-dependent peroxide metabolism [56,131]. Studies in freshly isolated rat enterocytes further revealed that intestinal metabolism of organic hydroperoxides subscribes to regulation by glucose availability for the production of NADPH that is pivotal for GR-catalyzed reduction of GSSG [153]. Significantly, NADPH generation from glucose flux through the pentose phosphate pathway was rate limiting in hydroperoxide catabolism, consistent with reductant supply as a major determinant of peroxide detoxication. Interestingly, the activities of enterocyte Gpx and GR did not appear to be limiting factors under these conditions [153] and in other pathophysiological conditions of diabetes [153], chronic hypoxia [153], and dietary peroxide intake [153]. Collectively, these findings underscore the centrality of GSH availability and/or the capacity for its regeneration, i.e., NADPH production, in intestinal hydroperoxide detoxication.
In colonic CaCo-2 or HT-29 cell lines, significant loss of cellular GSH either through oxidative or non-oxidative processes was associated with colonic cell apoptosis, and mitochondrial GSH (mtGSH) is a major player in colonic cell survival. A consistent observation was that an early spike in GSSG after peroxide (tert-butyl hydroperoxide, tBH) treatment was linked to the activation of the mitochondrial apoptotic cascade [154,155]. Importantly, apoptotic initiation occurred within a narrow window of GSH/GSSG redox shift (15–30 min post-oxidant exposure) that preceded cell death at 24h; subsequent restoration of GSH redox homeostasis did not prevent the apoptotic outcome [140,155,156]. The observation that an early loss of GSH/GSSG redox balance can trigger apoptotic signaling also characterizes tBH-induced apoptosis in other cell types, such as neuronal-like PC12 cells [157,158], which suggests that GSH oxidation is likely a common early event in oxidative stress mediated apoptosis in different cell types. Interestingly, induction of GSH loss by non-oxidants, such as staurosporine (STP), a broad spectrum inhibitor of protein kinases [156] can elicit colonic HT-29 cell apoptosis [156]. STP-induced GSH efflux and cell apoptosis was linked to caspase 3 activation, but independently of caspase 8 or 9 involvement [156], thus suggesting participation of a non classical pathway of apoptosis. Moreover, STP-induced GSH export was driven by γ-glutamyl transferase-mediated extracellular GSH hydrolysis without changes in the cellular GSH/GSSG ratio [156]. Precisely how GSH exit is coupled to the initiation of apoptotic signaling at the plasma membrane is an unresolved question that needs to be addressed.
Mitochondrial GSH control and intestinal integrity
Mitochondria involvement in oxidant-mediated cell apoptosis is well established [24,159], and early studies demonstrated that mitochondrial GSH (mtGSH) depletion sensitizes cells to oxidant-induced cell injury [160]. Mechanistically, the loss of mtGSH causes mitochondrial transition pore opening [161], inhibition of respiratory complexes [162], decreased ATP [155], and increased ROS production [163], that collectively lead to cell apoptosis [155,164]. Matrix GSH homeostasis is acquired through active GSH transport from the cytosolic compartment [31]. The DIC and OGC carriers are the main contributors [31,162,165], but a tricarboxylate carrier also accounted for mtGSH uptake in brain mitochondria [166]. Mitochondrial membrane dynamic plays an important role in the proper function of OGC and DIC carriers [165]; factors that impair membrane fluidity (e.g. cholesterol deposition, ceramide production, changes in phospholipid content) decreased membranal transport and induced mtGSH depletion [167,168]. This mechanism of selective mtGSH loss was implicated in acetaminophen- and TNF-α-induced apoptosis in hepatocytes [169,170] and amyloid β peptide-induced neuronal inflammation and toxicity [171]. In contrast, overexpression of DIC or OGC transporters, such as in rat renal proximal tubular NRK-52E cells, protected against tert-butylhydroperoxide- or S-(1,2-dichlorevinyl)-L-cysteine-induced cell apoptosis [172,173], thus underscoring the role of mtGSH in preserving cell survival.
Interestingly, OGC and DIC-mediated mtGSH transport in liver and kidney mitochondria is promoted by high mitochondrial ATP and energetic status [31,174], consistent with the role of these carriers in the transport of both mtGSH and respiratory substrates. Indeed, dicarboxylates such as malate, malonate, butylmalonate and phenylsuccinate are effective inhibitors of mtGSH uptake [31,175]. A close link between the matrix status of GSH and respiratory substrates is evidenced by a parallel increase in mtGSH levels and high mitochondrial flux of intermediary metabolites in the hypermetabolic remnant kidney following nephrectomy of the hypertrophied kidney; enhanced DIC and OGC activity was suggested to be a major cause of this high mtGSH uptake [176]. Mitochondrial respiratory substrates additionally regulate mitochondrial redox status through supply of reducing equivalents for NADPH-dependent reductive processes [177]. In isolated rat brain mitochondria, substrates such as succinate, malate and/or glutamate were shown to elevate mtGSH levels and NAD(P)H-dependent reduction of mitochondrial GSSG and S-glutathiolated proteins [177]. How these mechanisms contribute to preserving redox homeostasis and integrity of an oxidant-prone mitochondrial compartment [155] in intestinal cells remains unresolved.
In recent studies, we established the importance of mtGSH in preserving intestinal mitochondrial genomic [154] and functional integrity [155] in colonic NCM460 and HT29 cells against oxidative challenge by the redox cycling quinone, menadione (MQ). Enhancement of matrix GSH levels through promoting OGC and DIC expression and cytosolic GSH synthesis prevented against MQ-induced mitochondrial dysfunction, oxidative mitochondrial DNA damage and apoptosis [154,155]. Mitochondrial demise was accentuated by blockade of mtGSH transport and inhibition of cytosolic GSH production [155]. Interestingly, specific inhibition of DIC and OGC transport per se resulted in an increase in baseline mtGSH, suggesting that these carriers may function in mitochondria-to-cytosol GSH efflux [155], an intriguing notion that warrant further investigation. Significantly, the loss of mitochondrial GSH/GSSG redox balance was associated with a disrupted cellular pyridine nucleotides (NAD+/NADH, NADP+/NADPH) redox status that compromised cellular ATP, mitochondrial respiratory activity, and NADPH-dependent reducing capacity in colonic HT-29 cells [178]. Such redox and metabolic disruptions were corrected in part by the glucose-sparing mitochondrial substrates, succinate and glutamate [178], which underscores the quantitative importance of glucose in reductant (NADPH) supply.
Altered tissue redox status in intestinal inflammation and associated pathology
Intestinal GSH/GSSG redox state in tissue inflammation and pathology
An altered intestinal redox status is a recognized risk factor in the development of gut pathologies. Inflammatory bowel diseases, intestinal cancers, or oxidative conditions like diabetes are often associated with attenuated intraepithelial GSH status that contributes to disease progression and exacerbation of the pathological states. Chronic inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by chronic inflammation of the intestinal tract [179,180]. As such, excessive ROS generation by infiltrated inflammatory cells (macrophages and neutrophils) in the inflamed gut of IBD patients leads to oxidative stress, reportedly a pivotal factor in the onset and development of chronic gut inflammation. Elevated tissue GSSG have been correlated with the severity of mucosal inflammation [181,182], and decreased GSH synthetic enzymes and lower precursor Cys levels contributed to diminished mucosal GSH concentrations in IBD patients [183]. Significantly lower levels of GSH were similarly found in inflamed ileal mucosa from patients with CD [181], and in colonic mucosa of active UC disease state [184].
