Abstract
Galectins are a family of carbohydrate-binding proteins that modulate inflammation and immunity. This functional versatility prompted us to perform a histochemical study of their occurrence during wound healing using rat skin as an in vivo model. Wound healing is a dynamic process that exhibits three basic phases: inflammation, proliferation, and maturation. In this study antibodies against keratins-10 and -14, wide-spectrum cytokeratin, vimentin, and fibronectin, and non-cross-reactive antibodies to galectins-1, -2, and -3 were applied to frozen sections of skin specimens two days (inflammatory phase), seven days (proliferation phase), and twenty-one days (maturation phase) after wounding. The presence of binding sites for galectins-1, -2, -3, and -7 as a measure for assessing changes in reactivity was determined using labeled proteins as probes. Our study detected a series of alterations in galectin parameters during the different phases of wound healing. Presence of galectin-1, for example, increased during the early phase of healing, whereas galectin-3 rapidly decreased in newly formed granulation tissue. In addition, nuclear reactivity of epidermal cells for galectin-2 occurred seven days post-trauma. The dynamic regulation of galectins during re-epithelialization intimates a role of these proteins in skin wound healing, most notably for galectin-1 increasing during the early phases and galectin-3 then slightly increasing during later phases of healing. Such changes may identify a potential target for the development of novel drugs to aid in wound repair and patients’ care.
Keywords: differentiation, lectin, migration, proliferation, repair
I. Introduction
The integrity of skin is essential, because it forms a mechanically flexible barrier protecting higher organisms from infections. Replacement or repair of this barrier requires an intricate healing process that starts immediately after surgery and/or trauma. Successful wound healing involves an orchestration of several processes encompassing cell migration [47], proliferation [44], and differentiation [29]. Remodeling and formation of the extracellular matrix (ECM) [10] requires a sequence of molecular-, cellular-, and tissue-level events including cell-cell and cell-matrix interactions. On the molecular level, growth factors, chemo- and cytokines are known to play important roles in coordinating the events that lead to complete posttraumatic skin repair and finally to scar formation [3, 13, 49].
It is evident that the clinical presentation of non-healing wounds calls for better understanding of the basic biological mechanisms underlying the repair processes of higher organisms [41]. Hence, in addition to further exploring the regulatory pathways involved in wound healing, such studies can have clinical and socioeconomic implications. Toward this end, we focused on glycans as versatile biochemical signals and endogenous lectins as efficient signal-transduction elements, embodied by what is now called the sugar code: the transfer of information between cells via the shape and structure of glycan determinants [for recent reviews, see 16].
The recent application of array technology to study wound re-epithelialization has paved the way for discovering differential regulation of gene expression for enzymes involved in glycan remodeling and for the identification of distinct lectins such as galectins-1, -3, -4 and -7 [6, 7, 37]. In fact, members of this protein family are known to be potent regulators of cell adhesion, growth, and migration, via protein/glycan and protein/protein interactions [17, 40, 42, 45]. The marked effects of galectins on cell migration, observed in colon cancer cells and also in keratinocytes [20, 23], as well as the expression of galectins in malignancies of squamous epithelia, which in certain cases can correlate to tumor progression [4, 9, 28, 31, 38, 39], encouraged us to investigate galectin expression in skin during different phases of wound healing. Because galectin activity is also regulated on the level of ligand availability, e.g. by displaying distinct, highly reactive glycan epitopes on, to give examples, ganglioside GM1, the fibronectin receptor or CD7 in suited density through the action of a tumor suppressor or cell activation/differentiation [1, 14, 17, 26, 32, 35, 36, 46], parallel testing of accessibility of binding sites with galectins as tools provides insights into regulatory events on this level.
Wound healing is an intricately orchestrated cascade of events separated into the phases of inflammation, proliferation and maturation [2]. In our study, we systematically determined presence of adhesion/growth-regulatory galectins and the tissue reactivity to these proteins in Sprague-Dawley rats at three time points post-trauma, i.e. day two (inflammation), day seven (proliferation), and day twenty-one (maturation) after surgery. Tissue specimens were processed under identical conditions to exclude any factor other than the time-point that would affect signal occurrence and intensity.
In detail, we have monitored, the expression and reactivity of proto-type galectins-1 and -2 as well as the chimera-type galectin-3 using (immuno/galectin)histochemical techniques. This galectin-related work was flanked by examining keratin presence as marker to characterize the level of cell differentiation [15]. Among the keratins, keratin-10 is an indicator of early stages of keratinocyte differentiation [8, 19, 33], whereas keratin-14 is considered as a key feature of poorly differentiated epidermal cells located in the basal epidermal layer [30, 33]. In addition, to complete the study, wide-spectrum cytokeratin, vimentin and fibronectin were also localized.
II. Materials and Methods
Animal model
This study was approved by the State Veterinary and Food Administration of the Slovak Republic.