The mechanism by which an impaired epithelial redox environment signals inflammatory disease initiation and/or progression is unclear. It is generally thought that intestinal oxidative stress is secondary to the inflammatory process; however, we previously demonstrated that the loss of mucosal GSH/GSSG redox balance preceded the onset of colonic inflammation and manifestations of clinical colitis in SCID mice reconstituted with CD4+CD45RBhigh T-lymphocytes, an immune-based mouse model of experimental UC [185]. This finding suggests the involvement of redox dependent mechanisms in UC development. Supporting evidence comes from the finding that antioxidant therapy that counteracted ROS production and restored tissue redox homeostasis attenuated disease manifestations [185,186]. Unexpectedly, antioxidant therapy (vitamin C, vitamin E, GSH) in HLA-B27 transgenic rats, a model of IBD, was without beneficial effect despite low colonic mucosal GSH levels and high neutrophil infiltration [187]. One explanation could be that there was little evidence of tissue oxidative stress due to compromised neutrophilic respiratory burst in these mutant mice. Overall, these results support a link between oxidative stress, mucosal GSH redox status, and intestinal inflammation; however, establishment of causality between intestinal GSH/GSSG redox disruption and IBD development requires further study.
Precisely how altered redox signaling contributes to the onset of immunological response associated with chronic inflammation is not completely understood. Increasing evidence suggests that the redox milieu in the lamina propria is an important determinant of LP-T proliferation and of their responsiveness to luminal bacteria [188]. In normal gut, the hyporeactivity of LP-T against luminal flora is evidenced by their low proliferative capacity [189] due to a low intracellular GSH [190]. LP-T activation and proliferation rely on a reducing extracellular environment that is provided by antigen presenting cells [191]; resident lamina propria macrophages are unable to secrete cysteine, and are therefore unable to stimulate LP-T [192]. Furthermore, ROS-mediated NF-κB-dependent activation of an pro-inflammatory response was attenuated by a constitutively higher Trx1 redox status in LP-T cells as compared to peripheral blood T cells [111]. Oxidative damage to the epithelia caused the recruitment of cysteine-secreting blood-borne macrophages which increased LP-T GSH levels and induced LP-T proliferation [190]. The subsequent transition of T cells from a bacteria-tolerant to a reactive state creates and perpetuates a sustained inflammatory phenotype in the lamina propria [193]. Consistent with this scenario, IBD patients exhibit increased CD14+, cysteine positive macrophages as well as CD3+ LP-T cells with elevated intracellular GSH [190].
NF-kB signaling and intestinal inflammation
IBD is associated with activation of the NFκB pathway in intestinal mucosa [194,195] and lamina propria macrophages [196], as well as the overproduction of NF-κB-dependent pro-inflammatory cytokines [196,197]. In quiescent cells, members of the NF-κB family (RELA, RELB, REL, p50 and p52) exist as inactive dimers in association with the inhibitor of NF-κB protein (IκB). Inflammatory mediator induced NF-κB activation is achieved through IκB phosphorylation by the IκB kinase (IKK) complex (consists of IKKα, IKKβ catalytic subunits and NEMO regulatory subunit), dissociation of NF-κB-IκB interaction, nuclear translocation of NF-κB, and transcription of proinflammatory and/or anti-apoptotic genes. The relationship between NF-κB signaling and intestinal inflammation is highly complex and not fully resolved; the reader is referred to several excellent recent reviews on the subject [196–198]. As an example, while the inhibition of IKKβ-dependent NF-κB activation attenuated chronic inflammation, acute inflammation was enhanced [199], suggesting that duration of signaling is likely a pivotal factor in disease outcome. Other interesting observations support an opposing notion to a generally accepted paradigm for a pro-inflammatory function of NF-κB. For instance, NF-κB dysregulation in myeloid cells mediates inflammatory cell influx into the intestinal epithelia and elicits a pro-inflammatory response [199]. Mice deficient in intestinal epithelial cell (IEC) NEMO protein (NEMOIEC-KO) were characterized by excessive epithelial cell apoptosis, despite presenting with widespread colonic inflammation, innate immune cell infiltrate and spontaneous colitis early after birth [200]. Thus it appears that normal NF-κB signaling in these animals would be toward upregulation of anti-apoptotic genes and epithelial survival.
The redox mechanisms of NF-κB activation in intestinal epithelium are poorly characterized. ROS mediated oxidation or S-glutathiolation of redox-sensitive cysteines of NF-κB subunits can activate or inhibit signaling (see reviews [201,202]). Moreover, NF-κB-nuclear DNA binding depends on the redox status of Cys-62 of the p50 subunit; oxidation of this cysteine significantly decreased transcription of NF-κB-dependent genes [203] while its reduction by nuclear Trx1/Ref-1 system restored NF-κB binding affinity [204,205].
Emerging evidence implicates a role for ROS in the cross-talk between the microbiota and NF-κB redox signaling in intestinal epithelia [206]. Mitochondria-derived ROS caused by butyrate, a bacterial fermentation product of polysaccharides, was shown to mediate transient alteration of the redox status of cytosolic and mitochondrial Trx that promoted NF-κB inhibition [207]. Similarly, bacteria-intestinal epithelial cell co-culture resulted in oxidation of intestinal Trx and GSH by NADPH oxidase-derived ROS that modulated ubiquitin-like conjugating enzymes (cullin-1 and Ubc12) and NF-κB inactivation [206]. Specifically, H2O2-mediated oxidation of Ubc12 redox-active cysteine prevented TNFα–induced nuclear translocation of the p65 subunit and blocked the inflammatory response that was reversed by NAC treatment [206]. This means that commensal bacteria modulation of the ubiquitin-proteasome system through mucosal ROS could influence the epithelial outcome of NF-κB signaling. Furthermore, bacteria-induced intestinal ROS generation was shown to inactivate low molecular weight protein tyrosine phospatase (LMW-PTP) and SHP-2 and promoted epithelial restitution and recovery from dextran sulfate-mediated injury in mice [208]. The Lactobacillus strain exhibits a higher capacity for ROS generation, and their luminal abundance can be deleterious for the epithelial layer due to altered NF-κB protective function, and exacerbation of inflammatory processes secondary to invasion of luminal bacteria into the mucosa. Microbiota composition can alter tissue susceptibility to intestinal pathogens. For instance, an enriched Bacteroides microbiota from C57BL/6 resistant mice protected against Citrobacter rodentium-induced colitis in C3H/HeOuJ susceptible mice through GSH/GSSG-mediated changes in inflammatory cytokines and systemic pathogen load [209]. It is unclear what caused the change in epithelial GSH redox, but an oxidative stress response was important for microbiota to modulate inflammation-associated subset of genes that enhanced pathogen clearance. It is notable that greater pathogenic bacteria strains comprised the intestinal microbiota of IBD patients [210]; in the TNBS-induced injury rat model of colitis, the administration of probiotic bacteria can significantly enhance epithelial GSH and attenuate mucosal inflammation [211,212]. Loss of commensal bacteria induces epithelial apoptosis that was exacerbated by oxidative stress (ischemia/reperfusion) mediated by NF-κB-dependent transcription of pro-inflammatory cytokines [213]. The collective evidence underscore a role for intestinal GSH/GSSG and redox signaling through NF-κB in preserving the fragile balance between the luminal microenvironment and epithelial survival.
The delay in colitis onset in IL-10-deficient mice due to loss of IKKβ in macrophages/neutrophils but not in epithelial cells suggests that infiltrated immune cells mediate chronic inflammation [199], sustained by NF-κB-dependent inflammatory cytokine production [214]. Binding of the bacterial endotoxin, lipopolysaccharide (LPS) to toll-like receptors on immune cell surface triggers NADPH oxidase-catalyzed ROS generation and NF-κB-dependent pro-inflammatory cytokine production, indicating that redox signaling also governs NF-κB activation of immune cells. LPS-induced intestinal inflammation was exacerbated in Srx1 [215], Prx3 [216], or Prx2 [217]-deficient mice and those whose macrophages that are deficient in nuclear factor-E2-related factor 2 (Nrf2), master regulator of antioxidant responsive genes [218]. These results suggest that excessive immune cell generation of ROS and inflammatory cytokines contribute to mucosal damage [219], and that NF-κB redox signaling is central to immune cell-mediated intestinal inflammation.
Mucosal redox status and other inflammation associated intestinal disorders
Chronic gut inflammation increases cancer incidence, and cancer development is attributed to a persistent state of oxidative stress and diminished ROS elimination. Low GSH content and increased levels of 4-hydroxy-2-nonenal, malonyldialdehyde, and 8-hydroxy-2′-deoxy guanosine are associated with tissue malignancy [220–222]. The modulation of GSH- and/or Trx-dependent enzyme levels and activities was reported to enhance cancer cell proliferation, migration and metastasis while evading apoptosis [222,223], underscoring a role for redox involvement. Compelling evidence indicates that altered states of redox couples and/or redox-dependent systems are common in cancers of the intestinal tract although contribution of individual/combined redox systems at cancer stages has not been defined. Up-regulation of Se-insensitive Gpx2 has been correlated with increased cancer cell proliferation and rapid growth of intestinal tumors [86]. Interestingly, increases in Gpx2 protein occurred only in the early and not the advanced stages of human colorectal cancer, suggesting differential functional roles for Gpx2 during cancer progression [224]. Aberrant and reduced expression of proteins that are sensitive to Se deprivation, such as Gpx1, Gpx3, and selenoprotein P (SePP) in colon cancers is consistent with the sensitivity of Se-proteins to dietary Se and the susceptibility of the colonic tissue to oxidative stress [225].
Genetically engineered mice deficient in Gpx genes have provided useful experimental models to evaluate the role of these Se-proteins in the pathogenesis of intestinal inflammation, colitis and cancer. Homozygous mice lacking the wild-type (WT) Gpx1 or Gpx2 genes exhibit no overt phenotype for pathological symptoms in the gastrointestinal tract [226]. However, mice that express one wild-type allele of Gpx1 (Gpx1+/− Gpx2−/−) presented with low incidence of IBD symptoms that were exacerbated by Se-deficiency [88]. Regardless of Se content, Gpx1−/− Gpx2+/− mice seldom exhibit IBD pathology, indicating that Gpx2 afforded protection of the intestinal mucosa against inflammatory reactions [226]. Interestingly, the Gpx2 null mouse exhibited a compensatory increase in Gpx1 throughout the intestine, predominantly in colon and ileum which may explain why Gpx2 deficient animals do not develop IBD pathologies [227]. When Gpx2 deficient mice were fed Se-deficient diet, increased apoptosis was observed in colonic crypts [227], a region that normally supports cell proliferation [86] (see Figure 1) and expresses high levels of Gpx2 [224]. The double knockout mice (Gpx1/2-DKO) develop early ileocolitis and severe inflammation in the distal ileum at 6–9 month; ~25% of these mice exhibit microflora-associated cancer in the ileum and colon [226,228]. Taken together, these studies highlight a physiological role for Se-Gpx proteins in intestinal integrity and are therefore feasible target candidates in anti-cancer therapy.
The over-expression of Grx and Trx is a characteristic feature of colon cancer [229], and elevated Trx1 in primary colorectal cancer cells was associated with aggressive tumor growth and poor prognosis for survival [230]. In colon carcinoma, the expression of Grx3, an intestinal epithelial specific Grx isoform, was increased ~50-fold with no alteration in the other Grx proteins levels [231], consistent with Grx3 function in tumor growth and survival [232]. Indeed, the knock down of Grx3 in breast cancer cells promoted cellular ROS production and GSH oxidation; the resultant oxidized redox environment destabilized p65 and relB subunits and attenuated NF-κB survival signaling that inhibited tumor growth and migration [233]. Thus, targeting NF-κB signaling, a major promoter of tumorigenesis, tumor growth and metastasis as well as Grx3 expression in Grx3 overexpressing colon cancer cells, [233] would be a viable strategic approach in colon cancer therapy.
Specific intestinal pathologies that are directly attributed to the oxidative condition of the diabetic state have yet to be defined. However, diabetes associated hyperglycemia, oxidative and carbonyl stress can augment the susceptibility of the gastrointestinal system to oxidant- or ischemia/reperfusion-induced damage [234,235]. Significantly, diabetic rats exhibit impaired antioxidant defense capacity, evidenced by lower mucosal SOD activity and depressed GSH levels [234,236]. In accordance with an increased capacity to scavenge ROS, the administration of SOD or GSH was able to reverse oxidant-mediated gastrointestinal injury [234] while GSH depletion via starvation induced gastric lesions in streptozotocin-treated diabetic rats [237]. Importantly, the intestinal capacity for ROS elimination are majorly influenced by factors that modulate mucosal GSH regeneration, including glucose availability, cysteine supply, and NADPH production [237], rather than the activities of “core” redox enzymes such as Gpx, GR, and glucose-6-phosphate Surprisingly, glycemic control by short-term insulin treatment decreased mucosal GSH and suppressed activities of these core enzymes which were reversed by 7-day insulin treatment [235], suggesting a heightened vulnerability of the diabetic intestine to acute glycemic fluctuation. Significantly, the capacity of the diabetic intestine for peroxide (oxidant) detoxication is directly impacted by the GSH redox cycle function, regulated by cysteine supply for GSH synthesis and NADPH supply for GSH regeneration.
Concluding remarks
Intracellular and extracellular thiol-disulfide redox homeostasis is central to intestinal function and integrity. Maintenance of the intestinal epithelial redox environment is essential for the activities of key physiological processes that include digestion and absorption, cell proliferation and apoptosis, and immune response. Much is known of the GSH/GSSG and Cys/CySS redox systems at the apical epithelial surface from studies in animal models and in vitro intestinal cell culture systems. These two redox couples ensure a redox environment that supports the gut microflora, facilitates nutrient absorption, counteracts oxidant-induced epithelial injury, and regulates intestinal cell transformation and apoptosis. The fine tuning of the extracellular redox environment is also crucial in the intestinal stem cell niche that signals intestinal cell genesis. However, little is known of the redox mechanisms governing extracellular/membrane signaling events that mediate intracellular immunological responses that control pathogen-host interactions or inflammation and the contribution of the microbiota to these processes. Within intestinal cells, Trx/TrxSS and GSH/GSSG redox status as well as Trx- and GSH-dependent enzyme systems preserve a redox environment that supports redox signaling and regulation of cell metabolism, orchestrates antioxidant defense and initiates cell death-associated processes during oxidative stress. The distinctness of subcellular compartmentation of individual redox systems provides a unique level of independence and elegance in specific redox control of biological processes. Indeed, subcellular redox compartmentation could be a generalized underlying determinant of cell function. In the past decade, there is growing interest in redox involvement in the pathobiology of the intestine. Given the conceptual advances that shaped our current understanding of cellular redox regulation and signaling, the challenge for future research is to define the mechanistic relationship between disrupted regulation and communication of compartmental redox systems and luminal microbiota with altered intestinal processes such as enhanced inflammatory responses or aberrant cell proliferation, processes that fundamentally underlie intestinal disorders like cancer or IBD.
Acknowledgments
The cited research from the authors’ laboratory is supported by a grant from the National Institutes of Health, DK 44510.