One-year-old male Sprague-Dawley rats (n=17) were included into the experiment. In 15 rats, surgery was performed under general anesthesia induced by administration of ketamine (40 mg/kg; Narkamon a.u.v., Spofa, Prague, Czech Republic), xylazine (15 mg/kg; Rometar a.u.v., Spofa) and tramadol 5 mg/kg (Tramadol-K; Krka, Novo Mesto, Slovenia). Under aseptic conditions one round full-thickness skin wound, 10 mm in diameter, was inflicted to the back of each rat. Five rats were sacrificed at each time point by ether inhalation, i.e. after two, seven, and twenty-one days, respectively. Two rats that remained uninjured were included as control.
Histology
Either uninjured skin or skin-wound specimens were removed from rats sacrificed by ether inhalation at each evaluated time point and routinely processed for classical histological staining (fixation in 4% buffered formaldehyde, dehydration using increasing concentration of ethanol, paraffin embedding, sectioning, and staining). Deparaffinized sections were stained with hematoxylin-eosin (HE–basic staining) and Van Gieson (VG–non-specific collagen staining).
Immunohistochemistry and lectin histochemistry
In parallel, skin-wound specimens were cryoprotected by Tissue-Tek (Sakura, Zoeterwoude, Netherlands) and deeply frozen in liquid nitrogen. Ten-µm-thick cryocut sections obtained by microtome use (Reichert-Jung, Vienna, Austria) were first mounted on the surface of poly-L-lysine-treated supporting glass slides (Sigma-Aldrich, St. Louis, MO, USA), and then fixed using 2% (w/v) paraformaldehyde in phosphate-buffered saline (PBS; pH 7.2) for 10 min. Non-specific binding of the applied secondary antibody was precluded by a pre-incubation step of sections with normal swine serum (DakoCytomation, Glostrup, Denmark) diluted with PBS (1:100) for 30 min.
Primary and secondary antibodies as well as the biotinylated galectins used in this study are described in Table 1. The commercially available antibodies were diluted as recommended by supplier and antibodies against galectins as well as the biotinylated galectins were used at the concentration of 20 µg/ml in reaction medium. DNA in cell nuclei was stained by 4,6-diamino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO, USA). Controls for specificity of the immunohistochemical reaction included: 1) replacement of the specific by an irrelevant antibody (in the case of monoclonals of the same isotype), and 2) omission of the incubation step with the primary antibody to exclude antigen-independent signal generation. Involvement of the carbohydrate recognition domain in the lectin histochemical reaction was ascertained by pre-incubation of biotin-labeled lectins with 5 mM lactose (Sigma-Aldrich, St. Louis, MO, USA) as previously described [31]. Specimens were mounted by Vectashield (Vector Laboratories, Burlingame, CA, USA). The analyses of the specimens and data acquisition/storage were performed using a Nikon Eclipse-90i fluorescence microscope equipped by specific filter-blocks for DAPI, FITC, and TRITC (Nikon, Prague, Czech Republic) as well as by a Cool-1300Q CCD camera (Vosskühler, Osnabrück, Germany) and a computer-assisted image analysis system LUCIA 5.1 (Laboratory Imaging, Prague, Czech Republic).
Table 1.
Reagents for immunohistochemistry and lectin histochemistry
| primary antibody | abbrevation | host | produced by | secondary antibody | produced by | channel |
|---|---|---|---|---|---|---|
| vimentin | VIM | mouse monoclonal | DakoCytomation, Glostrup, Denmark | goat anti-mouse | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red |
| keratin-10 | K10 | mouse monoclonal | DakoCytomation, Glostrup, Denmark | goat anti-mouse | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red |
| keratin-14 | K14 | mouse monoclonal | Sigma-Aldrich, St. Louis, MO, USA | goat anti-mouse | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red |
| fibronectin | FIBR | rabbit polyclonal | Dakopatts, Glostrup, Denmark | swine anti-rabbit | (Santa Cruz Biotechnology, Santa Cruz, CA, USA) | FITC-green |
| wide spectrum cytokeratin | WSK | rabbit polyclonal | Abcam, Cambridge Science, Cambridge, UK | swine anti-rabbit | (Santa Cruz Biotechnology, Santa Cruz, CA, USA) | FITC-green |
| Galectin-1 | Gal-1 | rabbit polyclonal | house-made, Gabius laboratory | swine anti-rabbit | (Santa Cruz Biotechnology, Santa Cruz, CA, USA) | FITC-green |
| Galectin-2 | Gal-2 | rabbit polyclonal | house-made, Gabius laboratory | swine anti-rabbit | (Santa Cruz Biotechnology, Santa Cruz, CA, USA) | FITC-green |
| Galectin-3 | Gal-3 | rabbit polyclonal | house-made, Gabius laboratory | swine anti-rabbit | (Santa Cruz Biotechnology, Santa Cruz, CA, USA) | FITC-green |
| biotinylated lectin | abbrevation | produced by | second-step reagent | produced by | channel | |
|---|---|---|---|---|---|---|
| Galectin-1-binding site | Gal-1-BS | house-made, Gabius laboratory | ExtrAvidin | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red | |
| Galectin-2-binding site | Gal-2-BS | house-made, Gabius laboratory | ExtrAvidin | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red | |
| Galectin-3-binding site | Gal-3-BS | house-made, Gabius laboratory | ExtrAvidin | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red | |
| Galectin-7-binding site | Gal-7-BS | house-made, Gabius laboratory | ExtrAvidin | Sigma-Aldrich, St. Louis, MO, USA | TRITC-red |
Histological assessment
The status of re-epithelialization, the presence of polymorphonuclear leukocytes (PMNL), fibroblasts, newly formed vessels, and collagen were assessed according to the semi-quantitative scale system: –, +, ++, +++, and ++++ [18]. The extent of the immuno- and galectin histochemical reaction in injured epidermis and dermis was assessed by ranking the signal intensity according to the scale: –, +, ++, +++ [5]. Data are presented as median.