Abbreviations
- γ-GT
γ-glutamyl transferase
- Akt
serine/threonine protein kinase Akt
- BSO
L-buthionine-S,R-sulfoximine
- CD
Crohn’s disease
- Cys
cysteine
- CySS
cystine
- CyS-SG
cysteine-glutathione disulfide
- DIC
dicarboxylate transporter
- DP
dipeptidase
- ER
endoplasmic reticulum
- FAD
flavin adenine nucleotide
- FAE
follicle-associated epithelium
- GALT
gut-associated lymphoid tissue
- GCL
glutamate-cysteine ligase
- Gpx
glutathione peroxidase
- GR
glutathione reductase
- Grx
glutaredoxin
- GS
glutathione synthase
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione-S-transferases
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- hBD-1
human β-defensin 1
- IBD
inflammatory bowel diseases
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- IMS
mitochondrial intermembrane space
- ISC
intestinal crypt stem cells
- LP-T
lamina propria T lymphocytes
- LMW-PTP
low molecular weight protein tyrosine phosphatase
- Met
methionine, MQ, menadione
- mtGSH
mitochondrial GSH
- NF-κB
nuclear factor-κB
- NOX-1
NADPH oxidase 1
- NNT
nicotinamide nucleotide transhydrogenase
- OGC
2-oxoglutarate transporter
- PPs
Payer’s patches
- PPP
pentose phosphate pathway
- PI3K
phosphatidylinositol-3′-kinase
- Pr-SSG
protein disulfides
- Prx
peroxiredoxin
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SCFA
short chain fatty acids
- SQR
sulfide quinone reductase
- STP
staurosporine
- tBH
tert-butyl hydroperoxide
- Trx
reduced thioredoxin
- TrxR
Trx reductase
- TrxSS
oxidized thioredoxin
- TNBS
trinitrobenzensulfonic acid
- UC
ulcerative colitis
References
- 1.Lipkin M. Proliferation and differentiation of gastrointestinal cells. Physiol Rev. 1973;53:891–915. doi: 10.1152/physrev.1973.53.4.891. [DOI] [PubMed] [Google Scholar]
- 2.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat. 1974;141:521–535. doi: 10.1002/aja.1001410406. [DOI] [PubMed] [Google Scholar]
- 3.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 4.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 6.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–145. doi: 10.1016/j.ceb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- 9.Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–298. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- 10.Snoeck V, Goddeeris B, Cox E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005;7:997–1004. doi: 10.1016/j.micinf.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 12.Owen RL, Jones AL. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 13.Jung C, Hugot JP, Barreau F. Peyer’s Patches: The Immune Sensors of the Intestine. Int J Inflam. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 15.Jensen VB, Harty JT, Jones BD. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer’s patches. Infect Immun. 1998;66:3758–3766. doi: 10.1128/iai.66.8.3758-3766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52:2–12. doi: 10.1111/j.1574-695X.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 17.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 18.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab. 2010;12(Suppl 2):116–125. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 23.Sies H. Oxidative stress: Introductory remarks. In: Sies H, editor. Oxidative stress. London: Academic Press; 1985. pp. 1–8. [Google Scholar]
- 24.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meister A, Tate SS. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- 26.Ballatori N, Krance SM, Marchan R, Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med. 2009;30:13–28. doi: 10.1016/j.mam.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagen TM, Wierzbicka GT, Bowman BB, Aw TY, Jones DP. Fate of dietary glutathione: disposition in the gastrointestinal tract. Am J Physiol. 1990;259:G530–535. doi: 10.1152/ajpgi.1990.259.4.G530. [DOI] [PubMed] [Google Scholar]
- 28.Hagen TM, Aw TY, Jones DP. Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 1988;34:74–81. doi: 10.1038/ki.1988.147. [DOI] [PubMed] [Google Scholar]
- 29.Jocelyn PC, Kamminga A. The non-protein thiol of rat liver mitochondria. Biochim Biophys Acta. 1974;343:356–362. doi: 10.1016/0304-4165(74)90099-3. [DOI] [PubMed] [Google Scholar]
- 30.Schnellmann RG. Renal mitochondrial glutathione transport. Life Sci. 1991;49:393–398. doi: 10.1016/0024-3205(91)90447-j. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- 32.Rebrin I, Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehler CM, Beverly KN, Leverich EP. Redox pathways of the mitochondrion. Antioxid Redox Signal. 2006;8:813–822. doi: 10.1089/ars.2006.8.813. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann JM, Riemer J. The intermembrane space of mitochondria. Antioxid Redox Signal. 2010;13:1341–1358. doi: 10.1089/ars.2009.3063. [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard H, Tachibana C, Winther JR. Monitoring disulfide bond formation in the eukaryotic cytosol. J Cell Biol. 2004;166:337–345. doi: 10.1083/jcb.200402120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bass R, Ruddock LW, Klappa P, Freedman RB. A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J Biol Chem. 2004;279:5257–5262. doi: 10.1074/jbc.M304951200. [DOI] [PubMed] [Google Scholar]
- 37.Ho YF, Guenthner TM. Isolation of liver nuclei that retain functional trans-membrane transport. J Pharmacol Toxicol Methods. 1997;38:163–168. doi: 10.1016/s1056-8719(97)00082-8. [DOI] [PubMed] [Google Scholar]
- 38.Markovic J, Borras C, Ortega A, Sastre J, Vina J, Pallardo FV. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem. 2007;282:20416–20424. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 39.Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 40.Ahsan MK, Lekli I, Ray D, Yodoi J, Das DK. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxid Redox Signal. 2009;11:2741–2758. doi: 10.1089/ars.2009.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW, Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knoops B, Goemaere J, Van der Eecken V, Declercq JP. Peroxiredoxin 5: Structure, mechanism and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid Redox Signal. 2011;15:817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- 43.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci U S A. 2002;99:11599–11604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox AG, Pearson AG, Pullar JM, Jonsson TJ, Lowther WT, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin 3 is more resilient to hyperoxidation than cytoplasmic peroxiredoxins. Biochem J. 2009;421:51–58. doi: 10.1042/BJ20090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 46.Jonsson TJ, Lowther WT. The peroxiredoxin repair proteins. Subcell Biochem. 2007;44:115–141. doi: 10.1007/978-1-4020-6051-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 49.Park Y, Ziegler TR, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, Jones DP. Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J Nutr. 2010;140:760–765. doi: 10.3945/jn.109.116764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 51.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 52.Lash LH, Jones DP. Characteristics of cysteine uptake in intestinal basolateral membrane vesicles. Am J Physiol. 1984;247:G394–401. doi: 10.1152/ajpgi.1984.247.4.G394. [DOI] [PubMed] [Google Scholar]
- 53.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 54.Aw TY. Biliary glutathione promotes the mucosal metabolism of luminal peroxidized lipids by rat small intestine in vivo. J Clin Invest. 1994;94:1218–1225. doi: 10.1172/JCI117439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aw TY, Williams MW. Intestinal absorption and lymphatic transport of peroxidized lipids in rats: effect of exogenous GSH. Am J Physiol. 1992;263:G665–672. doi: 10.1152/ajpgi.1992.263.5.G665. [DOI] [PubMed] [Google Scholar]
- 56.Aw TY, Williams MW, Gray L. Absorption and lymphatic transport of peroxidized lipids by rat small intestine in vivo: role of mucosal GSH. Am J Physiol. 1992;262:G99–106. doi: 10.1152/ajpgi.1992.262.1.G99. [DOI] [PubMed] [Google Scholar]
- 57.Aw TY, Wierzbicka G, Jones DP. Oral glutathione increases tissue glutathione in vivo. Chem Biol Interact. 1991;80:89–97. doi: 10.1016/0009-2797(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 58.Shan XQ, Aw TY, Jones DP. Glutathione-dependent protection against oxidative injury. Pharmacol Ther. 1990;47:61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 59.Hagen TM, Jones DP. Transepithelial transport of glutathione in vascularly perfused small intestine of rat. Am J Physiol. 1987;252:G607–613. doi: 10.1152/ajpgi.1987.252.5.G607. [DOI] [PubMed] [Google Scholar]
- 60.Martensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990;87:1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincenzini MT, Iantomasi T, Favilli F. Glutathione transport across intestinal brush-border membranes: effects of ions, pH, delta psi, and inhibitors. Biochim Biophys Acta. 1989;987:29–37. doi: 10.1016/0005-2736(89)90451-3. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y, Feng Z, Hoos A, Klimberg VS. Glutamine enhances gut glutathione production. JPEN J Parenter Enteral Nutr. 1998;22:224–227. doi: 10.1177/0148607198022004224. [DOI] [PubMed] [Google Scholar]
- 63.Hagen TM, Wierzbicka GT, Sillau AH, Bowman BB, Jones DP. Bioavailability of dietary glutathione: effect on plasma concentration. Am J Physiol. 1990;259:G524–529. doi: 10.1152/ajpgi.1990.259.4.G524. [DOI] [PubMed] [Google Scholar]
- 64.Ballatori N, Rebbeor JF. Roles of MRP2 and oatp1 in hepatocellular export of reduced glutathione. Semin Liver Dis. 1998;18:377–387. doi: 10.1055/s-2007-1007171. [DOI] [PubMed] [Google Scholar]
- 65.Dahm LJ, Jones DP. Secretion of cysteine and glutathione from mucosa to lumen in rat small intestine. Am J Physiol. 1994;267:G292–300. doi: 10.1152/ajpgi.1994.267.2.G292. [DOI] [PubMed] [Google Scholar]
- 66.He M, Openo K, McCullough M, Jones DP. Total equivalent of reactive chemicals in 142 human food items is highly variable within and between major food groups. J Nutr. 2004;134:1114–1119. doi: 10.1093/jn/134.5.1114. [DOI] [PubMed] [Google Scholar]
- 67.Jones DP, Coates RJ, Flagg EW, Eley JW, Block G, Greenberg RS, Gunter EW, Jackson B. Glutathione in foods listed in the National Cancer Institute’s Health Habits and History Food Frequency Questionnaire. Nutr Cancer. 1992;17:57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- 68.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr. 2006;136:1242–1248. doi: 10.1093/jn/136.5.1242. [DOI] [PubMed] [Google Scholar]
- 69.Dahm LJ, Jones DP. Rat jejunum controls luminal thiol-disulfide redox. J Nutr. 2000;130:2739–2745. doi: 10.1093/jn/130.11.2739. [DOI] [PubMed] [Google Scholar]
- 70.Snary D, Allen A, Pain RH. Structural studies on gastric mucoproteins: lowering of molecular weight after reduction with 2-mercaptoethanol. Biochem Biophys Res Commun. 1970;40:844–851. doi: 10.1016/0006-291x(70)90980-0. [DOI] [PubMed] [Google Scholar]
- 71.Hudson VM. New insights into the pathogenesis of cystic fibrosis: pivotal role of glutathione system dysfunction and implications for therapy. Treat Respir Med. 2004;3:353–363. doi: 10.2165/00151829-200403060-00003. [DOI] [PubMed] [Google Scholar]
- 72.Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- 73.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G577–584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 74.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 76.Samiec PS, Dahm LJ, Jones DP. Glutathione S-transferase in mucus of rat small intestine. Toxicol Sci. 2000;54:52–59. doi: 10.1093/toxsci/54.1.52. [DOI] [PubMed] [Google Scholar]
- 77.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 78.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srigiridhar K, Nair KM, Subramanian R, Singotamu L. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Mol Cell Biochem. 2001;219:91–98. doi: 10.1023/a:1011023111048. [DOI] [PubMed] [Google Scholar]
- 80.Jin F, Frohman C, Thannhauser TW, Welch RM, Glahn RP. Effects of ascorbic acid, phytic acid and tannic acid on iron bioavailability from reconstituted ferritin measured by an in vitro digestion-Caco-2 cell model. Br J Nutr. 2009;101:972–981. doi: 10.1017/S0007114508055621. [DOI] [PubMed] [Google Scholar]
- 81.Espey MG, Chen P, Chalmers B, Drisko J, Sun AY, Levine M, Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011;50:1610–1619. doi: 10.1016/j.freeradbiomed.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 83.Haunhorst P, Berndt C, Eitner S, Godoy JR, Lillig CH. Characterization of the human monothiol glutaredoxin 3 (PICOT) as iron-sulfur protein. Biochem Biophys Res Commun. 2010;394:372–376. doi: 10.1016/j.bbrc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 84.Godoy JR, Funke M, Ackermann W, Haunhorst P, Oesteritz S, Capani F, Elsasser HP, Lillig CH. Redox atlas of the mouse Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Toppo S, Flohe L, Ursini F, Vanin S, Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta. 2009;1790:1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Chu FF, Esworthy RS, Doroshow JH. Role of Se-dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radic Biol Med. 2004;36:1481–1495. doi: 10.1016/j.freeradbiomed.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Chu FF, Esworthy RS, Ho YS, Bermeister M, Swiderek K, Elliott RW. Expression and chromosomal mapping of mouse Gpx2 gene encoding the gastrointestinal form of glutathione peroxidase, GPX-GI. Biomed Environ Sci. 1997;10:156–162. [PubMed] [Google Scholar]
- 88.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem. 1993;268:2571–2576. [PubMed] [Google Scholar]
- 89.Chu FF, Esworthy RS. The expression of an intestinal form of glutathione peroxidase (GSHPx-GI) in rat intestinal epithelium. Arch Biochem Biophys. 1995;323:288–294. doi: 10.1006/abbi.1995.9962. [DOI] [PubMed] [Google Scholar]
- 90.Wingler K, Bocher M, Flohe L, Kollmus H, Brigelius-Flohe R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur J Biochem. 1999;259:149–157. doi: 10.1046/j.1432-1327.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 91.Tham DM, Whitin JC, Kim KK, Zhu SX, Cohen HJ. Expression of extracellular glutathione peroxidase in human and mouse gastrointestinal tract. Am J Physiol. 1998;275:G1463–1471. doi: 10.1152/ajpgi.1998.275.6.G1463. [DOI] [PubMed] [Google Scholar]
- 92.Speckmann B, Bidmon HJ, Pinto A, Anlauf M, Sies H, Steinbrenner H. Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation. J Biol Chem. 2011;286:10764–10772. doi: 10.1074/jbc.M110.216028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoensch H, Peters WH, Roelofs HM, Kirch W. Expression of the glutathione enzyme system of human colon mucosa by localisation, gender and age. Curr Med Res Opin. 2006;22:1075–1083. doi: 10.1185/030079906X112480. [DOI] [PubMed] [Google Scholar]
- 94.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pool-Zobel B, Veeriah S, Bohmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens -- focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 96.Ebert MN, Klinder A, Peters WH, Schaferhenrich A, Sendt W, Scheele J, Pool-Zobel BL. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis. 2003;24:1637–1644. doi: 10.1093/carcin/bgg122. [DOI] [PubMed] [Google Scholar]
- 97.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 98.Scharrer E, Senn E, Wolffram S. Stimulation of mucosal uptake of selenium from selenite by some thiols at various sites of rat intestine. Biol Trace Elem Res. 1992;33:109–120. doi: 10.1007/BF02783999. [DOI] [PubMed] [Google Scholar]
- 99.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 100.Remond D, Buffiere C, Pouyet C, Papet I, Dardevet D, Savary-Auzeloux I, Williamson G, Faure M, Breuille D. Cysteine fluxes across the portal-drained viscera of enterally fed minipigs: effect of an acute intestinal inflammation. Amino Acids. 2011;40:543–552. doi: 10.1007/s00726-010-0672-6. [DOI] [PubMed] [Google Scholar]