III. Results
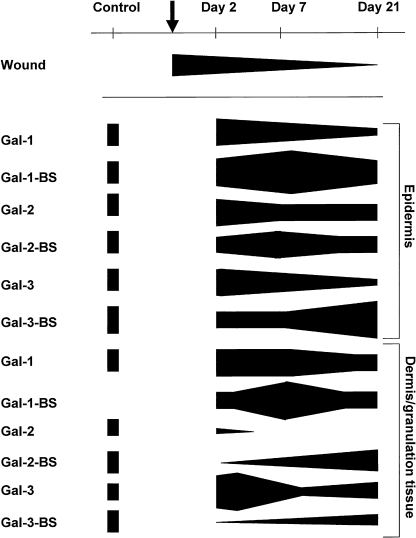
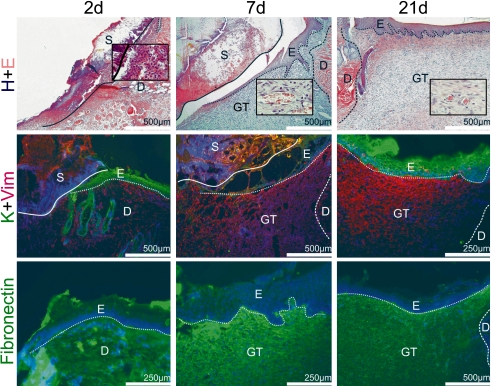
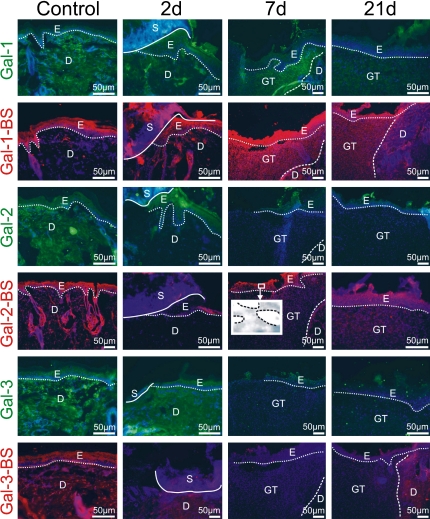
During the post-surgical period all animals remained healthy without clinical symptoms of infection. The data of the semi-quantitative analysis of the histological sections are summarized in Table 2 and Figure 3. The microphotographs presented are organized into two plates presenting hematoxilin-eosin stained sections (Fig. 1, top panel), and immunohistochemical localization profiles of marker proteins (Fig. 1 for fibronectin, vimentin and cytokeratin at all time points) as well as the galectin-related data (Fig. 2). In order to visually summarize the way galectin-related parameters are affected in the course of wound healing we present our data graphically in Figure 3, separating epidermis from dermis/granulation tissue. A graphical survey of these results for galectin presence and reactivity is given in Figure 3. A detailed account of the results obtained at each time point is reported below.
Table 2.
Results of the semi-quantitative assessment of selected cellular processes/structures
| re-epitheliliazation (WSK+) | PMNL | fibroblasts (Vim+) | new vessels | fibronectin | |
|---|---|---|---|---|---|
| 02d | + | +++ | – | – | + |
| 07d | + | ++ | ++++ | ++ | ++++ |
| 21d | ++++ | – | ++ | ++ | ++ |
Fig. 3.
Computation of the staining data on semi-quantitative scale for the tested galectins and respective binding sites in epidermis and in the dermis/granulation tissue at the three given time points during the healing process (top row) with respect to wound closure. Arrow indicates the moment of wounding.
Fig. 1.