- 101.Van Klinken BJ, Einerhand AW, Buller HA, Dekker J. Strategic biochemical analysis of mucins. Anal Biochem. 1998;265:103–116. doi: 10.1006/abio.1998.2896. [DOI] [PubMed] [Google Scholar]
- 102.Fang Z, Yao K, Zhang X, Zhao S, Sun Z, Tian G, Yu B, Lin Y, Zhu B, Jia G, Zhang K, Chen D, Wu D. Nutrition and health relevant regulation of intestinal sulfur amino acid metabolism. Amino Acids. 2010;39:633–640. doi: 10.1007/s00726-010-0502-x. [DOI] [PubMed] [Google Scholar]
- 103.Neil MW. The absorption of cystine and cysteine from rat small intestine. Biochem J. 1959;71:118–124. doi: 10.1042/bj0710118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bannai S. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta. 1984;779:289–306. doi: 10.1016/0304-4157(84)90014-5. [DOI] [PubMed] [Google Scholar]
- 105.Mannery YO, Ziegler TR, Hao L, Shyntum Y, Jones DP. Characterization of apical and basal thiol-disulfide redox regulation in human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G523–530. doi: 10.1152/ajpgi.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ookhtens M, Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin Liver Dis. 1998;18:313–329. doi: 10.1055/s-2007-1007167. [DOI] [PubMed] [Google Scholar]
- 108.Riedijk MA, Stoll B, Chacko S, Schierbeek H, Sunehag AL, van Goudoever JB, Burrin DG. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci U S A. 2007;104:3408–3413. doi: 10.1073/pnas.0607965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gasdaska JR, Gasdaska PY, Gallegos A, Powis G. Human thioredoxin reductase gene localization to chromosomal position 12q23–q24.1 and mRNA distribution in human tissue. Genomics. 1996;37:257–259. doi: 10.1006/geno.1996.0554. [DOI] [PubMed] [Google Scholar]
- 110.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 111.Sido B, Giese T, Autschbach F, Lasitschka F, Braunstein J, Meuer SC. Potential role of thioredoxin in immune responses in intestinal lamina propria T lymphocytes. Eur J Immunol. 2005;35:408–417. doi: 10.1002/eji.200424500. [DOI] [PubMed] [Google Scholar]
- 112.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 113.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 114.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vael C, Desager K. The importance of the development of the intestinal microbiota in infancy. Curr Opin Pediatr. 2009;21:794–800. doi: 10.1097/MOP.0b013e328332351b. [DOI] [PubMed] [Google Scholar]
- 116.Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood) 2004;229:586–597. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 117.Martin FP, Sprenger N, Yap IK, Wang Y, Bibiloni R, Rochat F, Rezzi S, Cherbut C, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Panorganismal gut microbiome-host metabolic crosstalk. J Proteome Res. 2009;8:2090–2105. doi: 10.1021/pr801068x. [DOI] [PubMed] [Google Scholar]
- 118.Martin FP, Wang Y, Yap IK, Sprenger N, Lindon JC, Rezzi S, Kochhar S, Holmes E, Nicholson JK. Topographical variation in murine intestinal metabolic profiles in relation to microbiome speciation and functional ecological activity. J Proteome Res. 2009;8:3464–3474. doi: 10.1021/pr900099x. [DOI] [PubMed] [Google Scholar]
- 119.Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 120.Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000;72:1488–1494. doi: 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- 121.Weisiger RA, Pinkus LM, Jakoby WB. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol. 1980;29:2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- 122.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta. 2005;1725:201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. Faseb J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 124.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 126.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 127.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 128.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 129.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 130.Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, Skurnik D, Grodet A, Fay M, Biard D, Lesuffleur T, Deffert C, Moreau R, Groyer A, Krause KH, Daniel F, Ogier-Denis E. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aw TY. Intestinal glutathione: determinant of mucosal peroxide transport, metabolism, and oxidative susceptibility. Toxicol Appl Pharmacol. 2005;204:320–328. doi: 10.1016/j.taap.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 132.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 133.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 134.Aw TY. Cellular redox: a modulator of intestinal epithelial cell proliferation. News Physiol Sci. 2003;18:201–204. doi: 10.1152/nips.01448.2003. [DOI] [PubMed] [Google Scholar]
- 135.Jonas CR, Gu LH, Nkabyo YS, Mannery YO, Avissar NE, Sax HC, Jones DP, Ziegler TR. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1421–1429. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- 136.Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY. Exogenous cysteine and cystine promote cell proliferation in CaCo-2 cells. Cell Prolif. 2002;35:117–129. doi: 10.1046/j.1365-2184.2002.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1069–1075. doi: 10.1152/ajpregu.00195.2007. [DOI] [PubMed] [Google Scholar]
- 138.Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gotoh Y, Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY. Lipid peroxide-induced redox imbalance differentially mediates CaCo-2 cell proliferation and growth arrest. Cell Prolif. 2002;35:221–235. doi: 10.1046/j.1365-2184.2002.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang TG, Gotoh Y, Jennings MH, Rhoads CA, Aw TY. Lipid hydroperoxide-induced apoptosis in human colonic CaCo-2 cells is associated with an early loss of cellular redox balance. Faseb J. 2000;14:1567–1576. doi: 10.1096/fj.14.11.1567. [DOI] [PubMed] [Google Scholar]
- 141.Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. Faseb J. 2001;15:2131–2139. doi: 10.1096/fj.01-0131com. [DOI] [PubMed] [Google Scholar]
- 142.Tabata K, Johnson LR. Ornithine decarboxylase and mucosal growth in response to feeding. Am J Physiol. 1986;251:G270–274. doi: 10.1152/ajpgi.1986.251.2.G270. [DOI] [PubMed] [Google Scholar]
- 143.Iwakiri R, Gotoh Y, Noda T, Sugihara H, Fujimoto K, Fuseler J, Aw TY. Programmed cell death in rat intestine: effect of feeding and fasting. Scand J Gastroenterol. 2001;36:39–47. doi: 10.1080/00365520150218048. [DOI] [PubMed] [Google Scholar]
- 144.Tsunada S, Iwakiri R, Fujimoto K, Aw TY. Chronic lipid hydroperoxide stress suppresses mucosal proliferation in rat intestine: potentiation of ornithine decarboxylase activity by epidermal growth factor. Dig Dis Sci. 2003;48:2333–2341. doi: 10.1023/b:ddas.0000007872.66693.6c. [DOI] [PubMed] [Google Scholar]
- 145.Tsunada S, Iwakiri R, Noda T, Fujimoto K, Fuseler J, Rhoads CA, Aw TY. Chronic exposure to subtoxic levels of peroxidized lipids suppresses mucosal cell turnover in rat small intestine and reversal by glutathione. Dig Dis Sci. 2003;48:210–222. doi: 10.1023/a:1021775524062. [DOI] [PubMed] [Google Scholar]
- 146.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289:G70–78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 147.Tian J, Washizawa N, Gu LH, Levin MS, Wang L, Rubin DC, Mwangi S, Srinivasan S, Gao Y, Jones DP, Ziegler TR. Stimulation of colonic mucosal growth associated with oxidized redox status in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1081–1091. doi: 10.1152/ajpregu.00050.2006. [DOI] [PubMed] [Google Scholar]
- 148.Tian J, Washizawa N, Gu LH, Levin MS, Wang L, Rubin DC, Mwangi S, Srinivasan S, Jones DP, Ziegler TR. Local glutathione redox status does not regulate ileal mucosal growth after massive small bowel resection in rats. J Nutr. 2007;137:320–325. doi: 10.1093/jn/137.2.320. [DOI] [PubMed] [Google Scholar]