H+E: Hematoxylin and eosin staining of skin wounds at three different stages of the healing process, starting at day 2: (2d): presence of tissue necrosis (S), formation of the demarcation line beneath the scab consisting mainly of polymorphonuclear leucocytes (see insert); seven-days healing wound (7d): migration of epidermal (E) cells over the wound, forming of the granulation tissue (GT) rich on fibroblasts and high-caliber vessels (see insert); 21-days healing wound (21d): completed epidermis regeneration, well-formed granulation tissue with decreased number of vessels and fibroblast (see insert) establishing into the scar. K+Vim: wide-spectrum cytokeratin+vimentin double-staining immunohistochemistry of healing skin wounds at the same time points: 2d: migration of epidermal cells beneath the scab; 7d: formation of the granulation tissue rich on vimentin-positive cells; 21d: completed epidermis regeneration, well-formed granulation tissue with decreased number of vimentin-positive cells. Fibronectin: simple staining immunohistochemical localization in the course of healing of skin wounds: 2d: wounds with low-level expression of fibronectin near the wound edge; 7d: granulation tissue rich on fibronectin; 21d: low-level presence of fibronectin in the developing scar. For orientation solid/dotted/broken lines are given separating distinct regions referred by the following abbreviations: E, epidermis; D, dermis; GT, granulation tissue; S, scab; in detail, a dotted line sets epidermis apart from dermis and/or granulation tissue; the broken line distinguishes dermis from granulation tissue and the solid line scab/necrosis from vital tissue.
Fig. 2.
Illustration of immunohistochemical galectin detection and localization of accessible binding sites (BS) for labeled galectins in the epidermis and in the dermis/granulation tissue during healing. Comparison between control data (first vertical panel) and specimens at each studied time point during healing (2d: second vertical panel; 7d: third vertical panel; 21d: fourth vertical panel) is thus made possible for each marker along each horizontal panel. In detail, the following assignments of type of probe and time point are given. First panel (Gal-1): strong signal intensity for galectin-1 two days after injury in both epidermis and dermis near the wound edge, decreasing over time to minimal presence in the dermis at day 21; second panel (Gal-1-BS): low level of galectin-1 reactivity in uninjured skin and wounds two and 21 days post wounding, increased reactivity to Gal-1-BS in the granulation tissue; third panel (Gal-2): galectin-2 detection in the epidermis, absence in granulation tissue; fourth panel (Gal-2-BS): galectin-2 reactivity in uninjured skin and wounds localized to epidermis, low-level presence of binding sites in the dermis, insert—galectin-2 nuclear reactivity in the epidermis (dashed line marks the nuclei of keratinocytes); fifth panel (Gal-3): presence in the suprabasal epidermal layer and dermis, low-level signal intensity in the granulation tissue; sixth panel Gal-3-BS: present in the suprabasal epidermal layer and in the surrounding dermis, low abundance presence in the scar forming. For orientation solid/dotted/broken lines are given separating distinct regions referred by the following abbreviations: E, epidermis; D, dermis; GT, granulation tissue; S, scab; in detail, a dotted line sets epidermis apart from dermis and/or granulation tissue; the broken line distinguishes dermis from granulation tissue and the solid line scab/necrosis from vital tissue.
Two days post-surgery
The analysis started with marker protein monitoring. Near the site of injury a slight increase for fibronectin deposition was seen (Fig. 1–2d Fibronectin). The epidermis was thickened at its cut edges (not shown). A demarcation line, rich in PMNL, was formed and separated the necrosis from vital tissue (Fig. 1–2d H+E insert). The number of fibroblasts was slightly increased in the dermis near the wounded area, as evidenced by vimentin-dependent staining (Fig. 1–2d K+Vim). Presence of both vimentin and cytokeratin was rarely observed in cells separated from the epithelial leading edge. Both keratin-10 and -14-positive cell populations were present in the epidermis and epidermal leading edge (data not shown).
The galectin-related data (presence of galectins-1, -2, and -3; reactivity to galectins-1, -2 and -3) are presented in pairs for each protein in horizontal panels in Figure 2. Each panel starts with the control to set the reference (first vertical panel in Fig. 2). Moving to the second vertical panel of Figure 2, the status after two days is exemplarily illustrated. Near the injury site a moderate level of galectin-1 expression and reactivity in both epidermis and dermis was observed. Galectin-2 was seen in all layers of the epidermis but not in the dermis. Reactivity to galectin-2 correlated with its expression; thus, it was confined to the epidermis. Galectin-3 positivity resembled the profile of galectin-1 in the dermis, but it was restricted to the suprabasal layer of epidermis. Rather weak signals were recorded for binding of labeled galectin-3 in the epidermis near the injury site.
Seven days post-surgery
By seven days after surgery, the skin edges separated by the open wound in vivo were not yet completely bridged by a new layer of epithelium (Fig. 1–7d H+E). The wounds were positive for keratins-10 and -14 and were only lightly infiltrated with PMNL (data not shown). The newly formed granulation tissue was rich on fibronectin (Fig. 1–7d Fibronectin), fibroblasts (Fig. 1–7d K+Vim), and high-caliber vessels (Fig. 1–7d H+E insert).
The galectin-related parameters presented similarities and conspicuous changes that are clearly illustrated in the third vertical panel of Figure 2. Increased expression of galectin-1 was maintained in the epidermis and granulation tissue, and the reactivity for this galectin was particularly strong during this time period of healing. Of note in view of the close homology between the two prototype galectins, the galectin-2 parameters were relatively unchanged, with evidence for nuclear reactivity in the epidermis (Fig. 2–insert, nuclei surrounded with dashed lines). There was no signal for galectin-3 presence and reactivity in the granulation tissue, but the expression of galectin-3 remained present suprabasally in the epidermis, excluding a false-negative result.
Twenty-one days post-surgery
At this stage, the presence of keratin layer in wounds demonstrated a normal course of keratinocyte differentiation and completed process of epidermis regeneration (Fig. 1–21d H+E). The number of luminized vessels in the granulation tissue decreased (Fig. 1–21d H+E insert). Equally typically, the level of presence of fibronectin in the granulation tissue had leveled off (Fig. 1–21d Fibronectin), while the content of collagen had increased (data not shown).
The galectin-related parameters are documented in the fourth vertical panel of Figure 2. At this stage, galectin-1 presence decreased to a minimum, signal intensity for binding sites of this lectin was also slightly reduced in both epidermis and granulation tissue and moderately in the surrounding dermis. The galectin-2-related parameters remained at relatively low levels, notably without nuclear reactivity for galectin-2 in the epidermis over the developing scar. Galectin-3 parameters also appeared to re-normalize, with slight increase observed in expression and reactivity. As a further specificity control for galectin binding we added analysis with biotinylated prototype galectin-7. As a result, no staining was detectable with this homodimeric protein, an indication for the specificity of the interaction among prototype galectins.
IV. Discussion
The proteins studied are versatile effectors in cell adhesion, growth regulation and other cellular processes, by virtue of binding distinct epitopes [17, 45, 48]. Of note, even the closely related prototype galectins-1 and -2 are known to have their characteristic activity profile, and functional competition between galectins-1 and -3 has also been documented [25, 35, 43]. By using non-cross-reactive antibodies and biotinylated galectins as probes we compared the expression and reactivity profiles for two prototype (Gal-1, -2) and the chimera-type (Gal-3) galectins. Our study resolved distinct aspects of the issue on galectin presence in wound healing: localization profiles and signal intensity were clearly different. Despite pronounced sequence homology each family member tested had its characteristic pattern during the course of wound healing.
Indications for a co-regulation of lectin expression/reactivity were discerned for galectin-1, first increasing, then leveling-off during scar formation. Of note, when compared to porcine skin such changes were less marked in rats [24], revealing interspecies differences. In functional terms, galectin-1 is known as a potent inductor of ECM formation and TGF-β-independent conversion of fibroblasts into myofibroblasts [12]. In addition, wound treatment with recombinant human galectin-1 resulted in significantly increased wound contraction in rats [12]. In contrast, galectins-3 and -7, but not galectin-1, have been shown to play a role in reepithelialization of murine corneal wounds [7]. From this point of view, the galectin-dependent regulation of wound healing might be different for epidermis and dermis.
Monitored in parallel in this model, the close relative of galectin-1, i.e. galectin-2, appeared to follow its own independent course. The proliferation phase was associated with nuclear reactivity to this galectin in the epidermis, adding to our previous observations of the nuclear galectin-2 presence following physical, chemical, and/or biological treatment modalities [11, 34]. The question whether galectin-2 joins the category of nuclear lectins has herewith been answered on the in vivo level as well.
In contrast to galectin-1, the expression pattern of the chimera-type galectin-3 was clearly different, with only a slight increase observed during the maturation phase. In semiquantitative terms, extent of galectin-3 signal intensity was lower than for galectin-1, and the presence in the dermis was confined to galectin-1, comparable to the situation in porcine skin [24]. The reported obvious differences in galectin regulation give this research a clear future direction. Because the rat epidermis is also known to express the tandem-repeat-type galectin-9 [27], galectin fingerprinting in skin wound healing, initiated here, can thus be extended to the members of this galectin group.
In summary, our study initiated characterization of galectin presence and reactivity in the course of healing of skin wounds in the rat model. Galectins-1 and -3 are differentially regulated during skin wound healing. Whereas galectin-1 seems to play a role in the early phases of healing and wound contraction, observations on the role of galectin-3 in hepatic [21] and renal [22] fibrosis combined with the presented evidence intimate that this lectin might be able to modulate scaring. An extrapolation from this experimental to the clinical situation is, however, not possible due to interspecies variability, but the general molecular regulation of wound healing should be similar. Respective investigations are thus encouraged by this study in the rat model.