- 149.Shyntum Y, Iyer SS, Tian J, Hao L, Mannery YO, Jones DP, Ziegler TR. Dietary sulfur amino acid supplementation reduces small bowel thiol/disulfide redox state and stimulates ileal mucosal growth after massive small bowel resection in rats. J Nutr. 2009;139:2272–2278. doi: 10.3945/jn.109.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Knoll N, Ruhe C, Veeriah S, Sauer J, Glei M, Gallagher EP, Pool-Zobel BL. Genotoxicity of 4-hydroxy-2-nonenal in human colon tumor cells is associated with cellular levels of glutathione and the modulation of glutathione S-transferase A4 expression by butyrate. Toxicol Sci. 2005;86:27–35. doi: 10.1093/toxsci/kfi171. [DOI] [PubMed] [Google Scholar]
- 151.Orihuela D, Meichtry V, Pregi N, Pizarro M. Short-term oral exposure to aluminium decreases glutathione intestinal levels and changes enzyme activities involved in its metabolism. J Inorg Biochem. 2005;99:1871–1878. doi: 10.1016/j.jinorgbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 152.Lash LH, Hagen TM, Jones DP. Exogenous glutathione protects intestinal epithelial cells from oxidative injury. Proc Natl Acad Sci U S A. 1986;83:4641–4645. doi: 10.1073/pnas.83.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aw TY, Rhoads CA. Glucose regulation of hydroperoxide metabolism in rat intestinal cells. Stimulation of reduced nicotinamide adenine dinucleotide phosphate supply. J Clin Invest. 1994;94:2426–2434. doi: 10.1172/JCI117610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Circu ML, Moyer MP, Harrison L, Aw TY. Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic Biol Med. 2009;47:1190–1198. doi: 10.1016/j.freeradbiomed.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med. 2008;44:768–778. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Circu ML, Stringer S, Rhoads CA, Moyer MP, Aw TY. The role of GSH efflux in staurosporine-induced apoptosis in colonic epithelial cells. Biochem Pharmacol. 2009;77:76–85. doi: 10.1016/j.bcp.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pias EK, Aw TY. Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death Differ. 2002;9:1007–1016. doi: 10.1038/sj.cdd.4401064. [DOI] [PubMed] [Google Scholar]
- 158.Pias EK, Aw TY. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production. Faseb J. 2002;16:781–790. doi: 10.1096/fj.01-0784com. [DOI] [PubMed] [Google Scholar]
- 159.Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21(Suppl 3):S3–6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 161.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kamga CK, Zhang SX, Wang Y. Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am J Physiol Cell Physiol. 299:C497–505. doi: 10.1152/ajpcell.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Caballero F, Fernandez A, Matias N, Martinez L, Fucho R, Elena M, Caballeria J, Morales A, Fernandez-Checa JC, Garcia-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. Faseb J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 165.Coll O, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 166.Wadey AL, Muyderman H, Kwek PT, Sims NR. Mitochondrial glutathione uptake: characterization in isolated brain mitochondria and astrocytes in culture. J Neurochem. 2009;109(Suppl 1):101–108. doi: 10.1111/j.1471-4159.2009.05936.x. [DOI] [PubMed] [Google Scholar]
- 167.Fernandez A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Cholesterol and sphingolipids in alcohol-induced liver injury. J Gastroenterol Hepatol. 2008;23(Suppl 1):S9–15. doi: 10.1111/j.1440-1746.2007.05280.x. [DOI] [PubMed] [Google Scholar]
- 168.Colell A, Fernandez A, Fernandez-Checa JC. Mitochondria, cholesterol and amyloid beta peptide: a dangerous trio in Alzheimer disease. J Bioenerg Biomembr. 2009;41:417–423. doi: 10.1007/s10863-009-9242-6. [DOI] [PubMed] [Google Scholar]
- 169.Fernandez-Checa JC, Ookhtens M, Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987;80:57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 171.Fernandez A, Llacuna L, Fernandez-Checa JC, Colell A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J Neurosci. 2009;29:6394–6405. doi: 10.1523/JNEUROSCI.4909-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lash LH, Putt DA, Matherly LH. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J Pharmacol Exp Ther. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- 173.Xu F, Putt DA, Matherly LH, Lash LH. Modulation of expression of rat mitochondrial 2-oxoglutarate carrier in NRK-52E cells alters mitochondrial transport and accumulation of glutathione and susceptibility to chemically induced apoptosis. J Pharmacol Exp Ther. 2006;316:1175–1186. doi: 10.1124/jpet.105.094599. [DOI] [PubMed] [Google Scholar]
- 174.Martensson J, Lai JC, Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990;87:7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Z, Putt DA, Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 176.Benipal B, Lash LH. Influence of renal compensatory hypertrophy on mitochondrial energetics and redox status. Biochem Pharmacol. 2011;81:295–303. doi: 10.1016/j.bcp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 177.Garcia J, Han D, Sancheti H, Yap LP, Kaplowitz N, Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Circu ML, Maloney RE, Aw TY. Disruption of pyridine nucleotide redox status during oxidative challenge at normal and low-glucose states: implications for cellular adenosine triphosphate, mitochondrial respiratory activity, and reducing capacity in colon epithelial cells. Antioxid Redox Signal. 2011;14:2151–2162. doi: 10.1089/ars.2010.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Arndt H, Palitzsch KD, Anderson DC, Rusche J, Grisham MB, Granger DN. Leucocyte-endothelial cell adhesion in a model of intestinal inflammation. Gut. 1995;37:374–379. doi: 10.1136/gut.37.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yamada T, Grisham MB. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klin Wochenschr. 1991;69:988–994. doi: 10.1007/BF01645144. [DOI] [PubMed] [Google Scholar]
- 181.Iantomasi T, Marraccini P, Favilli F, Vincenzini MT, Ferretti P, Tonelli F. Glutathione metabolism in Crohn’s disease. Biochem Med Metab Biol. 1994;53:87–91. doi: 10.1006/bmmb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 182.Holmes EW, Yong SL, Eiznhamer D, Keshavarzian A. Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci. 1998;43:1088–1095. doi: 10.1023/a:1018899222258. [DOI] [PubMed] [Google Scholar]
- 183.Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Droge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485–492. doi: 10.1136/gut.42.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karp SM, Koch TR. Oxidative stress and antioxidants in inflammatory bowel disease. Dis Mon. 2006;52:199–207. doi: 10.1016/j.disamonth.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 185.Tsunada S, Iwakiri R, Ootani H, Aw TY, Fujimoto K. Redox imbalance in the colonic mucosa of ulcerative colitis. Scand J Gastroenterol. 2003;38:1002–1003. doi: 10.1080/00365520310005055. [DOI] [PubMed] [Google Scholar]
- 186.Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, Panetta J, Morris CJ, Blake DR. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut. 1996;39:407–415. doi: 10.1136/gut.39.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schepens MA, Vink C, Schonewille AJ, Roelofs HM, Brummer RJ, van der Meer R, Bovee-Oudenhoven IM. Supplemental antioxidants do not ameliorate colitis development in HLA-B27 transgenic rats despite extremely low glutathione levels in colonic mucosa. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21584. in press. [DOI] [PubMed] [Google Scholar]
- 188.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 189.Qiao L, Schurmann G, Meuer SC, Wallich R, Schirren A, Autschbach F. Regulation of T cell reactivities by intestinal mucosa. Adv Exp Med Biol. 1995;371A:31–34. doi: 10.1007/978-1-4615-1941-6_6. [DOI] [PubMed] [Google Scholar]