V. Acknowledgments
We wish to thank Professor Richard L. Magin, Ph.D. (University of Illinois at Chicago) for critically reading this manuscript and for his valuable comments. Also, we are grateful to Iva Burdová, Vít Hajdúch, and Magdaléna Majnušová for expert technical assistance.
This study was supported in part by the Ministry of Education, Youth and Sport of the Czech Republic (No. M10538, No. MSM0021620806), by the Grant Agency of Ministry of Education, Science, Research, and Sport of the Slovak Republic (VEGA No. 1/1095/11), and the EC Glyco-HIT consortium (contract no. 260600).
VI. References
- 1.André S., Sanchez-Ruderisch H., Nakagawa H., Buchholz M., Kopitz J., Forberich P., Kemmner W., Böck C., Deguchi K., Detjen K. M., Wiedenmann B., von Knebel Doeberitz M., Gress T. M., Nishimura S-I., Rosewicz S., Gabius H.-J. Tumor suppressor p16INK4a: modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007;274:3233–3256. doi: 10.1111/j.1742-4658.2007.05851.x. [DOI] [PubMed] [Google Scholar]
- 2.Barbul A., Regan M. C. In “Surgical Basic Science”, ed. by J. A. Fischer. Mosby-Yearbook; St. Louis: 1993. Biology of wound healing; pp. 68–88. [Google Scholar]
- 3.Barrientos S., Stojadinovic O., Golinko M. S., Brem H., Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 4.Cada Z., Chovanec M., Smetana K., Betka J., Lacina L., Plzák J., Kodet R., Stork J., Lensch M., Kaltner H., André S., Gabius H.-J. Galectin-7: will the lectin’s activity establish clinical correlations in head and neck squamous cell and basal cell carcinomas? Histol. Histopathol. 2009;24:41–48. doi: 10.14670/HH-24.41. [DOI] [PubMed] [Google Scholar]
- 5.Cada Z., Smetana K., Jr., Lacina L., Plzáková Z., Stork J., Kaltner H., Russwurm R., Lensch M., André S., Gabius H.-J. Immunohistochemical fingerprinting of the network of seven adhesion/growth-regulatory lectins in human skin and detection of distinct tumour-associated alterations. Folia Biol. (Praha). 2009;55:145–152. [PubMed] [Google Scholar]
- 6.Cao Z., Wu H. K., Bruce A., Wollenberg K., Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest. Ophthalmol. Vis. Sci. 2002;43:2897–2904. [PubMed] [Google Scholar]
- 7.Cao Z., Said N., Amin S., Wu H. K., Bruce A., Garate M., Hsu D. K., Kuwabara I., Liu F. T., Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J. Biol. Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- 8.Carter C. A., Jolly D. G., Worden C. E. Sr., Hendren D. G., Kane C. J. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp. Mol. Pathol. 2003;74:244–255. doi: 10.1016/s0014-4800(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 9.Choufani G., Nagy N., Saussez S., Marchant H., Bisschop P., Burchert M., Danguy A., Louryan S., Salmon I., Gabius H. J., Kiss R., Hassid S. The levels of expression of galectin-1, galectin-3, and the Thomsen-Friedenreich antigen and their binding sites decrease as clinical aggressiveness increases in head and neck cancers. Cancer. 1999;86:2353–2363. doi: 10.1002/(sici)1097-0142(19991201)86:11<2353::aid-cncr25>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Clark R. A. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J. Invest. Dermatol. 1996;94 (Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- 11.Dvoránková B., Lacina L., Smetana K., Jr., Lensch M., Manning J. C., André S., Gabius H.-J. Human galectin-2: nuclear presence in vitro and its modulation by quiescence/stress factors. Histol. Histopathol. 2008;23:167–178. doi: 10.14670/HH-23.167. [DOI] [PubMed] [Google Scholar]
- 12.Dvoránková B., Szabo P., Lacina L., Gál P., Uhrova J., Zima T., Kaltner H., André S., Gabius H.-J., Syková E., Smetana K., Jr. Human galectins induce conversion of dermal fibroblasts into myofibroblasts and production of extracellular matrix: potential application in tissue engineering and wound repair. Cells Tissues Organs. 2011 doi: 10.1159/000324864. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Eslami A., Gallant-Behm C. L., Hart D. A., Wiebe C., Honardoust D., Gardner H., Häkkinen L., Larjava H. S. Expression of integrin αvβ6 and TGF-β in scarless vs scar-forming wound healing. J. Histochem. Cytochem. 2009;57:543–557. doi: 10.1369/jhc.2009.952572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer C., Sanchez-Ruderisch H., Welzel M., Wiedenmann B., Sakai T., André S., Gabius H.-J., Khachigian L., Detjen K. M., Rosewicz S. Galectin-1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 2005;280:37266–37277. doi: 10.1074/jbc.M411580200. [DOI] [PubMed] [Google Scholar]
- 15.Freedberg I. M., Tomic-Canic M., Komine M., Blumenberg M. Keratins and the keratinocyte activation cycle. J. Invest. Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- 16.Gabius H.-J., ed. Wiley-VCH; Weinheim: 2009. The Sugar Code. Fundamentals of Glycosciences. [Google Scholar]
- 17.Gabius H.-J., André S., Jiménez-Barbero J., Romero A., Solís D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem. Sci. 2011;36:298–313. doi: 10.1016/j.tibs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Gál P., Kilík R., Mokrý M., Vidinský B., Vasilenko T., Mozeš S., Bobrov N., Tomori Z., Bober J., Lenhardt L. Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: standardization of semi-quantitative and quantitative histological assessments. Vet. Med. 2008;53:652–659. [Google Scholar]
- 19.Gál P., Toporcer T., Grendel T., Vidová Z., Smetana K., Jr., Dvoránková B., Gál T., Mozeš S., Lenhardt L., Longauer F., Sabol M., Sabo J., Backor M. Effect of Atropa belladonna L. on skin wound healing: biomechanical and histological study in rats and in vitro study in keratinocytes, 3T3 fibroblasts, and human umbilical vein endothelial cells. Wound Repair Regen. 2009;17:378–386. doi: 10.1111/j.1524-475X.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 20.Gendronneau G., Sidhu S. S., Delacour D., Dang T., Calonne C., Houzelstein D., Magnaldo T., Poirier F. Galectin-7 in the control of epidermal homeostasis after injury. Mol. Biol. Cell. 2008;19:5541–5549. doi: 10.1091/mbc.E08-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson N. C., Mackinnon A. C., Farnworth S. L., Poirier F., Russo F. P., Iredale J. P., Haslett C., Simpson K. J., Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson N. C., Mackinnon A. C., Farnworth S. L., Kipari T., Haslett C., Iredale J. P., Liu F. T., Hughes J., Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hittelet A., Legendre H., Nagy N., Bronckart Y., Pector J. C., Salmon I., Yeaton P., Gabius H.-J., Kiss R., Camby I. Upregulation of galectins-1 and -3 in human colon cancer and their role in regulating cell migration. Int. J. Cancer. 2003;103:370–379. doi: 10.1002/ijc.10843. [DOI] [PubMed] [Google Scholar]
- 24.Klíma J., Lacina L., Dvoránková B., Herrmann D., Carnwath J. W., Niemann H., Kaltner H., André S., Motlík J., Gabius H.-J., Smetana K., Jr. Differential regulation of galectin expression/reactivity during wound healing in porcine skin and in cultures of epidermal cells with functional impact on migration. Physiol. Res. 2009;58:873–884. doi: 10.33549/physiolres.931624. [DOI] [PubMed] [Google Scholar]
- 25.Kopitz J., von Reitzenstein C., André S., Kaltner H., Uhl J., Ehemann V., Cantz M., Gabius H-J. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J. Biol. Chem. 2001;276:35917–35923. doi: 10.1074/jbc.M105135200. [DOI] [PubMed] [Google Scholar]
- 26.Kopitz J., Bergmann M., Gabius H.-J. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life. 2010;62:624–628. doi: 10.1002/iub.358. [DOI] [PubMed] [Google Scholar]
- 27.Lensch M., Lohr M., Russwurm R., Vidal M., Kaltner H., André S., Gabius H.-J. Unique sequence and expression profiles of rat galectins-5 and -9 as a result of species-specific gene divergence. Int. J. Biochem. Cell Biol. 2006;38:1741–1758. doi: 10.1016/j.biocel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Miranda F. A., Hassumi M. K., Guimarães M. C., Simões R. T., Silva T. G., Lira R. C., Rocha A. M., Mendes C. T., Jr., Donadi E. A., Soares C. P., Soares E. G. Galectin-3 overexpression in invasive laryngeal carcinoma, assessed by computer-assisted analysis. J. Histochem. Cytochem. 2009;57:665–673. doi: 10.1369/jhc.2009.952960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morasso M. I., Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol. Cell. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peryassu M. A., Cotta-Pereira G., Ramos-e-Silva M., Filgueira A. L. Expression of keratins 14, 10 and 16 in marginal keratoderma of the palms. Acta Dermatovenerol. Croat. 2005;13:206–211. [PubMed] [Google Scholar]
- 31.Plzák J., Betka J., Smetana K., Jr., Chovanec M., Kaltner H., André S., Kodet R., Gabius H.-J. Galectin-3: an emerging prognostic indicator in advanced head and neck carcinoma. Eur. J. Cancer. 2004;40:2324–2330. doi: 10.1016/j.ejca.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Rappl G., Abken H., Muche J. M., Sterry W., Tilgen W., André S., Kaltner H., Ugurel S., Gabius H.-J., Reinhold U. CD4+CD7– leukemic T cells from patients with Sézary syndrome are protected from galectin-1-triggered T cell death. Leukemia. 2002;16:840–845. doi: 10.1038/sj.leu.2402438. [DOI] [PubMed] [Google Scholar]