- 190.Sido B, Lasitschka F, Giese T, Gassler N, Funke B, Schroder-Braunstein J, Brunnemer U, Meuer SC, Autschbach F. A prominent role for mucosal cystine/cysteine metabolism in intestinal immunoregulation. Gastroenterology. 2008;134:179–191. doi: 10.1053/j.gastro.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 191.Yan Z, Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–1066. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sido B, Braunstein J, Breitkreutz R, Herfarth C, Meuer SC. Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes. J Exp Med. 2000;192:907–912. doi: 10.1084/jem.192.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Reyes BM, Danese S, Sans M, Fiocchi C, Levine AD. Redox equilibrium in mucosal T cells tunes the intestinal TCR signaling threshold. J Immunol. 2005;175:2158–2166. doi: 10.4049/jimmunol.175.4.2158. [DOI] [PubMed] [Google Scholar]
- 194.Ellis RD, Goodlad JR, Limb GA, Powell JJ, Thompson RP, Punchard NA. Activation of nuclear factor kappa B in Crohn’s disease. Inflamm Res. 1998;47:440–445. doi: 10.1007/s000110050358. [DOI] [PubMed] [Google Scholar]
- 195.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pasparakis M. IKK/NF-kappaB signaling in intestinal epithelial cells controls immune homeostasis in the gut. Mucosal Immunol. 2008;1(Suppl 1):S54–57. doi: 10.1038/mi.2008.53. [DOI] [PubMed] [Google Scholar]
- 197.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 198.Spehlmann ME, Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction. Curr Opin Gastroenterol. 2009;25:92–99. doi: 10.1097/MOG.0b013e328324f857. [DOI] [PubMed] [Google Scholar]
- 199.Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, Arkan MC, Greten FR. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 201.Brigelius-Flohe R, Flohe L. Basic Principles and Emerging Concepts in the Redox Control of Transcription Factors. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3534. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 204.Ando K, Hirao S, Kabe Y, Ogura Y, Sato I, Yamaguchi Y, Wada T, Handa H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 206.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, Pan ZQ, Jones DP, Neish AS. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. Embo J. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 2009;182:538–546. doi: 10.4049/jimmunol.182.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Swanson PA, 2nd, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, Klegeris A, Vallance BA, Gibson DL. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00509.2010. [DOI] [PubMed] [Google Scholar]
- 210.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Adrio JL, Olivares M, Xaus J, Zarzuelo A, Galvez J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int J Colorectal Dis. 2006;21:737–746. doi: 10.1007/s00384-005-0773-y. [DOI] [PubMed] [Google Scholar]
- 212.Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Diaz-Ropero MP, Olivares M, Xaus J, Zarzuelo A, Galvez J. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J Gastroenterol. 2005;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen LW, Chang WJ, Chen PH, Liu WC, Hsu CM. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock. 2008;30:563–570. doi: 10.1097/SHK.0b013e31816a3458. [DOI] [PubMed] [Google Scholar]
- 214.Wang Y, Rickman BH, Poutahidis T, Schlieper K, Jackson EA, Erdman SE, Fox JG, Horwitz BH. c-Rel is essential for the development of innate and T cell-induced colitis. J Immunol. 2008;180:8118–8125. doi: 10.4049/jimmunol.180.12.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Planson AG, Palais G, Abbas K, Gerard M, Couvelard L, Delaunay A, Baulande S, Drapier JC, Toledano MB. Sulfiredoxin protects mice from lipopolysaccharide-induced endotoxic shock. Antioxid Redox Signal. 2011;14:2071–2080. doi: 10.1089/ars.2010.3552. [DOI] [PubMed] [Google Scholar]
- 216.Li L, Shoji W, Takano H, Nishimura N, Aoki Y, Takahashi R, Goto S, Kaifu T, Takai T, Obinata M. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun. 2007;355:715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 217.Yang CS, Lee DS, Song CH, An SJ, Li S, Kim JM, Kim CS, Yoo DG, Jeon BH, Yang HY, Lee TH, Lee ZW, El-Benna J, Yu DY, Jo EK. Roles of peroxiredoxin II in the regulation of proinflammatory responses to LPS and protection against endotoxin-induced lethal shock. J Exp Med. 2007;204:583–594. doi: 10.1084/jem.20061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol. 2010;185:569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Victor VM, Rocha M, De la Fuente M. Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol. 2004;4:327–347. doi: 10.1016/j.intimp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 220.Mistry N, Bevan RJ, Cooke MS, Evans MD, Halligan EP, Lowes DA, Nichol K, Lunec J. Antiserum detection of reactive carbonyl species-modified DNA in human colonocytes. Free Radic Res. 2008;42:344–353. doi: 10.1080/10715760802008106. [DOI] [PubMed] [Google Scholar]
- 221.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 222.Brigelius-Flohe R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 223.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med. 2000;29:312–322. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 224.Florian S, Wingler K, Schmehl K, Jacobasch G, Kreuzer OJ, Meyerhof W, Brigelius-Flohe R. Cellular and subcellular localization of gastrointestinal glutathione peroxidase in normal and malignant human intestinal tissue. Free Radic Res. 2001;35:655–663. doi: 10.1080/10715760100301181. [DOI] [PubMed] [Google Scholar]
- 225.Murawaki Y, Tsuchiya H, Kanbe T, Harada K, Yashima K, Nozaka K, Tanida O, Kohno M, Mukoyama T, Nishimuki E, Kojo H, Matsura T, Takahashi K, Osaki M, Ito H, Yodoi J, Murawaki Y, Shiota G. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259:218–230. doi: 10.1016/j.canlet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 226.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 227.Florian S, Krehl S, Loewinger M, Kipp A, Banning A, Esworthy S, Chu FF, Brigelius-Flohe R. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic Biol Med. 2010;49:1694–1702. doi: 10.1016/j.freeradbiomed.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 229.Berggren M, Gallegos A, Gasdaska JR, Gasdaska PY, Warneke J, Powis G. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 1996;16:3459–3466. [PubMed] [Google Scholar]
- 230.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 231.Ohayon A, Babichev Y, Galperin M, Altman A, Isakov N. Widespread expression of PICOT in mouse and human tissues with predominant localization to epithelium. J Histochem Cytochem. 2010;58:799–806. doi: 10.1369/jhc.2010.956532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cha MK, Kim IH. Preferential overexpression of glutaredoxin3 in human colon and lung carcinoma. Cancer Epidemiol. 2009;33:281–287. doi: 10.1016/j.canep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 233.Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, Bukoye BA, Liu B, Lee AV, Lin X, Huang P, Martens JW, Giuliano AE, Zhang N, Cheng NH, Cui X. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-kappaB signaling. J Clin Invest. 2011;121:212–225. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tashima K, Fujita A, Takeuchi K. Aggravation of ischemia/reperfusion-induced gastric lesions in streptozotocin-diabetic rats. Life Sci. 2000;67:1707–1718. doi: 10.1016/s0024-3205(00)00754-2. [DOI] [PubMed] [Google Scholar]
- 235.Iwakiri R, Rhoads CA, Aw TY. Determinants of hydroperoxide detoxification in diabetic rat intestine: effect of insulin and fasting on the glutathione redox cycle. Metabolism. 1995;44:1462–1468. doi: 10.1016/0026-0495(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 236.Tunali S, Yanardag R. Effect of vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharmacol Res. 2006;53:271–277. doi: 10.1016/j.phrs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 237.Goldin E, Ardite E, Elizalde JI, Odriozola A, Panes J, Pique JM, Fernandez-Checa JC. Gastric mucosal damage in experimental diabetes in rats: role of endogenous glutathione. Gastroenterology. 1997;112:855–863. doi: 10.1053/gast.1997.v112.pm9041247. [DOI] [PubMed] [Google Scholar]