- 33.Reichelt J., Bussow H., Grund C., Magin T. M. Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol. Biol. Cell. 2001;12:1557–1568. doi: 10.1091/mbc.12.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saal I., Nagy N., Lensch M., Lohr M., Manning J. C., Decaestecker C., André S., Kiss R., Salmon I., Gabius H.-J. Human galectin-2: expression profiling by RT-PCR/immunohistochemistry and its introduction as histochemical tool for ligand localization. Histol. Histopathol. 2005;20:1191–1208. doi: 10.14670/HH-20.1191. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Ruderisch H., Fischer C., Detjen K. M., Welzel M., Wimmel A., Manning J. C., André S., Gabius H.-J. Tumor suppressor p16INK4a: downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010;277:3552–3563. doi: 10.1111/j.1742-4658.2010.07764.x. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Ruderisch H., Detjen K. M., Welzel M., André S., Fischer C., Gabius H.-J., Rosewicz S. Galectin-1 sensitizes carcinoma cells to anoikis via the fibronectin receptor α5β1-integrin. Cell Death Differ. 2011;18:806–816. doi: 10.1038/cdd.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saravanan C., Cao Z., Head S. R., Panjwani N. Analysis of differential expression of glycosyltransferases in healing corneas by glycogene microarrays. Glycobiology. 2010;20:13–23. doi: 10.1093/glycob/cwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saussez S., Decaestecker C., Lorfevre F., Chevalier D., Mortuaire G., Kaltner H., André S., Toubeau G., Gabius H.-J., Leroy X. Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology. 2008;52:483–493. doi: 10.1111/j.1365-2559.2008.02973.x. [DOI] [PubMed] [Google Scholar]
- 39.Saussez S., Decaestecker C., Mahillon V., Cludts S., Capouillez A., Chevalier D., Kaltner H., André S., Toubeau G., Leroy X., Gabius H.-J. Galectin-3 upregulation during tumor progression in head and neck cancer. Laryngoscope. 2008;118:1583–1590. doi: 10.1097/MLG.0b013e31817b0718. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz-Albiez R. In “The Sugar Code. Fundamentals of Glycosciences”, ed. by H.-J. Gabius. Wiley-VCH; Weinheim: 2009. Inflammation and glycosciences; pp. 447–467. [Google Scholar]
- 41.Sen C. K., Gordillo G. M., Roy S., Kirsner R., Lambert L., Hunt T. K., Gottrup F., Gurtner G. C., Longaker M. T. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smetana K., Jr., Dvoránková B., Chovanec M., Boucek J., Klíma J., Motlík J., Lensch M., Kaltner H., André S., Gabius H.-J. Nuclear presence of adhesion/growth-regulatory galectins in normal/malignant cells of squamous epithelial origin. Histochem. Cell Biol. 2006;125:171–182. doi: 10.1007/s00418-005-0074-0. [DOI] [PubMed] [Google Scholar]
- 43.Sturm A., Lensch M., André S., Kaltner H., Wiedenmann B., Rosewicz S., Dignass A. U., Gabius H.-J. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004;173:3825–3837. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- 44.Usui M. L., Mansbridge J. N., Carter W. G., Fujita M., Olerud J. E. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J. Histochem. Cytochem. 2008;56:687–696. doi: 10.1369/jhc.2008.951194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villalobo A., Nogales-Gonzalés A., Gabius H.-J. A guide to signaling pathways connecting protein-glycan interaction with the emerging versatile effector functionality of mammalian lectins. Trends Glycosci. Glycotechnol. 2006;18:1–37. [Google Scholar]
- 46.Wang J., Lu Z.-H., Gabius H.-J., Rohowsky-Kochan C., Ledeen R. W., Wu G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J. Immunol. 2009;182:4036–4045. doi: 10.4049/jimmunol.0802981. [DOI] [PubMed] [Google Scholar]
- 47.Woodley D. T., Chen J. D., Kim J. P., Sarret Y., Iwasaki T., Kim Y. H., O’Keefe E. J. Re-epithelialization. Human keratinocyte locomotion. Dermatol. Clin. 1993;11:641–646. [PubMed] [Google Scholar]
- 48.Wu A. M., Singh T., Liu J. H., André S., Lensch M., Siebert H.-C., Krzeminski M., Bonvin A. M. J. J., Kaltner H., Wu J. H., Gabius H.-J. Adhesion/growth-regulatory galectins: insights into their ligand selectivity using natural glycoproteins and glycotopes. Adv. Exp. Med. Biol. 2011;705:117–141. doi: 10.1007/978-1-4419-7877-6_7. [DOI] [PubMed] [Google Scholar]
- 49.Zaja-Milatovic S., Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol. Histopathol. 2008;23:1399–1407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]